Cadherin Signaling in Cancer and Autoimmune Diseases
Abstract
1. Introduction
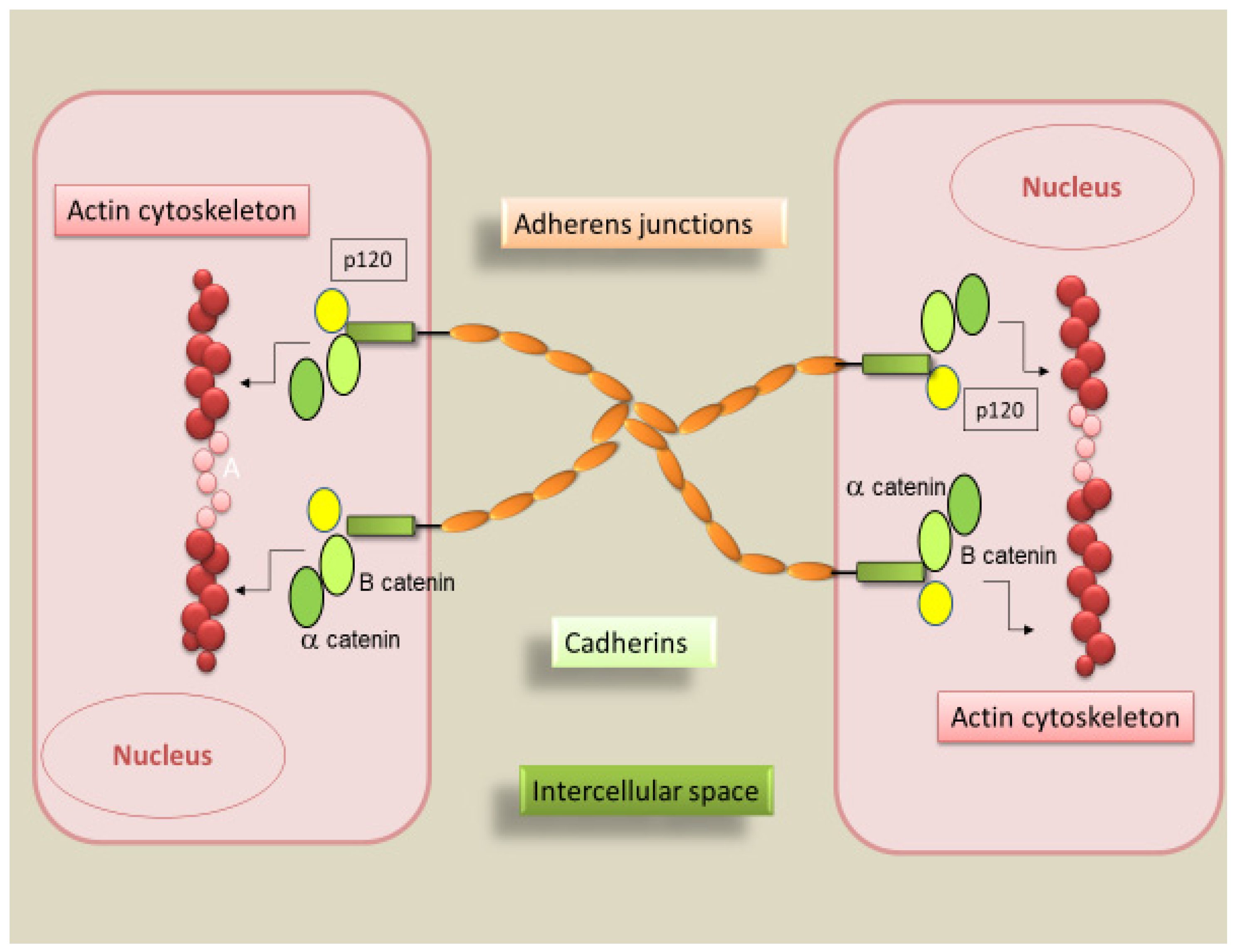
2. Cadherins
2.1. Types of Cadherins
2.2. Cadherin Trafficking Pathways
2.3. Physiological Role of Epithelial and Vascular-Endothelial Cadherins
3. Changes in Cadherins’ Expression during the Epithelial- and Endothelial- to Mesenchymal Transition
4. Cadherin-Mediated Signaling in the Context of Disease
4.1. Cadherin and Tumorigenesis
4.1.1. Interplay between Cadherin, Tumorigenesis and the EMT
4.1.2. E-cadherin and N-cadherin Deregulation and Cancer Development
4.1.3. Other Cadherins and Tumor Progression
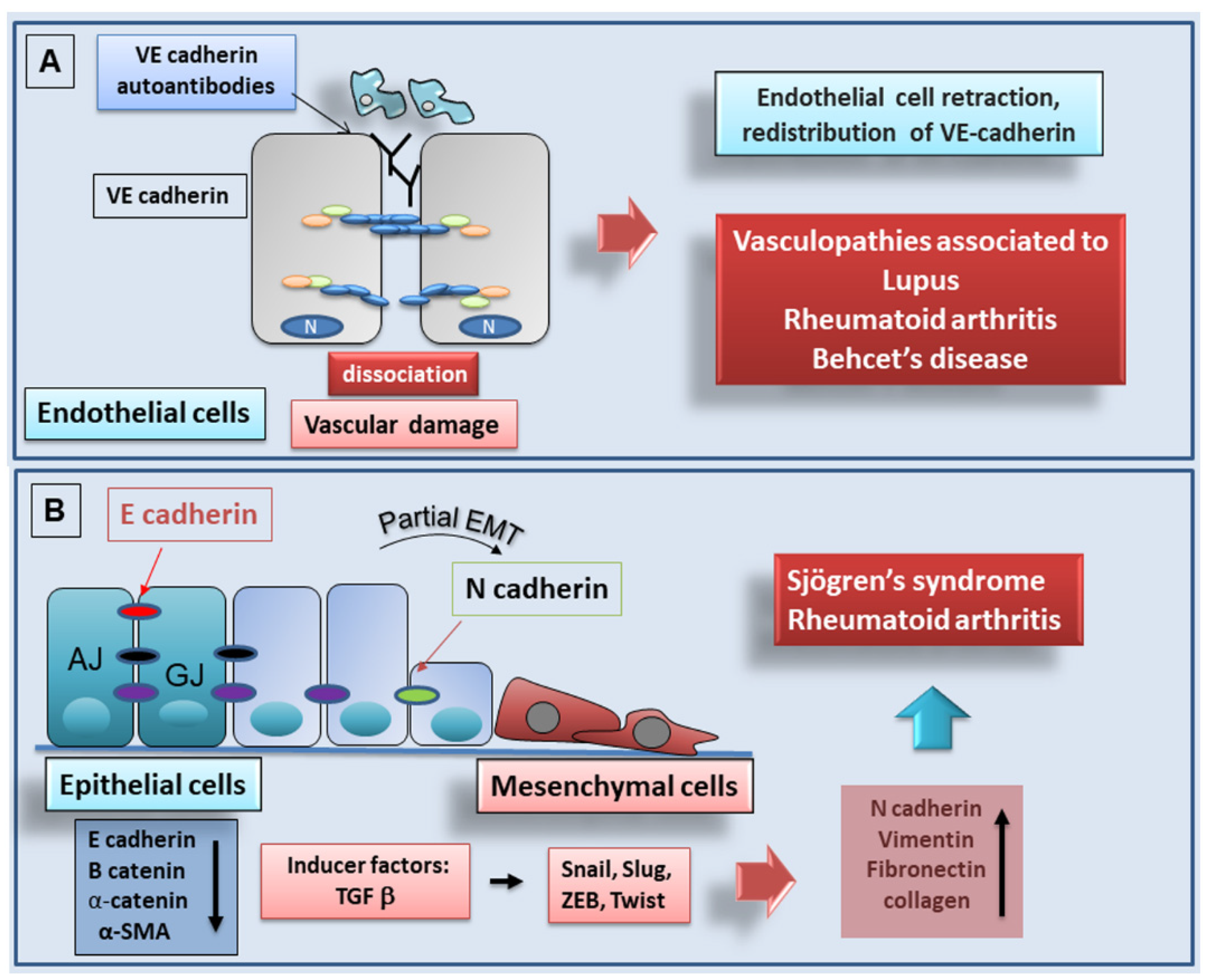
4.2. Autoantibodies against Cadherins as Markers for Autoimmune Diseases
4.2.1. Rheumatoid Arthritis
4.2.2. Systemic Lupus Erythematosus
Role of Ve-cadherin as Marker for SLE
Role of E-cadherin as Marker for SLE
4.2.3. Behçet’s Disease
4.2.4. Pemphigus
4.3. Cadherin-Dependent EMT in Autoimmune Diseases: Recent Advances
4.3.1. Rheumatoid Arthritis
4.3.2. Recent Advances in Understanding of the Role of Cadherins in Sjögren’s Syndrome
Soluble E-cadherin in Sjögren’s Syndrome: Murine Models
E-cadherin in EMT-Dependent Fibrosis in Sjögren’s Syndrome
5. Concluding Comments
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dieterle, M.P.; Husari, A.; Rolauffs, B.; Steinberg, T.; Tomakidi, P. Integrins, cadherins and channels in cartilage mechano transduction: Perspectives for future regeneration strategies. Expert Rev. Mol. Med. 2021, 23, e14. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.; Perl, A.L.; Svoboda, S.A.; Green, K.J. Desmosomal Cadherins in Health and Disease. Annu. Rev. Pathol. 2021, 17. [Google Scholar] [CrossRef]
- Bandyopadhyay, C.; Schecterson, L.; Gumbiner, B.M. E-cadherin activating antibodies limit barrier dysfunction and inflammation in mouse inflammatory bowel disease. Tissue Barriers 2021, 9, 1940741. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, M.; Yap, A.S. Classical cadherin adhesion molecules: Coordinating cell adhesion, signaling and the cytoskeleton. J. Mol. Histol. 2004, 35, 839–844. [Google Scholar] [CrossRef]
- Kottke, M.D.; Delva, E.; Kowalczyk, A.P. The desmosome: Cell science lessons from human diseases. J. Cell Sci. 2006, 119, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, M. Dynamic contacts: Rearranging adherens junctions to drive epithelial remodelling. Nat. Rev. Mol. Cell Biol. 2014, 15, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Peifer, M.; Yap, A.S. Traffic control: p120-catenin acts as a gatekeeper to control the fate of classical cadherins in mammalian cells. J. Cell Biol. 2003, 163, 437–440. [Google Scholar] [CrossRef]
- Yamada, S.; Nelson, W.J. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J. Cell Biol. 2007, 78, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Cadwell, C.M.; Su, W.; Kowalczyk, A.P. Cadherin tales: Regulation of cadherin function by endocytic membrane trafficking. Traffic 2016, 17, 1262–1271. [Google Scholar] [CrossRef]
- Kowalczyk, A.P.; Nanes, B.A. Adherens junction turnover: Regulating adhesion through cadherin endocytosis, degradation, and recycling. Adherens Junctions Mol. Mech. Tissue Dev. Dis. 2012, 60, 197–222. [Google Scholar]
- Traub, L.M.; Bonifacino, J.S. Cargo recognition in clathrin-mediated endocytosis. Cold Spring Harb. Perspect. Biol. 2013, 11, a016790. [Google Scholar] [CrossRef] [PubMed]
- Kirchhausen, T.; Owen, D.; Harrison, S.C. Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb. Perspect. Biol. 2014, 6, a016725. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef]
- Elkin, S.R.; Lakoduk, A.M.; Schmid, S.L. Endocytic pathways and endosomal trafficking: A primer. Wien. Med. Wochenschr. 2016, 166, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Hotchin, N.A. RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol. Biol. Cell 2001, 12, 847–862. [Google Scholar] [CrossRef]
- Lu, Z.; Ghosh, S.; Wang, Z.; Hunter, T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell 2003, 4, 499–515. [Google Scholar] [CrossRef]
- Bryant, D.M.; Kerr, M.C.; Hammond, L.A.; Joseph, S.R.; Mostov, K.E.; Teasdale, R.D.; Stow, J.L. EGF induces macropinocytosis and SNX1-modulated recycling of E-cadherin. J. Cell Sci. 2007, 120, 1818–1828. [Google Scholar] [CrossRef]
- Padmanabhan, R.; Taneyhill, L.A. Cadherin-6B undergoes macropinocytosis and clathrin-mediated endocytosis during cranial neural crest cell EMT. J. Cell Sci. 2015, 128, 1773–1786. [Google Scholar] [CrossRef]
- Paterson, A.D.; Parton, R.G.; Ferguson, C.; Stow, J.L.; Yap, A.S. Characterization of E-cadherin endocytosis in isolated MCF-7 and chinese hamster ovary cells: The initial fate of unbound E-cadherin. J. Biol. Chem. 2003, 278, 21050–21057. [Google Scholar] [CrossRef]
- Delva, E.; Jennings, J.M.; Calkins, C.C.; Kottke, M.D.; Faundez, V.; Kowalczyk, A.P. Pemphigus vulgaris IgG-induced desmoglein-3 endocytosis and desmosomal disassembly are mediated by a clathrin- and dynamin-independent mechanism. J. Biol. Chem. 2008, 283, 18303–18313. [Google Scholar] [CrossRef]
- Patel, S.D.; Chen, C.P.; Bahna, F.; Honig, B.; Shapiro, L. Cadherin-mediated cell-cell adhesion: Sticking together as a family. Curr. Opin. Struct. Biol. 2003, 1, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Getsios, S.; Huen, A.C.; Green, K.J. Working out the strength and flexibility of desmosomes. Nat. Rev. Mol. Cell Biol. 2004, 5, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Gumbiner, B.M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 2005, 6, 622–634. [Google Scholar] [CrossRef]
- Arslan, F.N.; Eckert, J.; Schmidt, T.; Heisenberg, C.P. Holding it together: When cadherin meets cadherin. Biophys J. 2021, 20, 4182–4192. [Google Scholar] [CrossRef]
- Raya-Sandino, A.; Luissint, A.C.; Kusters, D.H.M.; Narayanan, V.; Flemming, S.; Garcia-Hernandez, V.; Godsel, L.M.; Green, K.J.; Hagen, S.J.; Conway, D.E.; et al. Regulation of intestinal epithelial intercellular adhesion and barrier function by desmosomal cadherin desmocollin-2. Mol. Biol. Cell 2021, 32, 753–768. [Google Scholar] [CrossRef]
- Schafer, S.; Koch, P.J.; Franke, W.W. Identification of the ubiquitous human desmoglein, Dsg2, and the expression catalogue of the desmoglein subfamily of desmosomal cadherins. Exp. Cell Res. 1994, 211, 391–399. [Google Scholar] [CrossRef]
- Brennan, D.; Mahoney, M.G. Increased expression of Dsg2 in malignant skin carcinomas: A tissue-microarray based study. Cell Adhes. Migr. 2009, 3, 148–154. [Google Scholar] [CrossRef]
- Kljuic, A.; Bazzi, H.; Sundberg, J.P.; Martinez-Mir, A.; O’Shaughnessy, R.; Mahoney, M.G.; Levy, M.; Montagutelli, X.; Ahmad, W.; Aita, V.M.; et al. Desmoglein 4 in hair follicle differentiation and epidermal adhesion: Evidence from inherited hypotrichosis and acquired pemphigus vulgaris. Cell 2003, 113, 249–260. [Google Scholar] [CrossRef]
- Yamamoto, K.; Takagi, Y.; Ando, K.; Fukuhara, S. Rap1 Small GTPase Regulates Vascular Endothelial-Cadherin-Mediated Endothelial Cell-Cell Junctions and Vascular Permeability. Biol Pharm Bull. 2021, 44, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Rho, S.S.; Ando, K.; Fukuhara, S. Dynamic Regulation of Vascular Permeability by Vascular Endothelial Cadherin-Mediated Endothelial Cell-Cell Junctions. J. Nippon Med. Sch. 2017, 84, 148–159. [Google Scholar] [CrossRef]
- Schulte, D.; Küppers, V.; Dartsch, N.; Broermann, A.; Li, H.; Zarbock, A.; Kamenyeva, O.; Kiefer, F.; Khandoga, A.; Massberg, S.; et al. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J. 2011, 30, 4157–4170. [Google Scholar] [CrossRef]
- Vittet, D.; Buchou, T.; Schweitzer, A.; Dejana, E.; Huber, P. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc. Natl. Acad. Sci. USA 1997, 94, 6273–6278. [Google Scholar] [CrossRef]
- Vestweber, D. VE-cadherin: The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Rabiet, M.J.; Plantier, J.L.; Rival, Y.; Genoux, Y.; Lampugnani, M.G.; Dejana, E. Thrombin-induced increase in endothelial permeability is associated with changes in cell-to-cell junction organization. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 488–496. [Google Scholar] [CrossRef]
- Seynhaeve, A.L.; Rens, J.A.; Schipper, D.; Eggermont, A.M.; Ten Hagen, T.L. Exposing endothelial cells to tumor necrosis factor-α and peripheral blood mononuclear cells damage endothelial integrity via interleukin-1ß by degradation of vascular endothelial-cadherin. Surgery 2014, 155, 545–553. [Google Scholar] [CrossRef]
- Tinsley, J.H.; Ustinova, E.E.; Xu, W.; Yuan, S.Y. Src-dependent, neutrophil-mediated vascular hyperpermeability and beta-catenin modification. Am. J. Physiol. Cell Physiol. 2002, 283, C1745–C1751. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Turowski, P.; Martinelli, R.; Crawford, R.; Wateridge, D.; Papageorgiou, A.P.; Lampugnani, M.G.; Gamp, A.C.; Vestweber, D.; Adamson, P.; Dejana, E.; et al. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J. Cell Sci. 2008, 121, 29–37. [Google Scholar] [CrossRef]
- Chen, T.; You, Y.; Jiang, H.; Wang, Z.Z. Epithelial-mesenchymal transition (EMT): A biological process in the development, stem cell differentiation, and tumorigenesis. J. Cell Physiol. 2017, 232, 3261–3272. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Gheldof, A.; Berx, G. Cadherins and epithelial-to-mesenchymal transition. Prog. Mol. Biol. Transl. Sci. 2013, 116, 317–336. [Google Scholar]
- Wendt, M.K.; Allington, T.M.; Schiemann, W.P. Mechanisms of the epithelial-mesenchymal transition by TGF-beta. Future Oncol. 2009, 5, 1145–1168. [Google Scholar] [CrossRef] [PubMed]
- Aban, C.E.; Lombardi, A.; Neiman, G.; Biani, M.C.; La Greca, A.; Waisman, A.; Moro, L.N.; Sevlever, G.; Miriuka, S.; Luzzani, C. Downregulation of E-cadherin in pluripotent stem cells triggers partial EMT. Sci. Rep. 2021, 11, 2048. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Saha, P.; Samanta, A.; Bishayee, A. Emerging Concepts of Hybrid Epithelial-to-Mesenchymal Transition in Cancer Progression. Biomolecules 2020, 10, 1561. [Google Scholar] [CrossRef]
- Saxena, K.; Jolly, M.K.; Balamurugan, K. Hypoxia, Partial EMT and Collective Migration: Emerging Culprits in Metastasis. Transl. Oncol. 2020, 13, 100845. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Peinado, H.; Ballestar, E.; Esteller, M.; Cano, A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol. Cell Biol. 2004, 24, 306–319. [Google Scholar] [CrossRef]
- Drápela, S.; Bouchal, J.; Jolly, M.K.; Culig, Z.; Souček, K. ZEB1: A Critical Regulator of Cell Plasticity, DNA Damage Response, and Therapy Resistance. Front. Mol. Biosci. 2020, 7, 36. [Google Scholar] [CrossRef]
- Vannier, C.; Mock, K.; Brabletz, T.; Driever, W. Zeb1 regulates E-cadherin and Epcam (epithelial cell adhesion molecule) expression to control cell behavior in early zebrafish development. J. Biol. Chem. 2013, 288, 18643–18659. [Google Scholar] [CrossRef]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- McCormack, N.; O’Dea, S. Regulation of epithelial to mesenchymal transition by bone morphogenetic proteins. Cell. Signal. 2013, 25, 2856–2862. [Google Scholar] [CrossRef]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal. 2014, 7, re8. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Perez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Sancho, E.; Franci, C.; Domínguez, D.; Monfar, M.; Baulida, J.; García De Herreros, A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2000, 2, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Fan, Q.; Qiu, M.T.; Zhu, Z.; Zhou, J.H.; Chen, L.; Zhou, Y.; Gu, W.; Wang, L.H.; Li, Z.N.; Xu, Y.; et al. Twist induces epithelial-mesenchymal transition in cervical carcinogenesis by regulating the TGF-β/Smad3 signaling pathway. Oncol. Rep. 2015, 34, 1787–1794. [Google Scholar] [CrossRef]
- Platel, V.; Faure, S.; Corre, I.; Clere, N. Endothelial-to-Mesenchymal Transition (EndoMT): Roles in Tumorigenesis, Metastatic Extravasation and Therapy Resistance. J. Oncol. 2019, 2019, 8361945. [Google Scholar] [CrossRef]
- Dejana, E.; Hirschi, K.K.; Simons, M. The molecular basis of endothelial cell plasticity. Nat. Commun. 2017, 8, 14361. [Google Scholar] [CrossRef]
- Medici, D.; Shore, E.M.; Lounev, V.Y.; Kaplan, F.S.; Kalluri, R.; Olsen, B.R. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat. Med. 2010, 16, 1400. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Potenta, S.; Xie, L.; Zeisberg, M.; Kalluri, R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007, 67, 10123–10128. [Google Scholar] [CrossRef]
- Potenta, S.; Zeisberg, E.; Kalluri, R. The role of endothelial-to-mesenchymal transition in cancer progression. Br. J. Cancer 2008, 99, 1375–1379. [Google Scholar] [CrossRef]
- Heldin, C.H.; Vanlandewijck, M.; Moustakas, A. Regulation of EMT by TGFβ in cancer. FEBS Lett. 2012, 586, 1959–1970. [Google Scholar] [CrossRef]
- Labelle, M.; Schnittler, H.J.; Aust, D.E.; Friedrich, K.; Baretton, G.; Vestweber, D.; Breier, G. Vascular endothelial cadherin promotes breast cancer progression via transforming growth factor beta signaling. Cancer Res. 2008, 68, 1388–1397. [Google Scholar] [CrossRef]
- Ma, J.; Sanchez-Duffhues, G.; Goumans, M.J.; Ten Dijke, P. TGF-β-Induced Endothelial to Mesenchymal Transition in Disease and Tissue Engineering. Front. Cell Dev. Biol. 2020, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, Y.; Watabe, T. Roles of TGF-β signals in endothelial-mesenchymal transition during cardiac fibrosis. Int. J. Inflamm. 2011, 72, 4080. [Google Scholar]
- Espinoza, I.; Miele, L. Deadly crosstalk: Notch signaling at the intersection of EMT and cancer stem cells. Cancer Lett. 2013, 341, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chang, A.; Chang, L.; Niessen, K.; Eapen, S.; Setiadi, A.; Karsan, A. Differential regulation of transforming growth factor βsignaling pathways by Notch in human endothelial cells. J. Biol. Chem. 2009, 284, 19452–19462. [Google Scholar] [CrossRef] [PubMed]
- Taipale, J.; Beachy, P.A. The Hedgehog and Wnt signalling pathways in cancer. Nature 2001, 411, 349–354. [Google Scholar] [CrossRef]
- Roche, J. The Epithelial-to-Mesenchymal Transition in Cancer. Cancers 2018, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chakrabarti, R. Consequences of EMT-Driven Changes in the Immune Microenvironment of Breast Cancer and Therapeutic Response of Cancer Cells. J. Clin. Med. 2019, 8, 642. [Google Scholar] [CrossRef] [PubMed]
- Sauer, S.; Reed, D.R.; Ihnat, M.; Hurst, R.E.; Warshawsky, D.; Barkan, D. Innovative Approaches in the Battle Against Cancer Recurrence: Novel Strategies to Combat Dormant Disseminated Tumor Cells. Front. Oncol. 2021, 11, 659963. [Google Scholar] [CrossRef]
- Van Roy, F. Beyond E-cadherin: Roles of other cadherin superfamily members in cancer. Nat. Rev. Cancer 2014, 14, 121–134. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Onder, T.T.; Gupta, P.B.; Mani, S.A.; Yang, J.; Lander, E.S.; Weinberg, R.A. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008, 68, 3645–3654. [Google Scholar] [CrossRef]
- Casal, J.I.; Bartolome, R.A. Beyond N-cadherin, relevance of cadherins 5,6 and 17 in cancer progression and metastasis. Int. J. Mol. Sci. 2019, 20, 3373. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, S.; Alahari, S.K. Molecular mechanisms controlling E-cadherin expression in breast cancer. Biochem. Biophys. Res. Commun. 2009, 384, 6–11. [Google Scholar] [CrossRef]
- Kaszak, B.; NgosaToka, F.; Jurka, P. Role of Cadherins in Cancer-A Review. Int. J. Mol. Sci. 2020, 21, 7624. [Google Scholar] [CrossRef] [PubMed]
- Coradini, D.; Casarsa, C.; Oriana, S. Epithelial cell polarity and tumorigenesis: New perspectives for cancer detection and treatment. Acta Pharmacol. Sin. 2011, 32, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Yang, L.; Li, T.; Zhang, Y. Cadherin Signaling in Cancer: Its Functions and Role as a Therapeutic Target. Front. Oncol. 2019, 9, 989. [Google Scholar] [CrossRef]
- Cao, Z.Q.; Wang, Z.; Leng, P. Aberrant N-cadherin expression in cancer. Biomed. Pharmacother. 2019, 118, 109320. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, K.M.; Blaschuk, O.W.; Cheong, C.M.; Zannettino, A.C.W.; Vandyke, K. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer 2018, 18, 939. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; van Bokhoven, A.; van Leenders, G.J.; Ruijter, E.T.; Jansen, C.F.; Bussemakers, M.J.; Schalken, J.A. Cadherin switching in human prostate cancer progression. Cancer Res. 2000, 60, 3650–3654. [Google Scholar]
- Choi, Y.; Lee, H.J.; Jang, M.H.; Gwak, J.M.; Lee, K.S.; Kim, E.J.; Kim, H.J.; Lee, H.E.; Park, S.Y. Epithelial-mesenchymal transition increases during the progression of in situ to invasive basal-like breast cancer. Hum. Pathol. 2013, 44, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Lascombe, I.; Clairotte, A.; Fauconnet, S.; Bernardini, S.; Wallerand, H.; Kantelip, B.; Bittard, H. N-cadherin as a novel prognostic marker of progression in superficial urothelial tumors. Clin. Cancer Res. 2006, 12, 2780–2787. [Google Scholar] [CrossRef][Green Version]
- Nakajima, S.; Doi, R.; Toyoda, E.; Tsuji, S.; Wada, M.; Koizumi, M.; Tulachan, S.S.; Ito, D.; Kami, K.; Mori, T.; et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin. Cancer Res. 2004, 10, 4125–4133. [Google Scholar] [CrossRef]
- Liang, Z.; Sun, X.Y.; Xu, L.C.; Fu, R.Z. Abnormal expression of serum soluble E-cadherin is correlated with clinicopathological features and prognosis of breast cancer. Med. Sci. Monit. 2014, 20, 2776–2782. [Google Scholar] [PubMed]
- Li, K.; He, W.; Lin, N.; Wang, X.; Fan, Q.X. N-cadherin knock-down decreases invasiveness of esophageal squamous cell carcinoma in vitro. World J. Gastroenterol. 2009, 15, 697–704. [Google Scholar] [CrossRef]
- Li, Z.; Yin, S.; Zhang, L.; Liu, W.; Chen, B. Prognostic value of reduced E-cadherin expression in breast cancer: A meta-analysis. Oncotarget 2017, 8, 16445–16455. [Google Scholar] [CrossRef] [PubMed]
- Petrova, Y.I.; Schecterson, L.; Gumbiner, B.M. Roles for E-cadherin cell surface regulation in cancer. Mol. Biol. Cell 2016, 27, 3233–3244. [Google Scholar] [CrossRef]
- Carvalho, J.; Pinheiro, H.; Oliveira, C. E-cadherin germline mutations. In Spotlight on Familial and Hereditary Gastric Cancer; Corso, G., Roviello, F., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 35–49. [Google Scholar]
- Winter, J.M.; Ting, A.H.; Vilardell, F.; Gallmeier, E.; Baylin, S.B.; Hruban, R.H.; Kern, S.E.; Iacobuzio-Donahue, C.A. Absence of E-cadherin expression distinguishes noncohesive from cohesive pancreatic cancer. Clin. Cancer Res. 2008, 14, 412–418. [Google Scholar] [CrossRef]
- Mortazavi, F.; An, J.; Dubinett, S.; Rettig, M. p120-catenin is transcriptionally downregulated by FOXC2 in non-small cell lung cancer cells. Mol. Cancer Res. 2010, 8, 762–774. [Google Scholar] [CrossRef]
- Querzoli, P.; Coradini, D.; Pedriali, M.; Boracchi, P.; Ambrogi, F.; Raimondi, E.; La Sorda, R.; Lattanzio, R.; Rinaldi, R.; Lunardi, M.; et al. An immunohistochemically positive E-cadherin status is not always predictive for a good prognosis in human breast cancer. Br. J. Cancer. 2010, 103, 1835–1839. [Google Scholar] [CrossRef]
- Poletajew, S.; Fus, Ł.; Ilczuk, T.; Wojcieszak, P.; Sękowska, M.; Krajewski, W.; Wasiutyński, A.; Górnicka, B.; Radziszewski, P. Expression of E-cadherin, β-catenin, and epithelial membrane antigen does not predict survival in patients with high-risk non-muscle-invasive bladder cancer. Cent. Eur. J. Immunol. 2018, 43, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.G.; Adsay, N.V.; Grignon, D.J.; Osunkoya, A.O. E-cadherin expression in plasmacytoid, signet ring cell and micropapillary variants of urothelial carcinoma: Comparison with usual-type high-grade urothelial carcinoma. Mod. Pathol. 2011, 24, 241–247. [Google Scholar] [CrossRef]
- Liu, X.; Chu, K.M. E-cadherin and gastric cancer: Cause, consequence, and applications. BioMed Res. Int. 2014, 2014, 637308. [Google Scholar] [CrossRef]
- Li, Y.J.; Ji, X.R. Relationship between expression of E-cadherin-catenin complex and clinicopathologic characteristics of pancreatic cancer. World J. Gastroenterol. 2003, 9, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Aaltomaa, S.; Lipponen, P.; Ala-Opas, M.; Eskelinen, M.; Kosma, V.M. Alpha-catenin expression has prognostic value in local and locally advanced prostate cancer. Br. J. Cancer 1999, 80, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Na, T.Y.; Schecterson, L.; Mendonsa, A.M.; Gumbiner, B.M. The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proc. Natl. Acad. Sci USA 2020, 117, 5931–5937. [Google Scholar] [CrossRef]
- Kowalski, P.J.; Rubin, M.A.; Kleer, C.G. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003, 5, R217–R222. [Google Scholar] [CrossRef]
- Paredes, J.; Albergaria, A.; Oliveira, J.T.; Jeronimo, C.; Milanezi, F.; Schmitt, F.C. P-cadherin overexpression is an indicator of clinical outcome in invasive breast carcinomas and is associated with CDH3 promoter hypomethylation. Clin. Cancer Res. 2005, 11, 5869–5877. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Zhang, X.; Yang, Z.; Zhang, N.; Zhu, X.D.; Yang, K.Y.; Jin, F.; Shi, M.; Chen, Y.P.; Hu, W.H.; et al. Prognositic significance of P-cadherin expression in breast cancer: Protocol for a meta-analysis. Medicine 2019, 98, e14924. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.F.; Paredes, J. P-cadherin and the journey to cancer metastasis. Mol. Cancer 2015, 14, 178. [Google Scholar] [CrossRef]
- Van Marck, V.; Stove, C.; Jacobs, K.; Van den Eynden, G.; Bracke, M. P-cadherin in adhesion and invasion: Opposite roles in colon and bladder carcinoma. Int. J. Cancer 2011, 128, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.; Feys, L.; Vanhoecke, B.; Van Marck, V.; Bracke, M. P-cadherin expression reduces melanoma growth, invasion, and responsiveness to growth factors in nude mice. Eur. J. Cancer Prev. 2011, 20, 207–216. [Google Scholar] [CrossRef]
- Rudini, N.; Felici, A.; Giampietro, C.; Lampugnani, M.; Corada, M.; Swirsding, K.; Garrè, M.; Liebner, S.; Letarte, M.; ten Dijke, P.; et al. VE-cadherin is a critical endothelial regulator of TGF-beta signalling. EMBO J. 2008, 27, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Baudet, A.E.; Deroux, A.; Sidibé, A.; Dumestre-Perard, C.; Mannic, T.; Treillard, B.; Arboleas, M.A.; Chiquet, C.A.; Gulino-Debrac, D.G.; et al. Auto-antibodies to vascular endothelial cadherin in humans: Association with autoimmune diseases. Lab. Investig. 2013, 93, 1194–1202. [Google Scholar]
- Legrand, P.; Bibert, S.; Jaquinod, M.; Ebel, C.; Hewat, E.; Vincent, F.; Vanbelle, C.; Concord, E.; Vernet, T.; Gulino, D. Self-assembly of the vascular endothelial cadherin ectodomain in a Ca2+-dependent hexameric structure. J. Biol. Chem. 2001, 276, 3581–3588. [Google Scholar] [CrossRef]
- Hermant, B.; Bibert, S.; Concord, E.; Dublet, B.; Weidenhaupt, M.; Vernet, T.; Gulino-Debrac, D. Identification of proteases involved in the proteolysis of vascular endothelium cadherin during neutrophil transmigration. J. Biol. Chem. 2003, 278, 14002–14012. [Google Scholar] [CrossRef]
- Evangelista, F.; Roth, A.J.; Prisayanh, P.; Temple, B.R.; Li, N.; Qian, Y.; Culton, D.A.; Liu, Z.; Harrison, O.J.; Brasch, J.; et al. Pathogenic IgG4 autoantibodies from endemic pemphigus foliaceus recognize a desmoglein-1 conformational epitope. J. Autoimmun. 2018, 89, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, F.; Dasher, D.A.; Diaz, L.A.; Prisayanh, P.S.; Li, N. E-cadherin is an additional immunological target for pemphigus autoantibodies. J. Invest. Dermatol. 2008, 128, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A.; Prisayanh, P.S.; Dasher, D.A.; Li, N.; Evangelista, F.; Aoki, V.; Hans-Filho, G.; dos Santos, V.; Qaqish, B.F.; Rivitti, E.A.; et al. The IgM anti-desmoglein 1 response distinguishes Brazilian pemphigus foliaceus (fogoselvagem) from other forms of pemphigus. J. Investig. Dermatol. 2008, 128, 667–675. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Sidibé, A.; Mannic, T.; Arboleas, M.; Subileau, M.; Gulino-Debrac, D.; Bouillet, L.; Jan, M.; Vandhuick, T.; Le Loët, X.; Vittecoq, O.; et al. VE-cadherin in rheumatoid arthritis patients correlates with disease activity: Evidence for tumor necrosis factor α-induced VE-cadherin cleavage. Arthritis Rheum. 2012, 64, 77–87. [Google Scholar] [CrossRef]
- Banse, C.; Polena, H.; Stidder, B.; Khalil-Mgharbel, A.; Houivet, E.; Lequerré, T.; Fardellone, P.; Le-Loët, X.; Philippe, P.; Marcelli, C.; et al. Soluble vascular endothelial (VE) cadherin and autoantibodies to VE-cadherin in rheumatoid arthritis patients treated with etanercept or adalimumab. Jt. Bone Spine 2017, 84, 685–691. [Google Scholar] [CrossRef]
- Radic, M.; Martinovic Kaliterna, D.; Radic, J. Vascular manifestations of systemic lupus erythematosis. Neth. J. Med. 2013, 71, 10–16. [Google Scholar] [PubMed]
- Ding, Y.; Tan, Y.; Qu, Z.; Yu, F. Renal microvascular lesions in lupus nephritis. Ren. Fail. 2019, 42, 19–29. [Google Scholar] [CrossRef]
- Moscato, S.; Pratesi, F.; Bongiorni, F.; Scavuzzo, M.C.; Chimenti, D.; Bombardieri, S.; Migliorini, P. Endothelial cell binding by systemic lupus antibodies: Functional properties and relationship with anti-DNA activity. J. Autoimmun. 2002, 18, 231–238. [Google Scholar] [CrossRef]
- Jin, T.; Almehed, K.; Zhu, Y.; Carlsten, H.; Forsblad-d’Elia, H. Soluble E-cadherin in systemic lupus erythematosus. J. Rheumatol. 2013, 40, 1677–1682. [Google Scholar] [CrossRef]
- Pang, M.; Abe, T.; Fujihara, T.; Mori, S.; Tsuzaka, K.; Amano, K.; Koide, J.; Takeuchi, T. Up-regulation of alphaEbeta7, a novel integrin adhesion molecule, on T cells from systemic lupus erythematosus patients with specific epithelial involvement. Arthritis Rheum. 1998, 41, 1456–1463. [Google Scholar] [CrossRef]
- Löffek, S.; Schilling, O.; Franzke, C.W. Series “matrix metalloproteinases in lung health and disease”: Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef]
- De Wever, O.; Derycke, L.; Hendrix, A.; De Meerleer, G.; Godeau, F.; Depypere, H.; Bracke, M. Soluble cadherins as cancer biomarkers. Clin. Exp. Metastasis 2007, 24, 685–697. [Google Scholar] [CrossRef]
- Zeidan, M.J.; Saadoun, D.; Garrido, M.; Klatzmann, D.; Six, A.; Cacoub, P. Behçet’s disease physiopathology: A contemporary review. Autoimmun. Highlights 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, M. Historical review of the discovery of cadherin, in memory of Tokindo Okada. Dev Growth Differ. 2018, 60, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Corada, M.; Liao, F.; Lindgren, M.; Lampugnani, M.G.; Breviario, F.; Frank, R.; Muller, W.A.; Hicklin, D.J.; Bohlen, P.; Dejana, E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood 2001, 97, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.; Buckley, C.; Holness, C.L.; Bird, I.N.; Spragg, J.H.; Saunders, J.; Harris, A.; Simmons, D.L. Mapping the homotypic binding sites in CD31 and the role of CD31 adhesion in the formation of interendothelial cell contacts. J. Cell Biol. 1995, 128, 1229–1241. [Google Scholar] [CrossRef]
- Lampugnani, M.G.; Dejana, E.; Giampietro, C. Vascular Endothelial (VE)-Cadherin, Endothelial Adherens Junctions, and Vascular Disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029322. [Google Scholar] [CrossRef]
- Koirala, R.; Priest, A.V.; Yen, C.F.; Cheah, J.S.; Pannekoek, W.J.; Gloerich, M.; Yamada, S.; Sivasankar, S. Inside-out regulation of E-cadherin conformation and adhesion. Proc. Natl. Acad. Sci. USA 2021, 118, e2104090118. [Google Scholar] [CrossRef]
- Amagai, M.; Stanley, J.R. Desmoglein as a Target in Skin Disease and Beyond. J. Investig. Dermatol. 2012, 132, 776–784. [Google Scholar] [CrossRef]
- Hudemann, C.; Maglie, R.; Llamazares-Prada, M.; Beckert, B.; Didona, D.; Tikkanen, R.; Schmitt, T.; Hashimoto, T.; Waschke, J.; Hertl, M.; et al. Human Desmocollin 3‒Specific IgG Antibodies Are Pathogenic in a Humanized HLA Class II Transgenic Mouse Model of Pemphigus. J. Investig. Dermatol. 2021. [CrossRef]
- Roscoe, J.T.; Diaz, L.A.; Sampaio, S.A.P.; Castro, R.M.; Labib, R.S.; Takahashi, Y.; Patel, H.; Anhalt, G.J. Brazilian pemphigus foliaceus autoantibodies are pathogenic to BALB/C mice by passive transfer. J. Investig. Dermatol. 1985, 85, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Aoki, V.; Mascaro, J.M., Jr.; Lopez-Swiderski, A.; Diaz, L.A.; Fairley, J.A. Mucosal and mucocutaneous (generalized) pemphigus vulgaris show distinct autoantibody profiles. J. Investig. Dermatol. 1997, 109, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Amagai, M.; Tsunoda, K.; Zillikens, D.; Nagai, T.; Nishikawa, T. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J. Am. Acad. Dermatol. 1999, 40, 67–70. [Google Scholar] [CrossRef]
- Sekiguchi, M.; Futei, Y.; Fujii, Y.; Iwasaki, T.; Nishikawa, T.; Amagai, M. Dominant autoimmune epitopes recognized by pemphigus antibodies map to the N-terminal adhesive region of desmogleins. J. Immunol. 2001, 167, 5439–5448. [Google Scholar] [CrossRef]
- Li, N.; Aoki, V.; Hans-Filho, G.; Rivitti, E.A.; Diaz, L.A. The role of intramolecular epitope spreading in the pathogenesis of endemic pemphigus foliaceus (fogoselvagem). J. Exp. Med. 2003, 197, 1501–1510. [Google Scholar] [CrossRef]
- Spindler, V.; Waschke, J. Pemphigus-A Disease of Desmosome Dysfunction Caused by Multiple Mechanisms. Front. Immunol. 2018, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Oktarina, D.A.M.; Sokol, E.; Kramer, D.; Jonkman, M.F.; Pas, H.H. Endocytosis of IgG, Desmoglein 1, and Plakoglobin in Pemphigus Foliaceus Patient Skin. Front. Immunol. 2019, 10, 2635. [Google Scholar] [CrossRef]
- Hakuno, M.; Akiyama, M.; Shimizu, H.; Wheelock, M.J.; Nishikawa, T. Upregulation of P-cadherin expression in the lesional skin of pemphigus, Hailey-Hailey disease and Darier’s disease. J. Cutan. Pathol. 2001, 28, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Matsuyoshi, N.; Tanaka, T.; Toda, K.; Okamoto, H.; Furukawa, F.; Imamura, S. Soluble E-cadherin: A novel cutaneous disease marker. Br. J. Dermatol. 1995, 132, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Schmidt, E.; Bégány, A.; Hunyadi, J.; Szegedi, A. Immunohistochemical examination of P-cadherin in bullous and acantholytic skin diseases. Acta Derm. Venereol. 2004, 84, 116–119. [Google Scholar] [CrossRef]
- Berkowitz, P.; Hu, P.; Liu, Z.; Diaz, L.A.; Enghild, J.J.; Chua, M.P.; Rubenstein, D.S. Desmosome Signaling. Inhibition of p38MAPK prevents pemphigus vulgaris IgG-induced cytoskeleton reorganization. J. Biol. Chem. 2005, 280, 23778–23784. [Google Scholar] [CrossRef]
- Esaki, C.; Seishima, M.; Yamada, T.; Osada, K.; Kitajima, Y. Pharmacologic evidence for involvement of phospholipase C in pemphigus IgG-induced inositol 1,4,5-trisphosphate generation, intracellular calcium increase, and plasminogen activator secretion in DJM-1 cells, a squamous cell carcinoma line. J. Investig. Dermatol. 1995, 105, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Wallis, S.; Lloyd, S.; Wise, I.; Ireland, G.; Fleming, T.P.; Garrod, D. The alpha isoform of protein kinase C is involved in signaling the response of desmosomes to wounding in cultured epithelial cells. Mol. Biol. Cell 2000, 11, 1077–1092. [Google Scholar] [CrossRef]
- Thomason, H.A.; Scothern, A.; McHarg, S.; Garrod, D.R. Desmosomes: Adhesive strength and signalling in health and disease. Biochem. J. 2010, 429, 419–433. [Google Scholar] [CrossRef]
- Cirillo, N.; Lanza, A.; Prime, S.S. Induction of hyper-adhesion attenuates autoimmune-induced keratinocyte cell-cell detachment and processing of adhesion molecules via mechanisms that involve PKC. Exp. Cell Res. 2010, 316, 580–592. [Google Scholar] [CrossRef]
- Müller, E.J.; Williamson, L.; Kolly, C.; Suter, M.M. Outside-in signaling through integrins and cadherins: A central mechanism to control epidermal growth and differentiation? J. Investig. Dermatol. 2008, 128, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Heupel, W.M.; Zillikens, D.; Drenckhahn, D.; Waschke, J. Pemphigus vulgaris IgG directly inhibit desmoglein 3-mediated transinteraction. J. Immunol. 2008, 181, 1825–1834. [Google Scholar] [CrossRef]
- Spindler, V.; Rötzer, V.; Dehner, C.; Kempf, B.; Gliem, M.; Radeva, M.; Hartlieb, E.; Harms, G.S.; Schmidt, E.; Waschke, J. Peptide-mediated desmoglein 3 crosslinking prevents pemphigus vulgaris autoantibody-induced skin blistering. J. Clin. Investig. 2013, 123, 800–811. [Google Scholar] [CrossRef]
- Tsang, S.M.; Brown, L.; Lin, K.; Liu, L.; Piper, K.; O’Toole, E.A.; Grose, R.; Hart, I.R.; Garrod, D.R.; Fortune, F.; et al. Non-junctional human desmoglein 3 acts as an upstream regulator of Src in E-cadherin adhesion, a pathway possibly involved in the pathogenesis of pemphigus vulgaris. J. Pathol. 2012, 227, 81–93. [Google Scholar] [CrossRef]
- Garrod, D.R.; Berika, M.Y.; Bardsley, W.F.; Holmes, D.; Tabernero, L. Hyper-adhesion in desmosomes: Its regulation in wound healing and possible relationship to cadherin crystal structure. J. Cell Sci. 2005, 118, 5743–5754. [Google Scholar] [CrossRef] [PubMed]
- Seishima, M.; Esaki, C.; Osada, K.; Mori, S.; Hashimoto, T.; Kitajima, Y. Pemphigus IgG, but not bullous pemphigoid IgG, causes a transient increase in intracellular calcium and inositol 1,4,5-triphosphate in DJM-1 cells, a squamous cell carcinoma line. J. Invest. Dermatol. 1995, 104, 33–37. [Google Scholar] [CrossRef]
- Mousavi, M.J.; Karami, J.; Aslani, S.; Tahmasebi, M.N.; Vaziri, A.S.; Jamshidi, A.; Farhadi, E.; Mahmoudi, M. Transformation of fibroblast-like synoviocytes in rheumatoid arthritis; from a friend to foe. Autoimmun. Highlights 2021, 12, 3. [Google Scholar] [CrossRef]
- Neidhart, M.; Seemayer, C.A.; Hummel, K.M.; Michel, B.A.; Gay, R.E.; Gay, S. Functional characterization of adherent synovial fluid cells in rheumatoid arthritis: Destructive potential in vitro and in vivo. Arthritis Rheum. 2003, 48, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Kiener, H.P.; Watts, G.F.; Cui, Y.; Wright, J.; Thornhill, T.S.; Sköld, M.; Behar, S.M.; Niederreiter, B.; Lu, J.; Cernadas, M.; et al. Synovial fibroblasts self-direct multicellular lining architecture and synthetic function in three-dimensional organ culture. Arthritis Rheum. 2010, 62, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Ospelt, C.; Gay, S. The role of resident synovial cells in destructive arthritis. Best Pract. Res. Clin. Rheumatol. 2008, 22, 239–252. [Google Scholar] [CrossRef]
- Steenvoorden, M.M.; Tolboom, T.C.; van der Pluijm, G.; Lowik, C.; Visser, C.P.; DeGroot, J.; DeRuiter, M.C.; Wisse, B.J.; Huizinga, T.W.; Toes, R.E. Transition of healthy to diseased synovial tissue in rheumatoid arthritis is associated with gain of mesenchymal/fibrotic characteristics. Arthritis Res. Ther. 2006, 8, R165. [Google Scholar] [CrossRef]
- Li, G.Q.; Zhang, Y.; Liu, D.; Qian, Y.Y.; Zhang, H.; Guo, S.Y.; Sunagawa, M.; Hisamitsu, T.; Liu, Y.Q. PI3 kinase/Akt/HIF-1α pathway is associated with hypoxia-induced epithelial-mesenchymal transition in fibroblast-like synoviocytes of rheumatoid arthritis. Mol. Cell Biochem. 2013, 372, 221–331. [Google Scholar] [CrossRef]
- Van der Kraan, P.M. Differential Role of Transforming Growth Factor-beta in an Osteoarthritic or a Healthy Joint. J. Bone Metab. 2018, 25, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, H.; Park, E.J.; Hwang, J.W.; Bae, E.K.; Ahn, J.K.; Ahn, K.S.; Koh, E.M.; Cha, H.S. A role for benzo[a]pyrene and Slug in invasive properties of fibroblast-like synoviocytes in rheumatoid arthritis: A potential molecular link between smoking and radiographic progression. Jt. Bone Spine 2013, 80, 621–625. [Google Scholar] [CrossRef]
- Dou, C.; Yan, Y.; Dong, S. Role of cadherin-11 in synovial joint formation and rheumatoid arthritis pathology. Mod. Rheumatol. 2013, 23, 1037–1044. [Google Scholar] [CrossRef]
- Park, Y.E.; Woo, Y.J.; Park, S.H.; Moon, Y.M.; Oh, H.J.; Kim, J.I.; Jin, H.S.; Baek, S.H.; Kim, G.T.; Lee, J.H.; et al. IL-17 increases cadherin-11 expression in a model of autoimmune experimental arthritis and in rheumatoid arthritis. Immunol. Lett. 2011, 140, 97–103. [Google Scholar] [CrossRef]
- Kiener, H.P.; Karonitsch, T. The synovium as a privileged site in rheumatoid arthritis: Cadherin-11 as a dominant player in synovial pathology. Best Pract. Res. Clin. Rheumatol. 2011, 25, 767–777. [Google Scholar] [CrossRef]
- Chang, S.K.; Gu, Z.; Brenner, M.B. Fibroblast-like synoviocytes in inflammatory arthritis pathology: The emerging role of cadherin-11. Immunol. Rev. 2010, 233, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yang, H.; Liu, N.; Li, Y.; Chen, J. Advances in Pathogenesis of Sjögren’s Syndrome. J. Immunol. Res. 2021, 2021, 5928232. [Google Scholar] [CrossRef]
- Jonsson, M.V.; Salomonsson, S.; Øijordsbakken, G.; Skarstein, K. Elevated serum levels of soluble E-cadherin in patients with primary Sjögren’s syndrome. Scand. J. Immunol. 2005, 62, 552–559. [Google Scholar] [CrossRef]
- Goicovich, E.; Molina, C.; Perez, P.; Aguilera, S.; Fernández, J.; Olea, N.; Alliende, C.; Leyton, C.; Romo, R.; Leyton, L.; et al. Enhanced degradation of proteins of the basal lamina and stroma by matrix metalloproteinases from the salivary glands of Sjögren’s syndrome patients: Correlation with reduced structural integrity of acini and ducts. Arthritis Rheum. 2003, 48, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- Perez, P.; Goicovich, E.; Alliende, C.; Aguilera, S.; Leyton, C.; Molina, C.; Pinto, R.; Romo, R.; Martinez, B.; González, M.J. Differential expression of matrix metalloproteinases in labial salivary glands of patients with primary Sjögren’s syndrome. Arthritis Rheum. 2000, 43, 2807–2817. [Google Scholar] [CrossRef]
- Gao, C.Y.; Yao, Y.; Li, L.; Yang, S.H.; Chu, H.; Tsuneyama, K.; Li, X.M.; Gershwin, M.E.; Lian, Z.X. Tissue-Resident Memory CD8+ T Cells Acting as Mediators of Salivary Gland Damage in a Murine Model of Sjögren’s Syndrome. Arthritis Rheumatol. 2019, 71, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Esch, T.R.; Jonsson, M.V.; Levanos, V.A.; Poveromo, J.D.; Sorkin, B.C. Leukocytes infiltrating the submandibular glands of NOD mice express E-cadherin. J. Autoimmun. 2000, 15, 387–393. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, J.; Tian, J.; Wang, S. CD8+ T Lymphocytes: Crucial Players in Sjögren’s Syndrome. Front. Immunol. 2021, 11, 602823. [Google Scholar] [CrossRef] [PubMed]
- Sisto, M.; Ribatti, D.; Lisi, S. ADAM 17 and Epithelial-to-Mesenchymal Transition: The Evolving Story and Its Link to Fibrosis and Cancer. J. Clin. Med. 2021, 10, 3373. [Google Scholar] [CrossRef] [PubMed]
- Sisto, M.; Ribatti, D.; Lisi, S. Organ Fibrosis and Autoimmunity: The Role of Inflammation in TGFβ-Dependent EMT. Biomolecules 2021, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Sisto, M.; Ribatti, D.; Lisi, S. SMADS-Mediate Molecular Mechanisms in Sjögren’s Syndrome. Int. J. Mol. Sci. 2021, 22, 3203. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sisto, M.; Ribatti, D.; Lisi, S. Cadherin Signaling in Cancer and Autoimmune Diseases. Int. J. Mol. Sci. 2021, 22, 13358. https://doi.org/10.3390/ijms222413358
Sisto M, Ribatti D, Lisi S. Cadherin Signaling in Cancer and Autoimmune Diseases. International Journal of Molecular Sciences. 2021; 22(24):13358. https://doi.org/10.3390/ijms222413358
Chicago/Turabian StyleSisto, Margherita, Domenico Ribatti, and Sabrina Lisi. 2021. "Cadherin Signaling in Cancer and Autoimmune Diseases" International Journal of Molecular Sciences 22, no. 24: 13358. https://doi.org/10.3390/ijms222413358
APA StyleSisto, M., Ribatti, D., & Lisi, S. (2021). Cadherin Signaling in Cancer and Autoimmune Diseases. International Journal of Molecular Sciences, 22(24), 13358. https://doi.org/10.3390/ijms222413358







