A Rat Model of Post-Traumatic Stress Syndrome Causes Phenotype-Associated Morphological Changes and Hypofunction of the Adrenal Gland
Abstract
:1. Introduction
2. Results
2.1. PSS Affects the Anxiety of Rats in an Elevated Plus-Maze Test
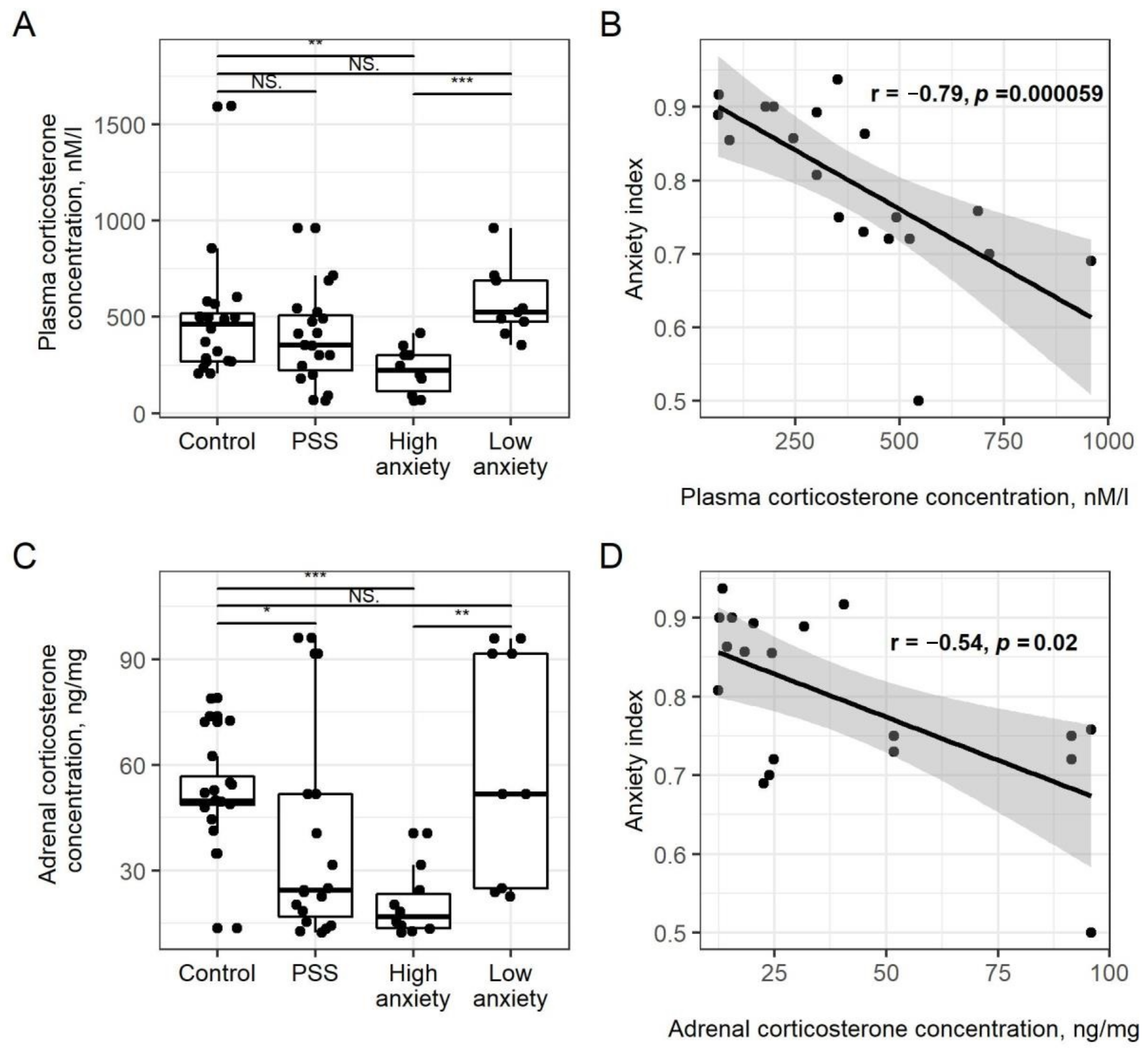
2.2. PSS Causes a Decrease in Plasma CORT Concentrations in High-Anxiety Rats
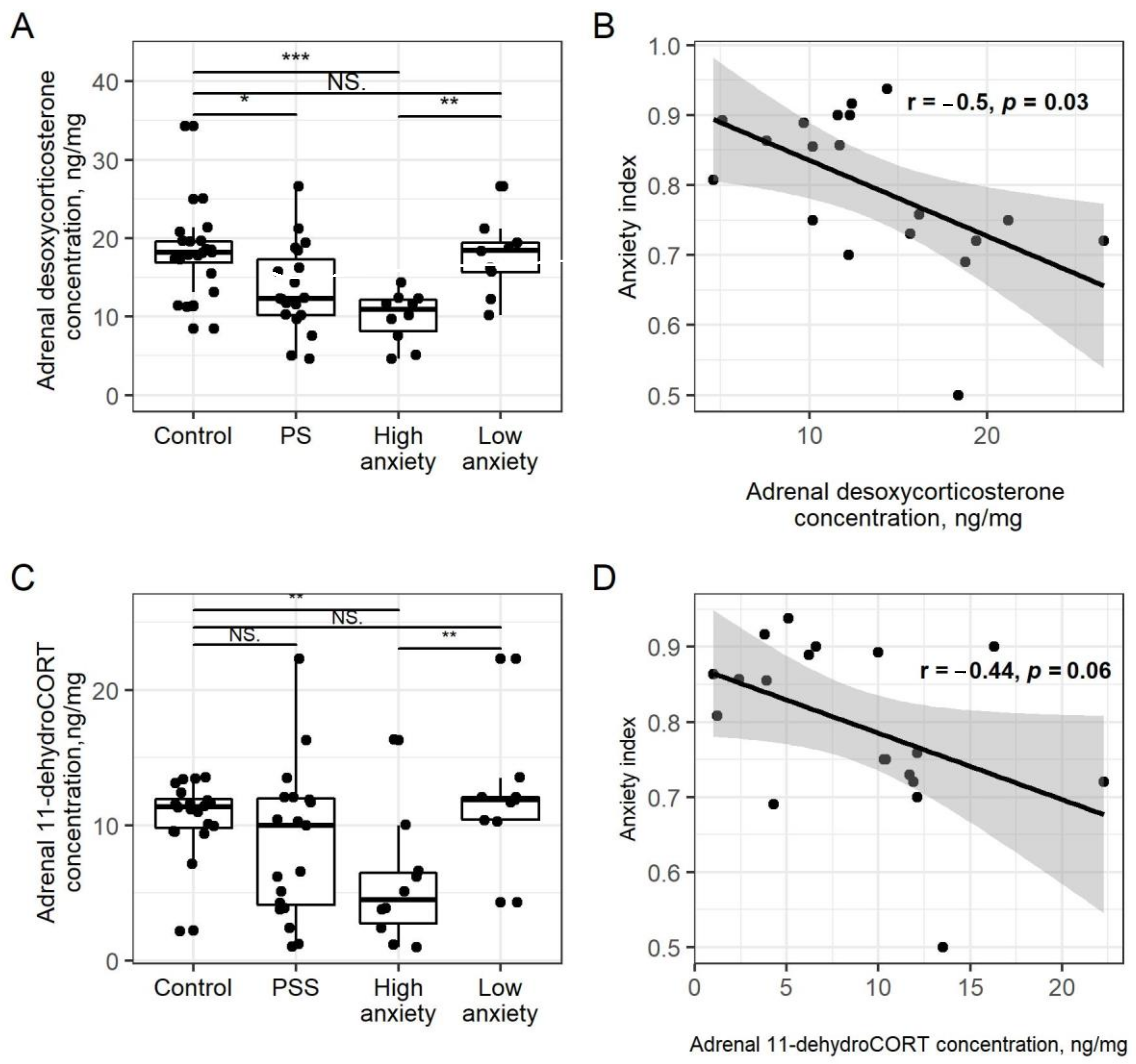
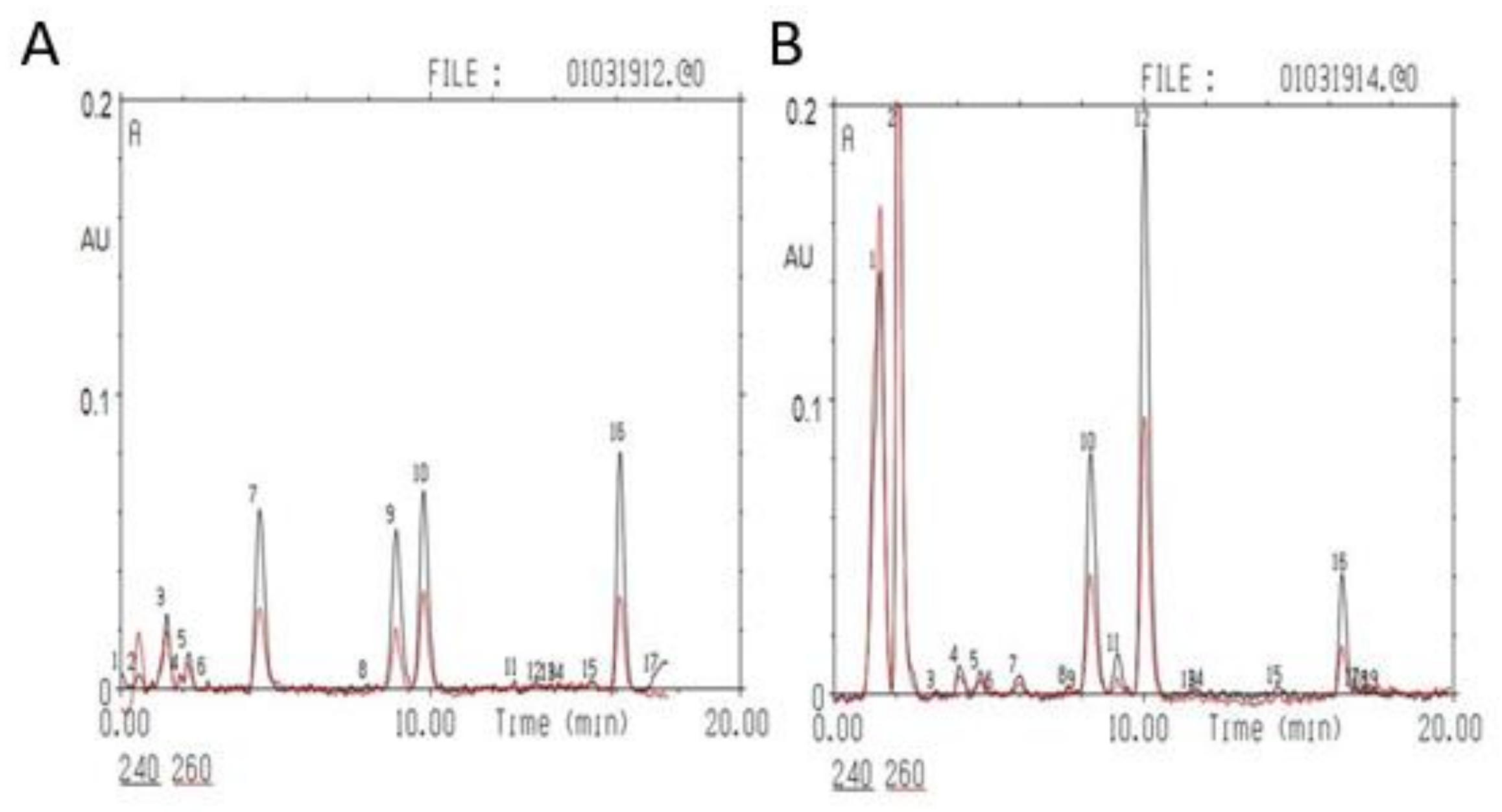
2.3. PSS Causes a Decrease in Adrenal Corticosteroids
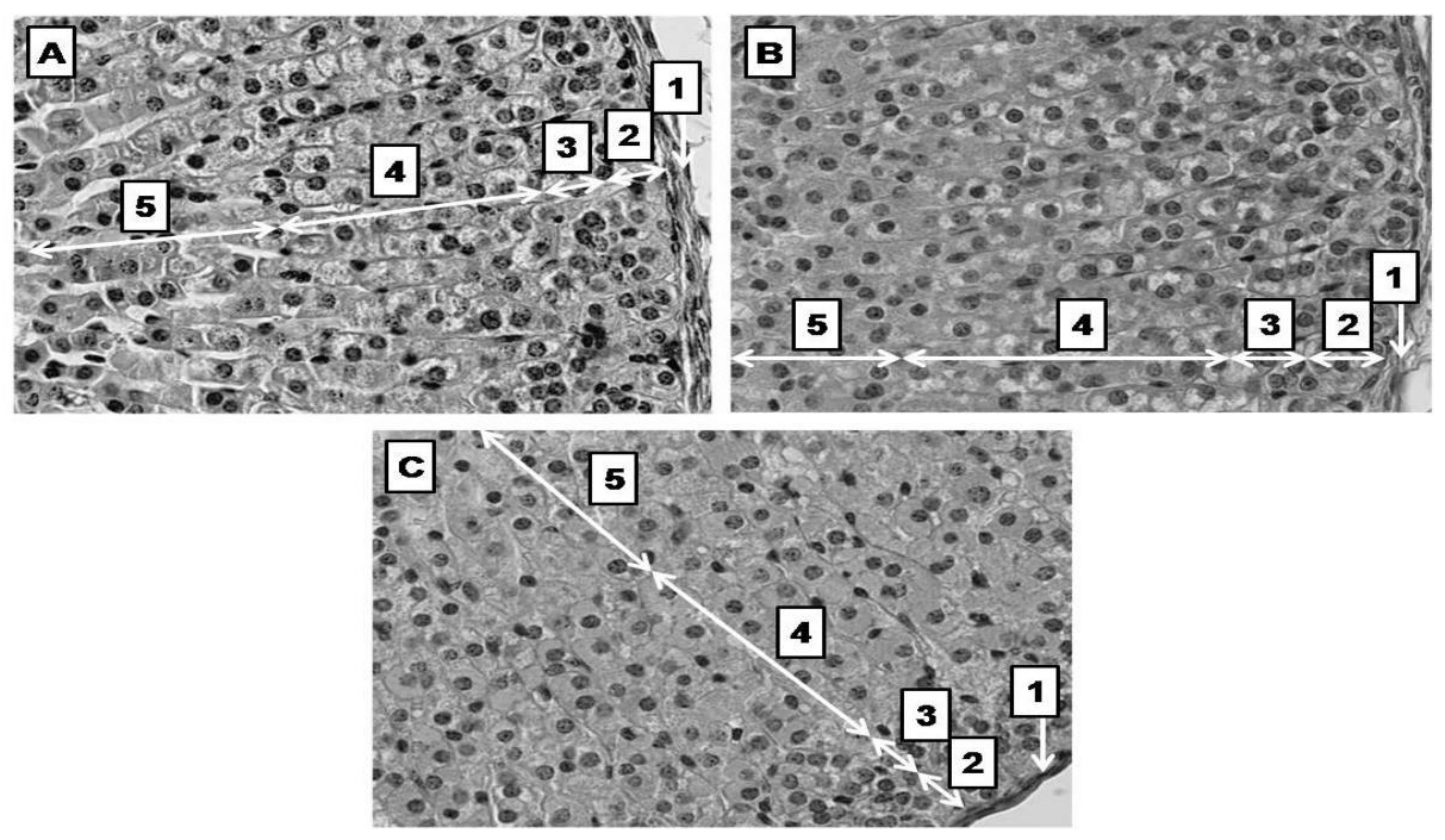
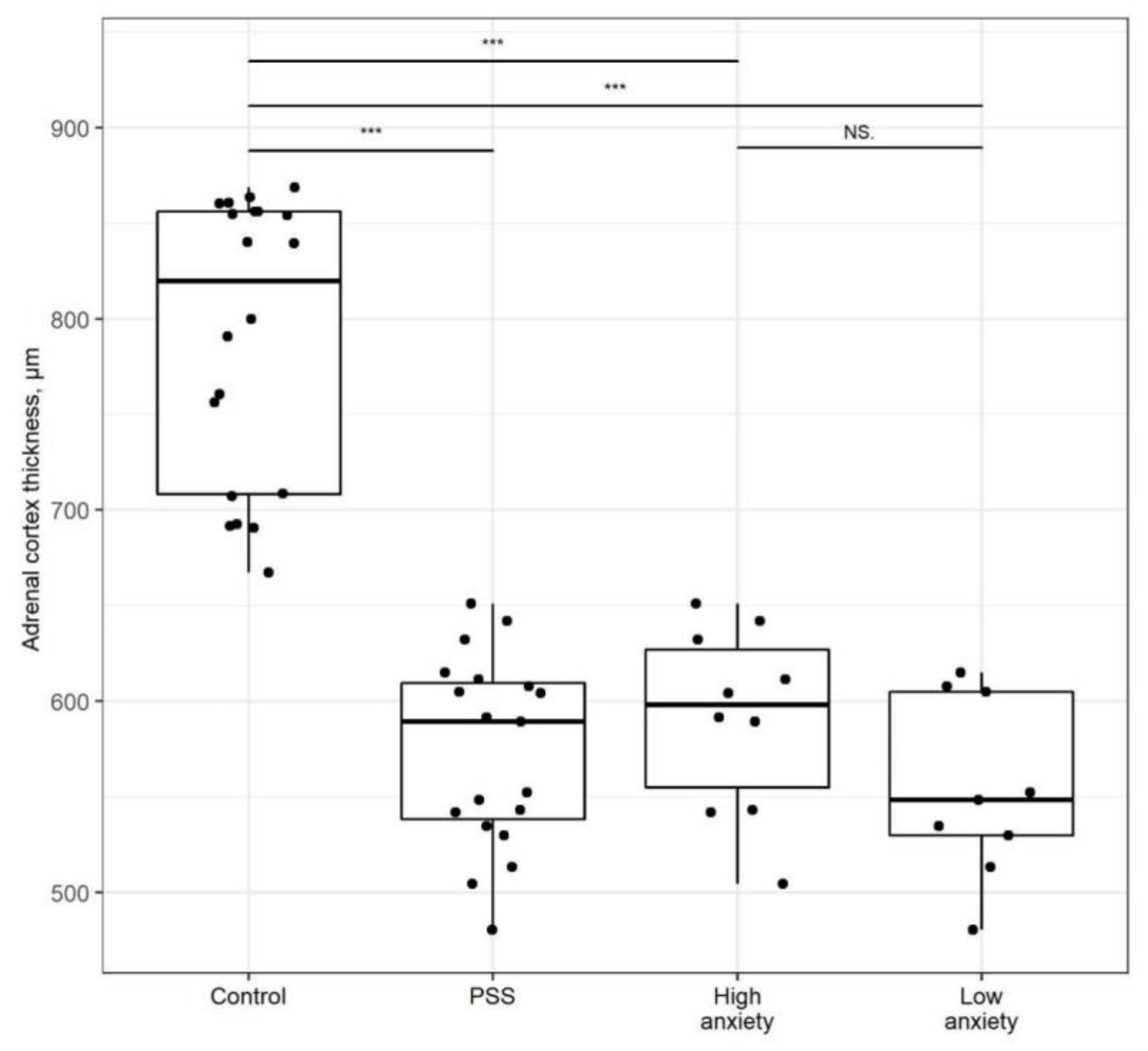
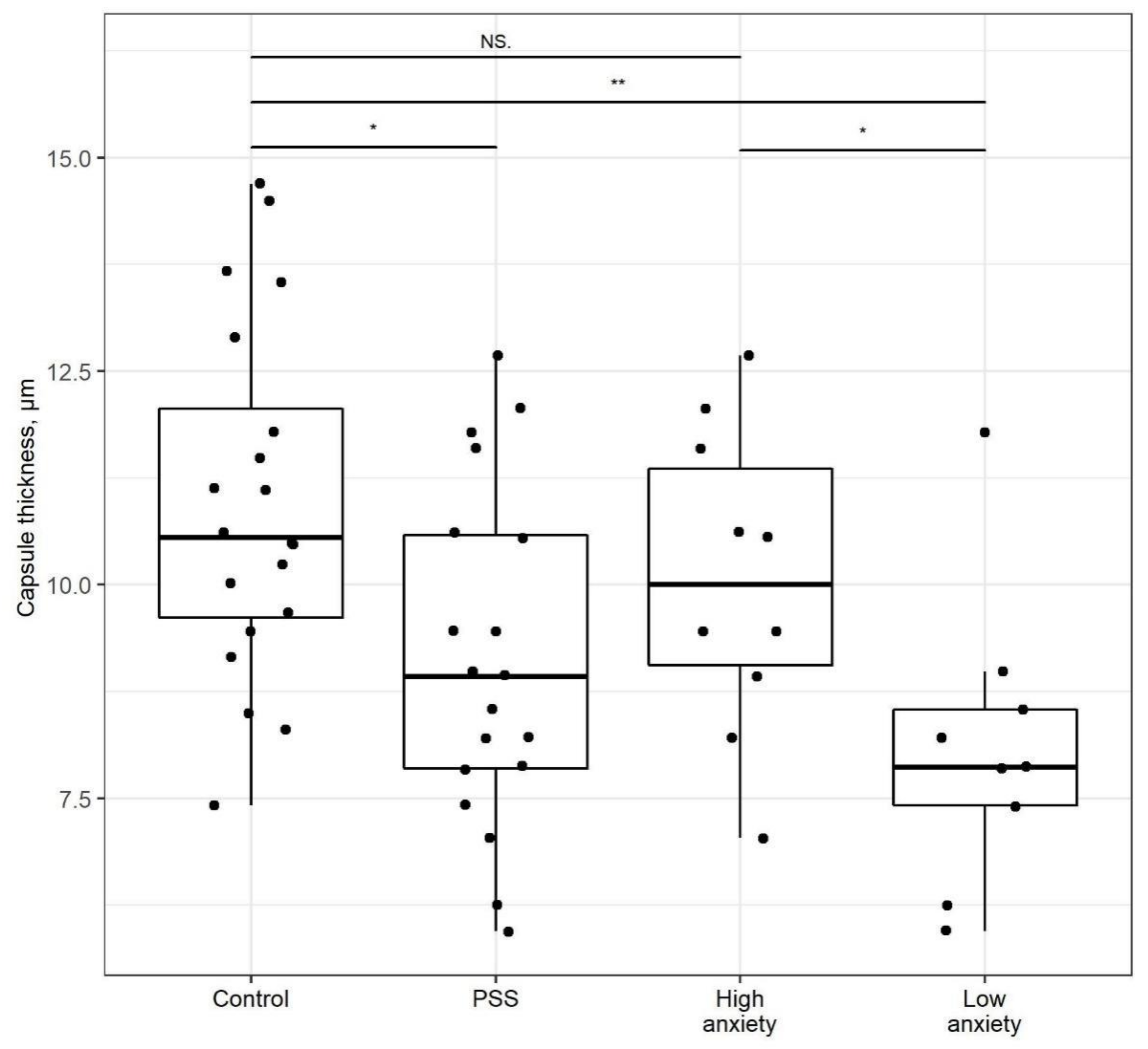
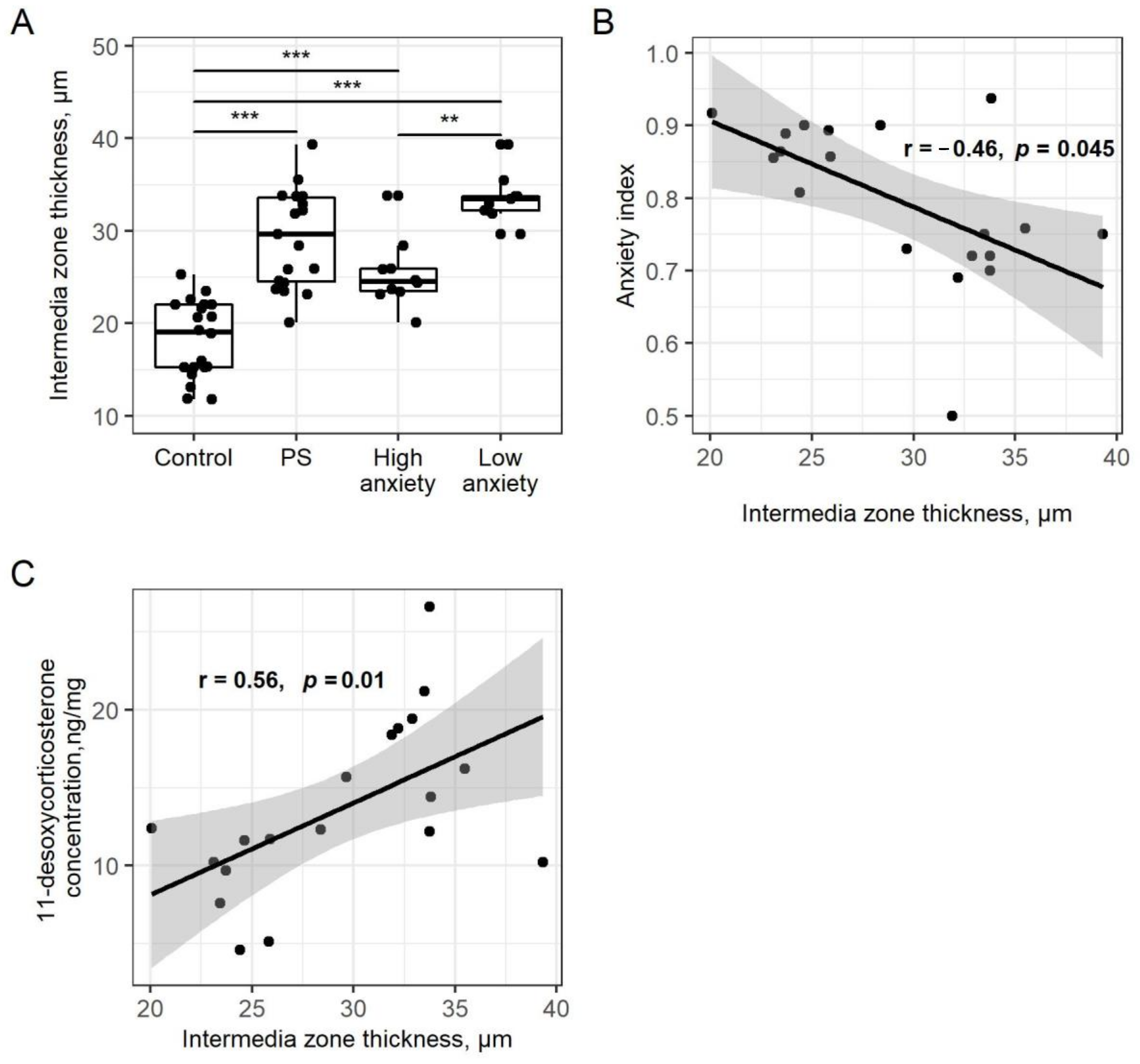
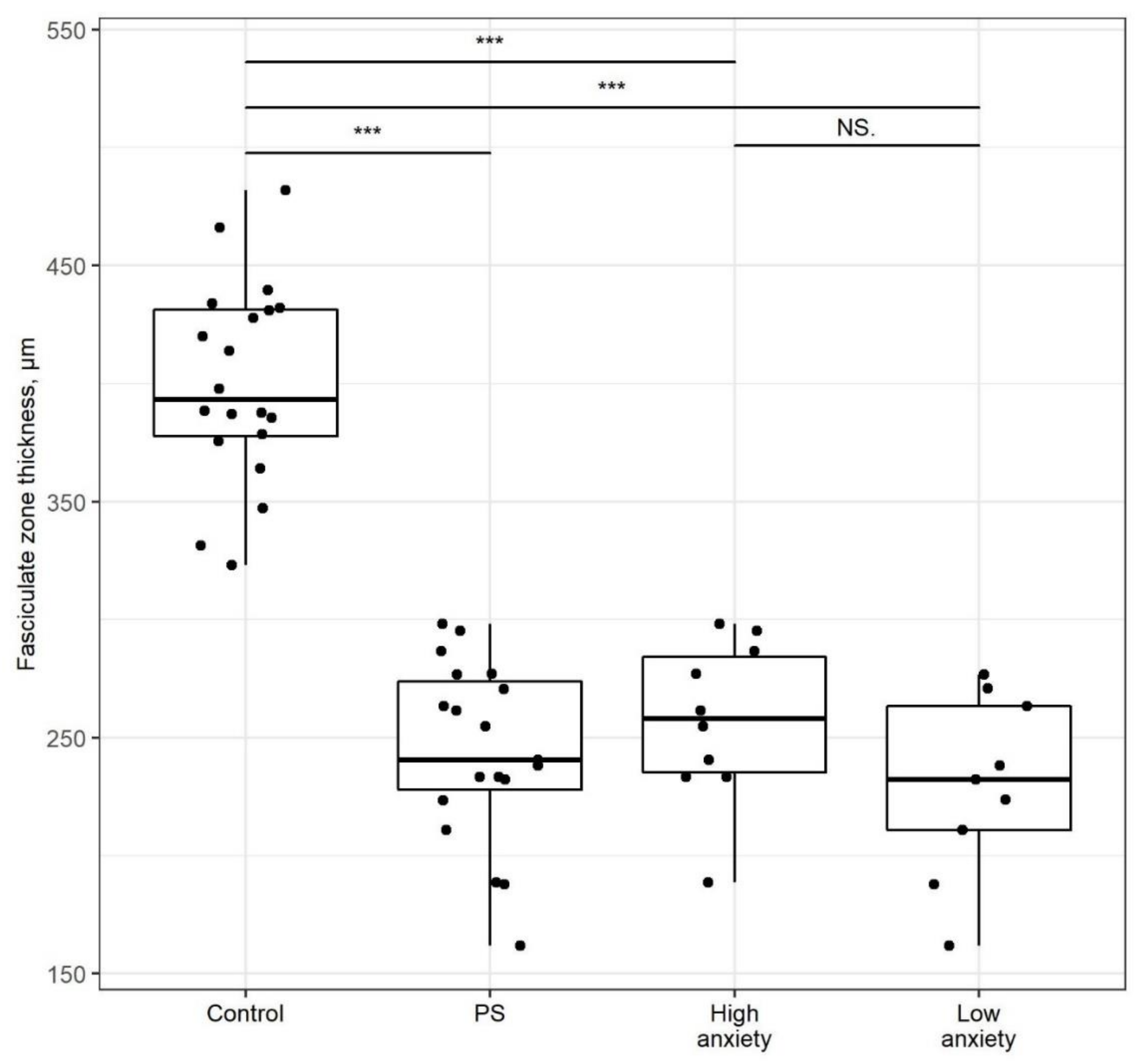
2.4. PSS Affects the Thickness of the Adrenal Cortex
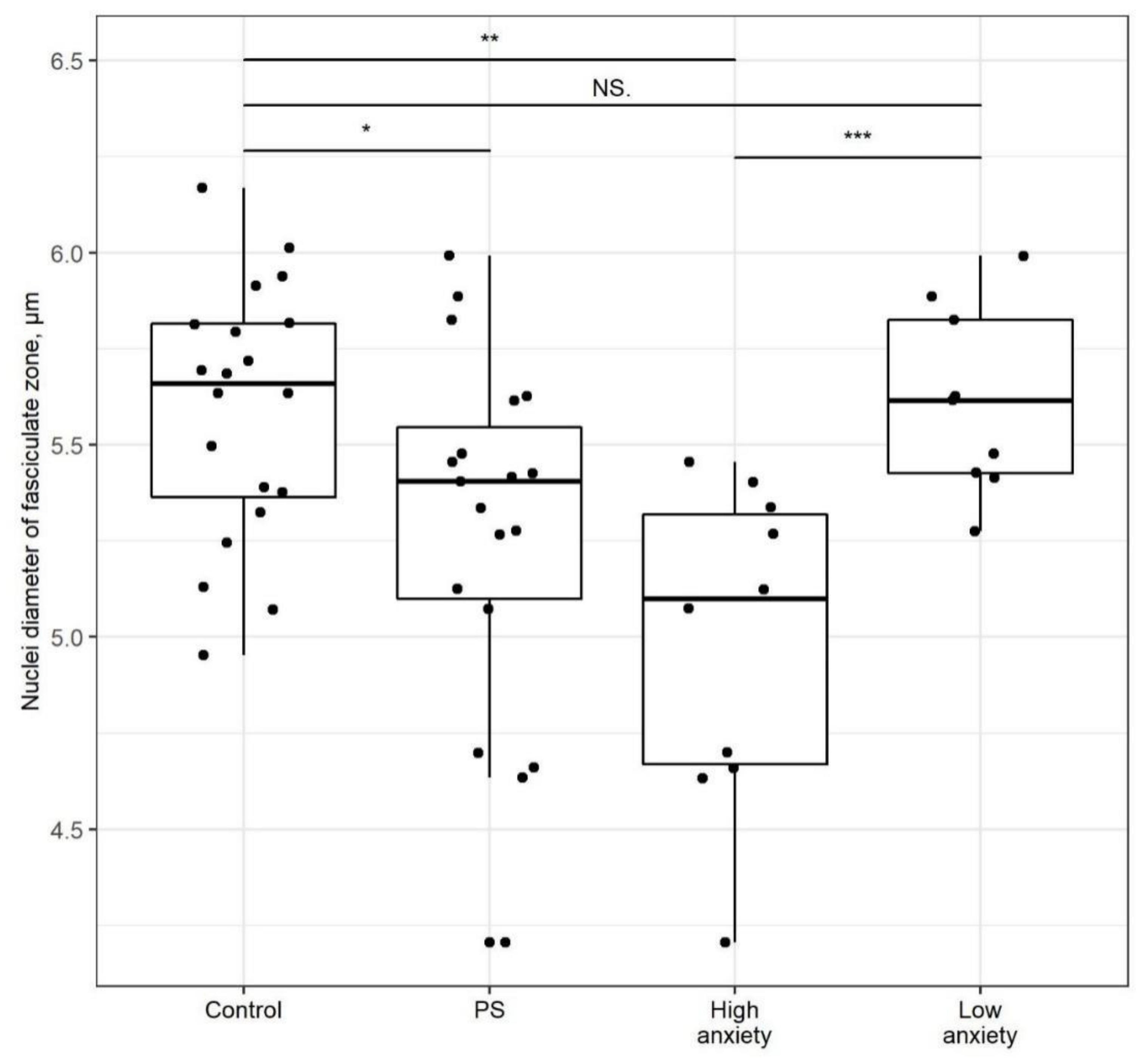
2.5. PSS Affects Nucleus Diameter in the Zona Fasciculata
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Modeling PTSD
4.3. Behavioral Testing
4.4. Blood and Tissue Collection and Storage
4.5. Plasma Corticosterone and Adrenal Corticosteroid Measurements
4.6. Histomorphology
5. Data Analyses
6. Conclusions
7. Limitations of This Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berger, I.; Werdermann, M.; Bornstein, S.R.; Steenblock, C. The adrenal gland in stress—Adaptation on a cellular level. J. Steroid Biochem. Mol. Biol. 2019, 190, 198–206. [Google Scholar] [CrossRef]
- Gunnar, M.R.; Vazquez, D.M. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Dev. Psychopathol. 2001, 13, 515–538. [Google Scholar] [CrossRef] [PubMed]
- Fries, E.; Hesse, J.; Hellhammer, J.; Hellhammer, D.H. A new view on hypocortisolism. Psychoneuroendocrinology 2005, 30, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Dauvermann, M.R.; Donohoe, G. Cortisol stress response in psychosis from the high-risk to the chronic stage: A systematic review. Ir. J. Psychol. Med. 2019, 36, 305–315. [Google Scholar] [CrossRef]
- Zohar, J.; Yahalom, H.; Kozlovsky, N.; Cwikel-Hamzany, S.; Matar, M.A.; Kaplan, Z.; Yehuda, R.; Cohen, H. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: Interplay between clinical and animal studies. Eur. Neuropsychopharmacol. 2011, 21, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Schweingruber, N.; Reichardt, S.D.; Luhder, F.; Reichardt, H.M. Mechanisms of Glucocorticoids in the Control of Neuroinflammation. J. Neuroendocr. 2011, 24, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Vitellius, G.; Trabado, S.; Bouligand, J.; Delemer, B.; Lombès, M. Pathophysiology of Glucocorticoid Signaling. Ann. Endocrinol. 2018, 79, 98–106. [Google Scholar] [CrossRef]
- Manukhina, E.B.; Tseilikman, V.E.; Tseilikman, O.B.; Komelkova, M.; Kondashevskaya, M.; Goryacheva, A.V.; Lapshin, M.S.; Platkovskii, P.O.; Alliluev, A.V.; Downey, H.F. Intermittent hypoxia improves behavioral and adrenal gland dysfunction induced by posttraumatic stress disorder in rats. J. Appl. Physiol. 2018, 125, 931–937. [Google Scholar] [CrossRef]
- Ullmann, E.; Chrousos, G.; Perry, S.W.; Wong, M.L.; Licinio, J.; Bornstein, S.R.; Tseilikman, O.; Komelkova, M.; Lapshin, M.S.; Vasilyeva, M.; et al. Offensive Behavior, Striatal Glutamate Metabolites, and Lim-bic-Hypothalamic-Pituitary-Adrenal Responses to Stress in Chronic Anxiety. Int. J. Mol. Sci. 2020, 21, 7440. [Google Scholar] [CrossRef]
- Schwendt, M.; Shallcross, J.; Hadad, N.A.; Namba, M.D.; Hiller, H.; Wu, L.; Krause, E.; Knackstedt, L.A. A novel rat model of comorbid PTSD and addiction reveals intersections between stress susceptibility and enhanced cocaine seeking with a role for mGlu5 receptors. Transl. Psychiatry 2018, 8, 209. [Google Scholar] [CrossRef]
- Skórzewska, A.; Lehner, M.; Wisłowska-Stanek, A.; Turzyńska, D.; Sobolewska, A.; Krząścik, P.; Szyndler, J.; Maciejak, P.; Chmielewska, N.; Kołosowska, K.; et al. Individual susceptibility or resistance to posttraumatic stress disorder-like behaviours. Behav. Brain Res. 2020, 386, 112591. [Google Scholar] [CrossRef]
- Shimojo, M.; Whorwood, C.B.; Stewart, P.M. 11 beta-Hydroxysteroid dehydrogenase in the rat adrenal. J. Mol. Endocrinol. 1996, 17, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Cozza, E.N.; Zhou, M.Y.; Gomez-Sanchez, E.P.; Romero, D.G.; Gomez-Sanchez, C.E. Regulation of the 11 be-ta-hydroxysteroid dehydrogenase in the rat adrenal. Decrease enzymatic activity induced by ACTH. Endocrine 1997, 7, 331–335. [Google Scholar] [CrossRef]
- Lecybyl, R.; Jagodzinski, P.P.; Krozowski, Z.S.; Trzeciak, W.H. Regulation of 11beta-hydroxysteroid dehydrogenase type II in rat adrenocortical cells. Endocr. Res. 1998, 24, 643–647. [Google Scholar] [CrossRef]
- Kanczkowski, W.; Sue, M.; Bornstein, S.R. The adrenal gland microenvironment in health, disease and during regeneration. Hormones 2017, 16, 251–265. [Google Scholar] [CrossRef]
- Lyraki, R.; Schedl, A. Adrenal cortex renewal in health and disease. Nat. Rev. Endocrinol. 2021, 17, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Grabek, A.; Dolfi, B.; Klein, B.; Jian-Motamedi, F.; Chaboissier, M.-C.; Schedl, A. The Adult Adrenal Cortex Undergoes Rapid Tissue Renewal in a Sex-Specific Manner. Cell Stem Cell 2019, 25, 290–296.e2. [Google Scholar] [CrossRef] [PubMed]
- Finco, I.; Lerario, A.; Hammer, G.D. Sonic Hedgehog and WNT Signaling Promote Adrenal Gland Regeneration in Male Mice. Endocrinology 2018, 159, 579–596. [Google Scholar] [CrossRef] [Green Version]
- Bornstein, S.R.; Steenblock, C.; Chrousos, G.P.; Schally, A.V.; Beuschlein, F.; Kline, G.; Krone, N.P.; Licinio, J.; Wong, M.L.; Ullmann, E.; et al. Stress-inducible-stem cells: A new view on endocrine, metabolic and mental disease? Mol. Psychiatry 2019, 24, 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandiera, R.; Vidal, V.P.; Motamedi, F.J.; Clarkson, M.; Sahut-Barnola, I.; von Gise, A.; Pu, W.T.; Hohenstein, P.; Martinez, A.; Schedl, A. WT1 Maintains Adrenal-Gonadal Primordium Identity and Marks a Population of AGP-like Progenitors within the Adrenal Gland. Dev. Cell 2013, 27, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Steenblock, C.; de Celis, M.F.R.; Silva, L.F.D.; Pawolski, V.; Brennand, A.; Werdermann, M.; Berger, I.; Santambrogio, A.; Peitzsch, M.; Andoniadou, C.L.; et al. Isolation and characterization of adrenocortical progenitors involved in the adaptation to stress. Proc. Natl. Acad. Sci. USA 2018, 115, 12997–13002. [Google Scholar] [CrossRef] [Green Version]
- Bornstein, S.R.; Berger, I.; Steenblock, C. Are Nestin-positive cells responsive to stress? Stress 2020, 23, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Werdermann, M.; Berger, I.; Scriba, L.D.; Santambrogio, A.; Schlinkert, P.; Brendel, H.; Morawietz, H.; Schedl, A.; Peitzsch, M.; King, A.J.; et al. Insulin and obesity transform hypothalamic-pituitary-adrenal axis stemness and function in a hyperactive state. Mol. Metab. 2021, 43, 101112. [Google Scholar] [CrossRef]
- Cohen, H.; Zohar, J. An Animal Model of Posttraumatic Stress Disorder: The Use of Cut-Off Behavioral Criteria. Ann. N. Y. Acad. Sci. 2004, 1032, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Serova, L.I.; Laukova, M.; Alaluf, L.G.; Pucillo, L.; Sabban, E.L. Intranasal neuropeptide Y reverses anxiety and depressive-like be-havior impaired by single prolonged stress PTSD model. Eur. Neuropsychopharmacol. 2014, 24, 142–147. [Google Scholar] [CrossRef] [PubMed]
| Control (n = 20) | PSS (n = 19) | High Anxiety (n = 10) | Low Anxiety (n = 9) | |
|---|---|---|---|---|
| Time spent in open arms (s) | 115 ± 14 | 89 ± 12 | 43 ± 7 * | 108 ± 20 ### |
| Time spent in closed arms (s) | 485 ± 14 | 511 ± 14 | 557 ± 7 * | 492 ± 20 ### |
| Entries to open arms | 3.3 ± 0.4 | 2.7 ± 0.2 | 2.4 ± 0.4 | 2.7 ± 0.4 |
| Entries to closed arms | 6.9 ± 0.7 | 6.6 ± 0.6 | 8.0 ± 0.7 | 2.7 ± 0.4 *** ### |
| AI | 0.74 ± 0.02 | 0.78 ± 0.01 | 0.85 ± 0.03 ** | 0.66 ± 0.03 ### |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseilikman, V.; Komelkova, M.; Kondashevskaya, M.V.; Manukhina, E.; Downey, H.F.; Chereshnev, V.; Chereshneva, M.; Platkovskii, P.; Goryacheva, A.; Pashkov, A.; et al. A Rat Model of Post-Traumatic Stress Syndrome Causes Phenotype-Associated Morphological Changes and Hypofunction of the Adrenal Gland. Int. J. Mol. Sci. 2021, 22, 13235. https://doi.org/10.3390/ijms222413235
Tseilikman V, Komelkova M, Kondashevskaya MV, Manukhina E, Downey HF, Chereshnev V, Chereshneva M, Platkovskii P, Goryacheva A, Pashkov A, et al. A Rat Model of Post-Traumatic Stress Syndrome Causes Phenotype-Associated Morphological Changes and Hypofunction of the Adrenal Gland. International Journal of Molecular Sciences. 2021; 22(24):13235. https://doi.org/10.3390/ijms222413235
Chicago/Turabian StyleTseilikman, Vadim, Maria Komelkova, Marina V. Kondashevskaya, Eugenia Manukhina, H. Fred Downey, Valerii Chereshnev, Margarita Chereshneva, Pavel Platkovskii, Anna Goryacheva, Anton Pashkov, and et al. 2021. "A Rat Model of Post-Traumatic Stress Syndrome Causes Phenotype-Associated Morphological Changes and Hypofunction of the Adrenal Gland" International Journal of Molecular Sciences 22, no. 24: 13235. https://doi.org/10.3390/ijms222413235
APA StyleTseilikman, V., Komelkova, M., Kondashevskaya, M. V., Manukhina, E., Downey, H. F., Chereshnev, V., Chereshneva, M., Platkovskii, P., Goryacheva, A., Pashkov, A., Fedotova, J., Tseilikman, O., Maltseva, N., Cherkasova, O., Steenblock, C., Bornstein, S. R., Ettrich, B., Chrousos, G. P., & Ullmann, E. (2021). A Rat Model of Post-Traumatic Stress Syndrome Causes Phenotype-Associated Morphological Changes and Hypofunction of the Adrenal Gland. International Journal of Molecular Sciences, 22(24), 13235. https://doi.org/10.3390/ijms222413235







