Regenerative Potential of Platelet Concentrate Lysate in Mechanically Injured Cartilage and Matrix-Associated Chondrocyte Implantation In Vitro
Abstract
1. Introduction
2. Results
2.1. Apoptosis in Mechanically Injured Cartilage
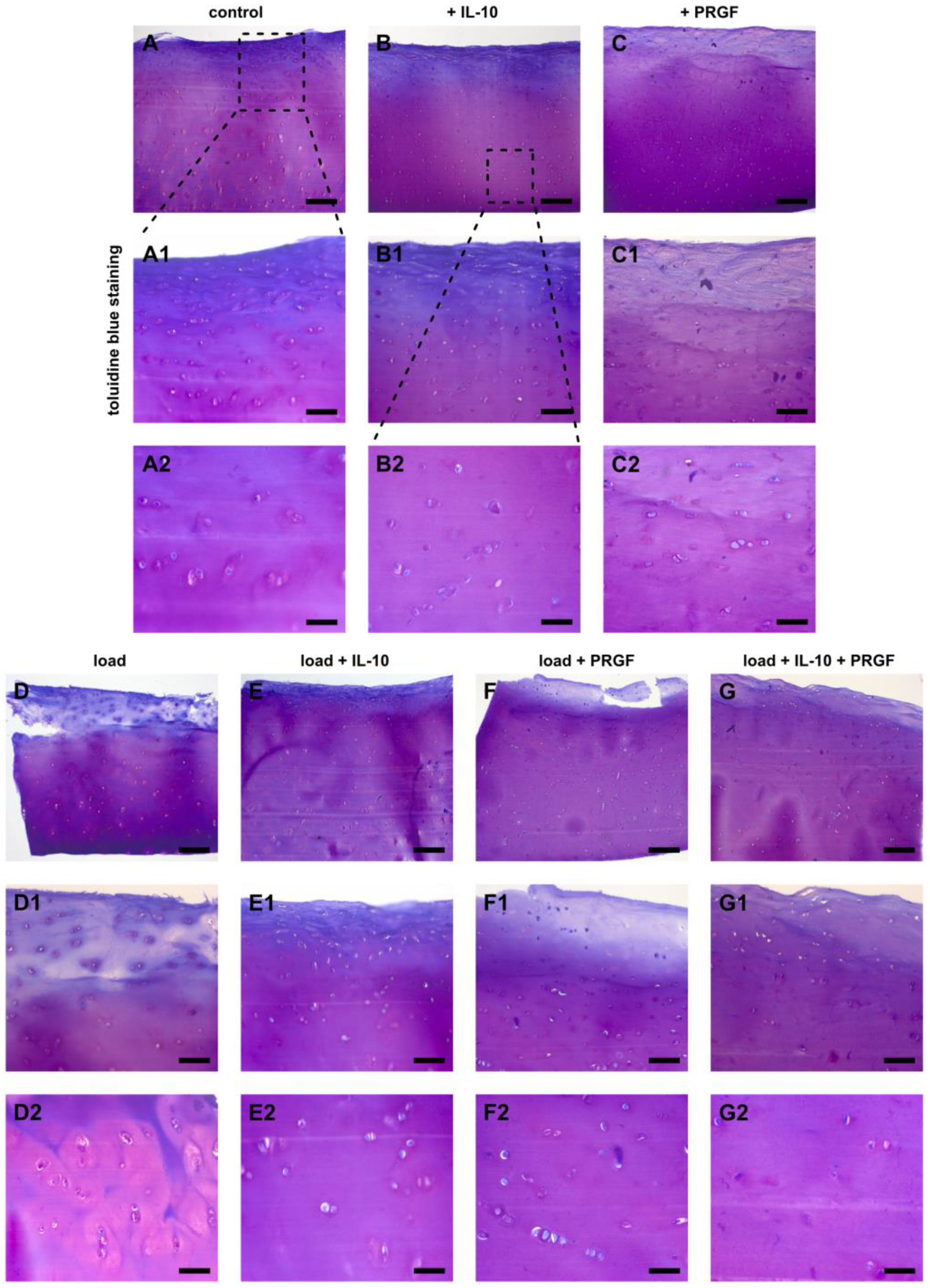
2.2. Alterations of the Chondrogenic Phenotype in Mechanically Injured Chondrocytes
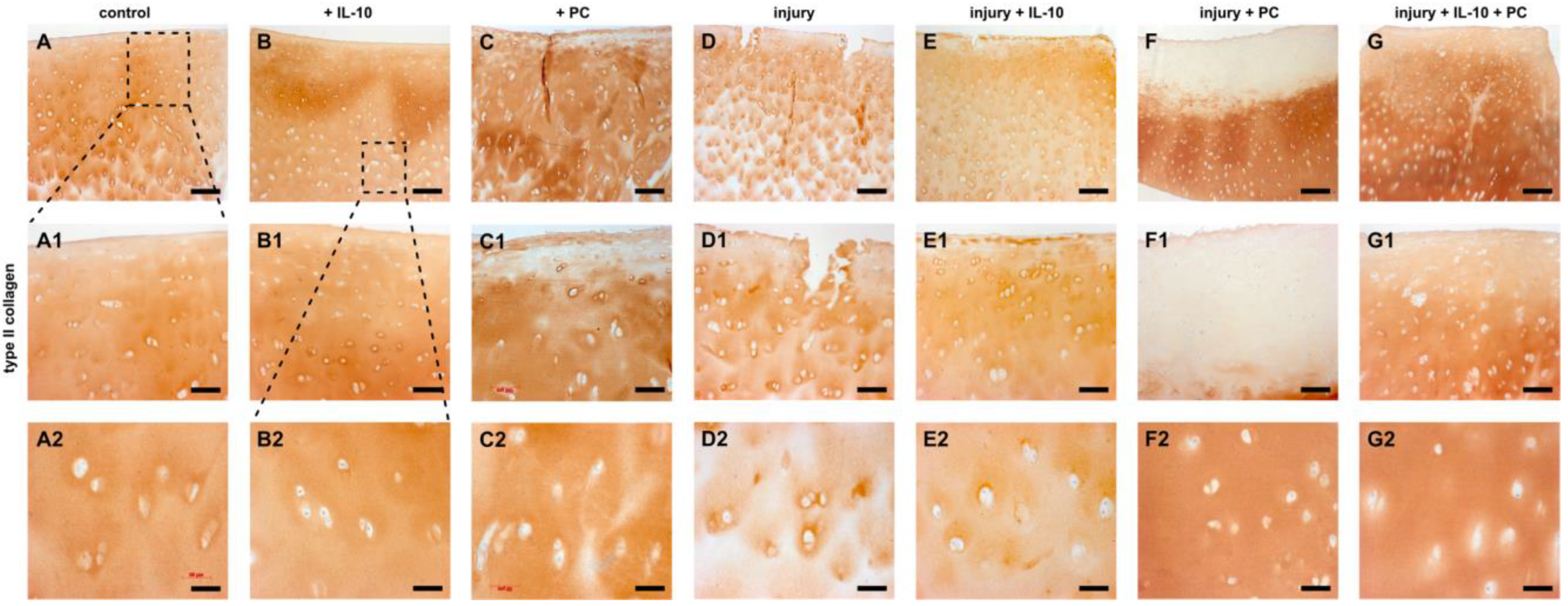
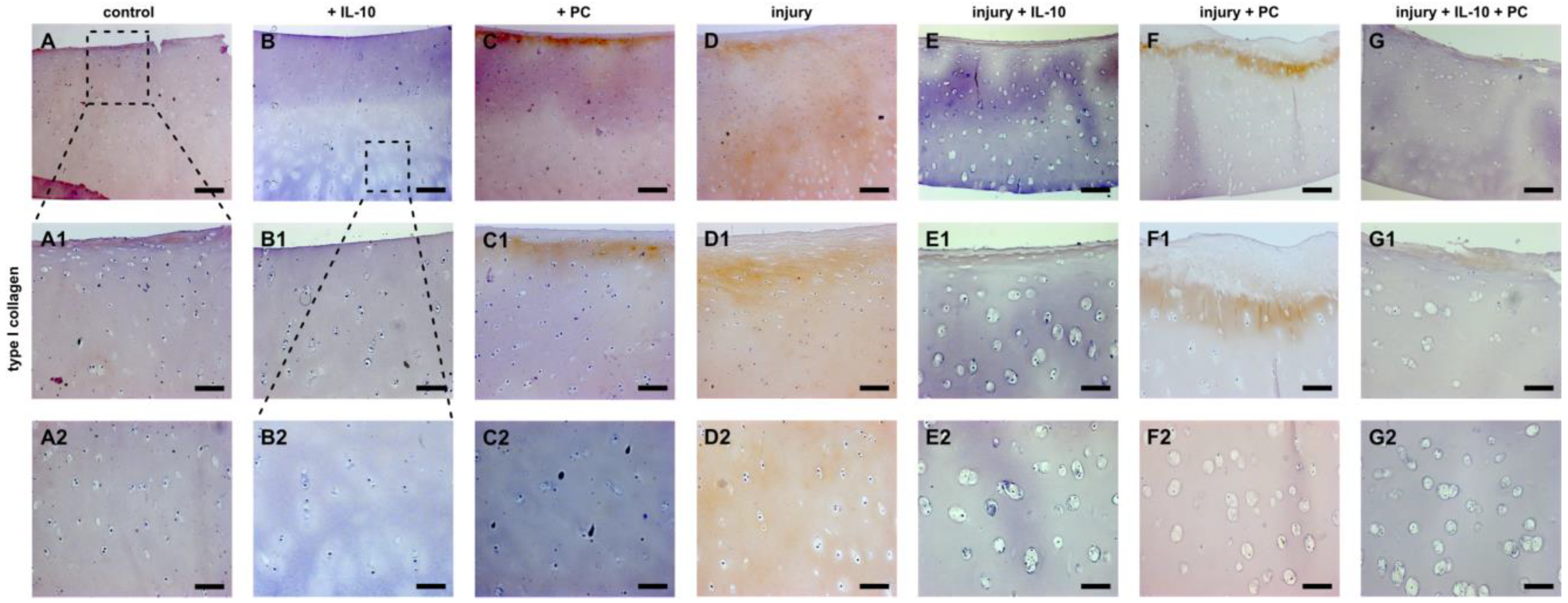
2.3. Post-Injurious Changes of Extracellular Matrix Compounds in Human Cartilage Explants
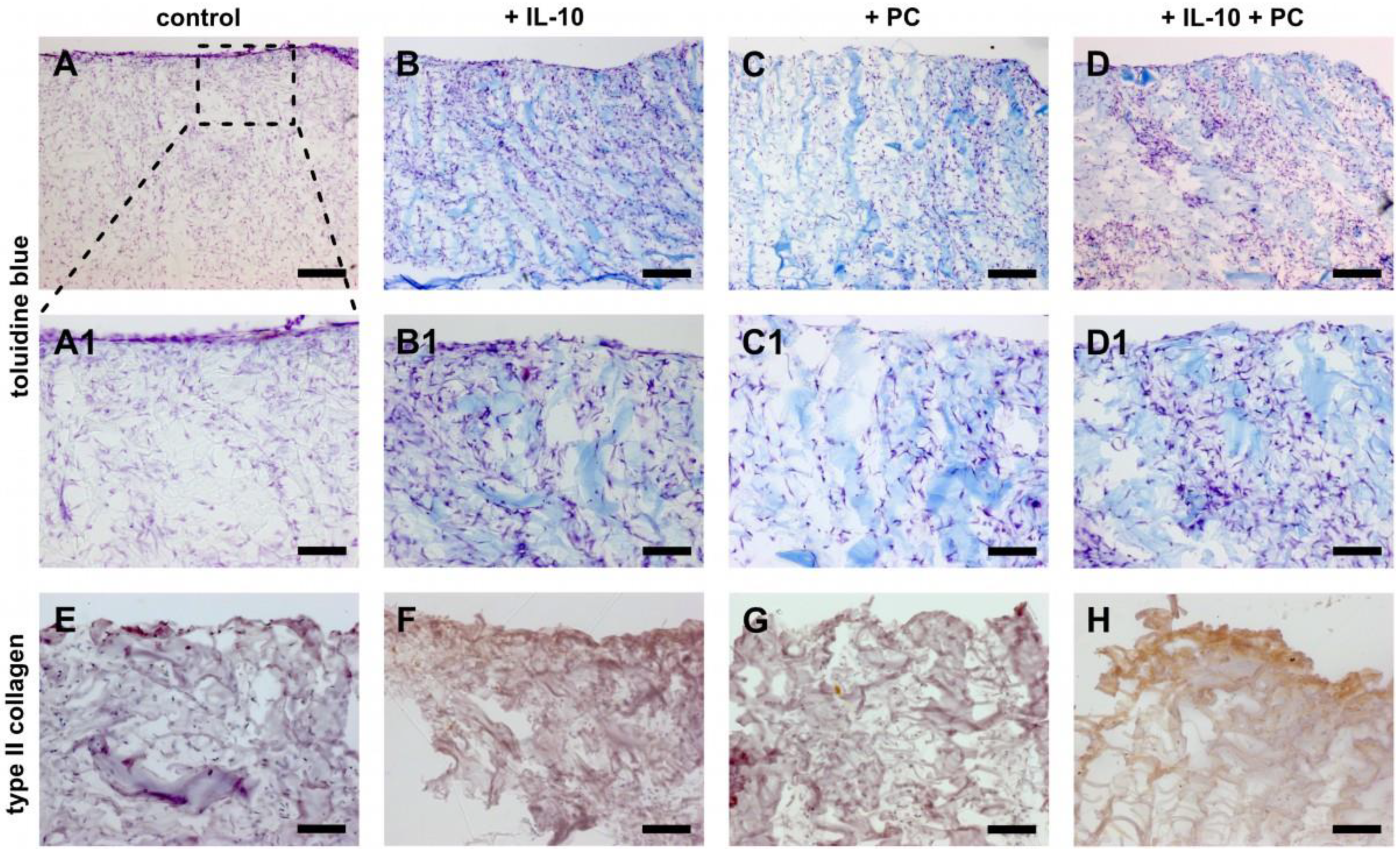
2.4. Chondrogenic Potential of IL-10 and PC Treatment in Cellularized ACI Grafts
3. Discussion
4. Materials and Methods
4.1. Preparation of PC
4.2. Isolation of Articular Cartilage Explants and Preparation of Human Chondrocyte-Laden Collagen Scaffolds
4.3. Injurious Compression and Treatment with PC and IL-10
4.4. DNA Quantification and Glycosaminoglycan Synthesis
4.5. Relative Quantification PCR (qPCR)
4.6. Histology—Detection of Cell Death, Toluidine Blue Staining and Immunohistochemistry
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Everhart, J.S.; Abouljoud, M.M.; Kirven, J.C.; Flanigan, D.C. Full-Thickness Cartilage Defects Are Important Independent Predictive Factors for Progression to Total Knee Arthroplasty in Older Adults with Minimal to Moderate Osteoarthritis: Data from the Osteoarthritis Initiative. J. Bone Jt. Surg. Am. 2019, 101, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Khella, C.M.; Asgarian, R.; Horvath, J.M.; Rolauffs, B.; Hart, M.L. An Evidence-Based Systematic Review of Human Knee Post-Traumatic Osteoarthritis (PTOA): Timeline of Clinical Presentation and Disease Markers, Comparison of Knee Joint PTOA Models and Early Disease Implications. Int. J. Mol. Sci. 2021, 22, 1996. [Google Scholar] [CrossRef]
- Hagemans, F.J.A.; Larsson, S.; Reijman, M.; Frobell, R.B.; Struglics, A.; Meuffels, D.E. An Anterior Cruciate Ligament Rupture Increases Levels of Urine N-terminal Cross-linked Telopeptide of Type I Collagen, Urine C-terminal Cross-linked Telopeptide of Type II Collagen, Serum Aggrecan ARGS Neoepitope, and Serum Tumor Necrosis Factor-α. Am. J. Sports Med. 2021, 49, 3534–3543. [Google Scholar] [CrossRef]
- Anitua, E.; Alkhraisat, M.H.; Orive, G. Perspectives and challenges in regenerative medicine using plasma rich in growth factors. J. Control Release 2012, 157, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Pauly, S.; Klatte-Schulz, F.; Stahnke, K.; Scheibel, M.; Wildemann, B. The effect of autologous platelet rich plasma on tenocytes of the human rotator cuff. BMC Musculoskelet. Disord. 2018, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Law, J.X.; Chowdhury, S.R.; Saim, A.B.; Idrus, R.B.H. Platelet-rich plasma with keratinocytes and fibroblasts enhance healing of full-thickness wounds. J. Tissue Viability 2017, 26, 208–215. [Google Scholar] [CrossRef]
- Lana, J.F.; Weglein, A.; Sampson, S.E.; Vicente, E.F.; Huber, S.C.; Souza, C.V.; Ambach, M.A.; Vincent, H.; Urban-Paffaro, A.; Onodera, C.M.; et al. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J. Stem Cells Regen. Med. 2016, 12, 69–78. [Google Scholar] [PubMed]
- Filardo, G.; Previtali, D.; Napoli, F.; Candrian, C.; Zaffagnini, S.; Grassi, A. PRP Injections for the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Cartilage 2020. [Google Scholar] [CrossRef]
- Hahn, O.; Kieb, M.; Jonitz-Heincke, A.; Bader, R.; Peters, K.; Tischer, T. Dose-Dependent Effects of Platelet-Rich Plasma Powder on Chondrocytes In Vitro. Am. J. Sports Med. 2020, 48, 1727–1734. [Google Scholar] [CrossRef]
- Lippross, S.; Loibl, M.; Hoppe, S.; Meury, T.; Benneker, L.; Alini, M.; Verrier, S. Platelet released growth factors boost expansion of bone marrow derived CD34(+) and CD133(+) endothelial progenitor cells for autologous grafting. Platelets 2011, 22, 422–432. [Google Scholar] [CrossRef]
- Anitua, E. Plasma rich in growth factors: Preliminary results of use in the preparation of future sites for implants. Int. J. Oral. Maxillofac. Implant. 1999, 14, 529–535. [Google Scholar]
- Gulliksson, H. Platelets from platelet-rich-plasma versus buffy-coat-derived platelets: What is the difference? Rev. Bras. Hematol. Hemoter. 2012, 34, 76–77. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, P.; Preusse-Prange, A.; Klüter, T.; Haake, M.; Rolauffs, B.; Grodzinsky, A.J.; Lippross, S.; Kurz, B. IL-10 reduces apoptosis and extracellular matrix degradation after injurious compression of mature articular cartilage. Osteoarthr. Cartil. 2016, 24, 1981–1988. [Google Scholar] [CrossRef]
- Behrendt, P.; Feldheim, M.; Preusse-Prange, A.; Weitkamp, J.T.; Haake, M.; Eglin, D.; Rolauffs, B.; Fay, J.; Seekamp, A.; Grodzinsky, A.J.; et al. Chondrogenic potential of IL-10 in mechanically injured cartilage and cellularized collagen ACI grafts. Osteoarthr. Cartil. 2018, 26, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Rolauffs, B.; Muehleman, C.; Li, J.; Kurz, B.; Kuettner, K.E.; Frank, E.; Grodzinsky, A.J. Vulnerability of the superficial zone of immature articular cartilage to compressive injury. Arthritis Rheum. 2010, 62, 3016–3027. [Google Scholar] [CrossRef] [PubMed]
- Kurz, B.; Domm, C.; Jin, M.; Sellckau, R.; Schünke, M. Tissue engineering of articular cartilage under the influence of collagen I/III membranes and low oxygen tension. Tissue Eng. 2004, 10, 1277–1286. [Google Scholar] [CrossRef]
- Rolauffs, B.; Kurz, B.; Felka, T.; Rothdiener, M.; Uynuk-Ool, T.; Aurich, M.; Frank, E.; Bahrs, C.; Badke, A.; Stockle, U.; et al. Stress-vs-time signals allow the prediction of structurally catastrophic events during fracturing of immature cartilage and predetermine the biomechanical, biochemical, and structural impairment. J. Struct. Biol. 2013, 183, 501–511. [Google Scholar] [CrossRef]
- Kurz, B.; Lemke, A.K.; Fay, J.; Pufe, T.; Grodzinsky, A.J.; Schunke, M. Pathomechanisms of cartilage destruction by mechanical injury. Ann. Anat. Anat. Anz. Off. Organ Anat. Ges. 2005, 187, 473–485. [Google Scholar] [CrossRef]
- Loening, A.M.; James, I.E.; Levenston, M.E.; Badger, A.M.; Frank, E.H.; Kurz, B.; Nuttall, M.E.; Hung, H.H.; Blake, S.M.; Grodzinsky, A.J.; et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch. Biochem. Biophys 2000, 381, 205–212. [Google Scholar] [CrossRef]
- Imgenberg, J.; Rolauffs, B.; Grodzinsky, A.J.; Schunke, M.; Kurz, B. Estrogen reduces mechanical injury-related cell death and proteoglycan degradation in mature articular cartilage independent of the presence of the superficial zone tissue. Osteoarthr. Cartil. 2013, 21, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Kurz, B.; Jin, M.; Patwari, P.; Cheng, D.M.; Lark, M.W.; Grodzinsky, A.J. Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J. Orthop. Res. 2001, 19, 1140–1146. [Google Scholar] [CrossRef]
- Aurich, M.; Hofmann, G.O.; Best, N.; Rolauffs, B. Induced Redifferentiation of Human Chondrocytes from Articular Cartilage Lesion in Alginate Bead Culture After Monolayer Dedifferentiation: An Alternative Cell Source for Cell-Based Therapies? Tissue Eng. Part A 2018, 24, 275–286. [Google Scholar] [CrossRef]
- Aurich, M.; Hofmann, G.O.; Gras, F.; Rolauffs, B. Human osteochondritis dissecans fragment-derived chondrocyte characteristics ex vivo, after monolayer expansion-induced de-differentiation, and after re-differentiation in alginate bead culture. BMC Musculoskelet. Disord. 2018, 19, 168. [Google Scholar] [CrossRef]
- Aurich, M.; Hofmann, G.O.; Muckley, T.; Mollenhauer, J.; Rolauffs, B. In vitro phenotypic modulation of chondrocytes from knees of patients with osteochondritis dissecans: Implications for chondrocyte implantation procedures. J. Bone Jt. Surg. Br. 2012, 94, 62–67. [Google Scholar] [CrossRef]
- Assirelli, E.; Filardo, G.; Mariani, E.; Kon, E.; Roffi, A.; Vaccaro, F.; Marcacci, M.; Facchini, A.; Pulsatelli, L. Effect of two different preparations of platelet-rich plasma on synoviocytes. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2690–2703. [Google Scholar] [CrossRef]
- Cavallo, C.; Filardo, G.; Mariani, E.; Kon, E.; Marcacci, M.; Pereira Ruiz, M.T.; Facchini, A.; Grigolo, B. Comparison of platelet-rich plasma formulations for cartilage healing: An in vitro study. J. Bone Jt. Surg. Am. 2014, 96, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.; Wijaya, B.; Möbus, L.; Rademacher, F.; Rodewald, M.; Tohidnezhad, M.; Pufe, T.; Drücke, D.; Gläser, R.; Harder, J. Platelet-Released Growth Factors and Platelet-Rich Fibrin Induce Expression of Factors Involved in Extracellular Matrix Organization in Human Keratinocytes. Int. J. Mol. Sci. 2020, 21, 4404. [Google Scholar] [CrossRef]
- Patwari, P.; Cook, M.N.; DiMicco, M.A.; Blake, S.M.; James, I.E.; Kumar, S.; Cole, A.A.; Lark, M.W.; Grodzinsky, A.J. Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: Interaction with exogenous cytokines. Arthritis Rheum. 2003, 48, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Ramezanifard, R.; Kabiri, M.; Hanaee Ahvaz, H. Effects of platelet rich plasma and chondrocyte co-culture on MSC chondrogenesis, hypertrophy and pathological responses. EXCLI J. 2017, 16, 1031–1045. [Google Scholar]
- Davies, R.L.; Kuiper, N.J. Regenerative Medicine: A Review of the Evolution of Autologous Chondrocyte Implantation (ACI) Therapy. Bioengineering 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Hulme, C.H.; Wilson, E.L.; Peffers, M.J.; Roberts, S.; Simpson, D.M.; Richardson, J.B.; Gallacher, P.; Wright, K.T. Autologous chondrocyte implantation-derived synovial fluids display distinct responder and non-responder proteomic profiles. Arthritis Res. 2017, 19, 150. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020, 2020, 8810813. [Google Scholar] [CrossRef]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Lu, L.; Bao, C. Human adipose-derived mesenchymal stem cells for osteoarthritis: A pilot study with long-term follow-up and repeated injections. Regen. Med. 2018, 13, 295–307. [Google Scholar] [CrossRef]
- Saris, T.F.F.; de Windt, T.S.; Kester, E.C.; Vonk, L.A.; Custers, R.J.H.; Saris, D.B.F. Five-Year Outcome of 1-Stage Cell-Based Cartilage Repair Using Recycled Autologous Chondrons and Allogenic Mesenchymal Stromal Cells: A First-in-Human Clinical Trial. Am. J. Sports Med. 2021, 49, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.; Lammel, J.; Rademacher, F.; Groß, J.; Siggelkow, M.; Lippross, S.; Klüter, T.; Varoga, D.; Tohidnezhad, M.; Pufe, T.; et al. Platelet-released growth factors induce the antimicrobial peptide human beta-defensin-2 in primary keratinocytes. Exp. Derm. 2016, 25, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Patwari, P.; Gaschen, V.; James, I.E.; Berger, E.; Blake, S.M.; Lark, M.W.; Grodzinsky, A.J.; Hunziker, E.B. Ultrastructural quantification of cell death after injurious compression of bovine calf articular cartilage. Osteoarthr. Cartil. 2004, 12, 245–252. [Google Scholar] [CrossRef][Green Version]
- Gille, J.; Kunow, J.; Boisch, L.; Behrens, P.; Bos, I.; Hoffmann, C.; Köller, W.; Russlies, M.; Kurz, B. Cell-Laden and Cell-Free Matrix-Induced Chondrogenesis versus Microfracture for the Treatment of Articular Cartilage Defects: A Histological and Biomechanical Study in Sheep. Cartilage 2010, 1, 29–42. [Google Scholar] [CrossRef] [PubMed]



| COL2A1 | Mean | ±SD # | p† | |
| a | +IL-10 | 1.24 | 0.65 | ** e, ** f |
| b | +PC | 2.1 | 1.4 | ** e, * f |
| c | injury | 1.53 | 0.9 | ** e, * f |
| d | injury + IL-10 | 3.74 | 2.19 | ns |
| e | injury + PC | 7.56 | 5.5 | ns |
| f | injury + IL-10 + PC | 7.44 | 3.91 | ns |
| ACAN | Mean | ±SD | p | |
| a | +IL-10 | 1.14 | 0.64 | ** e, * f |
| b | +PC | 2.74 | 2 | * e |
| c | injury | 2.13 | 1.34 | ** e |
| d | injury + IL-10 | 4.44 | 2.68 | ns |
| e | injury + PC | 7.22 | 5 | ns |
| f | injury + IL-10 + PC | 5.88 | 2.87 | ns |
| SOX9 | Mean | ±SD | p | |
| a | +IL-10 | 2.21 | 0.81 | ns |
| b | +PC | 5.88 | 5.79 | ** c |
| c | injury | 0.63 | 0.37 | ns |
| d | injury + IL-10 | 1.94 | 1.74 | ns |
| e | injury + PC | 4.24 | 2.8 | ns |
| f | injury + IL-10 + PC | 2.33 | 0.97 | ns |
| COL1A1 | Mean | ±SD | p | |
| a | +IL-10 | 1.02 | 1.3 | * c |
| b | +PC | 1.88 | 1.59 | ns |
| c | injury | 8.89 | 9.07 | ns |
| d | injury + IL-10 | 4.19 | 4.42 | ns |
| e | injury + PC | 4.53 | 5.53 | ns |
| f | injury + IL-10 + PC | 2.03 | 2.01 | ns |
| COL10A1 | Mean | ±SD | p | |
| a | +IL-10 | 0.61 | 0.51 | **** c |
| b | +PC | 0.9 | 0.58 | **** c |
| c | injury | 2.84 | 1.38 | ns |
| d | injury + IL-10 | 0.59 | 0.43 | **** c |
| e | injury + PC | 0.67 | 0.62 | **** c |
| f | injury + IL-10 + PC | 0.25 | 0.36 | **** c |
| COL2/1 ratio | Mean | ±SD | p | |
| a | +IL-10 | 1.98 | 2.27 | ns |
| b | +PC | 1.87 | 1.48 | ns |
| c | injury | 0.78 | 0.82 | ns |
| d | injury + IL-10 | 3.9 | 4.05 | ns |
| e | injury + PC | 2.35 | 2.71 | ns |
| f | injury + IL-10 + PC | 2.95 | 2.34 | ns |
| COL2A1 | Mean | ±SD # | p † | |
| a | +IL-10 | 0.82 | 0.24 | ** c |
| b | +PC | 1.09 | 0.16 | * c |
| c | +IL-10 + PC | 1.66 | 0.29 | ns |
| ACAN | Mean | ±SD | p | |
| a | +IL-10 | 2.8 | 0.66 | ns |
| b | +PC | 2.78 | 1.49 | ns |
| c | +IL-10 + PC | 2.41 | 1.34 | ns |
| COL1A1 | Mean | ±SD | p | |
| a | +IL-10 | 0.81 | 0.21 | ns |
| b | +PC | 1.04 | 0.53 | ** c |
| c | +IL-10 + PC | 0.31 | 0.1 | ns |
| COL10A1 | Mean | ±SD | p | |
| a | +IL-10 | 1.6 | 0.66 | ns |
| b | +PC | 2.12 | 1.49 | ns |
| c | +IL-10 + PC | 1.04 | 1.34 | ns |
| COL2/1 ratio | Mean | ±SD | p | |
| a | +IL-10 | 0.94 | 0.12 | ** c |
| b | +PC | 1.3 | 0.8 | ** c |
| c | +IL-10 + PC | 5.91 | 1.77 | ns |
| Human Target | Sequence (5′–3′) Sense | Sequence (5′–3′) Antisense |
|---|---|---|
| ACAN | GAGGCCAGCAGAGAAGATTCTG | GACGCCTCGCCTTCTTGAA |
| COL2A1 | CAACACTGCCAACGTCCAGAT | CTGCTTCGTCCAGATAGGCAAT |
| SOX9 | CTCGGAGACTTCTGAACGAGAG | CGTTCTTCACCGACTTCCTCC |
| COL1A1 COL10A1 | AATTCCAAGGCCAAGAAGCATG CCCTTTTTGCTGCTAGTATCCTTGA | GGTAGCCATTTCCTTGGTGGTT AACTGTGTCTTGGTGTTGGGTAGTG |
| GAPDH | GCCTCAAGATCATCAGCAATGC | TGGTCATGAGTCCTTCCACGAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weitkamp, J.-T.; Rolauffs, B.; Feldheim, M.; Bayer, A.; Lippross, S.; Weuster, M.; Smeets, R.; Naujokat, H.; Grodzinsky, A.J.; Kurz, B.; et al. Regenerative Potential of Platelet Concentrate Lysate in Mechanically Injured Cartilage and Matrix-Associated Chondrocyte Implantation In Vitro. Int. J. Mol. Sci. 2021, 22, 13179. https://doi.org/10.3390/ijms222413179
Weitkamp J-T, Rolauffs B, Feldheim M, Bayer A, Lippross S, Weuster M, Smeets R, Naujokat H, Grodzinsky AJ, Kurz B, et al. Regenerative Potential of Platelet Concentrate Lysate in Mechanically Injured Cartilage and Matrix-Associated Chondrocyte Implantation In Vitro. International Journal of Molecular Sciences. 2021; 22(24):13179. https://doi.org/10.3390/ijms222413179
Chicago/Turabian StyleWeitkamp, Jan-Tobias, Bernd Rolauffs, Moritz Feldheim, Andreas Bayer, Sebastian Lippross, Matthias Weuster, Ralf Smeets, Hendrik Naujokat, Alan Jay Grodzinsky, Bodo Kurz, and et al. 2021. "Regenerative Potential of Platelet Concentrate Lysate in Mechanically Injured Cartilage and Matrix-Associated Chondrocyte Implantation In Vitro" International Journal of Molecular Sciences 22, no. 24: 13179. https://doi.org/10.3390/ijms222413179
APA StyleWeitkamp, J.-T., Rolauffs, B., Feldheim, M., Bayer, A., Lippross, S., Weuster, M., Smeets, R., Naujokat, H., Grodzinsky, A. J., Kurz, B., & Behrendt, P. (2021). Regenerative Potential of Platelet Concentrate Lysate in Mechanically Injured Cartilage and Matrix-Associated Chondrocyte Implantation In Vitro. International Journal of Molecular Sciences, 22(24), 13179. https://doi.org/10.3390/ijms222413179








