Anti-Inflammatory Therapeutic Approaches to Prevent or Delay Post-Traumatic Osteoarthritis (PTOA) of the Knee Joint with a Focus on Sustained Delivery Approaches
Abstract
1. Introduction
2. Methodology
3. A Brief Description of the Cells and Tissues That Participate in the Pathogenesis and Progression of PTOA within the Knee Joint
4. Anti-Inflammatory Therapeutic Interventions to Prevent or Treat PTOA of the Knee Joint
4.1. Dexamethasone
4.2. Triamcinolone Acetonide (TCA)
4.3. Hyaluronic Acid (HA)
4.4. Inhibitors of TNF-α and Interleukin-1 Receptor Antagonist (IL-1ra)
4.5. Anti-IL-6 Receptor Antibody
4.6. Anti-Inflammatory Cytokines IL-4, IL-10 and IL-13
4.7. Complement Inhibitors
4.8. Tranexamic Acid (TXA)
5. Possible Sustained Delivery Approaches to Prevent or Delay Knee PTOA
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACAN | Aggrecan |
| ACLT | Anterior cruciate ligament transection |
| ACS | autologous conditioned serum |
| ADAMTS | A disintegrin and metalloproteinase with thrombospondin motifs |
| bFGF | Basic fibroblast growth factor |
| C1INH | C1 inhibitor |
| COLI | Collagen I |
| COLII | Collagen II |
| COMP | Cartilage oligomeric matrix protein |
| CTX-I | C-terminal crosslinked telopeptide type I collagen |
| CTX-II | C-terminal crosslinked telopeptide type II collagen |
| CXCL | Chemokine ligand |
| ECM | Extracellular matrix |
| ELP | Elastine-like polypeptide |
| GAG | Glycosaminoglycan |
| GlcNAc | N-acetylglucosamine |
| gp130 | Glycoprotein 130 |
| HA | Hyaluronic acid (HA) |
| IFN-ɣ | Interferon-gamma |
| IGF-1 | Insulin-like growth factor 1 |
| IL | Interleukin |
| IL-1ra | IL-1 receptor antagonist |
| IL-6R | IL-6 receptor |
| i.a. | Intra-articular |
| i.p. | Intraperitoneal |
| i.v. | Intravenous |
| iNOS | Inducible NO synthase |
| IGF-1 | Insulin-like growth factor 1 |
| kDa | Kilodalton |
| KOOS | Knee Injury and Osteoarthritis Outcome Score |
| LPS | Lipopolysaccharide |
| MAC | Membrane attack complex |
| MCLT | medial collateral ligament transection |
| MSC | Mesenchymal stem/stromal cells |
| MCP-1 | Monocyte chemoattractant protein-1, also known as CCL2 |
| MIP-1 | Macrophage inflammatory protein-1 |
| MMPs | Matrix metalloproteinases |
| MW | molecular weight |
| MRI | Magnetic resonance imaging |
| NO | Nitric oxide |
| NOS2 | Nitric Oxide Synthase 2 |
| NSAIDS | nonsteroidal anti-inflammatory drugs |
| OA | Osteoarthritis |
| PCM | Pericellular matrix |
| PDGF | Platelet-derived growth factor |
| PEA | Polyester amide |
| PGPS | peptidoglycan-polysaccharide streptococcal |
| PEG | Polyethylene glycol |
| PGE2 | Prostaglandin E₂ |
| PLGA | Poly (lactic-co-glycolic acid) |
| PTOA | Post-traumatic osteoarthritis |
| RA | Rheumatoid arthritis |
| s.c. | subcutaneous |
| sIL-6R | Soluble IL-6 receptor |
| sTNFRII | Soluble TNF receptor II |
| TCA | Triamcinolone acetonide |
| TCC | Terminal complement complex |
| TACE | Tumor necrosis factor-α converting enzyme |
| TGF-β | transforming growth factor-beta |
| TIMP | Tissue inhibitor of metalloproteinases |
| TNF-α | Tumor necrosis factor-alpha |
| Th | T helper |
| TSG-6 | TNF-stimulated gene 6 protein |
| TXA | Tranexamic Acid |
| WOMAC | The Western Ontario and McMaster Universities Osteoarthritis |
References
- Brown, T.D.; Johnston, R.C.; Saltzman, C.L.; Marsh, J.L.; Buckwalter, J.A. Posttraumatic osteoarthritis: A first estimate of incidence, prevalence, and burden of disease. J. Orthop. Trauma 2006, 20, 739–744. [Google Scholar] [CrossRef]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. E. Clin. Med. 2020, 29, 100587. [Google Scholar]
- Khella, C.M.; Asgarian, R.; Horvath, J.M.; Rolauffs, B.; Hart, M.L. An evidence-based systematic review of human knee post-traumatic osteoarthritis (ptoa): Timeline of clinical presentation and disease markers, comparison of knee joint ptoa models and early disease implications. Int. J. Mol. Sci. 2021, 22, 1996. [Google Scholar] [CrossRef]
- Schenker, M.L.; Mauck, R.L.; Ahn, J.; Mehta, S. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J. Am. Acad. Orthop. Surg. 2014, 22, 20–28. [Google Scholar] [CrossRef]
- Lohmander, L.S.; Englund, P.M.; Dahl, L.L.; Roos, E.M. The long-term consequence of anterior cruciate ligament and meniscus injuries: Osteoarthritis. Am. J. Sports Med. 2007, 35, 1756–1769. [Google Scholar] [CrossRef] [PubMed]
- Gelber, A.C.; Hochberg, M.C.; Mead, L.A.; Wang, N.Y.; Wigley, F.M.; Klag, M.J. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann. Intern. Med. 2000, 133, 321–328. [Google Scholar] [CrossRef]
- Gillquist, J.; Messner, K. Anterior cruciate ligament reconstruction and the long-term incidence of gonarthrosis. Sports Med. 1999, 27, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Roos, H.; Lauren, M.; Adalberth, T.; Roos, E.M.; Jonsson, K.; Lohmander, L.S. Knee osteoarthritis after meniscectomy: Prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum 1998, 41, 687–693. [Google Scholar] [CrossRef]
- Laird, A.; Keating, J.F. Acetabular fractures: A 16-year prospective epidemiological study. J. Bone Jt. Surg. Br. 2005, 87, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Weigel, D.P.; Marsh, J.L. High-energy fractures of the tibial plateau. Knee function after longer follow-up. J. Bone Jt. Surg. 2002, 84, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Zhang, Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum 1998, 41, 1343–1355. [Google Scholar] [CrossRef]
- Muthuri, S.G.; McWilliams, D.F.; Doherty, M.; Zhang, W. History of knee injuries and knee osteoarthritis: A meta-analysis of observational studies. Osteoarthr. Cartil. 2011, 19, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Lohmander, L.S.; Struglics, A. Synovial fluid level of aggrecan args fragments is a more sensitive marker of joint disease than glycosaminoglycan or aggrecan levels: A cross-sectional study. Arthritis Res. 2009, 11, R92. [Google Scholar] [CrossRef] [PubMed]
- Panina, S.B.; Krolevets, I.V.; Milyutina, N.P.; Sagakyants, A.B.; Kornienko, I.V.; Ananyan, A.A.; Zabrodin, M.A.; Plotnikov, A.A.; Vnukov, V.V. Circulating levels of proinflammatory mediators as potential biomarkers of post-traumatic knee osteoarthritis development. J. Orthop. Traumatol. 2017, 18, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Roos, E.M. Joint injury causes knee osteoarthritis in young adults. Curr. Opin. Rheumatol. 2005, 17, 195–200. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Roos, E.M. A pragmatic approach to prevent post-traumatic osteoarthritis after sport or exercise-related joint injury. Best Pract. Res. Clin. Rheumatol. 2019, 33, 158–171. [Google Scholar] [CrossRef]
- Smith, J.K. Exercise as an adjuvant to cartilage regeneration therapy. Int. J. Mol. Sci. 2020, 21, 9471. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.; Evans, M.; Tucker, M.; Quilty, B.; Dieppe, P.; Donovan, J.L. Why don’t patients do their exercises? Understanding non-compliance with physiotherapy in patients with osteoarthritis of the knee. J. Epidemiol. Community Health 2001, 55, 132–138. [Google Scholar] [CrossRef]
- Larsen, C.; Ostergaard, J.; Larsen, S.W.; Jensen, H.; Jacobsen, S.; Lindegaard, C.; Andersen, P.H. Intra-articular depot formulation principles: Role in the management of postoperative pain and arthritic disorders. J. Pharm. Sci. 2008, 97, 4622–4654. [Google Scholar] [CrossRef]
- Testa, G.; Giardina, S.M.C.; Culmone, A.; Vescio, A.; Turchetta, M.; Cannavo, S.; Pavone, V. Intra-articular injections in knee osteoarthritis: A review of literature. J. Funct. Morphol. Kinesiol. 2021, 6, 15. [Google Scholar] [CrossRef]
- Urech, D.M.; Feige, U.; Ewert, S.; Schlosser, V.; Ottiger, M.; Polzer, K.; Schett, G.; Lichtlen, P. Anti-inflammatory and cartilage-protecting effects of an intra-articularly injected anti-tnf{alpha} single-chain fv antibody (esba105) designed for local therapeutic use. Ann. Rheum. Dis. 2010, 69, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Khodoun, M.; Lewis, C.C.; Yang, J.Q.; Orekov, T.; Potter, C.; Wynn, T.; Mentink-Kane, M.; Hershey, G.K.; Wills-Karp, M.; Finkelman, F.D. Differences in expression, affinity, and function of soluble (s)il-4ralpha and sil-13ralpha2 suggest opposite effects on allergic responses. J. Immunol. 2007, 179, 6429–6438. [Google Scholar] [CrossRef]
- Huhn, R.D.; Radwanski, E.; O’Connell, S.M.; Sturgill, M.G.; Clarke, L.; Cody, R.P.; Affrime, M.B.; Cutler, D.L. Pharmacokinetics and immunomodulatory properties of intravenously administered recombinant human interleukin-10 in healthy volunteers. Blood 1996, 87, 699–705. [Google Scholar] [CrossRef]
- Huhn, R.D.; Yurkow, E.J.; Kuhn, J.G.; Clarke, L.; Gunn, H.; Resta, D.; Shah, R.; Myers, L.A.; Seibold, J.R. Pharmacodynamics of daily subcutaneous recombinant human interleukin-3 in normal volunteers. Clin. Pharm. 1995, 57, 32–41. [Google Scholar] [CrossRef]
- Kraus, V.B.; Birmingham, J.; Stabler, T.V.; Feng, S.; Taylor, D.C.; Moorman, C.T., 3rd; Garrett, W.E.; Toth, A.P. Effects of intraarticular il1-ra for acute anterior cruciate ligament knee injury: A randomized controlled pilot trial (nct00332254). Osteoarthr. Cartil. 2012, 20, 271–278. [Google Scholar] [CrossRef]
- Chou, C.H.; Jain, V.; Gibson, J.; Attarian, D.E.; Haraden, C.A.; Yohn, C.B.; Laberge, R.M.; Gregory, S.; Kraus, V.B. Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci. Rep. 2020, 10, 10868. [Google Scholar] [CrossRef]
- Sebastian, A.; McCool, J.L.; Hum, N.R.; Murugesh, D.K.; Wilson, S.P.; Christiansen, B.A.; Loots, G.G. Single-cell rna-seq reveals transcriptomic heterogeneity and post-traumatic osteoarthritis-associated early molecular changes in mouse articular chondrocytes. Cells 2021, 10, 1462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, N.; Huang, R.; Wang, L.; Ke, Q.; Cai, L.; Wu, S. Single-cell rna seq analysis identifies the biomarkers and differentiation of chondrocyte in human osteoarthritis. Am. J. Transl. Res. 2020, 12, 7326–7339. [Google Scholar]
- Ji, Q.; Zheng, Y.; Zhang, G.; Hu, Y.; Fan, X.; Hou, Y.; Wen, L.; Li, L.; Xu, Y.; Wang, Y.; et al. Single-cell rna-seq analysis reveals the progression of human osteoarthritis. Ann. Rheum. Dis. 2019, 78, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Culemann, S.; Gruneboom, A.; Nicolas-Avila, J.A.; Weidner, D.; Lammle, K.F.; Rothe, T.; Quintana, J.A.; Kirchner, P.; Krljanac, B.; Eberhardt, M.; et al. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature 2019, 572, 670–675. [Google Scholar] [CrossRef]
- Belluzzi, E.; Stocco, E.; Pozzuoli, A.; Granzotto, M.; Porzionato, A.; Vettor, R.; De Caro, R.; Ruggieri, P.; Ramonda, R.; Rossato, M.; et al. Contribution of infrapatellar fat pad and synovial membrane to knee osteoarthritis pain. BioMed. Res. Int. 2019, 2019, 6390182. [Google Scholar] [CrossRef]
- Faust, H.J.; Zhang, H.; Han, J.; Wolf, M.T.; Jeon, O.H.; Sadtler, K.; Pena, A.N.; Chung, L.; Maestas, D.R., Jr.; Tam, A.J.; et al. Il-17 and immunologically induced senescence regulate response to injury in osteoarthritis. J. Clin. Investig. 2020, 130, 5493–5507. [Google Scholar] [CrossRef]
- Xu, H.; Edwards, J.; Banerji, S.; Prevo, R.; Jackson, D.G.; Athanasou, N.A. Distribution of lymphatic vessels in normal and arthritic human synovial tissues. Ann. Rheum. Dis. 2003, 62, 1227–1229. [Google Scholar] [CrossRef]
- Wechalekar, M.D.; Smith, M.D. Utility of arthroscopic guided synovial biopsy in understanding synovial tissue pathology in health and disease states. World J. Orthop. 2014, 5, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tang, Y.; Song, B.; Yu, M.; Li, Q.; Zhang, C.; Hou, J.; Yang, R. Nomenclature clarification: Synovial fibroblasts and synovial mesenchymal stem cells. Stem. Cell Res. Ther. 2019, 10, 260. [Google Scholar] [CrossRef]
- Berkelaar, M.H.M.; Korthagen, N.M.; Jansen, G.; van Spil, W.E. Synovial macrophages: Potential key modulators of cartilage damage, osteophyte formation and pain in knee osteoarthritis. J. Rheum. Dis. Treat. 2018, 4. [Google Scholar] [CrossRef][Green Version]
- Macchi, V.; Stocco, E.; Stecco, C.; Belluzzi, E.; Favero, M.; Porzionato, A.; De Caro, R. The infrapatellar fat pad and the synovial membrane: An anatomo-functional unit. J. Anat. 2018, 233, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Greif, D.N.; Kouroupis, D.; Murdock, C.J.; Griswold, A.J.; Kaplan, L.D.; Best, T.M.; Correa, D. Infrapatellar fat pad/synovium complex in early-stage knee osteoarthritis: Potential new target and source of therapeutic mesenchymal stem/stromal cells. Front. Bioeng. Biotechnol. 2020, 8, 860. [Google Scholar] [CrossRef] [PubMed]
- Ioan-Facsinay, A.; Kloppenburg, M. An emerging player in knee osteoarthritis: The infrapatellar fat pad. Arthritis Res 2013, 15, 225. [Google Scholar] [CrossRef]
- Heilmeier, U.; Mamoto, K.; Amano, K.; Eck, B.; Tanaka, M.; Bullen, J.A.; Schwaiger, B.J.; Huebner, J.L.; Stabler, T.V.; Kraus, V.B.; et al. Infrapatellar fat pad abnormalities are associated with a higher inflammatory synovial fluid cytokine profile in young adults following acl tear. Osteoarthr. Cartil. 2020, 28, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.F.; Fang, J.H.; Wu, L.D. Role of infrapatellar fat pad in pathological process of knee osteoarthritis: Future applications in treatment. World J. Clin. Cases 2019, 7, 2134–2142. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretation. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Zappia, J.; Sanchez, C.; Florin, A.; Dubuc, J.E.; Henrotin, Y. The damage-associated molecular patterns (damps) as potential targets to treat osteoarthritis: Perspectives from a review of the literature. Front. Med. 2020, 7, 607186. [Google Scholar] [CrossRef]
- Relja, B.; Land, W.G. Damage-associated molecular patterns in trauma. Eur. J. Trauma Emerg. Surg. 2020, 46, 751–775. [Google Scholar] [CrossRef]
- Riegger, J.; Huber-Lang, M.; Brenner, R.E. Crucial role of the terminal complement complex in chondrocyte death and hypertrophy after cartilage trauma. Osteoarthr. Cartil. 2020, 28, 685–697. [Google Scholar] [CrossRef]
- Rosshirt, N.; Trauth, R.; Platzer, H.; Tripel, E.; Nees, T.A.; Lorenz, H.M.; Tretter, T.; Moradi, B. Proinflammatory T cell polarization is already present in patients with early knee osteoarthritis. Arthritis Res. 2021, 23, 37. [Google Scholar] [CrossRef]
- Moradi, B.; Schnatzer, P.; Hagmann, S.; Rosshirt, N.; Gotterbarm, T.; Kretzer, J.P.; Thomsen, M.; Lorenz, H.M.; Zeifang, F.; Tretter, T. Cd4(+)cd25(+)/highcd127low/(-) regulatory t cells are enriched in rheumatoid arthritis and osteoarthritis joints-analysis of frequency and phenotype in synovial membrane, synovial fluid and peripheral blood. Arthritis Res. 2014, 16, R97. [Google Scholar] [CrossRef] [PubMed]
- Attur, M.; Belitskaya-Levy, I.; Oh, C.; Krasnokutsky, S.; Greenberg, J.; Samuels, J.; Smiles, S.; Lee, S.; Patel, J.; Al-Mussawir, H.; et al. Increased interleukin-1beta gene expression in peripheral blood leukocytes is associated with increased pain and predicts risk for progression of symptomatic knee osteoarthritis. Arthritis Rheum. 2011, 63, 1908–1917. [Google Scholar] [CrossRef]
- Bojarski, K.K.; Karczynska, A.S.; Samsonov, S.A. Role of glycosaminoglycans in procathepsin b maturation: Molecular mechanism elucidated by a computational study. J. Chem. Inf. Model 2020, 60, 2247–2256. [Google Scholar] [CrossRef]
- Grodzinsky, A.J.; Wang, Y.; Kakar, S.; Vrahas, M.S.; Evans, C.H. Intra-articular dexamethasone to inhibit the development of post-traumatic osteoarthritis. J. Orthop. Res. 2017, 35, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Chubinskaya, S.; Schoeberl, B.; Florine, E.; Kopesky, P.; Grodzinsky, A.J. Effects of insulin-like growth factor-1 and dexamethasone on cytokine-challenged cartilage: Relevance to post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 266–274. [Google Scholar] [CrossRef]
- Lu, Y.C.; Evans, C.H.; Grodzinsky, A.J. Effects of short-term glucocorticoid treatment on changes in cartilage matrix degradation and chondrocyte gene expression induced by mechanical injury and inflammatory cytokines. Arthritis Res. 2011, 13, R142. [Google Scholar] [CrossRef]
- Bajpayee, A.G.; Quadir, M.A.; Hammond, P.T.; Grodzinsky, A.J. Charge based intra-cartilage delivery of single dose dexamethasone using avidin nano-carriers suppresses cytokine-induced catabolism long term. Osteoarthr. Cartil. 2016, 24, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Mapp, P.I.; Walsh, D.A. Contributions of angiogenesis to inflammation, joint damage, and pain in a rat model of osteoarthritis. Arthritis Rheum. 2011, 63, 2700–2710. [Google Scholar] [CrossRef] [PubMed]
- Huebner, K.D.; Shrive, N.G.; Frank, C.B. Dexamethasone inhibits inflammation and cartilage damage in a new model of post-traumatic osteoarthritis. J. Orthop. Res. 2014, 32, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Heard, B.J.; Barton, K.I.; Chung, M.; Achari, Y.; Shrive, N.G.; Frank, C.B.; Hart, D.A. Single intra-articular dexamethasone injection immediately post-surgery in a rabbit model mitigates early inflammatory responses and post-traumatic osteoarthritis-like alterations. J. Orthop. Res. 2015, 33, 1826–1834. [Google Scholar] [CrossRef]
- Black, R.; Grodzinsky, A.J. Dexamethasone: Chondroprotective corticosteroid or catabolic killer? Eur. Cells Mater. 2019, 38, 246–263. [Google Scholar] [CrossRef]
- Malfait, A.M.; Tortorella, M.; Thompson, J.; Hills, R.; Meyer, D.M.; Jaffee, B.D.; Chinn, K.; Ghoreishi-Haack, N.; Markosyan, S.; Arner, E.C. Intra-articular injection of tumor necrosis factor-alpha in the rat: An acute and reversible in vivo model of cartilage proteoglycan degradation. Osteoarthr. Cartil. 2009, 17, 627–635. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boehme, K.A.; Rolauffs, B. Onset and progression of human osteoarthritis-can growth factors, inflammatory cytokines, or differential mirna expression concomitantly induce proliferation, ecm degradation, and inflammation in articular cartilage? Int. J. Mol. Sci. 2018, 19, 2282. [Google Scholar] [CrossRef]
- Richardson, D.W.; Dodge, G.R. Dose-dependent effects of corticosteroids on the expression of matrix-related genes in normal and cytokine-treated articular chondrocytes. Inflamm. Res. 2003, 52, 39–49. [Google Scholar] [CrossRef]
- Shalom-Barak, T.; Quach, J.; Lotz, M. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and nf-kappab. J. Biol. Chem. 1998, 273, 27467–27473. [Google Scholar] [CrossRef]
- Bauer, C.; Niculescu-Morzsa, E.; Jeyakumar, V.; Kern, D.; Spath, S.S.; Nehrer, S. Chondroprotective effect of high-molecular-weight hyaluronic acid on osteoarthritic chondrocytes in a co-cultivation inflammation model with m1 macrophages. J. Inflamm. 2016, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Bajpayee, A.G.; Scheu, M.; Grodzinsky, A.J.; Porter, R.M. Electrostatic interactions enable rapid penetration, enhanced uptake and retention of intra-articular injected avidin in rat knee joints. J. Orthop. Res. 2014, 32, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Bajpayee, A.G.; Scheu, M.; Grodzinsky, A.J.; Porter, R.M. A rabbit model demonstrates the influence of cartilage thickness on intra-articular drug delivery and retention within cartilage. J. Orthop. Res. 2015, 33, 660–667. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Zhang, C.; Vedadghavami, A.; Mehta, S.; Clark, H.A.; Porter, R.M.; Bajpayee, A.G. Multi-arm avidin nano-construct for intra-cartilage delivery of small molecule drugs. J. Control. Release Off. J. Control. Release Soc. 2020, 318, 109–123. [Google Scholar] [CrossRef]
- Bajpayee, A.G.; De la Vega, R.E.; Scheu, M.; Varady, N.H.; Yannatos, I.A.; Brown, L.A.; Krishnan, Y.; Fitzsimons, T.J.; Bhattacharya, P.; Frank, E.H.; et al. Sustained intra-cartilage delivery of low dose dexamethasone using a cationic carrier for treatment of post traumatic osteoarthritis. Eur. Cells Mater. 2017, 34, 341–364. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.; Chang, C.; Wei, J.; Lin, H.; Brown, C.; Shih, S. Single intra-articular injection of tlc599 provided sustained pain relief through 24 weeks in participants with symptomatic knee osteoarthritis. Osteoarthr. Cartil. 2019, 27, S87–S88. [Google Scholar] [CrossRef]
- Wernecke, C.; Braun, H.J.; Dragoo, J.L. The effect of intra-articular corticosteroids on articular cartilage: A systematic review. Orthop. J. Sports. Med. 2015, 3, 2325967115581163. [Google Scholar] [CrossRef]
- Zeng, C.; Lane, N.E.; Hunter, D.J.; Wei, J.; Choi, H.K.; McAlindon, T.E.; Li, H.; Lu, N.; Lei, G.; Zhang, Y. Intra-articular corticosteroids and the risk of knee osteoarthritis progression: Results from the osteoarthritis initiative. Osteoarthr. Cartil. 2019, 27, 855–862. [Google Scholar] [CrossRef]
- Paik, J.; Duggan, S.T.; Keam, S.J. Triamcinolone acetonide extended-release: A review in osteoarthritis pain of the knee. Drugs 2019, 79, 455–462. [Google Scholar] [CrossRef]
- McAlindon, T.E.; La Valley, M.P.; Harvey, W.F.; Price, L.L.; Driban, J.B.; Zhang, M.; Ward, R.J. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: A randomized clinical trial. JAMA 2017, 317, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.S. Systemic effects of intra-articular corticosteroids. Clin. Rheumatol. 2009, 28, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Lattermann, C.; Jacobs, C.A.; Proffitt Bunnell, M.; Huston, L.J.; Gammon, L.G.; Johnson, D.L.; Reinke, E.K.; Huebner, J.L.; Kraus, V.B.; Spindler, K.P. A multicenter study of early anti-inflammatory treatment in patients with acute anterior cruciate ligament tear. Am. J. Sports Med. 2017, 45, 325–333. [Google Scholar] [CrossRef]
- Blaschke, S.; Koziolek, M.; Schwarz, A.; Benohr, P.; Middel, P.; Schwarz, G.; Hummel, K.M.; Muller, G.A. Proinflammatory role of fractalkine (cx3cl1) in rheumatoid arthritis. J. Rheumatol. 2003, 30, 1918–1927. [Google Scholar]
- Sieker, J.T.; Ayturk, U.M.; Proffen, B.L.; Weissenberger, M.H.; Kiapour, A.M.; Murray, M.M. Immediate administration of intraarticular triamcinolone acetonide after joint injury modulates molecular outcomes associated with early synovitis. Arthritis Rheumatol. 2016, 68, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Frank, E.; Hung, H.H.; Krishnan, Y.; Senter, B.; Bodick, N.; Grodzinsky, A. Dose-dependent chondroprotective effects of traimincinolone acetonide on inflammaed and injured cartlage using an in vitro model. Osteoarthr. Cartil. 2019, 27. [Google Scholar] [CrossRef]
- Rudnik-Jansen, I.; Colen, S.; Berard, J.; Plomp, S.; Que, I.; van Rijen, M.; Woike, N.; Egas, A.; van Osch, G.; van Maarseveen, E.; et al. Prolonged inhibition of inflammation in osteoarthritis by triamcinolone acetonide released from a polyester amide microsphere platform. J. Control. Release Off. J. Control. Release Soc. 2017, 253, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Rudnik-Jansen, I.; Schrijver, K.; Woike, N.; Tellegen, A.; Versteeg, S.; Emans, P.; Mihov, G.; Thies, J.; Eijkelkamp, N.; Tryfonidou, M.; et al. Intra-articular injection of triamcinolone acetonide releasing biomaterial microspheres inhibits pain and inflammation in an acute arthritis model. Drug Deliv. 2019, 26, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bendele, A.M.; Blanks, R.C.; Bodick, N. Sustained efficacy of a single intra-articular dose of fx006 in a rat model of repeated localized knee arthritis. Osteoarthr. Cartil. 2015, 23, 151–160. [Google Scholar] [CrossRef]
- Woodard, L.N.; Grunlan, M.A. Hydrolytic degradation and erosion of polyester biomaterials. ACS Macro Lett. 2018, 7, 976–982. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Mattia, T.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Kraus, V.B.; Conaghan, P.G.; Aazami, H.A.; Mehra, P.; Kivitz, A.J.; Lufkin, J.; Hauben, J.; Johnson, J.R.; Bodick, N. Synovial and systemic pharmacokinetics (pk) of triamcinolone acetonide (ta) following intra-articular (ia) injection of an extended-release microsphere-based formulation (fx006) or standard crystalline suspension in patients with knee osteoarthritis (oa). Osteoarthr. Cartil. 2018, 26, 34–42. [Google Scholar] [CrossRef]
- Spitzer, A.I.; Richmond, J.C.; Kraus, V.B.; Gomoll, A.; Jones, D.G.; Huffman, K.M.; Peterfy, C.; Cinar, A.; Lufkin, J.; Kelley, S.D. Safety and efficacy of repeat administration of triamcinolone acetonide extended-release in osteoarthritis of the knee: A phase 3b, open-label study. Rheumatol. Ther. 2019, 6, 109–124. [Google Scholar] [CrossRef]
- Bowman, S.; Awad, M.E.; Hamrick, M.W.; Hunter, M.; Fulzele, S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin. Transl. Med. 2018, 7, 6. [Google Scholar] [CrossRef]
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Cowman, M.K.; Schmidt, T.A.; Raghavan, P.; Stecco, A. Viscoelastic properties of hyaluronan in physiological conditions. F1000Res 2015, 4, 622. [Google Scholar] [CrossRef]
- Strauss, E.J.; Hart, J.A.; Miller, M.D.; Altman, R.D.; Rosen, J.E. Hyaluronic acid viscosupplementation and osteoarthritis: Current uses and future directions. Am. J. Sports Med. 2009, 37, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Lee-Sayer, S.S.; Dong, Y.; Arif, A.A.; Olsson, M.; Brown, K.L.; Johnson, P. The where, when, how, and why of hyaluronan binding by immune cells. Front. Immunol. 2015, 6, 150. [Google Scholar] [CrossRef]
- Knudson, W.; Ishizuka, S.; Terabe, K.; Askew, E.B.; Knudson, C.B. The pericellular hyaluronan of articular chondrocytes. Matrix Biol. J. Int. Soc. Matrix Biol. 2019, 78–79, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Guidolin, D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: Are the effects molecular weight dependent? Semin. Arthritis Rheum. 2002, 32, 10–37. [Google Scholar] [CrossRef]
- Culty, M.; Nguyen, H.A.; Underhill, C.B. The hyaluronan receptor (cd44) participates in the uptake and degradation of hyaluronan. J. Cell Biol. 1992, 116, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Rai, M.F.; Holguin, N.; Silva, M.J.; Patra, D.; Liao, W.; Sandell, L.J. Early changes in the knee of healer and non-healer mice following non-invasive mechanical injury. J. Orthop. Res. 2017, 35, 524–536. [Google Scholar] [CrossRef]
- Lyman, J.R.; Chappell, J.D.; Morales, T.I.; Kelley, S.S.; Lee, G.M. Response of chondrocytes to local mechanical injury in an ex vivo model. Cartilage 2012, 3, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size matters: Molecular weight specificity of hyaluronan effects in cell biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef] [PubMed]
- Seror, J.; Zhu, L.; Goldberg, R.; Day, A.J.; Klein, J. Supramolecular synergy in the boundary lubrication of synovial joints. Nat. Commun. 2015, 6, 6497. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, W.; Fan, Y.; Kampf, N.; Wang, Y.; Klein, J. Effects of hyaluronan molecular weight on the lubrication of cartilage-emulating boundary layers. Biomacromolecules 2020, 21, 4345–4354. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Sarabia, F.; Coronel, P.; Collantes, E.; Navarro, F.J.; de la Serna, A.R.; Naranjo, A.; Gimeno, M.; Herrero-Beaumont, G. A 40-month multicentre, randomised placebo-controlled study to assess the efficacy and carry-over effect of repeated intra-articular injections of hyaluronic acid in knee osteoarthritis: The amelia project. Ann. Rheum. Dis. 2011, 70, 1957–1962. [Google Scholar] [CrossRef]
- Listrat, V.; Ayral, X.; Patarnello, F.; Bonvarlet, J.P.; Simonnet, J.; Amor, B.; Dougados, M. Arthroscopic evaluation of potential structure modifying activity of hyaluronan (hyalgan) in osteoarthritis of the knee. Osteoarthr. Cartil. 1997, 5, 153–160. [Google Scholar] [CrossRef]
- Pasquali Ronchetti, I.; Guerra, D.; Taparelli, F.; Boraldi, F.; Bergamini, G.; Mori, G.; Zizzi, F.; Frizziero, L. Morphological analysis of knee synovial membrane biopsies from a randomized controlled clinical study comparing the effects of sodium hyaluronate (hyalgan) and methylprednisolone acetate (depomedrol) in osteoarthritis. Rheumatology 2001, 40, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Hsu, H.C.; Yang, K.C.; Yao, C.H.; Lin, F.H. Effect of different molecular weight hyaluronans on osteoarthritis-related protein production in fibroblast-like synoviocytes from patients with tibia plateau fracture. J. Trauma 2010, 68, 146–152. [Google Scholar] [CrossRef]
- Duan, X.; Sandell, L.J.; Chinzei, N.; Holguin, N.; Silva, M.J.; Schiavinato, A.; Rai, M.F. Therapeutic efficacy of intra-articular hyaluronan derivative and platelet-rich plasma in mice following axial tibial loading. PLoS ONE 2017, 12, e0175682. [Google Scholar] [CrossRef]
- Kikuchi, T.; Yamada, H.; Shimmei, M. Effect of high molecular weight hyaluronan on cartilage degeneration in a rabbit model of osteoarthritis. Osteoarthr. Cartil. 1996, 4, 99–110. [Google Scholar] [CrossRef]
- Wiig, M.E.; Amiel, D.; VandeBerg, J.; Kitabayashi, L.; Harwood, F.L.; Arfors, K.E. The early effect of high molecular weight hyaluronan (hyaluronic acid) on anterior cruciate ligament healing: An experimental study in rabbits. J. Orthop. Res. 1990, 8, 425–434. [Google Scholar] [CrossRef]
- Schiavinato, A.; Lini, E.; Guidolin, D.; Pezzoli, G.; Botti, P.; Martelli, M.; Cortivo, R.; De Galateo, A.; Abatangelo, G. Intraarticular sodium hyaluronate injections in the pond-nuki experimental model of osteoarthritis in dogs. Ii. Morphological findings. Clin. Orthop. Relat. Res. 1989, 241, 286–299. [Google Scholar]
- Zhou, P.H.; Liu, S.Q.; Peng, H. The effect of hyaluronic acid on il-1beta-induced chondrocyte apoptosis in a rat model of osteoarthritis. J. Orthop. Res. 2008, 26, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Fedak, K.M.; Bernal, A.; Capshaw, Z.A.; Gross, S. Applying the bradford hill criteria in the 21st century: How data integration has changed causal inference in molecular epidemiology. Emerg. Themes Epidemiol. 2015, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Bongartz, T.; Sutton, A.J.; Sweeting, M.J.; Buchan, I.; Matteson, E.L.; Montori, V. Anti-tnf antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: Systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006, 295, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Kimmerling, K.A.; Furman, B.D.; Mangiapani, D.S.; Moverman, M.A.; Sinclair, S.M.; Huebner, J.L.; Chilkoti, A.; Kraus, V.B.; Setton, L.A.; Guilak, F.; et al. Sustained intra-articular delivery of il-1ra from a thermally-responsive elastin-like polypeptide as a therapy for post-traumatic arthritis. Eur. Cells Mater. 2015, 29, 124–139. [Google Scholar] [CrossRef]
- Furman, B.D.; Mangiapani, D.S.; Zeitler, E.; Bailey, K.N.; Horne, P.H.; Huebner, J.L.; Kraus, V.B.; Guilak, F.; Olson, S.A. Targeting pro-inflammatory cytokines following joint injury: Acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis Res. 2014, 16, R134. [Google Scholar] [CrossRef]
- Maksymowych, W.P.; Russell, A.S.; Chiu, P.; Yan, A.; Jones, N.; Clare, T.; Lambert, R.G. Targeting tumour necrosis factor alleviates signs and symptoms of inflammatory osteoarthritis of the knee. Arthritis Res. 2012, 14, R206. [Google Scholar] [CrossRef]
- Wang, J. Efficacy and safety of adalimumab by intra-articular injection for moderate to severe knee osteoarthritis: An open-label randomized controlled trial. J. Int. Med. Res. 2018, 46, 326–334. [Google Scholar] [CrossRef]
- Lindsley, H.; Schue, J.; Tawfik, O.; Bolce, R.; Smith, D.; Hinson, G.; Wick, J. Treatment of knee osteoarthritis with intra-articular infliximab improves total womac score. High baseline levels of synovial cellularity predict improvement. Ann. Rheuamatic Dis. 2013, 71, 411–417. [Google Scholar] [CrossRef]
- van der Bijl, A.E.; Teng, Y.K.; van Oosterhout, M.; Breedveld, F.C.; Allaart, C.F.; Huizinga, T.W. Efficacy of intraarticular infliximab in patients with chronic or recurrent gonarthritis: A clinical randomized trial. Arthritis Rheum. 2009, 61, 974–978. [Google Scholar] [CrossRef]
- Burmester, G.R.; Panaccione, R.; Gordon, K.B.; McIlraith, M.J.; Lacerda, A.P. Adalimumab: Long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and crohn’s disease. Ann. Rheum. Dis. 2013, 72, 517–524. [Google Scholar] [CrossRef]
- Melsheimer, R.; Geldhof, A.; Apaolaza, I.; Schaible, T. Remicade((r)) (infliximab): 20 years of contributions to science and medicine. Biologics 2019, 13, 139–178. [Google Scholar]
- Fields, J.K.; Gunther, S.; Sundberg, E.J. Structural basis of il-1 family cytokine signaling. Front. Immunol. 2019, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Arend, W.P.; Guthridge, C.J. Biological role of interleukin 1 receptor antagonist isoforms. Ann. Rheum. Dis. 2000, 59, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Swellam, M.; Mahmoud, M.S.; Samy, N.; Gamal, A.A. Potential influence of interleukin-1 receptor antagonist gene polymorphism on knee osteoarthritis risk. Dis. Markers 2010, 28, 299–305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruzickova, S.; Senolt, L.; Gatterova, J.; Vencovsky, J.; Pavelka, K. The lack of correlation between the increased frequency of allele il-1rn*2 of interleukin-1 receptor antagonist gene in czech patients with knee osteoarthritis and the markers of cartilage degradation. Folia Biol. 2008, 54, 115–120. [Google Scholar]
- Chevalier, X.; Goupille, P.; Beaulieu, A.D.; Burch, F.X.; Bensen, W.G.; Conrozier, T.; Loeuille, D.; Kivitz, A.J.; Silver, D.; Appleton, B.E. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009, 61, 344–352. [Google Scholar] [CrossRef]
- Scallon, B.; Cai, A.; Solowski, N.; Rosenberg, A.; Song, X.Y.; Shealy, D.; Wagner, C. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J. Pharm. Exp. 2002, 301, 418–426. [Google Scholar] [CrossRef]
- Chamberlain, C.S.; Leiferman, E.M.; Frisch, K.E.; Brickson, S.L.; Murphy, W.L.; Baer, G.S.; Vanderby, R. Interleukin expression after injury and the effects of interleukin-1 receptor antagonist. PLoS ONE 2013, 8, e71631. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, K.A.; Ubhe, A.; Shaman, Z.; D’Souza, G. Intra-articular interleukin-1 receptor antagonist (il1-ra) microspheres for posttraumatic osteoarthritis: In vitro biological activity and in vivo disease modifying effect. J. Exp. Orthop. 2016, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Gong, M.; He, Q.T.; Zhou, P.H. Controlled release of interleukin-1 receptor antagonist from hyaluronic acid-chitosan microspheres attenuates interleukin-1beta-induced inflammation and apoptosis in chondrocytes. BioMed. Res. Int. 2016, 2016, 6290957. [Google Scholar] [CrossRef]
- Chamberlain, C.S.; Leiferman, E.M.; Frisch, K.E.; Duenwald-Kuehl, S.E.; Brickson, S.L.; Murphy, W.L.; Baer, G.S.; Vanderby, R. Interleukin-1 receptor antagonist modulates inflammation and scarring after ligament injury. Connect. Tissue Res. 2014, 55, 177–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mehta, S.; Akhtar, S.; Porter, R.M.; Onnerfjord, P.; Bajpayee, A.G. Interleukin-1 receptor antagonist (il-1ra) is more effective in suppressing cytokine-induced catabolism in cartilage-synovium co-culture than in cartilage monoculture. Arthritis Res. 2019, 21, 238. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cabello, J.C.; Arias, F.J.; Rodrigo, M.A.; Girotti, A. Elastin-like polypeptides in drug delivery. Adv. Drug Deliv. Rev. 2016, 97, 85–100. [Google Scholar] [CrossRef]
- Meijer, H.; Reinecke, J.; Becker, C.; Tholen, G.; Wehling, P. The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Inflamm. Res. 2003, 52, 404–407. [Google Scholar] [CrossRef]
- Barreto, A. A short report on the effect of decreased incubation time on the architectural profile of autologous conditioned serum (acs). Cytokine 2017, 94, 52–54. [Google Scholar] [CrossRef]
- Baltzer, A.W.; Moser, C.; Jansen, S.A.; Krauspe, R. Autologous conditioned serum (orthokine) is an effective treatment for knee osteoarthritis. Osteoarthr. Cartil. 2009, 17, 152–160. [Google Scholar] [CrossRef]
- Auw Yang, K.G.; Raijmakers, N.J.; van Arkel, E.R.; Caron, J.J.; Rijk, P.C.; Willems, W.J.; Zijl, J.A.; Verbout, A.J.; Dhert, W.J.; Saris, D.B. Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial. Osteoarthr. Cartil. 2008, 16, 498–505. [Google Scholar] [CrossRef]
- Fox, B.A.; Stephens, M.M. Treatment of knee osteoarthritis with orthokine-derived autologous conditioned serum. Expert Rev. Clin. Immunol. 2010, 6, 335–345. [Google Scholar] [CrossRef]
- Vitali, M.; Ometti, M.; Drossinos, A.; Pironti, P.; Santoleri, L.; Salini, V. Autologous conditioned serum: Clinical and functional results using a novel disease modifying agent for the management of knee osteoarthritis. J. Drug. Assess 2020, 9, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Taheri, M.; Adlkhoo, H.; Dadkhah, P.; Abbasian, M.R. Comparison of the effect of intra-articular injection of autologous (orthokine) interleukin-1 receptor antagonist (il-1ra) and hyaluronic acid in pain control of knee osteoarthritis. Nov. Biomed. 2019, 7, 210–217. [Google Scholar]
- Baselga Garcia-Escudero, J.; Miguel Hernandez Trillos, P. Treatment of osteoarthritis of the knee with a combination of autologous conditioned serum and physiotherapy: A two-year observational study. PLoS ONE 2015, 10, e0145551. [Google Scholar] [CrossRef]
- Tassara, M.; De Ponti, A.; Barzizza, L.; Zambelli, M.; Parisi, C.; Milani, R.; Santoleri, L. Autologous conditioned serum (acs) for intra-articular treatment in osteoarthritis: Retrospective report of 28 cases. Transfus. Apher. Sci. 2018, 57, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Lasarzik, J.; Bondzio, A.; Rettig, M.; Estrada, R.; Klaus, C.; Ehrle, A.; Einspanier, R.; Lischer, C. Evaluation of two protocols using autologous conditioned serum for intra-articular therapy of equine osteoarthritis-a pilot study monitoring cytokines and cartilage-specific biomarkers. J. Equine Vet. Sci. 2018, 60, 35–42. [Google Scholar] [CrossRef]
- Fjordbakk, C.T.; Johansen, G.M.; Løvås, A.C.; Oppegård, K.L.; Storset, A.K. Surgical stress influences cytokine content in autologous conditioned serum. Equine Vet. J. 2015, 47, 212–217. [Google Scholar] [CrossRef]
- Barreto, A.; Braun, T.R. A method to induce interleukin-1 receptor antagonist protein from autologous whole blood. Cytokine 2016, 81, 137–141. [Google Scholar] [CrossRef]
- Akeson, G.; Malemud, C.J. A role for soluble il-6 receptor in osteoarthritis. J. Funct. Morphol. Kinesiol. 2017, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Wiegertjes, R.; van de Loo, F.A.J.; Blaney Davidson, E.N. A roadmap to target interleukin-6 osteoarthritis. Rheumatology 2020, 59, 2681–2694. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chanalaris, A.; Troeberg, L. Adamts and adam metalloproteinases in osteoarthritis - looking beyond the ’usual suspects’. Osteoarthr. Cartil. 2017, 25, 1000–1009. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Yip, R.M.L.; Yim, C.W. Role of interleukin 6 inhibitors in the management of rheumatoid arthritis. J. Clin. Rheumatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Rafique, A.; Potocky, T.; Paccaly, A.; Nolain, P.; Lu, Q.; Iglesias-Rodriguez, M.; St John, G.; Nivens, M.C.; Kanamaluru, V.; et al. Differential binding of sarilumab and tocilizumab to il-6ralpha and effects of receptor occupancy on clinical parameters. J. Clin. Pharm. 2021, 61, 714–724. [Google Scholar] [CrossRef]
- Singh, J.A.; Furst, D.E.; Bharat, A.; Curtis, J.R.; Kavanaugh, A.F.; Kremer, J.M.; Moreland, L.W.; O’Dell, J.; Winthrop, K.L.; Beukelman, T.; et al. 2012 update of the 2008 american college of rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012, 64, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Latourte, A.; Cherifi, C.; Maillet, J.; Ea, H.K.; Bouaziz, W.; Funck-Brentano, T.; Cohen-Solal, M.; Hay, E.; Richette, P. Systemic inhibition of il-6/stat3 signalling protects against experimental osteoarthritis. Ann. Rheum Dis. 2017, 76, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cao, L.; Li, F.; Ma, C.; Liu, G.; Wang, Q. Interleukin-6 from subchondral bone mesenchymal stem cells contributes to the pathological phenotypes of experimental osteoarthritis. Am. J. Transl. Res. 2018, 10, 1143–1154. [Google Scholar]
- Sui, Y.; Lee, J.H.; DiMicco, M.A.; Vanderploeg, E.J.; Blake, S.M.; Hung, H.H.; Plaas, A.H.; James, I.E.; Song, X.Y.; Lark, M.W.; et al. Mechanical injury potentiates proteoglycan catabolism induced by interleukin-6 with soluble interleukin-6 receptor and tumor necrosis factor alpha in immature bovine and adult human articular cartilage. Arthritis Rheum. 2009, 60, 2985–2996. [Google Scholar] [CrossRef]
- Byun, S.; Sinskey, Y.L.; Lu, Y.C.; Ort, T.; Kavalkovich, K.; Sivakumar, P.; Hunziker, E.B.; Frank, E.H.; Grodzinsky, A.J. Transport of anti-il-6 antigen binding fragments into cartilage and the effects of injury. Arch. Biochem. Biophys. 2013, 532, 15–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meszaros, E.C.; Dahoud, W.; Mesiano, S.; Malemud, C.J. Blockade of recombinant human il-6 by tocilizumab suppresses matrix metalloproteinase-9 production in the c28/i2 immortalized human chondrocyte cell line. Integr. Mol. Med. 2015, 2, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wu, X.; Wang, Z.; Ge, J.; Chen, F. Interleukin-6 enhances acid-induced apoptosis via upregulating acid-sensing ion channel 1a expression and function in rat articular chondrocytes. Int. Immunopharmacol. 2015, 29, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Pluddemann, A. Macrophage clearance of apoptotic cells: A critical assessment. Front. Immunol. 2018, 9, 127. [Google Scholar] [CrossRef]
- Steen, E.H.; Wang, X.; Balaji, S.; Butte, M.J.; Bollyky, P.L.; Keswani, S.G. The role of the anti-inflammatory cytokine interleukin-10 in tissue fibrosis. Adv. Wound Care 2020, 9, 184–198. [Google Scholar] [CrossRef]
- Barker, T.; Rogers, V.E.; Henriksen, V.T.; Trawick, R.H.; Momberger, N.G.; Lynn Rasmussen, G. Circulating il-10 is compromised in patients predisposed to developing and in patients with severe knee osteoarthritis. Sci. Rep. 2021, 11, 1812. [Google Scholar] [CrossRef]
- Schwarz, S.; Mrosewski, I.; Silawal, S.; Schulze-Tanzil, G. The interrelation of osteoarthritis and diabetes mellitus: Considering the potential role of interleukin-10 and in vitro models for further analysis. Inflamm. Res. 2018, 67, 285–300. [Google Scholar] [CrossRef]
- Rai, M.F.; Sandell, L.J. Regeneration of articular cartilage in healer and non-healer mice. Matrix Biol. J. Int. Soc. Matrix Biol. 2014, 39, 50–55. [Google Scholar] [CrossRef]
- Diekman, B.O.; Wu, C.L.; Louer, C.R.; Furman, B.D.; Huebner, J.L.; Kraus, V.B.; Olson, S.A.; Guilak, F. Intra-articular delivery of purified mesenchymal stem cells from c57bl/6 or mrl/mpj superhealer mice prevents posttraumatic arthritis. Cell Transpl. 2013, 22, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J. Enhanced cartilage repair in ’healer’ mice-new leads in the search for better clinical options for cartilage repair. Semin. Cell Dev. Biol. 2017, 62, 78–85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ward, B.D.; Furman, B.D.; Huebner, J.L.; Kraus, V.B.; Guilak, F.; Olson, S.A. Absence of posttraumatic arthritis following intraarticular fracture in the mrl/mpj mouse. Arthritis Rheum. 2008, 58, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Wallis, S.K.; Cooney, L.A.; Endres, J.L.; Lee, M.J.; Ryu, J.; Somers, E.C.; Fox, D.A. A polymorphism in the interleukin-4 receptor affects the ability of interleukin-4 to regulate th17 cells: A possible immunoregulatory mechanism for genetic control of the severity of rheumatoid arthritis. Arthritis Res. 2011, 13, R15. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, A.I.; Beekhuizen, M.; t Hart, M.C.; Radstake, T.R.; Dhert, W.J.; Saris, D.B.; van Osch, G.J.; Creemers, L.B. Cytokine profiles in the joint depend on pathology, but are different between synovial fluid, cartilage tissue and cultured chondrocytes. Arthritis Res. 2014, 16, 441. [Google Scholar] [CrossRef]
- Struglics, A.; Larsson, S.; Kumahashi, N.; Frobell, R.; Lohmander, L.S. Changes in cytokines and aggrecan args neoepitope in synovial fluid and serum and in c-terminal crosslinking telopeptide of type ii collagen and n-terminal crosslinking telopeptide of type i collagen in urine over five years after anterior cruciate ligament rupture: An exploratory analysis in the knee anterior cruciate ligament, nonsurgical versus surgical treatment trial. Arthritis Rheumatol. 2015, 67, 1816–1825. [Google Scholar] [PubMed]
- Haller, J.M.; McFadden, M.; Kubiak, E.N.; Higgins, T.F. Inflammatory cytokine response following acute tibial plateau fracture. J. Bone Jt. Surg. 2015, 97, 478–483. [Google Scholar] [CrossRef]
- Bigoni, M.; Sacerdote, P.; Turati, M.; Franchi, S.; Gandolla, M.; Gaddi, D.; Moretti, S.; Munegato, D.; Augusti, C.A.; Bresciani, E.; et al. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J. Orthop. Res. 2013, 31, 315–321. [Google Scholar] [CrossRef]
- van Meegeren, M.E.; Roosendaal, G.; Jansen, N.W.; Wenting, M.J.; van Wesel, A.C.; van Roon, J.A.; Lafeber, F.P. Il-4 alone and in combination with il-10 protects against blood-induced cartilage damage. Osteoarthr. Cartil. 2012, 20, 764–772. [Google Scholar] [CrossRef]
- Assirelli, E.; Pulsatelli, L.; Dolzani, P.; Platano, D.; Olivotto, E.; Filardo, G.; Trisolino, G.; Facchini, A.; Borzi, R.M.; Meliconi, R. Human osteoarthritic cartilage shows reduced in vivo expression of il-4, a chondroprotective cytokine that differentially modulates il-1beta-stimulated production of chemokines and matrix-degrading enzymes in vitro. PLoS ONE 2014, 9, e96925. [Google Scholar] [CrossRef]
- Iannone, F.; De Bari, C.; Dell’Accio, F.; Covelli, M.; Cantatore, F.P.; Patella, V.; Lo Bianco, G.; Lapadula, G. Interleukin-10 and interleukin-10 receptor in human osteoarthritic and healthy chondrocytes. Clin. Exp. Rheumatol. 2001, 19, 139–145. [Google Scholar] [PubMed]
- Guicheux, J.; Palmer, G.; Relic, B.; Mezin, F.; Caverzasio, J.; Apostolides, P.; Gauchat, J.F.; Gabay, C.; Guerne, P.A. Primary human articular chondrocytes, dedifferentiated chondrocytes, and synoviocytes exhibit differential responsiveness to interleukin-4: Correlation with the expression pattern of the common receptor gamma chain. J. Cell Physiol. 2002, 192, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Millward-Sadler, S.J.; Khan, N.S.; Bracher, M.G.; Wright, M.O.; Salter, D.M. Roles for the interleukin-4 receptor and associated jak/stat proteins in human articular chondrocyte mechanotransduction. Osteoarthr. Cartil. 2006, 14, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Steen-Louws, C.; Popov-Celeketic, J.; Mastbergen, S.C.; Coeleveld, K.; Hack, C.E.; Eijkelkamp, N.; Tryfonidou, M.; Spruijt, S.; van Roon, J.A.G.; Lafeber, F. Il4-10 fusion protein has chondroprotective, anti-inflammatory and potentially analgesic effects in the treatment of osteoarthritis. Osteoarthr. Cartil. 2018, 26, 1127–1135. [Google Scholar] [CrossRef]
- Dolzani, P.; Assirelli, E.; Pulsatelli, L.; Meliconi, R.; Mariani, E.; Neri, S. Ex vivo physiological compression of human osteoarthritis cartilage modulates cellular and matrix components. PLoS ONE 2019, 14, e0222947. [Google Scholar] [CrossRef]
- Feng, N.; Lugli, S.M.; Schnyder, B.; Gauchat, J.F.; Graber, P.; Schlagenhauf, E.; Schnarr, B.; Wiederkehr-Adam, M.; Duschl, A.; Heim, M.H.; et al. The interleukin-4/interleukin-13 receptor of human synovial fibroblasts: Overexpression of the nonsignaling interleukin-13 receptor alpha2. Lab. Investig. J. Tech. Methods Pathol. 1998, 78, 591–602. [Google Scholar]
- Ritchlin, C.; Haas-Smith, S.A. Expression of interleukin 10 mrna and protein by synovial fibroblastoid cells. J. Rheumatol. 2001, 28, 698–705. [Google Scholar]
- Hart, P.H.; Bonder, C.S.; Balogh, J.; Dickensheets, H.L.; Donnelly, R.P.; Finlay-Jones, J.J. Differential responses of human monocytes and macrophages to il-4 and il-13. J. Leukoc. Biol. 1999, 66, 575–578. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.M.; Heller, N.M. Commentary: Il-4 and il-13 receptors and signaling. Cytokine 2015, 75, 38–50. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Bao, K.; Reinhardt, R.L. The differential expression of il-4 and il-13 and its impact on type-2 immunity. Cytokine 2015, 75, 25–37. [Google Scholar] [CrossRef]
- Schlaak, J.F.; Pfers, I.; Meyer Zum Buschenfelde, K.H.; Marker-Hermann, E. Different cytokine profiles in the synovial fluid of patients with osteoarthritis, rheumatoid arthritis and seronegative spondylarthropathies. Clin. Exp. Rheumatol. 1996, 14, 155–162. [Google Scholar] [PubMed]
- Ishii, H.; Tanaka, H.; Katoh, K.; Nakamura, H.; Nagashima, M.; Yoshino, S. Characterization of infiltrating t cells and th1/th2-type cytokines in the synovium of patients with osteoarthritis. Osteoarthr. Cartil. 2002, 10, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, T.; Pulsatelli, L.; Dolzani, P.; Facchini, A.; Meliconi, R. Elevated serum levels of soluble interleukin-4 receptor in osteoarthritis. Osteoarthr. Cartil. 2006, 14, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Fritz, P.; Einsele, H.; Sell, S.; Saal, J.G. Evaluation of synovial cytokine patterns in rheumatoid arthritis and osteoarthritis by quantitative reverse transcription polymerase chain reaction. Rheumatol. Int. 1997, 16, 191–196. [Google Scholar] [CrossRef]
- Ding, J.; Niu, X.; Su, Y.; Li, X. Expression of synovial fluid biomarkers in patients with knee osteoarthritis and meniscus injury. Exp. Med. 2017, 14, 1609–1613. [Google Scholar] [CrossRef]
- Nees, T.A.; Rosshirt, N.; Zhang, J.A.; Reiner, T.; Sorbi, R.; Tripel, E.; Walker, T.; Schiltenwolf, M.; Hagmann, S.; Moradi, B. Synovial cytokines significantly correlate with osteoarthritis-related knee pain and disability: Inflammatory mediators of potential clinical relevance. J. Clin. Med. 2019, 8, 1343. [Google Scholar] [CrossRef]
- Imamura, M.; Ezquerro, F.; Marcon Alfieri, F.; Vilas Boas, L.; Tozetto-Mendoza, T.R.; Chen, J.; Ozcakar, L.; Arendt-Nielsen, L.; Rizzo Battistella, L. Serum levels of proinflammatory cytokines in painful knee osteoarthritis and sensitization. Int. J. Inflam. 2015, 2015, 329792. [Google Scholar] [CrossRef]
- Behrendt, P.; Feldheim, M.; Preusse-Prange, A.; Weitkamp, J.T.; Haake, M.; Eglin, D.; Rolauffs, B.; Fay, J.; Seekamp, A.; Grodzinsky, A.J.; et al. Chondrogenic potential of il-10 in mechanically injured cartilage and cellularized collagen aci grafts. Osteoarthr. Cartil. 2018, 26, 264–275. [Google Scholar] [CrossRef]
- Behrendt, P.; Preusse-Prange, A.; Kluter, T.; Haake, M.; Rolauffs, B.; Grodzinsky, A.J.; Lippross, S.; Kurz, B. Il-10 reduces apoptosis and extracellular matrix degradation after injurious compression of mature articular cartilage. Osteoarthr. Cartil. 2016, 24, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, P.; Hafelein, K.; Preusse-Prange, A.; Bayer, A.; Seekamp, A.; Kurz, B. Il-10 ameliorates tnf-alpha induced meniscus degeneration in mature meniscal tissue in vitro. BMC Musculoskelet. Disord. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Chernoff, A.E.; Granowitz, E.V.; Shapiro, L.; Vannier, E.; Lonnemann, G.; Angel, J.B.; Kennedy, J.S.; Rabson, A.R.; Wolff, S.M.; Dinarello, C.A. A randomized, controlled trial of il-10 in humans. Inhibition of inflammatory cytokine production and immune responses. J. Immunol. 1995, 154, 5492–5499. [Google Scholar] [PubMed]
- Wang, X.; Wong, K.; Ouyang, W.; Rutz, S. Targeting il-10 family cytokines for the treatment of human diseases. Cold Spring Harb. Perspect. Biol. 2019, 11, a028548. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lou, S. Direct protective effect of interleukin-10 on articular chondrocytes in vitro. Chin. Med. J. 2001, 114, 723–725. [Google Scholar]
- Muller, R.D.; John, T.; Kohl, B.; Oberholzer, A.; Gust, T.; Hostmann, A.; Hellmuth, M.; Laface, D.; Hutchins, B.; Laube, G.; et al. Il-10 overexpression differentially affects cartilage matrix gene expression in response to tnf-alpha in human articular chondrocytes in vitro. Cytokine 2008, 44, 377–385. [Google Scholar] [CrossRef] [PubMed]
- John, T.; Muller, R.D.; Oberholzer, A.; Zreiqat, H.; Kohl, B.; Ertel, W.; Hostmann, A.; Tschoeke, S.K.; Schulze-Tanzil, G. Interleukin-10 modulates pro-apoptotic effects of tnf-alpha in human articular chondrocytes in vitro. Cytokine 2007, 40, 226–234. [Google Scholar] [CrossRef]
- Silawal, S.; Willauschus, M.; Schulze-Tanzil, G.; Gogele, C.; Gesslein, M.; Schwarz, S. Il-10 could play a role in the interrelation between diabetes mellitus and osteoarthritis. Int. J. Mol. Sci. 2019, 20, 768. [Google Scholar] [CrossRef]
- Park, E.; Hart, M.L.; Rolauffs, B.; Stegemann, J.P.; Annamalai, R.T. Bioresponsive microspheres for on-demand delivery of anti-inflammatory cytokines for articular cartilage repair. J. Biomed. Mater. Res. A 2020, 108, 722–733. [Google Scholar] [CrossRef]
- Chowdhury, T.T.; Bader, D.L.; Lee, D.A. Anti-inflammatory effects of il-4 and dynamic compression in il-1beta stimulated chondrocytes. Biochem. Biophys. Res. Commun. 2006, 339, 241–247. [Google Scholar] [CrossRef]
- Yorimitsu, M.; Nishida, K.; Shimizu, A.; Doi, H.; Miyazawa, S.; Komiyama, T.; Nasu, Y.; Yoshida, A.; Watanabe, S.; Ozaki, T. Intra-articular injection of interleukin-4 decreases nitric oxide production by chondrocytes and ameliorates subsequent destruction of cartilage in instability-induced osteoarthritis in rat knee joints. Osteoarthr. Cartil. 2008, 16, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Schuerwegh, A.J.; Dombrecht, E.J.; Stevens, W.J.; Van Offel, J.F.; Bridts, C.H.; De Clerck, L.S. Influence of pro-inflammatory (il-1 alpha, il-6, tnf-alpha, ifn-gamma) and anti-inflammatory (il-4) cytokines on chondrocyte function. Osteoarthr. Cartil. 2003, 11, 681–687. [Google Scholar] [CrossRef]
- Yeh, L.A.; Augustine, A.J.; Lee, P.; Riviere, L.R.; Sheldon, A. Interleukin-4, an inhibitor of cartilage breakdown in bovine articular cartilage explants. J. Rheumatol. 1995, 22, 1740–1746. [Google Scholar] [PubMed]
- Lubberts, E.; Joosten, L.A.; van de Loo, F.A.; van den Gersselaar, L.A.; van den Berg, W.B. Reduction of interleukin-17-induced inhibition of chondrocyte proteoglycan synthesis in intact murine articular cartilage by interleukin-4. Arthritis Rheum. 2000, 43, 1300–1306. [Google Scholar] [CrossRef]
- Shimizu, A.; Watanabe, S.; Iimoto, S.; Yamamoto, H. Interleukin-4 protects matrix synthesis in chondrocytes under excessive mechanical stress in vitro. Mod. Rheumatol. 2004, 14, 296–300. [Google Scholar] [CrossRef]
- Sward, P.; Frobell, R.; Englund, M.; Roos, H.; Struglics, A. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis)-a cross-sectional analysis. Osteoarthr. Cartil. 2012, 20, 1302–1308. [Google Scholar] [CrossRef]
- Struglics, A.; Okroj, M.; Sward, P.; Frobell, R.; Saxne, T.; Lohmander, L.S.; Blom, A.M. The complement system is activated in synovial fluid from subjects with knee injury and from patients with osteoarthritis. Arthritis Res. 2016, 18, 223. [Google Scholar] [CrossRef]
- Olsson, O.; Isacsson, A.; Englund, M.; Frobell, R.B. Epidemiology of intra- and peri-articular structural injuries in traumatic knee joint hemarthrosis - data from 1145 consecutive knees with subacute mri. Osteoarthr. Cartil. 2016, 24, 1890–1897. [Google Scholar] [CrossRef]
- Watt, F.E.; Paterson, E.; Freidin, A.; Kenny, M.; Judge, A.; Saklatvala, J.; Williams, A.; Vincent, T.L. Acute molecular changes in synovial fluid following human knee injury: Association with early clinical outcomes. Arthritis Rheumatol. 2016, 68, 2129–2140. [Google Scholar] [CrossRef]
- van Meegeren, M.E.; Roosendaal, G.; Jansen, N.W.; Lafeber, F.P.; Mastbergen, S.C. Blood-induced joint damage: The devastating effects of acute joint bleeds versus micro-bleeds. Cartilage 2013, 4, 313–320. [Google Scholar] [CrossRef] [PubMed]
- van Vulpen, L.F.D.; Popov-Celeketic, J.; van Meegeren, M.E.R.; Coeleveld, K.; van Laar, J.M.; Hack, C.E.; Schutgens, R.E.G.; Mastbergen, S.C.; Lafeber, F. A fusion protein of interleukin-4 and interleukin-10 protects against blood-induced cartilage damage in vitro and in vivo. J. Thromb. Haemost. JTH 2017, 15, 1788–1798. [Google Scholar] [CrossRef]
- Jansen, N.W.; Roosendaal, G.; Hooiveld, M.J.; Bijlsma, J.W.; van Roon, J.A.; Theobald, M.; Lafeber, F.P. Interleukin-10 protects against blood-induced joint damage. Br. J. Haematol. 2008, 142, 953–961. [Google Scholar] [CrossRef] [PubMed]
- van Meegeren, M.E.; Roosendaal, G.; Coeleveld, K.; Nieuwenhuizen, L.; Mastbergen, S.C.; Lafeber, F.P. A single intra-articular injection with il-4 plus il-10 ameliorates blood-induced cartilage degeneration in haemophilic mice. Br. J. Haematol. 2013, 160, 515–520. [Google Scholar] [CrossRef] [PubMed]
- van Helvoort, E.M.; Popov-Celeketic, J.; Eijkelkamp, N.; Coeleveld, K.; Tryfonidou, M.A.; Wijne, C.D.; Hack, C.E.; Lafeber, F.; Mastbergen, S.C. Canine il4-10 fusion protein provides disease modifying activity in a canine model of oa; an exploratory study. PLoS ONE 2019, 14, e0219587. [Google Scholar] [CrossRef] [PubMed]
- Nabbe, K.C.; van Lent, P.L.; Holthuysen, A.E.; Sloetjes, A.W.; Koch, A.E.; Radstake, T.R.; van den Berg, W.B. Local il-13 gene transfer prior to immune-complex arthritis inhibits chondrocyte death and matrix-metalloproteinase-mediated cartilage matrix degradation despite enhanced joint inflammation. Arthritis Res. 2005, 7, R392–R401. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, D.; Pelletier, J.P.; Alaaeddine, N.; Mineau, F.; Geng, C.; Ranger, P.; Martel-Pelletier, J. Effect of il-13 on cytokines, cytokine receptors and inhibitors on human osteoarthritis synovium and synovial fibroblasts. Osteoarthr. Cartil. 1998, 6, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mao, Z.; Yu, C. Suppression of early experimental osteoarthritis by gene transfer of interleukin-1 receptor antagonist and interleukin-10. J. Orthop. Res. 2004, 22, 742–750. [Google Scholar] [CrossRef]
- Broeren, M.G.; de Vries, M.; Bennink, M.B.; Arntz, O.J.; Blom, A.B.; Koenders, M.I.; van Lent, P.L.; van der Kraan, P.M.; van den Berg, W.B.; van de Loo, F.A. Disease-regulated gene therapy with anti-inflammatory interleukin-10 under the control of the cxcl10 promoter for the treatment of rheumatoid arthritis. Hum. Gene 2016, 27, 244–254. [Google Scholar] [CrossRef]
- Broeren, M.G.; de Vries, M.; Bennink, M.B.; Arntz, O.J.; van Lent, P.L.; van der Kraan, P.M.; van den Berg, W.B.; van den Hoogen, F.H.; Koenders, M.I.; van de Loo, F.A. Suppression of the inflammatory response by disease-inducible interleukin-10 gene therapy in a three-dimensional micromass model of the human synovial membrane. Arthritis Res. 2016, 18, 186. [Google Scholar] [CrossRef]
- Annamalai, R.T.; Turner, P.A.; Carson, W.F.t.; Levi, B.; Kunkel, S.; Stegemann, J.P. Harnessing macrophage-mediated degradation of gelatin microspheres for spatiotemporal control of bmp2 release. Biomaterials 2018, 161, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Lehar, J.; Krueger, A.S.; Avery, W.; Heilbut, A.M.; Johansen, L.M.; Price, E.R.; Rickles, R.J.; Short, G.F., 3rd; Staunton, J.E.; Jin, X.; et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat. Biotechnol. 2009, 27, 659–666. [Google Scholar] [CrossRef]
- Hart, M.L.; Walsh, M.C.; Stahl, G.L. Initiation of complement activation following oxidative stress. In vitro and in vivo observations. Mol. Immunol. 2004, 41, 165–171. [Google Scholar] [CrossRef]
- Walsh, M.C.; Hart, M.L.; Bourcier, T.M.; Bhole, D.; Takahashi, M.; Stahl, G.L. The Complement System: Novel Roles in Health and Disease; Role of Complement in Myocardial Ischemia and Infarction; Szebeni, J.E., Ed.; Springer Science + Business Media: New York, NY, USA, 2004; pp. 421–435. [Google Scholar]
- Melin Furst, C.; Ahrman, E.; Bratteby, K.; Waldemarson, S.; Malmstrom, J.; Blom, A.M. Quantitative mass spectrometry to study inflammatory cartilage degradation and resulting interactions with the complement system. J. Immunol. 2016, 197, 3415–3424. [Google Scholar] [CrossRef]
- Sohn, D.H.; Sokolove, J.; Sharpe, O.; Erhart, J.C.; Chandra, P.E.; Lahey, L.J.; Lindstrom, T.M.; Hwang, I.; Boyer, K.A.; Andriacchi, T.P.; et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via toll-like receptor 4. Arthritis Res. 2012, 14, R7. [Google Scholar] [CrossRef]
- Colten, H.R.; Ooi, Y.M.; Edelson, P.J. Synthesis and secretion of complement proteins by macrophages. Ann. N. Y. Acad. Sci. 1979, 332, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, R.; van Schaarenburg, R.A.; Kwekkeboom, J.C.; Levarht, E.W.N.; Bakker, A.M.; Mahdad, R.; Monteagudo, S.; Cherifi, C.; Lories, R.J.; Toes, R.E.M.; et al. Complement component c1q is produced by isolated articular chondrocytes. Osteoarthr. Cartil. 2020, 28, 675–684. [Google Scholar] [CrossRef]
- Bradley, K.; North, J.; Saunders, D.; Schwaeble, W.; Jeziorska, M.; Woolley, D.E.; Whaley, K. Synthesis of classical pathway complement components by chondrocytes. Immunology 1996, 88, 648–656. [Google Scholar]
- Schulze-Tanzil, G.; Kohl, B.; El Sayed, K.; Arens, S.; Ertel, W.; Stolzel, K.; John, T. Anaphylatoxin receptors and complement regulatory proteins in human articular and non-articular chondrocytes: Interrelation with cytokines. Cell Tissue Res. 2012, 350, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Ignatius, A.; Schoengraf, P.; Kreja, L.; Liedert, A.; Recknagel, S.; Kandert, S.; Brenner, R.E.; Schneider, M.; Lambris, J.D.; Huber-Lang, M. Complement c3a and c5a modulate osteoclast formation and inflammatory response of osteoblasts in synergism with il-1beta. J. Cell. Biochem. 2011, 112, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Assirelli, E.; Mariani, E.; Meliconi, R. Complement factor expression in osteoarthritis joint compartments. Osteoarthr. Cartil. 2016, 24, S383–S384. [Google Scholar] [CrossRef]
- Ritter, S.Y.; Subbaiah, R.; Bebek, G.; Crish, J.; Scanzello, C.R.; Krastins, B.; Sarracino, D.; Lopez, M.F.; Crow, M.K.; Aigner, T.; et al. Proteomic analysis of synovial fluid from the osteoarthritic knee: Comparison with transcriptome analyses of joint tissues. Arthritis Rheum. 2013, 65, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Rozelle, A.L.; Lepus, C.M.; Scanzello, C.R.; Song, J.J.; Larsen, D.M.; Crish, J.F.; Bebek, G.; Ritter, S.Y.; Lindstrom, T.M.; et al. Identification of a central role for complement in osteoarthritis. Nat. Med. 2011, 17, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Gobezie, R.; Kho, A.; Krastins, B.; Sarracino, D.A.; Thornhill, T.S.; Chase, M.; Millett, P.J.; Lee, D.M. High abundance synovial fluid proteome: Distinct profiles in health and osteoarthritis. Arthritis Res. 2007, 9, R36. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Puente, P.; Mateos, J.; Fernandez-Costa, C.; Oreiro, N.; Fernandez-Lopez, C.; Ruiz-Romero, C.; Blanco, F.J. Identification of a panel of novel serum osteoarthritis biomarkers. J. Proteome Res. 2011, 10, 5095–5101. [Google Scholar] [CrossRef] [PubMed]
- Corvetta, A.; Pomponio, G.; Rinaldi, N.; Luchetti, M.M.; Di Loreto, C.; Stramazzotti, D. Terminal complement complex in synovial tissue from patients affected by rheumatoid arthritis, osteoarthritis and acute joint trauma. Clin. Exp. Rheumatol. 1992, 10, 433–438. [Google Scholar]
- Bollmann, M.; Colombo, F.; Marco, P.; Brandstaedter, K.; Lohmann, C.H.; Bertrand, J. Inhibition of the complement system component c5 as possible treatment in OA. Osteoarthr. Cartil. 2018, 26, S108. [Google Scholar] [CrossRef]
- Trouw, L.A.; Pickering, M.C.; Blom, A.M. The complement system as a potential therapeutic target in rheumatic disease. Nat. Rev. Rheumatol. 2017, 13, 538–547. [Google Scholar] [CrossRef]
- Horiuchi, T.; Tsukamoto, H. Complement-targeted therapy: Development of c5- and c5a-targeted inhibition. Inflamm. Regen. 2016, 36, 11. [Google Scholar] [CrossRef] [PubMed]
- Macor, P.; Durigutto, P.; De Maso, L.; Garrovo, C.; Biffi, S.; Cortini, A.; Fischetti, F.; Sblattero, D.; Pitzalis, C.; Marzari, R.; et al. Treatment of experimental arthritis by targeting synovial endothelium with a neutralizing recombinant antibody to c5. Arthritis Rheum. 2012, 64, 2559–2567. [Google Scholar] [CrossRef]
- Durigutto, P.; Macor, P.; Ziller, F.; De Maso, L.; Fischetti, F.; Marzari, R.; Sblattero, D.; Tedesco, F. Prevention of arthritis by locally synthesized recombinant antibody neutralizing complement component c5. PLoS ONE 2013, 8, e58696. [Google Scholar] [CrossRef]
- Jansen, N.W.; Roosendaal, G.; Bijlsma, J.W.; Degroot, J.; Lafeber, F.P. Exposure of human cartilage tissue to low concentrations of blood for a short period of time leads to prolonged cartilage damage: An in vitro study. Arthritis Rheum. 2007, 56, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.L.; Brandt, K.D.; O’Connor, B.L.; Visco, D.M.; Albrecht, M.E. Synovitis and osteoarthritic changes in canine articular cartilage after anterior cruciate ligament transection. Effect of surgical hemostasis. Arthritis Rheum. 1990, 33, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.L.; Letson, H.L.; Morris, J.L.; McEwen, P.; Hazratwala, K.; Wilkinson, M.; Dobson, G.P. Tranexamic acid is associated with selective increase in inflammatory markers following total knee arthroplasty (tka): A pilot study. J. Orthop. Surg. Res. 2018, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Schmal, H.; Salzmann, G.M.; Niemeyer, P.; Langenmair, E.; Guo, R.; Schneider, C.; Habel, M.; Riedemann, N. Early intra-articular complement activation in ankle fractures. BioMed. Res. Int. 2014, 2014, 426893. [Google Scholar] [CrossRef]
- Thing, M.; Mertz, N.; Ågårdh, L.; Larsen, S.; Østergaard, J.; Larsen, C. Simulated synovial fluids for in vitro drug and prodrug release testing of depot injectables intended for joint injection. J. Drug Deliv. Sci. Tech. 2019, 49, 169–176. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Woodhouse, L.J.; Nettel-Aguirre, A.; Emery, C.A. Outcomes associated with early post-traumatic osteoarthritis and other negative health consequences 3-10 years following knee joint injury in youth sport. Osteoarthr. Cartil. 2015, 23, 1122–1129. [Google Scholar] [CrossRef]
- Group, M.K.; Jones, M.H.; Oak, S.R.; Andrish, J.T.; Brophy, R.H.; Cox, C.L.; Dunn, W.R.; Flanigan, D.C.; Fleming, B.C.; Huston, L.J.; et al. Predictors of radiographic osteoarthritis 2 to 3 years after anterior cruciate ligament reconstruction: Data from the moon on-site nested cohort. Orthop. J. Sports Med. 2019, 7, 2325967119867085. [Google Scholar]
- Tschaikowsky, M.; Selig, M.; Brander, S.; Balzer, B.N.; Hugel, T.; Rolauffs, B. Proof-of-concept for the detection of early osteoarthritis pathology by clinically applicable endomicroscopy and quantitative ai-supported optical biopsy. Osteoarthr. Cartil. 2020, 29, 269–279. [Google Scholar] [CrossRef]
- Longo, S.K.; Guo, M.G.; Ji, A.L.; Khavari, P.A. Integrating single-cell and spatial transcriptomics to elucidate intercellular tissue dynamics. Nat. Rev. Genet. 2021. [Google Scholar] [CrossRef]
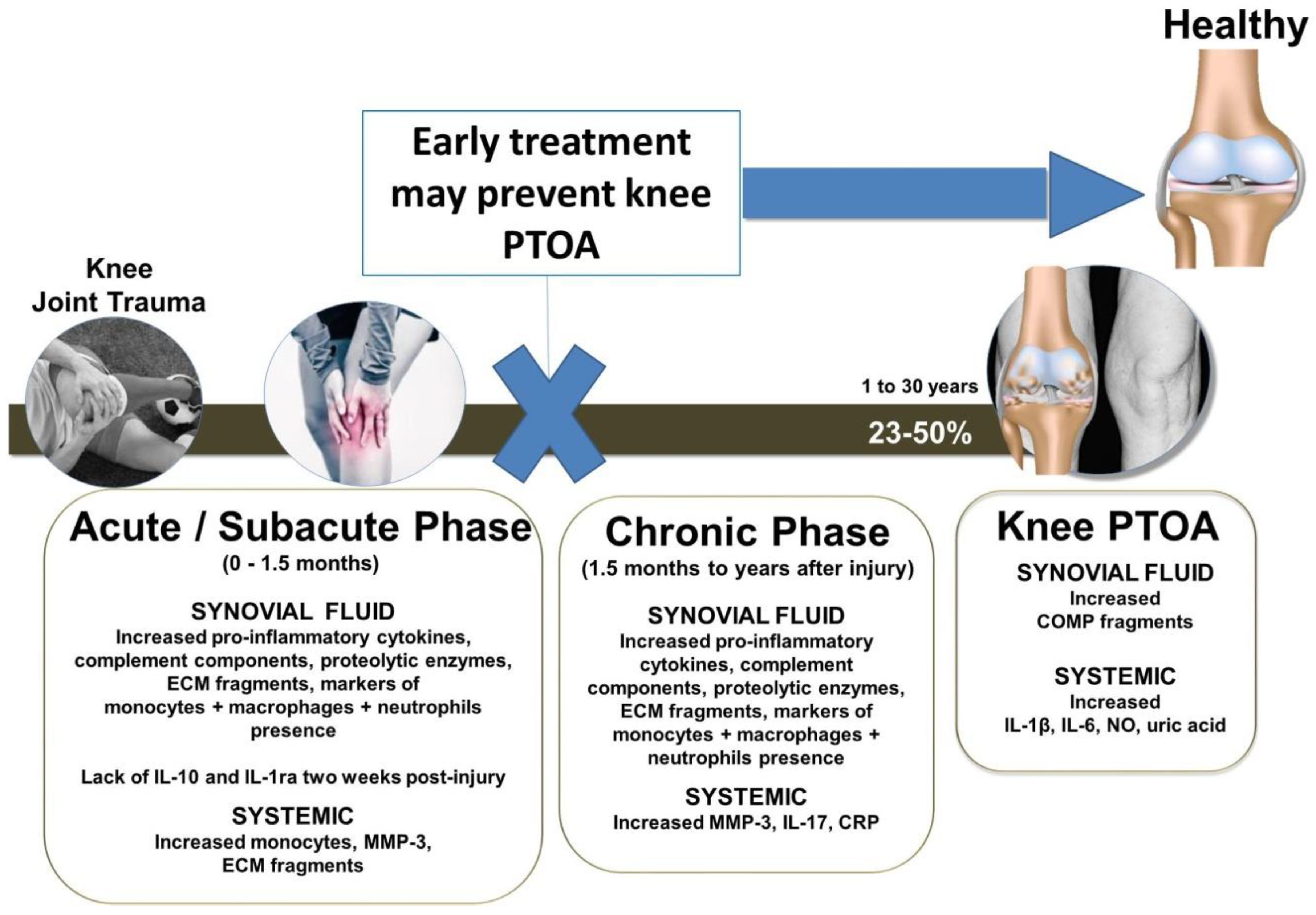
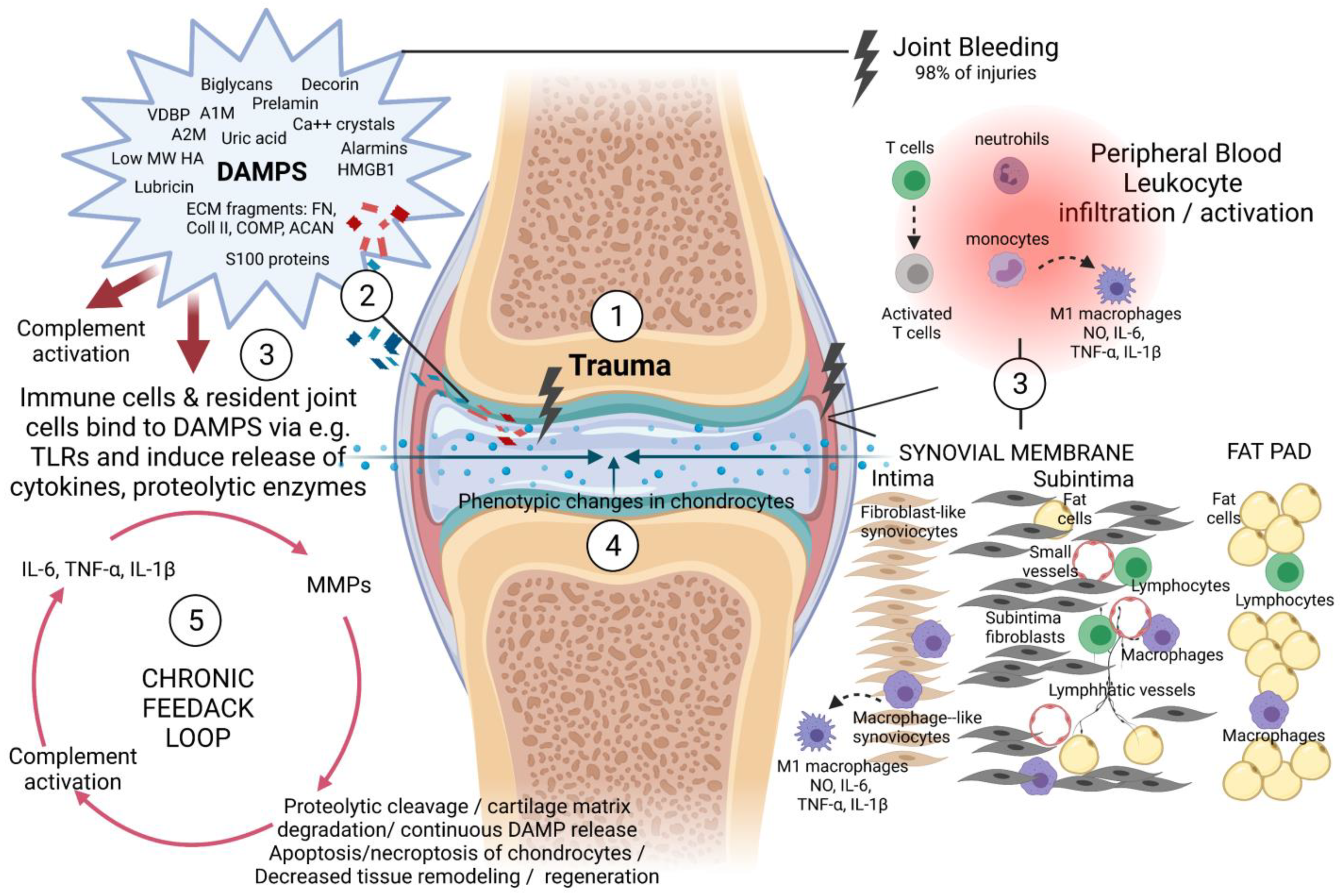
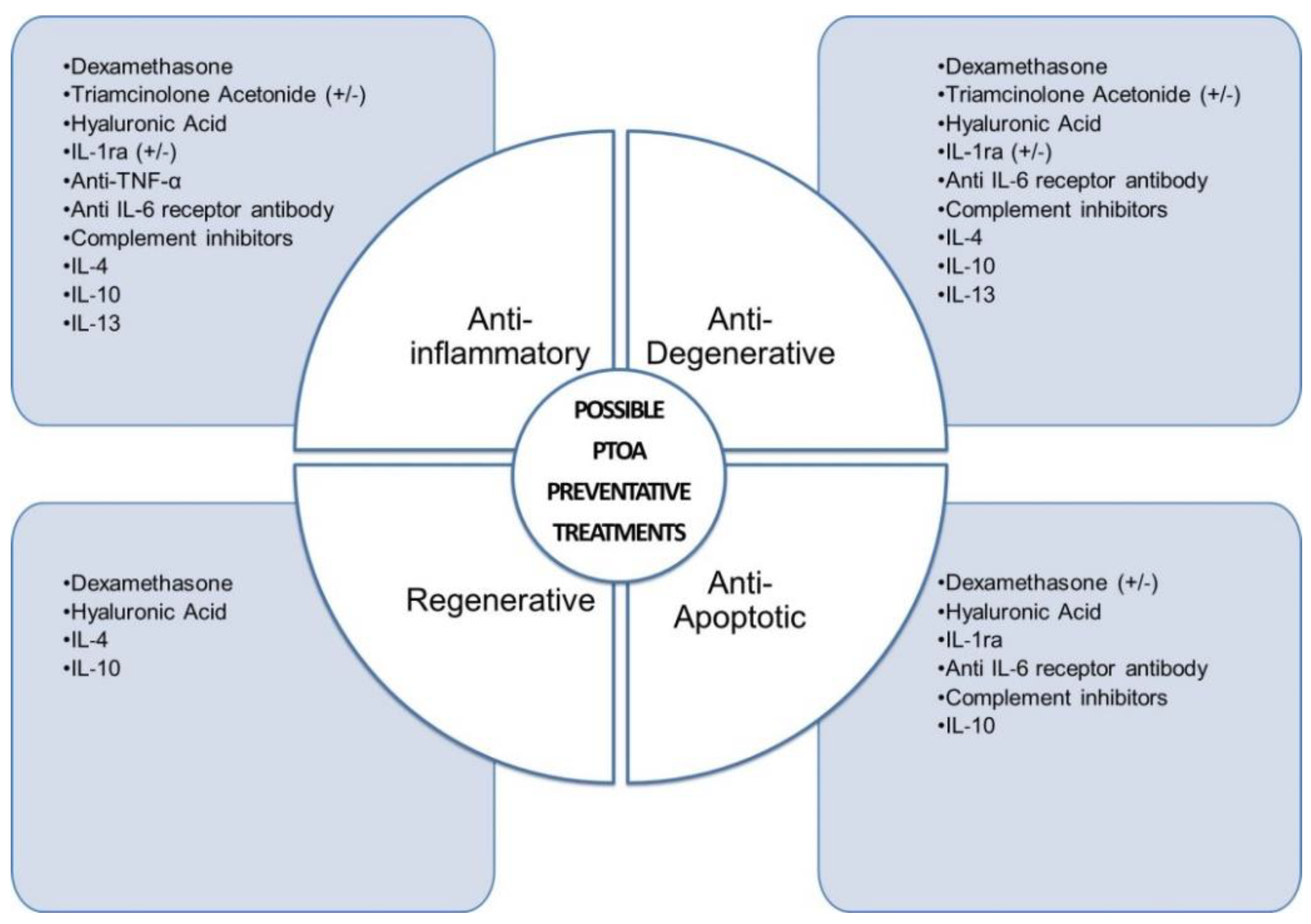
| Clinical Anti-Inflammatory Approaches to Prevent or Delay Knee PTOA | |||||
|---|---|---|---|---|---|
| Intervention | Trial Name | Patient Criteria | Study Design | Outcome | Benefit Observed |
| Triamcinolone acetonide from PLGA microspheres (FX006, Zilretta®) | Success of Long-acting Anti-inflammatories After Anterior Cruciate Ligament and Meniscal Injury (SLAM) (NCT04331002) | Elevated synovial fluid IL-6 remaining 4 weeks after ACL reconstruction with meniscal involvement (Age 18–40 years) | Single i.a. injection 8 weeks after ACL reconstruction in a Phase 2, randomized, quadruple blinded, parallel assignment, placebo-controlled study | Bone Shape, IKDC, KOOS Global ICOAP at baseline, 4 months, 1 year and 2 years after intervention; CTX-II levels only at baseline and 4 months | Recruiting |
| Triamcinolone acetonide alone (Kenalog®-40) vs. Triamcinolone acetonide from PLGA microspheres (FX006, Zilretta®) | Proof of Concept Study Comparing FX006 to Kenalog®-40 in Patients With Post-Traumatic Osteoarthritis of the Knee (NCT02468583) | Kellgren-Lawrence (KL) Grade 2 or 3 PTOA (Age 20–50 years) | Single i.a. injection in a Phase 2, randomized, quadruple blinded, parallel assignment study | Pain intensity score using NRS, WOMAC—(A1, B, C and Total), KOOS, PGIC, CGIC, % of responders according to OMERACT-OARSI criteria, time to onset of pain relief and average weekly and total consumption of rescue medication over 12 weeks after intervention | Data not yet available |
| Recombinant human IL-1ra (Anakinra®) | Study to Prevent Cartilage Damage Following Acute Knee Injury (NCT00332254) [25] | Onset of a sports-related ACL tear requiring surgery (Age 18–30 years) | Single i.a. injection within 4 weeks (a mean 15 ± 7 days) of knee injury in a Phase 1/2, randomized, quadruple blinded, parallel assignment, placebo-controlled study involving 11 patients | KOOS at baseline before treatment, 4 and 14 days after intervention; SF IL-1α, IL-1β and IL-1ra levels and serum HA at baseline and a mean of 35 days after treatment | Improvement in KOOS, decrease in SF IL-1α within 2 weeks of treatment |
| Recombinant human IL-1ra (Anakinra®) | Study to Early PTOA Following Acute Knee Injury (NCT02930122) | ACL tear and painful effusions (Age 14–40 years) | Single i.a. injection within 4 weeks of injury in a Phase 2 prospective, single-center, randomized, triple-blinded, placebo-controlled study | CTX-II levels from injury to time of surgery, KOOS scores and quantitative T1rho MRI 1 year after injury | Data not yet available |
| Therapeutic Agent | Drug Delivery | Pre-Clinical and Clinical Studies | Effect | Company/ References |
|---|---|---|---|---|
| Dexamethasone | Lipid-based microspheres (TLC599, BioSeizer®) | Phase 2 (OA-associated knee pain) | Reduced pain | TLC, [67] |
| Dexamethasone | Avidin | Bovine cartilage explant (IL-1α) model PTOA (ACLT) rabbit model | Rescued IL-1α induced cell death and decreased cartilage degeneration Decreased inflammation and cartilage degeneration | [53,65,66] |
| Triamcinolone Acetonide | PLGA microspheres (FX006, Zilretta®) | FDA approved (OA-associated knee pain); Phase 2 (PTOA, ACL injury) Acute synovitis (PGPS) rat model | Reduced pain Results not yet published Decreased inflammation and cartilage degeneration | Flexion Therapeutics [70] NCT04331002 NCT02468583 [79] |
| Triamcinolone Acetonide | PEA microspheres | OA (type II collagenase) rat model; Acute synovitis (bacterial PGPS) rat model | Decreased inflammation but was not capable of decreasing cartilage degeneration | [77,78] |
| IL-1rawith or withoutsTNFRII | Elastin-like polypeptide (Anakinra® vs. Etanercept®) | PTOA (MCLT) mouse model | IL-1ra decreased inflammation and cartilage degeneration sTNFRII had adverse effects on cartilage and bone and caused synovial inflammation | [109] |
| IL-1ra | PLGA microspheres (Anakinra®) | PTOA (ACL tear) rat model | Decreased inflammation and cartilage degeneration | [124] |
| IL-1ra | Gene therapy Adenovirus vector under with NF-kB-responsive promoter (FX201) | Phase 1 (Knee OA) | Results not yet published | NCT04119687 |
| IL-1ra | HA-chitosan microspheres | Rat chondrocyte (IL-1β) model | Decreased inflammation and chondrocyte apoptosis | [125] |
| IL-1ra and IL-10 | Gene therapy retrovirus vector | PTOA (MCL+MM) rabbit model | Decreased cartilage degeneration | [217] |
| IL-4 and IL-10 | Fusion protein | Human OA knee cartilage and synovium; PTOA (groove) canine model | Decreased inflammation, improved PG turnover and reduced pain | [174] |
| IL-4 and IL-10 | Fusion protein | Human healthy cartilage (50% v/v blood-injury model); PTOA (joint bleeding) hemophilic mouse model | Decreased cartilage degeneration | [212,213] |
| IL-10 | Gene therapy lentivirus vector with CXCL10-responsive promoter | RA synovial cell and THP-1 monocyte cell line (LPS) models; Human OA synovial membrane/Matrigel 3D culture (TNF-α or LPS) model | Decreased inflammation | [218,219] |
| IL-4, IL-13 or IL-10 | Gelatin microspheres | ATDC-5 mouse chondrocyte (IL-1β or LPS) model | IL-4 and IL-13 decreased NO production | [199] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khella, C.M.; Horvath, J.M.; Asgarian, R.; Rolauffs, B.; Hart, M.L. Anti-Inflammatory Therapeutic Approaches to Prevent or Delay Post-Traumatic Osteoarthritis (PTOA) of the Knee Joint with a Focus on Sustained Delivery Approaches. Int. J. Mol. Sci. 2021, 22, 8005. https://doi.org/10.3390/ijms22158005
Khella CM, Horvath JM, Asgarian R, Rolauffs B, Hart ML. Anti-Inflammatory Therapeutic Approaches to Prevent or Delay Post-Traumatic Osteoarthritis (PTOA) of the Knee Joint with a Focus on Sustained Delivery Approaches. International Journal of Molecular Sciences. 2021; 22(15):8005. https://doi.org/10.3390/ijms22158005
Chicago/Turabian StyleKhella, Christine M., Judith M. Horvath, Rojiar Asgarian, Bernd Rolauffs, and Melanie L. Hart. 2021. "Anti-Inflammatory Therapeutic Approaches to Prevent or Delay Post-Traumatic Osteoarthritis (PTOA) of the Knee Joint with a Focus on Sustained Delivery Approaches" International Journal of Molecular Sciences 22, no. 15: 8005. https://doi.org/10.3390/ijms22158005
APA StyleKhella, C. M., Horvath, J. M., Asgarian, R., Rolauffs, B., & Hart, M. L. (2021). Anti-Inflammatory Therapeutic Approaches to Prevent or Delay Post-Traumatic Osteoarthritis (PTOA) of the Knee Joint with a Focus on Sustained Delivery Approaches. International Journal of Molecular Sciences, 22(15), 8005. https://doi.org/10.3390/ijms22158005








