Metal-Specificity Divergence between Metallothioneins of Nerita peloronta (Neritimorpha, Gastropoda) Sets the Starting Point for a Novel Chemical MT Classification Proposal
Abstract
:1. Introduction
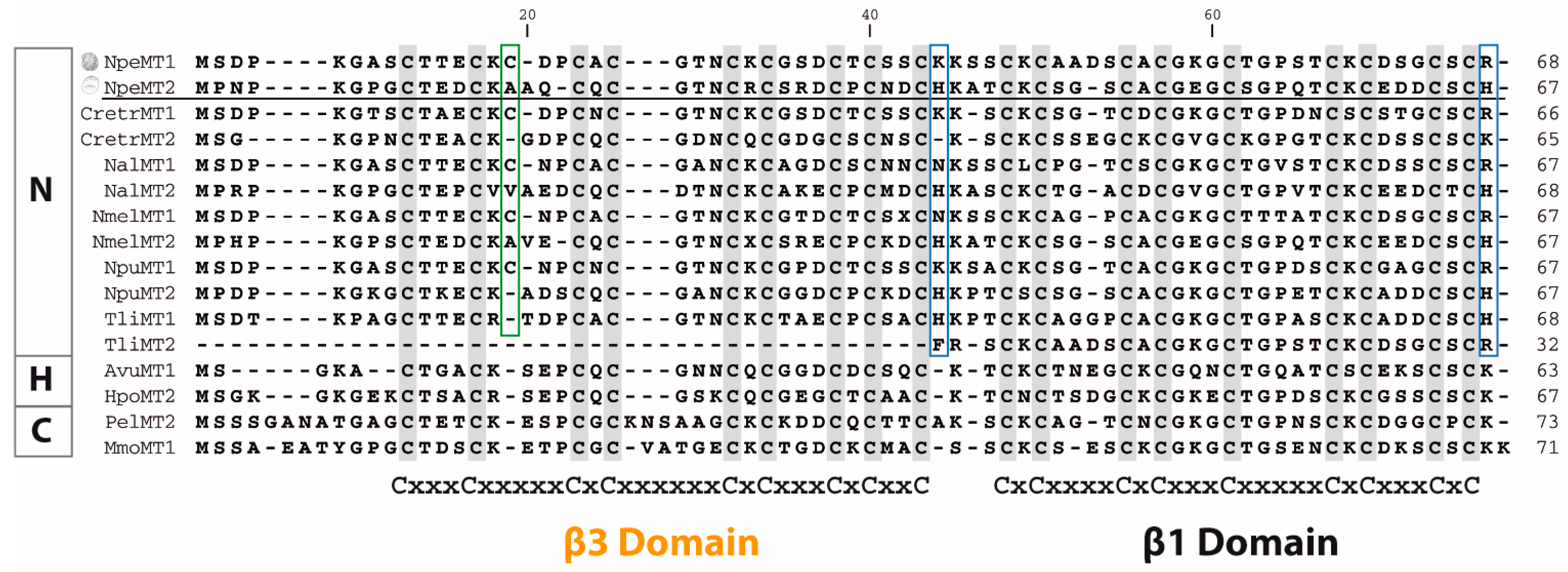
2. Results and Discussion
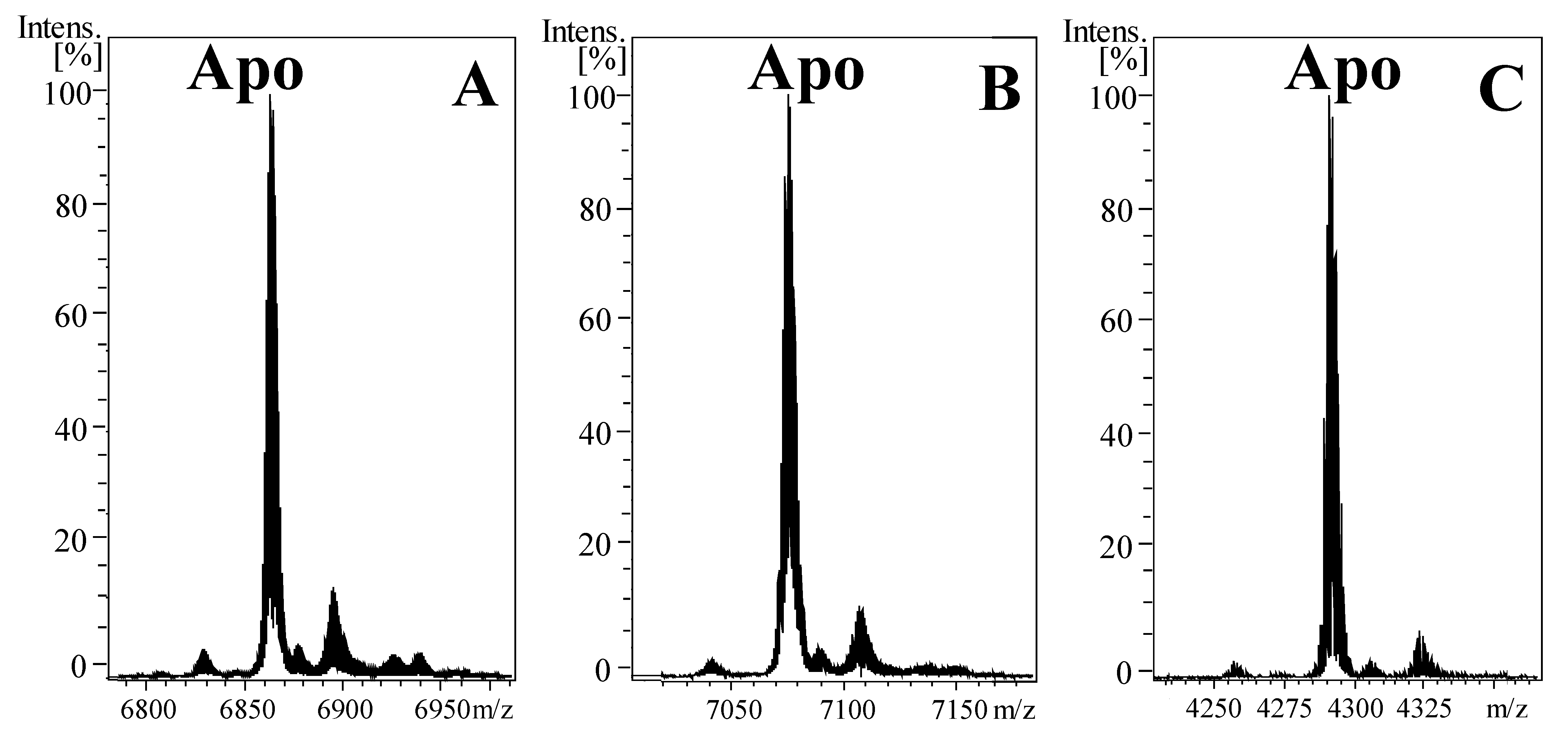
2.1. Heterologous Expression and Production of Metal-NpeMT Complexes
2.2. The Metal-Binding Abilities of NpeMT Isoforms towards Divalent Metal Ions Confirms a Cd-Selective Origin in Snail MTs
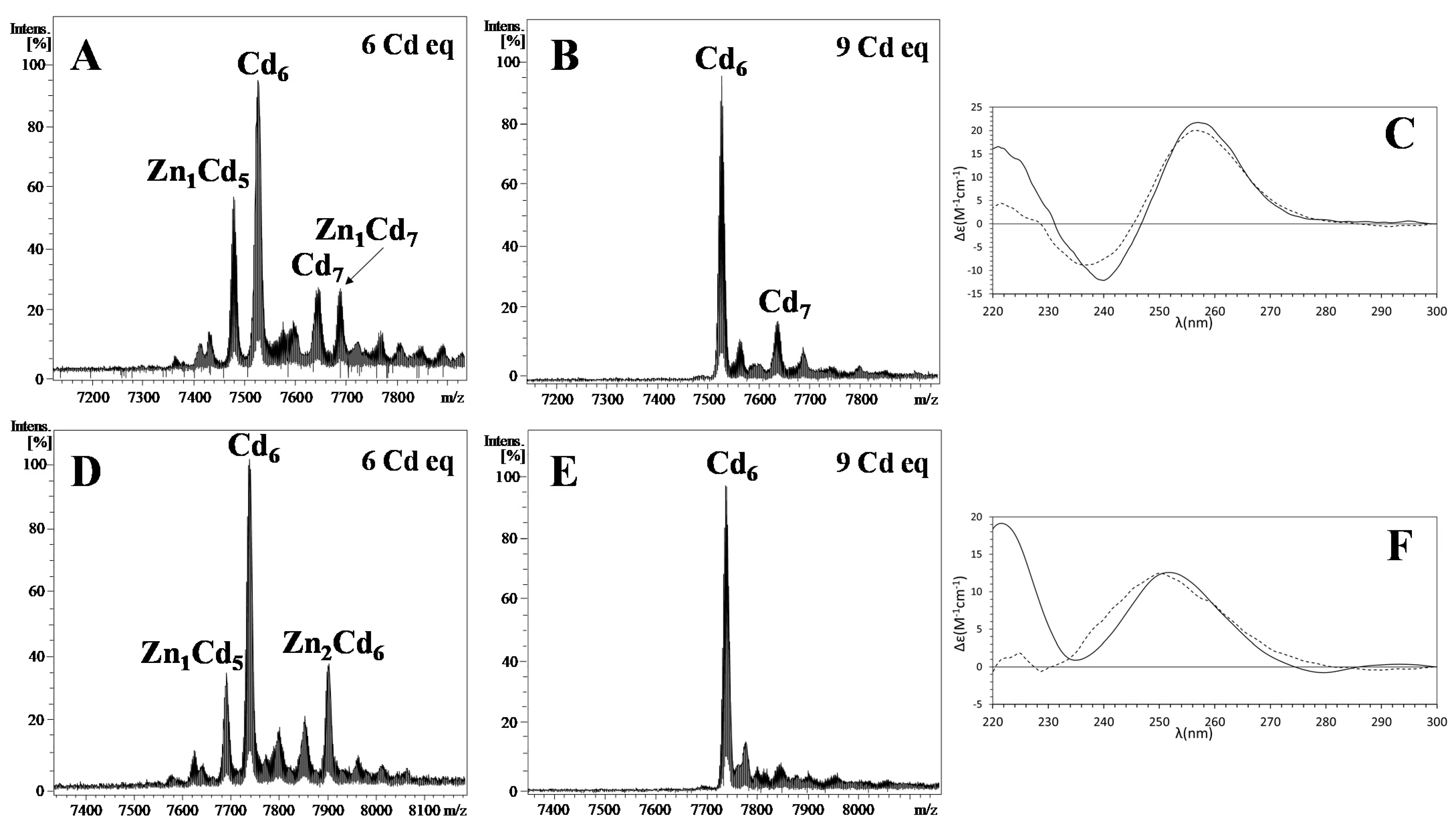
2.3. Cd Selectivity in NpeMTs Makes Way to a Gradual Transition towards Zn Specificity
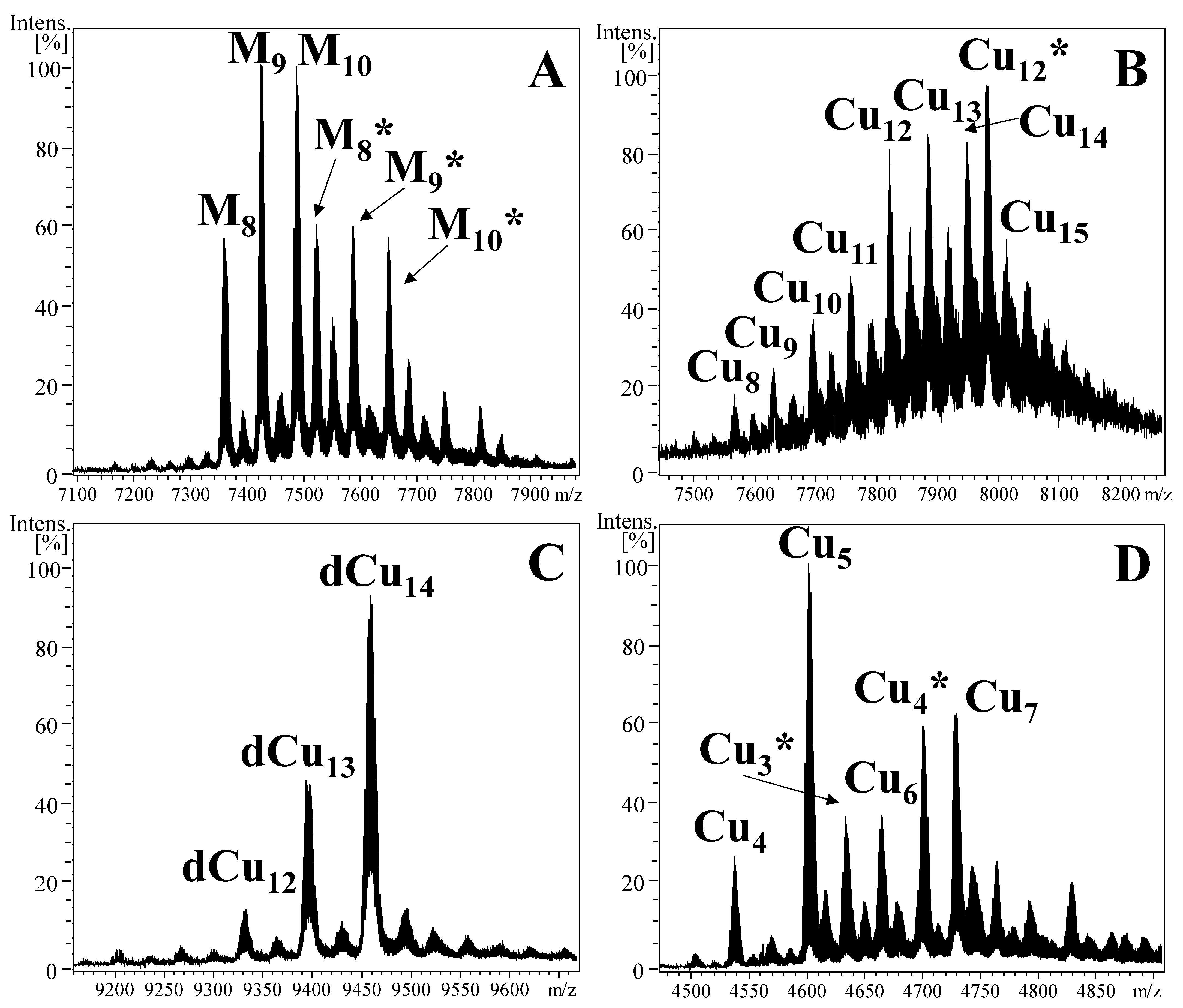
2.4. NpeMT Isoforms Reveal Important Differences in Their CuI Binding Features
2.5. NpeMT Isoforms Provide the Key to Set Up a Novel MT Classification Scheme
2.6. Biological Implications
3. Materials and Methods
3.1. Cloning, Production, and Purification of Recombinant Metal–MT Complexes
3.2. Characterization of the Metal-NpeMT1 and Metal-NpeMT2 Complexes
3.3. Metal-Protein Binding Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nordberg, M.; Nordberg, G.F. Metallothioneins: Historical development and overview. In Metallothioneins and Related Chelators; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2009; Volume 5, pp. 1–29. [Google Scholar]
- Capdevila, M.; Bofill, R.; Palacios, O.; Atrian, S. State-of-the-art of metallothioneins at the beginning of the 21st century. Coord. Chem. Rev. 2012, 256, 46–62. [Google Scholar] [CrossRef]
- Capdevila, M.; Atrian, S. Metallothionein protein evolution: A miniassay. J. Biol. Inorg. Chem. 2011, 16, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.D.; Liu, J.; Choudhuri, S. Metallothionein: An intracellular protein to protect against cadmium toxicity. Annu Rev Pharmacol. Toxicol. 1999, 39, 267–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandbichler, A.M.; Höckner, M. Cadmium Protection Strategies—A Hidden Trade-Off? Int. J. Mol. Sci. 2016, 17, 139. [Google Scholar] [CrossRef] [Green Version]
- Moulis, J.M. Cellular mechanisms of cadmium toxicity related to the homeostasis of essential metals. Biometals 2010, 23, 877–896. [Google Scholar] [CrossRef]
- Janssens, T.K.S.; Roelofs, D.; van Straalen, N.M. Molecular mechanisms of heavy metal tolerance and evolution in invertebrates. Insect Sci. 2009, 16, 3–18. [Google Scholar] [CrossRef]
- Faddeeva-Vakhrusheva, A.; Derks, M.F.L.; Anvar, S.Y.; Agamennone, V.; Suring, W.; Smit, S.; van Straalen, N.M.; Roelofs, D. Gene Family Evolution Reflects Adaptation to Soil Environmental Stressors in the Genome of the Collembolan Orchesella cincta. Genome Biol. Evol. 2016, 7, 2106–2117. [Google Scholar] [CrossRef] [Green Version]
- Egg, M.; Höckner, M.; Chabicovsky, M.; Brandstätter, A.; Schuler, D.; Dallinger, R. Structural and bioinformatic analysis of the Roman snail Cd-Metallothionein gene uncovers molecular adaptation towards plasticity in coping with multifarious environmental stress. Mol. Ecol. 2009, 18, 2426–2443. [Google Scholar] [CrossRef] [PubMed]
- Dallinger, R.; Zerbe, O.; Baumann, C.; Egger, B.; Capdevila, M.; Palacios, Ò.; Albalat, R.; Calatayud, S.; Ladurner, P.; Schlick-Steiner, B.C.; et al. Metallomics reveals a persisting impact of cadmium on the evolution of metal-selective snail metallothioneins. Metallomics 2020, 12, 702–720. [Google Scholar] [CrossRef]
- Calatayud, S.; Garcia-Risco, M.; Pedrini-Martha, V.; Eernisse, D.J.; Dallinger, R.; Palacios, Ò.; Capdevila, M.; Albalat, R. Modularity in protein evolution: Modular organization and de novo domain evolution in mollusc metallothioneins. Mol. Biol. Evol. 2020, 38, 424–436. [Google Scholar] [CrossRef]
- Calatayud, S.; Garcia-Risco, M.; Palacios, Ò.; Capdevila, M.; Cañestro, C.; Albalat, R. Tunicates illuminate the enigmatic evolution of chordate metallothioneins by gene gains and losses, independent modular expansions and functional convergences. Mol. Biol. Evol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Schmielau, L.; Dvorak, M.; Niederwanger, M.; Dobieszewski, N.; Pedrini-Martha, V.; Ladurner, P.; Pedregal, J.R.; Maréchal, J.D.; Dallinger, R. Differential response to Cadmium exposure by expression of a two and a three-domain metallothionein isoform in the land winkle Pomatias elegans: Valuating the marine heritage of a land snail. Sci. Total Environ. 2019, 648, 561–571. [Google Scholar] [CrossRef]
- Dvorak, M.; Lackner, R.; Niederwanger, M.; Rotondo, C.; Schnegg, R.; Ladurner, P.; Pedrini-Martha, V.; Salvenmoser, W.; Kremser, L.; Lindner, H.; et al. Metal binding functions of metallothioneins in the slug Arion vulgaris differ from metal-specific isoforms of terrestrial snails. Metallomics 2018, 10, 1638–1654. [Google Scholar] [CrossRef]
- Pérez-Rafael, S.; Monteiro, F.; Dallinger, R.; Atrian, S.; Palacios, Ò.; Capdevila, M. Cantareus aspersus metallothionein metal binding abilities: The unspecific CaCd/CuMT isoform provides hints about the metal preference determinants in metallothioneins. BBA 2014, 1844, 1694–1707. [Google Scholar] [CrossRef] [PubMed]
- Bofill, R.; Capdevila, M.; Atrian, S. Independent metal-binding features of recombinant metallothioneins convergently draw a step gradation between Zn- and Cu–thioneins. Metallomics 2009, 1, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Binz, P.A.; Kägi, J.H.R. Metallothionein: Molecular evolution and classification. In Metallothionein IV; Klassen, C.D., Ed.; Birkhäuser Verlag: Basel, Switzerland, 1999; pp. 7–13. [Google Scholar]
- Valls, M.; Bofill, R.; Gonzalez-Duarte, R.; Gonzalez-Duarte, P.; Capdevila, M.; Atrian, S. A new insight into metallothionein (MT) classification and evolution. The in vivo and in vitro metal binding features of Homarus americanus recombinant MT. J. Biol. Chem. 2001, 276, 32835–32843. [Google Scholar] [CrossRef] [Green Version]
- Palacios, Ò.; Atrian, S.; Capdevila, M. Zn- and Cu–thioneins: A functional classification for metallothioneins? J. Biol. Inorg. Chem. 2011, 16, 991–1009. [Google Scholar] [CrossRef]
- Palacios, Ò.; Pérez-Rafael, S.; Pagani, A.; Dallinger, R.; Atrian, S.; Capdevila, M. Cognate and noncognate metal ion coordination in metal-specific metallothioneins: The Helix pomatia system as a model. J. Biol. Inorg. Chem. 2014, 19, 923–935. [Google Scholar] [CrossRef]
- Höckner, M.; Stefanon, K.; de Vaufleury, A.; Monteiro, F.; Pérez-Rafael, S.; Palacios, Ò.; Capdevila, M.; Atrian, S.; Dallinger, R. Physiological relevance and contribution to metal balance of specific and non-specific metallothionein isoforms in the garden snail, Cantareus aspersus. Biometals 2011, 24, 1079–1092. [Google Scholar] [CrossRef]
- Cunha, T.J.; Giribet, G. A congruent topology for deep gastropod relationships. Proc. Biol. Sci. 2019, 286, 20182776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uribe, J.E.; Colgan, D.; Castro, L.R.; Kano, Y.; Zardoya, R. Phylogenetic relationships among superfamilies of Neritimorpha (Mollusca: Gastropoda)”. Mol. Phylogen. Evol. 2016, 104, 21–31. [Google Scholar] [CrossRef]
- Strong, E.E.; Gargominy, O.; Ponder, W.F.; Bouchet, P. Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. Hydrobiologia 2008, 595, 149–166. [Google Scholar] [CrossRef]
- Ekdale, A.A. Marine molluscs from shallow water environments (0 to 60 m) off the northeast Yucatan coast, Mexico. Bull. Mar. Sci. 1974, 24, 638–668. [Google Scholar]
- Frey, M.A.; Vermeij, G.J. Molecular phylogenies and historical biogeography of a circumtropical group of gastropods (Genus: Nerita): Implications for regional diversity patterns in the marine tropics. Mol. Phyl. Evol. 2008, 48, 1067–1086. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.L.; Homan, W.C.; Swanson, J.; Weldon, P.J. Flight responses of three congeneric species of intertidal gastropods to sympatric predatory gastropods from Barbados. Veliger 1978, 21, 293–296. [Google Scholar]
- Hughes, R.N. Ecological energetics of Nerita (Archaeogastropoda, Neritacea) populations on Barbados, West Indies. Mar. Biol. 1971, 11, 12–22. [Google Scholar] [CrossRef]
- Yap, C.K.; Cheng, W.H.; Ismail, A.; Ismail, A.R.; Tan, S.G. Biomonitoring of heavy metal (Cd, Cu, Pb, and Zn) concentrations in the west intertidal area of Peninsular Malaysia by using Nerita lineata. Toxicol. Environ. Chem. 2009, 91, 29–41. [Google Scholar] [CrossRef]
- Miskon, M.F.; Schazili, N.A.M.; Mohamad, F.; Yunus, K. Nerita chameleon as Biomonitoring Agent for Pb, Cd, Cu and Zn in Malaysian Intertidal Rocky Shore Environment. Oriental J. Chem. 2015, 31, 1013–1020. [Google Scholar] [CrossRef]
- Azratul, A.N.M.D.; Akbar John, B.; Kamaruzzaman, B.Y.; Hassan, S.I.; Jalal, K.C.A.; Noor Faizul, H.N. Bio-Monitoring Selected Heavy Metal Concentration In Nerita Sp. Collected From Tanjung Lumpur Mangrove Forest. Environ. Ecosyst. Sci. 2017, 1, 04–07. [Google Scholar]
- Cols, N.; Romero-Isart, N.; Capdevila, M.; Oliva, B.; Gonzàlez-Duarte, P.; Gonzàlez-Duarte, R.; Atrian, S. Binding of excess cadmium(II) to Cd7-metallothionein from recombinant mouse Zn7-metallothionein 1. UV-VIS absorption and circular dichroism studies and theoretical location approach by surface accessibility analysis. J. Inor. Biochem. 1997, 68, 157–166. [Google Scholar] [CrossRef]
- Palacios, Ò.; Pagani, A.; Pérez-Rafael, S.; Egg, M.; Höckner, M.; Brandstätter, A.; Capdevila, M.; Atrian, S.; Dallinger, R. Shaping mechanisms of metal specificity in a family of metazoan metallothioneins: Evolutionary differentiation of mollusc metallothioneins. BMC Biol. 2011, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallinger, R.; Berger, B.; Hunziker, P.E.; Kägi, J.H.R. Metallothionein in snail Cd and Cu metabolism. Nature 1997, 388, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Dallinger, R.; Chabicovsky, M.; Hödl, E.; Prem, C.; Hunziker, P.; Manzl, C. Copper in Helix pomatia (Gastropoda) is regulated by one single cell type: Differently responsive metal pools in rhogocytes. Am. J. Physiol. 2005, 189, R1185–R1195. [Google Scholar] [CrossRef] [Green Version]
- García-Risco, M.; Calatayud, S.; Niederwanger, M.; Albalat, R.; Palacios, Ò.; Capdevila, M.; Dallinger, R. Two unconventional metallothioneins in the apple snail Pomacea bridgesii have lost their metal specificity during adaptation to freshwater habitats. Int. J. Mol. Sci. 2021, 22, 95. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, S.; Garcia-Risco, M.; Capdevila, M.; Cañestro, C.; Palacios, Ò.; Albalat, R. Modular Evolution and Population Variability of Oikopleura dioicaa Metallothioneins. Front. Cell Dev. Biol. 2021, 9, 702688, eCollection 2021. [Google Scholar] [CrossRef]
- Gruber, C.; Stürzenbaum, S.; Gehrig, P.; Sack, R.; Hunziker, P.; Berger, B.; Dallinger, R. (Cd)-Metallothionein from Eisenia foetida: Isolation and characterization of a self-sufficient one-domain protein. Eur. J. Biochem. 2000, 267, 573–582. [Google Scholar] [CrossRef]
- Baumann, C.; Beil, A.; Jurt, S.; Niederwanger, M.; Palacios, O.; Capdevila, M.; Atrian, S.; Dallinger, R.; Zerbe, O. Structural adaptation of a protein to increased metal stress: NMR structure of a marine snail metallothionein with an additional domain. Angew. Chem. Int. 2017, 56, 4617–4622. [Google Scholar] [CrossRef]
- Beil, A.; Jurt, S.; Walser, R.; Schönhut, T.; Güntert, P.; Palacios, Ò.; Atrian, S.; Capdevila, M.; Dallinger, R.; Zerbe, O. The solution structure and dynamics of Cd-metallothionein from Helix pomatia reveal optimization for binding Cd over Zn. Biochemistry 2019, 58, 4570–4581. [Google Scholar] [CrossRef] [Green Version]
- Villarreal, L.; Tío, L.; Capdevila, M.; Atrian, S. Comparative metal binding and genomic analysis of the avian (chicken) and mammalian metallothionein. FEBS J. 2006, 273, 523–535. [Google Scholar] [CrossRef]
- Leszczyszyn, O.I.; Schmid, R.; Blindauer, C.A. Toward a property/function relationship for metallothioneins: Histidine coordination and unusual cluster composition in a zinc-metallothionein from plants. Proteins 2007, 68, 922–935. [Google Scholar] [CrossRef]
- Pérez-Rafael, S.; Pagani, A.; Palacios, Ò.; Dallinger, R.; Capdevila, M.; Atrian, S. The Role of Histidine in a Copper-Specific Metallothionein. Z. Anorg. Allg. Chem. 2013, 639, 1356–1360. [Google Scholar] [CrossRef]
- Ejnik, J.; Robinson, J.; Zhu, J.; Försterling, H.; Shaw, C.F.; Petering, D.H. Folding pathway of apo-metallothionein induced by Zn2+, Cd2+ and Co2+. J. Inorg. Biochem. 2002, 88, 144–152. [Google Scholar] [CrossRef]
- Chen, S.H.; Chen, L.; Russell, D.H. Metal-induced conformational changes of human metallothionein-2A: A combined theoretical and experimental study of metal-free and partially metalated intermediates. J. Am. Chem. Soc. 2014, 136, 9499–9508. [Google Scholar] [CrossRef]
- Schaeffer, R.D.; Daggett, V. Protein folds and protein folding. Protein Eng. Des. Sel. 2011, 1-2, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Calatayud, S.; Garcia-Risco, M.; Rojas, N.S.; Espinosa-Sánchez, L.; Artime, S.; Palacios, S.; Cañestro, C.; Albalat, R. Metallothioneins of the urochordate Oikopleura dioica have Cys-rich tandem repeats, large size and cadmium-binding preference. Metallomics 2018, 10, 1585–1594. [Google Scholar] [CrossRef] [Green Version]
- MolluscaBase (Ed.) 2021. Available online: http://www.molluscabase.org (accessed on 2 February 2021).
- Amin, B.; Ismail, A.; Arshad, A.; Yap, C.K.; Kamarudin, M.S. A comparative study of heavy metal concentrations in Nerita lineata from the intertidal zone between Indonesia and Johor Malaysia. J. Coastal Developm. 2013, 10, 19–32. [Google Scholar]
- Yap, C.K.; Cheng, W.H.; Zakaria, M.P.; Zaharin, A.; Tan, S.G. Cd and Zn in Nerita lineata collected from selected areas of the south west coast of peninsular Malaysia. J. Sustainab. Sci. Managem. 2013, 8, 207–211. [Google Scholar]
- Benito, D.; Niederwanger, M.; Izagirre, U.; Dallinger, R.; Soto, M. Successive onset of molecular, cellular and tissue-specific responses in midgut gland of Littorina littorea exposed to sub-lethal cadmium concentrations. Int. J. Mol. Sci. 2017, 18, 1815. [Google Scholar] [CrossRef] [Green Version]
- Bongers, J.; Walton, C.D.; Richardson, D.E.; Bell, J.U. Micromolar protein concentrations and metalloprotein stoichiometries obtained by inductively coupled plasma. Atomic emission spectrometric determination of sulfur. Anal. Chem. 1988, 60, 2683–2686. [Google Scholar] [CrossRef] [PubMed]
- Fabris, D.; Zaia, J.; Hathout, Y.; Fenselau, C. Retention of thiol protons in two classes of protein zinc ion coordination centers. J. Am. Chem. Soc. 1996, 118, 12242–12243. [Google Scholar] [CrossRef]
- Capdevila, M.; Cols, N.; Romero-Isart, N.; Gonzàlez-Duarte, R.; Atrian, S.; Gonzàlez-Duarte, P. Recombinant synthesis of mouse Zn3-beta and Zn4-alpha metallothionein 1 domains and characterization of their cadmium(II) binding capacity. Cell. Mol. Life. Sci. 1997, 53, 681–688. [Google Scholar] [CrossRef] [PubMed]


| Isoform | Supplemented Metal Ion a | Protein Concentration (10−4 M) | Metal-to-Protein Ratio b |
|---|---|---|---|
| NpeMT1 (19 Cys) | ZnII | 2.1 | 5.9 Zn |
| CdII | 1.0 | 5.5 Cd | |
| CuII | 1.0 | 1.9 Zn; 7.1 Cu | |
| NpeMT2 (18 Cys + 2 His) | ZnII | 2.9 | 5.9 Zn |
| CdII | 1.4 | 5.5 Cd | |
| CuII | 0.3 | 12.0 Cu | |
| β3NpeMT2 (9 Cys + 1 His) | ZnII | 3.7 | 2.8 Zn |
| CdII | 3.7 | 2.6 Cd | |
| CuII (Dimers) | 0.6 | 5.3 Cu | |
| CuII (Monomers) | 0.6 | 5.6 Cu |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Risco, M.; Calatayud, S.; Pedrini-Martha, V.; Albalat, R.; Dallinger, R.; Palacios, Ò.; Capdevila, M. Metal-Specificity Divergence between Metallothioneins of Nerita peloronta (Neritimorpha, Gastropoda) Sets the Starting Point for a Novel Chemical MT Classification Proposal. Int. J. Mol. Sci. 2021, 22, 13114. https://doi.org/10.3390/ijms222313114
García-Risco M, Calatayud S, Pedrini-Martha V, Albalat R, Dallinger R, Palacios Ò, Capdevila M. Metal-Specificity Divergence between Metallothioneins of Nerita peloronta (Neritimorpha, Gastropoda) Sets the Starting Point for a Novel Chemical MT Classification Proposal. International Journal of Molecular Sciences. 2021; 22(23):13114. https://doi.org/10.3390/ijms222313114
Chicago/Turabian StyleGarcía-Risco, Mario, Sara Calatayud, Veronika Pedrini-Martha, Ricard Albalat, Reinhard Dallinger, Òscar Palacios, and Mercè Capdevila. 2021. "Metal-Specificity Divergence between Metallothioneins of Nerita peloronta (Neritimorpha, Gastropoda) Sets the Starting Point for a Novel Chemical MT Classification Proposal" International Journal of Molecular Sciences 22, no. 23: 13114. https://doi.org/10.3390/ijms222313114
APA StyleGarcía-Risco, M., Calatayud, S., Pedrini-Martha, V., Albalat, R., Dallinger, R., Palacios, Ò., & Capdevila, M. (2021). Metal-Specificity Divergence between Metallothioneins of Nerita peloronta (Neritimorpha, Gastropoda) Sets the Starting Point for a Novel Chemical MT Classification Proposal. International Journal of Molecular Sciences, 22(23), 13114. https://doi.org/10.3390/ijms222313114









