Transcriptomics Reveals the ERF2-bHLH2-CML5 Module Responses to H2S and ROS in Postharvest Calcium Deficiency Apples
Abstract
:1. Introduction
2. Results
2.1. Calcium Deficiency Significantly Affects the Phenotype of Apple Peels and Reduces H2S Production
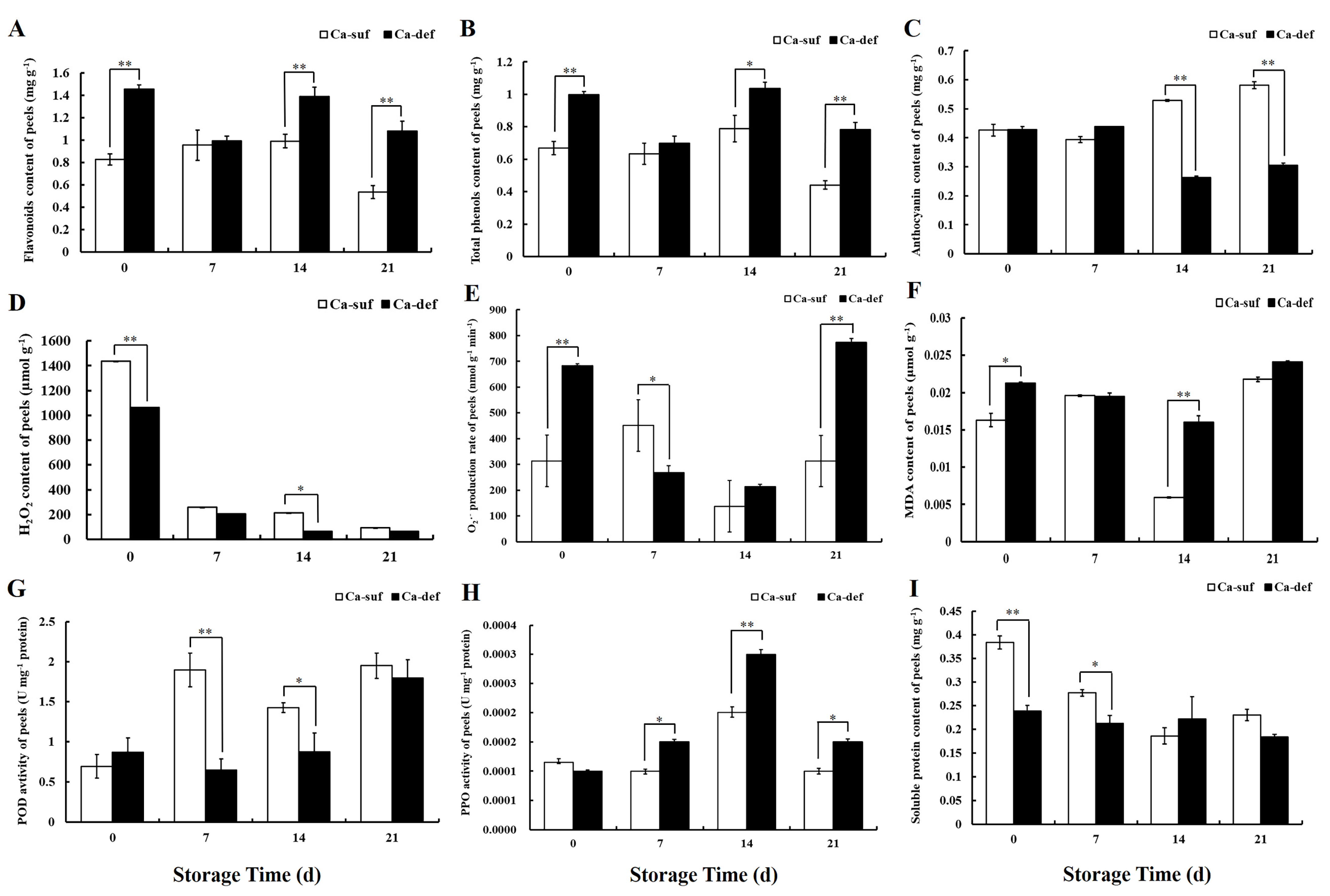
2.2. Calcium Deficiency Increases the Contents of Flavonoids and Total Phenols but Decreases Anthocyanin Contents in Apple Peels
2.3. Calcium Deficiency Induces the Production of ROS in Apple Peels
2.4. Changes in POD Activity, PPO Activity Soluble Protein Content between Calcium Deficiency and Sufficiency Conditions
2.5. PCA Analysis of the Bioactive Substance Changes in Apple Peels
2.6. Identification and GO Classification and KEGG Enrichment Analysis of DEGs between Calcium-Deficient and Calcium-Sufficient Apple Peels
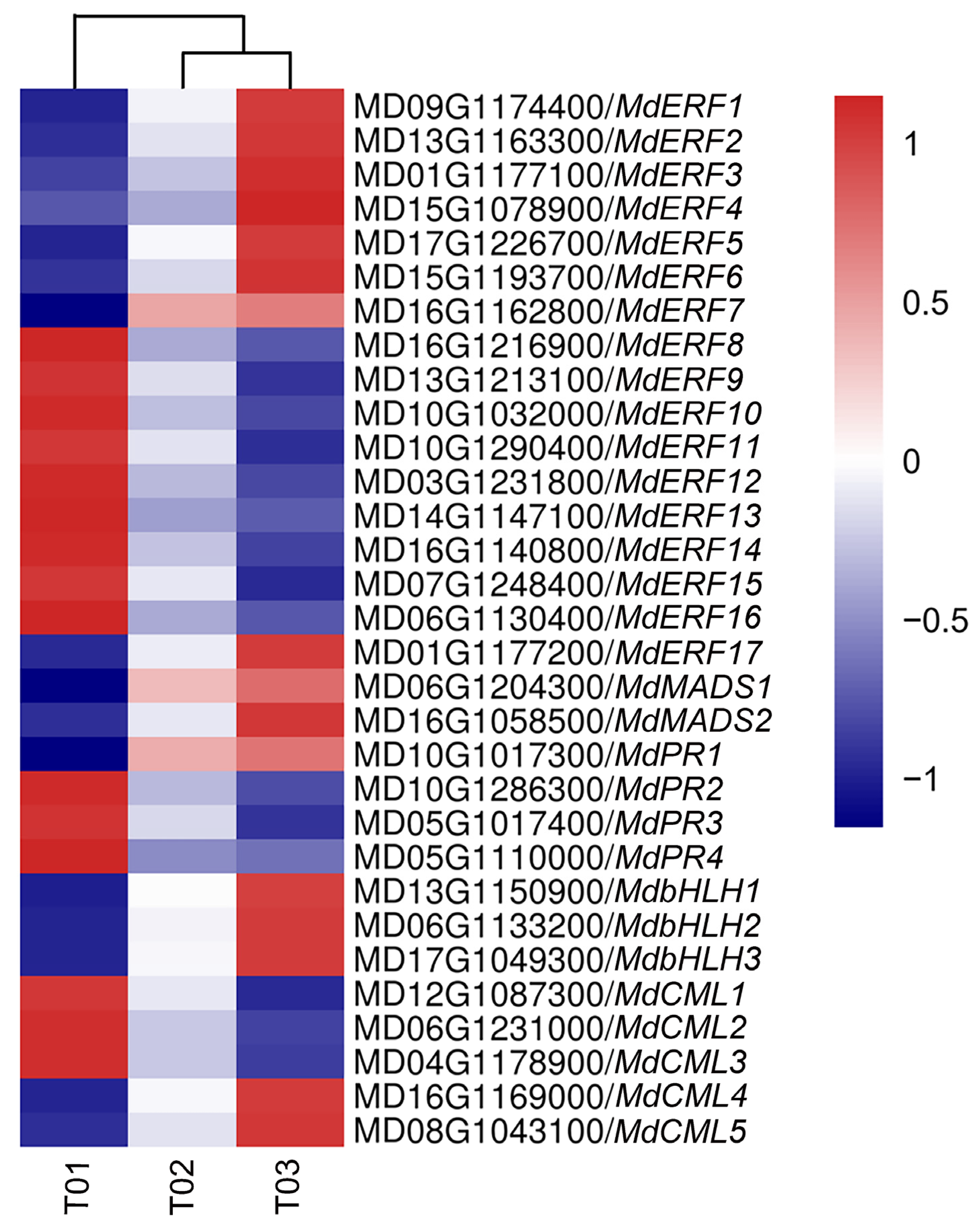
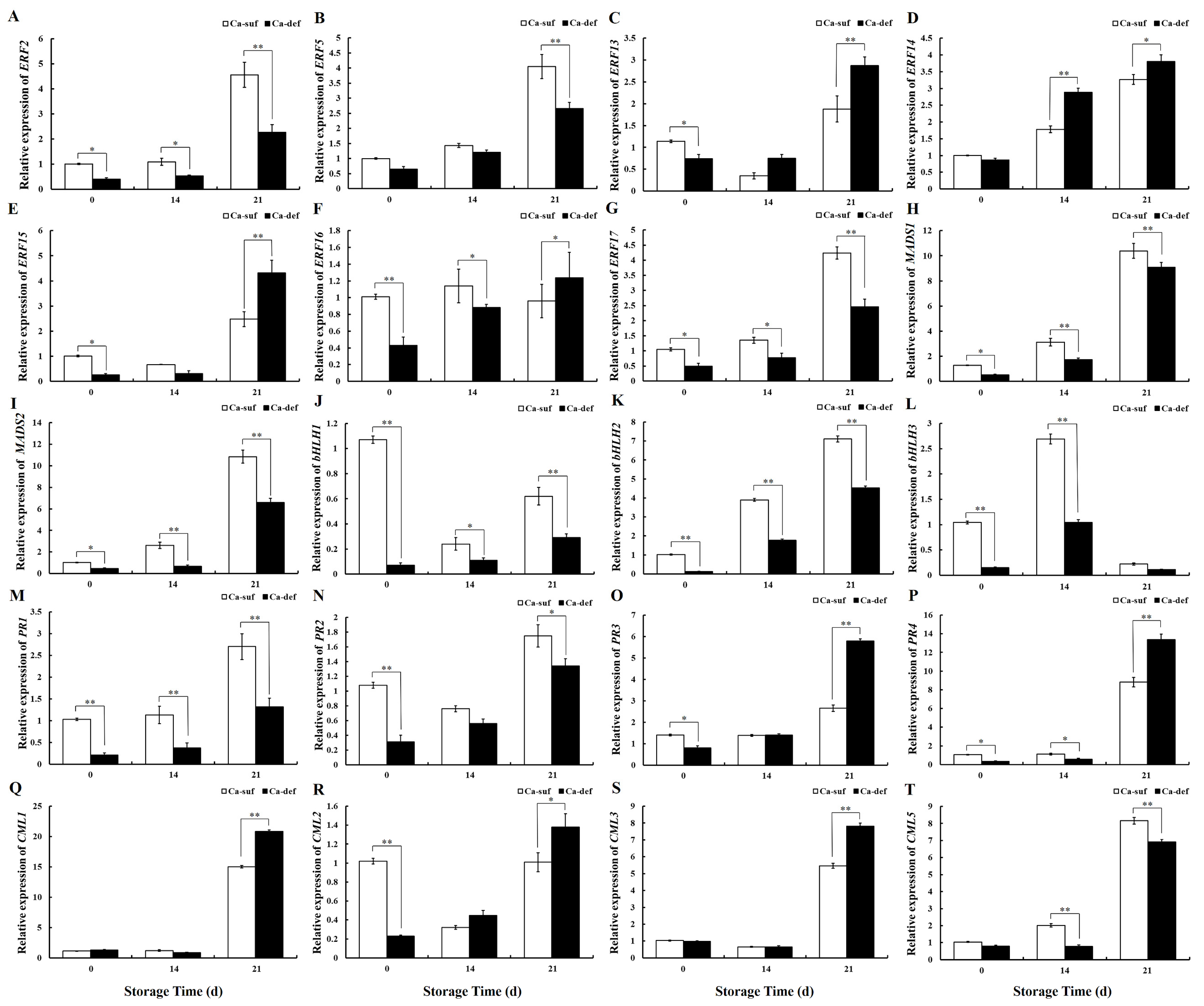
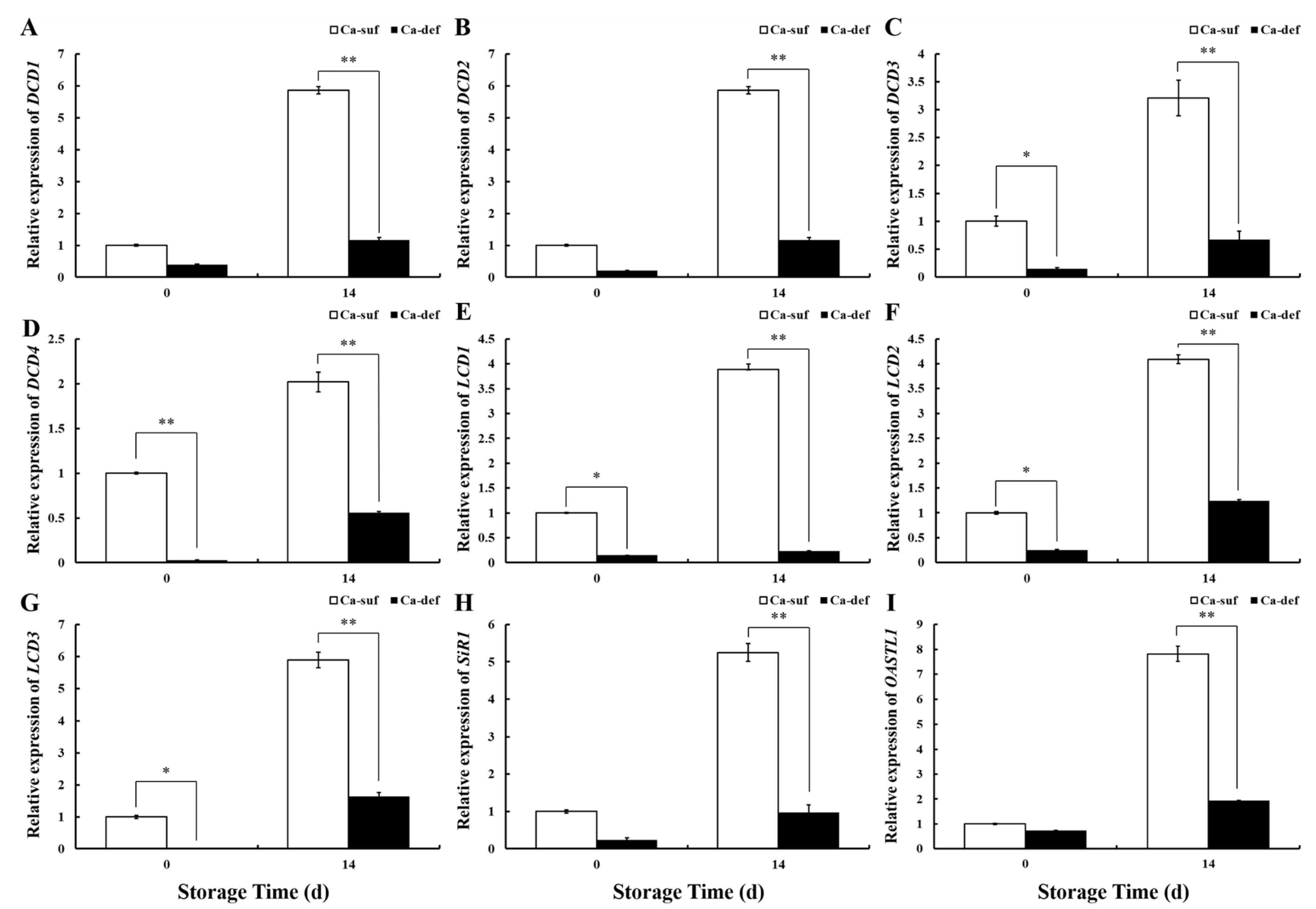
2.7. Analysis of Transcriptomics on Calcium-Deficient Peels and Gene Expression Validation
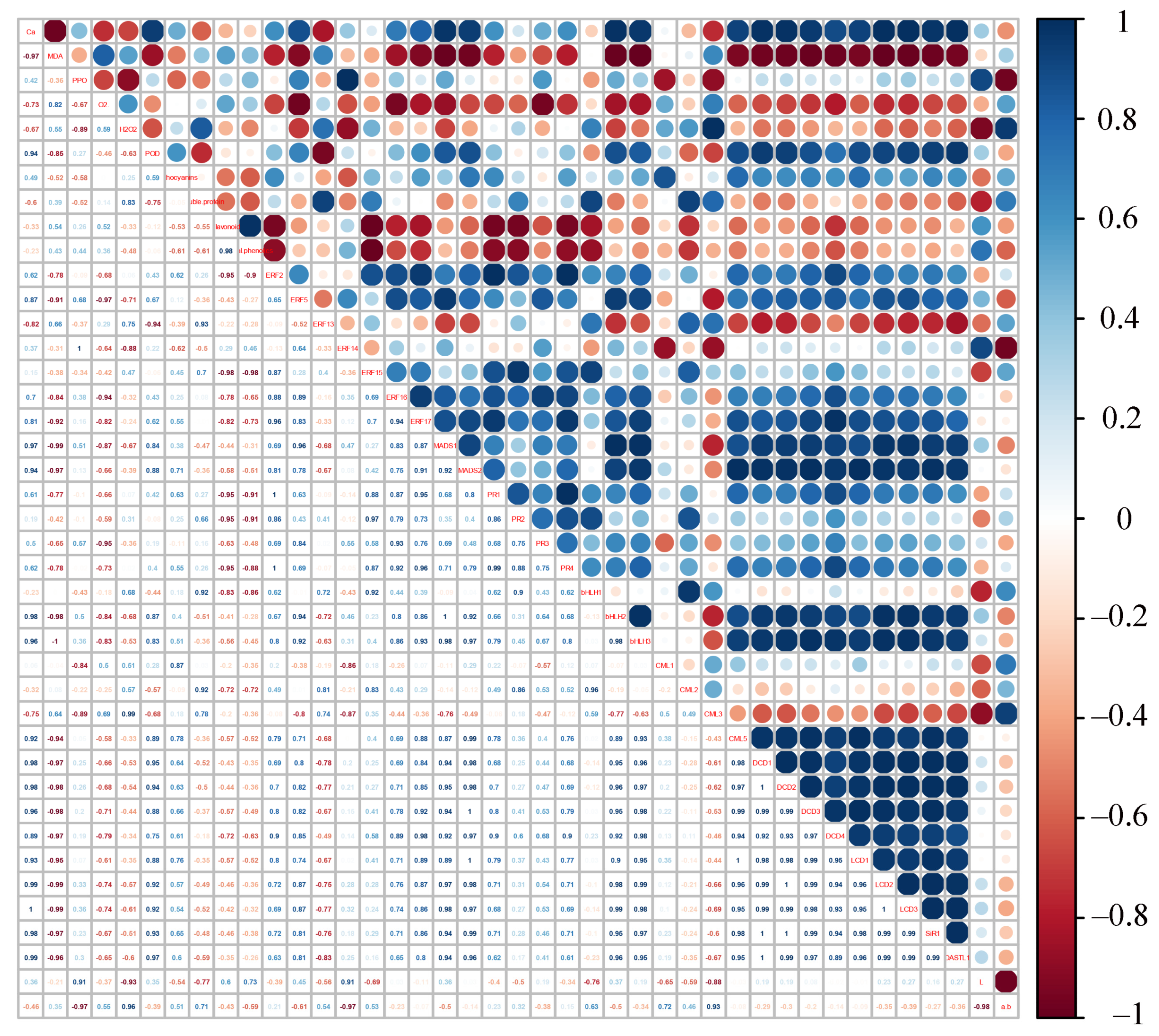
2.8. Correlation Analysis between Genes Expression and Physiological Parameters in Apple Peels
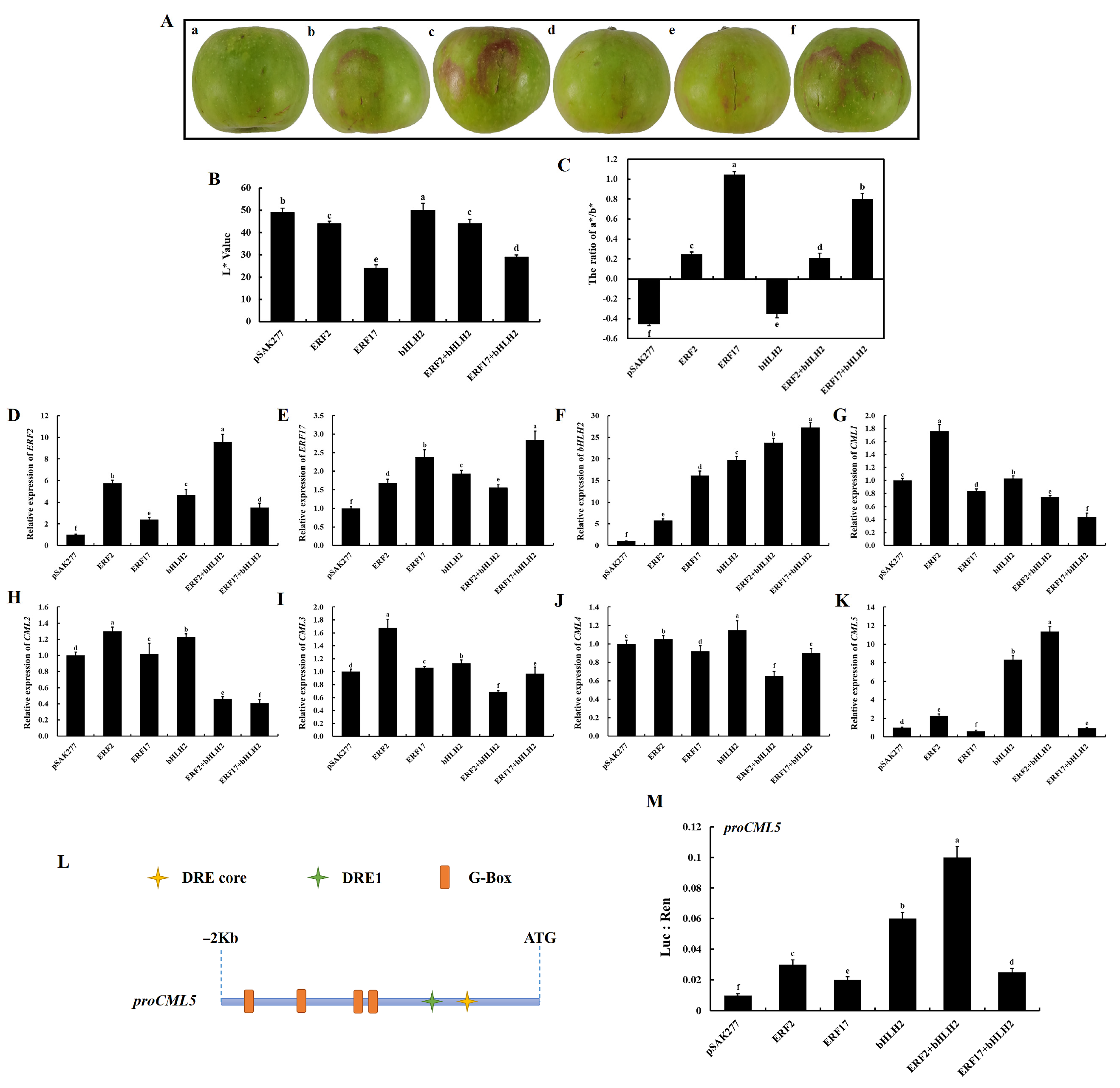
2.9. ERF2-bHLH2 Coregulation Promotes CML5 Expression in Apple Peels
3. Discussion
3.1. Calcium Deficiency Increases ROS Generation, Reduces H2S Production and Postharvest Storage Quality in Apple Peels
3.2. The ERF2-bHLH2 Coactivate the CML5 Expression and Coregulate Downstream Responses in Calcium-Deficient Apple Peels
4. Conclusions
5. Materials and Methods
5.1. Plant Materials and Treatment
5.2. Determination of Calcium Content
5.3. Determination of H2S Production in Apple Peels
5.4. Determination of Total Phenol, Flavonoid, and Anthocyanin Contents
5.5. Assay of ROS (Superoxide Anion and Hydrogen Peroxide) and Malondialdehyde (MDA)
5.6. Determination of POD, PPO Activity, and Soluble Protein Content
5.7. Transcriptome Data Analysis
5.8. RNA Extraction and Real-Time Quantitative PCR Analysis
5.9. Prediction of Cis-Acting Elements in Gene Promoters
5.10. Transient Expression Assays in Apple Peels
5.11. Dual-Luciferase Reporter Assay of Tobacco Leaves
5.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Delian, E.; Chira, A.; Dulescu, L.B.; Chira, L. Calcium alleviates stress in plants: Insight into regulatory mechanisms. AgroLife Sci. J. 2014, 3, 19–28. [Google Scholar] [CrossRef]
- Reddy, A.S.N.; Ali, G.S.; Celesnik, H.; Day, I.S. Coping with stresses: Roles of calcium-and calcium/calmodulin-regulated gene expression. Plant Cell 2011, 23, 2010–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.Y.; Zhang, Z.B.; Shao, H.B.; Guo, X.L.; Cao, H.X.; Zhao, H.B.; Hu, X.J. Relationship between calcium decoding elements and plant abiotic-stress resistance. Int. J. Biol. Sci. 2008, 4, 116–125. [Google Scholar] [CrossRef]
- Jiang, T.; Zhan, X.; Xu, Y.; Zhou, L.; Zong, L. Roles of calcium in stress-tolerance of plants and its ecological significance. J. Appl. Ecol. 2005, 16, 971–976. [Google Scholar] [CrossRef]
- Kowalik, P.; Lipa, T.; Michałojć, Z.; Chwil, M. Ultrastructure of cells and microanalysis in Malus domestica Borkh. ‘Szampion’ fruit in relation to varied calcium foliar feeding. Molecules 2020, 25, 4622. [Google Scholar] [CrossRef]
- He, L.; Li, B.; Lu, X.; Yuan, L.; Yang, Y.; Yuan, Y.; Du, J.; Guo, S. The effect of exogenous calcium on mitochondria, respiratory metabolism enzymes and ion transport in cucumber roots under hypoxia. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuteja, N.; Mahajan, S. Calcium signalling network in plants: An overview. Plant Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.L.D.; Prado, R.D.M.; Tenesaca, L.F.L.; Silva, J.L.F.D.; Mattiuz, B.H. Silicon attenuates calcium deficiency by increasing ascorbic acid content, growth and quality of cabbage leaves. Sci. Rep. 2021, 11, 1770. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Chawla, S.; Choudhary, P.; Jain, S. Post-harvest calcium chloride treatments influence fruit firmness, cell wall components and cell wall hydrolyzing enzymes of Ber (Ziziphus mauritiana Lamk.) fruits during storage. J. Food Sci. Technol. 2019, 56, 4535–4542. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J.; Wei, Q.; Li, B.; Zhong, X.; Hu, T.; Hu, H.; Bao, C. Transcriptome-wide identification and characterization of circular RNAs in leaves of Chinese cabbage (Brassica rapa L. ssp. pekinensis) in response to calcium deficiency-induced tip-burn. Sci. Rep. 2019, 9, 14544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Habbasha, S.F.; Faten, M.I. Calcium: Physiological function, deficiency and absorption. Inter. J. Chem. Tech. Res. 2015, 8, 196–202. [Google Scholar]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Moradinezhad, F.; Dorostkar, M. Pre-harvest foliar application of calcium chloride and potassium nitrate influences growth and quality of apricot (Prunus armeniaca L.) fruit cv. ‘Shahroudi’. J. Soil Sci. Plant Nutr. 2021, 21, 1642–1652. [Google Scholar] [CrossRef]
- Rosenberger, D.A.; Schupp, J.R.; Hoying, S.A.; Cheng, L.; Watkins, C.B. Controlling bitter pit in ‘Honeycrisp’ apples. Hort. Technol. 2004, 14, 342–349. [Google Scholar] [CrossRef] [Green Version]
- McAinsh, M.R.; Pittman, J.K. Shaping the calcium signature. New Phytol. 2009, 181, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Dodd, A.N.; Kudla, J.; Sanders, D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.K.; Yadav, D.; Khan, A.L.; Hashem, A.; Al-Harrasi, A. Molecular players of EF-hand containing calcium signaling event in plants. Int. J. Mol. Sci. 2019, 20, 1476. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Liu, W.; Li, W.; Liu, Y.; Wang, W.; Xie, P.; Kang, Y.; Liao, L.; Qian, L.; Liu, Z.; et al. Genome-wide identification and expression analysis of CaM/CML genes in Brassica napus under abiotic stress. J. Plant Physiol. 2020, 255, 153251. [Google Scholar] [CrossRef]
- Shen, L.; Yang, S.; Guan, D.; He, S. CaCML13 acts positively in pepper immunity against Ralstonia solanacearum infection forming feedback loop with CabZIP63. Int. J. Mol. Sci. 2020, 21, 4186. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Liu, H.; Xing, Y.; Hussain, S.; Ouyang, B.; Zhang, Y.; Li, H. Overexpression of calmodulin-like (ShCML44) stress-responsive gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses. Sci. Rep. 2016, 6, 31772. [Google Scholar] [CrossRef]
- Jung, H.; Chung, P.J.; Park, S.H.; Redillas, M.C.F.R.; Kim, Y.S.; Suh, J.W.; Kim, J.K. Overexpression of OsERF48 causes regulation of OsCML16, a calmodulin-like protein gene that enhances root growth and drought tolerance. Plant Biotech. J. 2017, 15, 1295–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, L.; Li, J.; Wang, X.; Wang, C. Crosstalk between hydrogen sulfide and other signal molecules regulates plant growth and development. Int. J. Mol. Sci. 2020, 21, 4593. [Google Scholar] [CrossRef]
- Peng, R.Y.; Bian, Z.Y.; Zhou, L.N.; Cheng, W.; Hai, N.; Yang, C.Q.; Yang, T.; Wang, X.Y.; Wang, C.Y. Hydrogen sulfide enhances nitric oxide-induced tolerance of hypoxia in maize (Zea mays L.). Plant Cell Rep. 2016, 35, 2325–2340. [Google Scholar] [CrossRef]
- Li, Z.G.; Gong, M.; Xie, H.; Yang, L.; Li, J. Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells and involvement of Ca2+ and calmodulin. Plant Sci. 2012, 185, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.T. Harnessing evolutionary toxins for signaling: Reactive oxygen species, nitric oxide and hydrogen sulfide in plant cell regulation. Front. Plant Sci. 2016, 8, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamasaki, H.; Cohen, M.F. Biological consilience of hydrogen sulfide and nitric oxide in plants: Gases of primordial earth linking plant, microbial and animal physiologies. Nitric Oxide 2016, 55, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Papenbrock, J.; Riemenschneider, A.; Kamp, A.; Schulz-Vogt, H.N.; Schmidt, A. Characterization of cysteine-degrading and H2S-releasingenzymes of higher plants—From the field to the test tube and back. Plant Biol. 2007, 9, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xiao, T.; Zhou, H.; Xie, Y.; Shen, W. Hydrogen sulfide: A versatile regulator of environmental stress in plants. Acta Physiol. Plant 2016, 38, 16. [Google Scholar] [CrossRef]
- Ali, S.; Nawaz, A.; Ejaz, S.; Haider, S.T.A.; Alam, M.W.; Javed, H.U. Effects of hydrogen sulfide on postharvest physiology of fruits and vegetables: An overview. Sci. Hortic. 2019, 243, 290–299. [Google Scholar] [CrossRef]
- Yao, G.F.; Wei, Z.Z.; Li, T.T.; Tang, J.; Huang, Z.Q.; Yang, F.Y.; Li, Y.H.; Han, Z.; Hu, F.; Hu, L.Y.; et al. Enhanced antioxidant activity is modulated by hydrogen sulfide antagonizing ethylene in tomato fruit ripening. J. Agric. Food Chem. 2018, 66, 10380–10387. [Google Scholar] [CrossRef]
- Molinett, S.A.; Alfaro, J.F.; Sáez, F.A.; Elgueta, S.; Moya-León, M.A.; Figueroa, C.R. Postharvest treatment of hydrogen sulfide delays the softening of Chilean strawberry fruit by downregulating the expression of key genes involved in pectin catabolism. Int. J. Mol. Sci. 2021, 22, 10008. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Q.; Wang, W.; Shi, J.Y.; Zhang, W.; Shen, Y.G.; Du, H.Y.; Wu, S.F. Hydrogen sulfide extends the postharvest life and enhances antioxidant activity of kiwifruit during storage. J. Sci. Food Agric. 2014, 94, 2699–2704. [Google Scholar] [CrossRef] [PubMed]
- Kolupaev, Y.E.; Karpets, Y.V. The role of reactive oxygen species and calcium ions in the implementation of the stress-protective effect of brassinosteroids on plant cells. Ukr. Biochem. J. 2014, 86, 18–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, A.; Parida, A.K.; Rangani, J.; Panda, A. Antioxidant activities, metabolic profiling, proximate analysis, mineral nutrient composition of Salvadora persica fruit unravel a potential functional food and a natural source of pharmaceuticals. Front. Pharmacol. 2017, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Falchi, R.; Bonghi, C.; Drincovich, M.F.; Famiani, F.; Lara, M.V.; Walker, R.P. Sugar metabolism in stone fruit: Source-sink relationships and environmental and agronomical effects. Front. Plant Sci. 2020, 11, 573982. [Google Scholar] [CrossRef]
- Gao, Q.; Xiong, T.; Li, X.; Chen, W.; Zhu, X. Calcium and calcium sensors in fruit development and ripening. Sci. Hortic. 2019, 253, 412–421. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.W.; Zhou, B.; Li, G.D.; Liu, Y.F.; Xia, X.L.; Xiao, Z.G.; Lu, F.; Ji, S.J. Calcium inhibited peel browning by regulating enzymes in membrane metabolism of ‘Nanguo’ pears during post-ripeness after refrigerated storage. Sci. Hortic. 2019, 244, 15–21. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, M.; Zhang, P.; Liu, Z.; Dong, T.; Zhang, S.; Ren, Y.; Jia, H.; Fang, J. Transcriptional and metabolite analysis reveal a shift in fruit quality in response to calcium chloride treatment on ‘Kyoho’ grapevine. J. Food Sci. Technol. 2021, 58, 2246–2257. [Google Scholar] [CrossRef]
- Tang, M.; Xu, C.; Cao, H.; Shi, Y.; Chen, J.; Chai, Y.; Li, Z. Tomato calmodulin-like protein SlCML37 is a calcium (Ca2+) sensor that interacts with proteasome maturation factor SlUMP1 and plays a role in tomato fruit chilling stress tolerance. J. Plant Physiol. 2021, 153373, 258–259. [Google Scholar] [CrossRef]
- Yang, S.; Xiong, X.; Arif, S.; Gao, L.; Zhao, L.; Shah, I.H.; Zhang, Y. A calmodulin-like CmCML13 from Cucumis melo improved transgenic Arabidopsis salt tolerance through reduced shoot’s Na+, and also improved drought resistance. Plant Physiol. Biochem. 2020, 155, 271–283. [Google Scholar] [CrossRef]
- Aleynova, O.A.; Kiselev, K.V.; Ogneva, Z.V.; Dubrovina, A.S. The grapevine calmodulin-like protein gene CML21 is regulated by alternative splicing and involved in abiotic stress response. Int. J. Mol. Sci. 2020, 21, 7939. [Google Scholar] [CrossRef]
- Siddiqui, M.W.; Homa, F.; Lata, D.; Mir, H.; Aftab, T.; Mishra, P. Hydrogen sulphide infiltration downregulates oxidative metabolism and extends postharvest life of banana. Plant Biol. 2021, 11. [Google Scholar] [CrossRef]
- Dou, Y.; Chang, C.M.; Wang, J.; Cai, Z.P.; Zhang, W.; Du, H.Y.; Gan, Z.Y.; Wan, C.P.; Chen, J.Y.; Zhu, L.Q. Hydrogen sulfide inhibits enzymatic browning of fresh-cut Chinese water chestnuts. Front Nutr. 2021, 8, 165. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Wang, J.; Cao, Q.; Wen, Z. Hydrogen sulfide is involved in maintaining ion homeostasis via regulating plasma membrane Na+/H+ antiporter system in the hydrogen peroxide-dependent manner in salt-stress Arabidopsis thaliana root. Protoplasma 2014, 251, 899–912. [Google Scholar] [CrossRef]
- Kim, T.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.H.; Liu, Z.Q.; Long, Y.P.; Liang, Y.L.; Jin, Z.P.; Zhang, L.P.; Liu, D.M.; Pei, Y.X. The Ca2+/calmodulin2-binding transcription factor TGA3 elevates LCD expression and H2S production to bolster Cr6+ tolerance in Arabidopsis. Plant J. 2017, 91, 1038–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suliburska, J.; Krejpcio, Z. Evaluation of the content and bioaccessibility of iron, zinc, calcium and magnesium from groats, rice, leguminous grains and nuts. J. Food Sci. Technol. 2014, 51, 589–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hine, C.; Harputlugil, E.; Zhang, Y.; Ruckenstuhl, C.; Lee, B.C.; Brace, L.; Longchamp, A. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 2015, 160, 132–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.Y.; Hu, S.L.; Wu, J.; Li, Y.H.; Zheng, J.L.; Wei, Z.J.; Liu, J.; Wang, H.L.; Liu, Y.S.; Zhang, H. Hydrogen sulfide prolongs postharvest shelf life of strawberry and plays an antioxidative role in fruits. J. Agric. Food Chem. 2012, 60, 8684–8693. [Google Scholar] [CrossRef]
- Pirie, A.; Mullins, M.G. Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissues treated with sucrose, nitrate and abscisic acid. Plant Physiol. 1976, 58, 468–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.P.; Hu, K.D.; Hu, L.Y.; Li, Y.H.; Jiang, A.M.; Xiao, F. Hydrogen sulfide alleviates postharvest senescence of broccoli by modulating antioxidant defense and senescence-related gene expression. J. Agric. Food Chem. 2014, 62, 1119–1129. [Google Scholar] [CrossRef]
- Lee, H.S.; Wicker, L. Anthocyanin pigments in the skin of lychee fruit. J. Food Sci. 2006, 56, 466–468. [Google Scholar] [CrossRef]
- Benjamin, N.D.; Montgomery, M.W. Polyphenol oxidase of royal ann cherries: Purification and characterization. J. Food Sci. 1973, 38, 799–806. [Google Scholar] [CrossRef]
- Beaudoin-Eagan, L.D.; Thorpe, T.A. Tyrosine and phenyl-alanine ammonia lyase activities during shoot initiation in tobacco callus cultures. Plant Physiol. 1985, 78, 438–441. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Qiu, L.N.; Hu, S.S.; Wang, Y.Z.; Qu, H.Y. Accumulation of abnormal amyloplasts in pulp cells induces bitter pit in Malus domestica. Front. Plant Sci. 2021, 12, 2052. [Google Scholar] [CrossRef]
- Voinnet, O.; Rivas, S.; Mestre, P.; Baulcombe, D. Retracted: An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 2003, 33, 949–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]

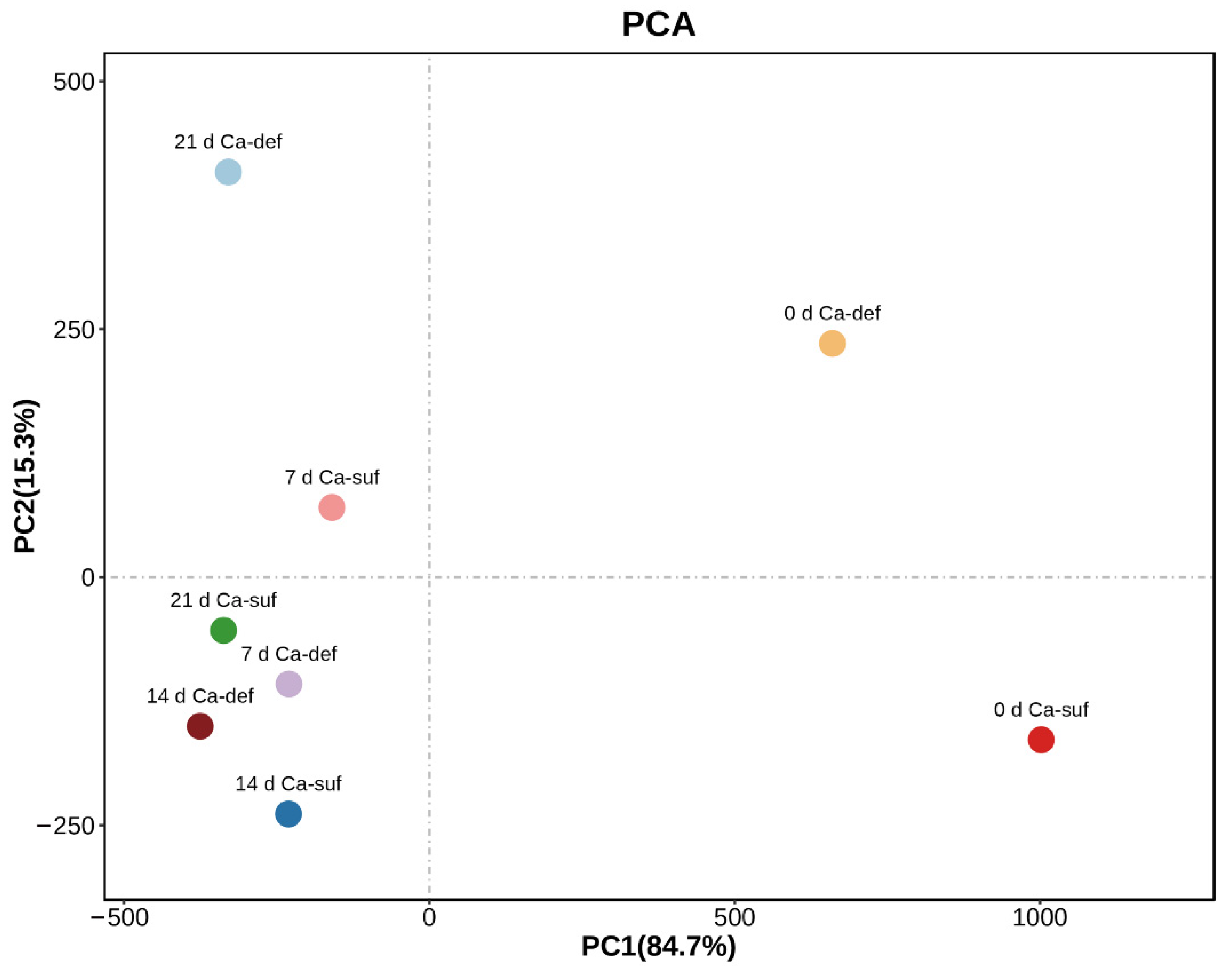
| Component Name | PC1 (84.7%) | PC2 (15.3%) |
|---|---|---|
| H2O2 content | 9.97 × 10−1 | −8.25 × 10−2 |
| O2•− production rate | 8.25 × 10−2 | 9.97 × 10−1 |
| Calcium content | 2.01 × 10−4 | −2.49 × 10−4 |
| Ratio of a*/b* | 1.99 × 10−4 | −1.64 × 10−4 |
| Soluble protein content | 9.53 × 10−5 | −7.17 × 10−5 |
| Flavonoid content | 6.48 × 10−5 | 3.87 × 10−4 |
| Total phenolics content | 4.70 × 10−5 | 1.37 × 10−4 |
| Anthocyanins content | 1.46 × 10−5 | −1.74 × 10−4 |
| MDA content | 5.56 × 10−8 | 1.86 × 10−5 |
| PPO activity | −5.74 × 10−5 | −1.19 × 10−4 |
| POD activity | −5.61 × 10−4 | 8.97 × 10−4 |
| L* value | −8.94 × 10−3 | 9.04 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.-Y.; Zhang, W.-W.; Qu, H.-Y.; Gou, S.-S.; Li, L.-X.; Song, H.-H.; Yang, H.-Q.; Li, W.-J.; Zhang, H.; Hu, K.-D.; et al. Transcriptomics Reveals the ERF2-bHLH2-CML5 Module Responses to H2S and ROS in Postharvest Calcium Deficiency Apples. Int. J. Mol. Sci. 2021, 22, 13013. https://doi.org/10.3390/ijms222313013
Sun H-Y, Zhang W-W, Qu H-Y, Gou S-S, Li L-X, Song H-H, Yang H-Q, Li W-J, Zhang H, Hu K-D, et al. Transcriptomics Reveals the ERF2-bHLH2-CML5 Module Responses to H2S and ROS in Postharvest Calcium Deficiency Apples. International Journal of Molecular Sciences. 2021; 22(23):13013. https://doi.org/10.3390/ijms222313013
Chicago/Turabian StyleSun, Hong-Ye, Wei-Wei Zhang, Hai-Yong Qu, Sha-Sha Gou, Li-Xia Li, Hui-Hui Song, Hong-Qiang Yang, Wan-Jie Li, Hua Zhang, Kang-Di Hu, and et al. 2021. "Transcriptomics Reveals the ERF2-bHLH2-CML5 Module Responses to H2S and ROS in Postharvest Calcium Deficiency Apples" International Journal of Molecular Sciences 22, no. 23: 13013. https://doi.org/10.3390/ijms222313013
APA StyleSun, H.-Y., Zhang, W.-W., Qu, H.-Y., Gou, S.-S., Li, L.-X., Song, H.-H., Yang, H.-Q., Li, W.-J., Zhang, H., Hu, K.-D., & Yao, G.-F. (2021). Transcriptomics Reveals the ERF2-bHLH2-CML5 Module Responses to H2S and ROS in Postharvest Calcium Deficiency Apples. International Journal of Molecular Sciences, 22(23), 13013. https://doi.org/10.3390/ijms222313013








