H2O2 Functions as a Downstream Signal of IAA to Mediate H2S-Induced Chilling Tolerance in Cucumber
Abstract
:1. Introduction
2. Results
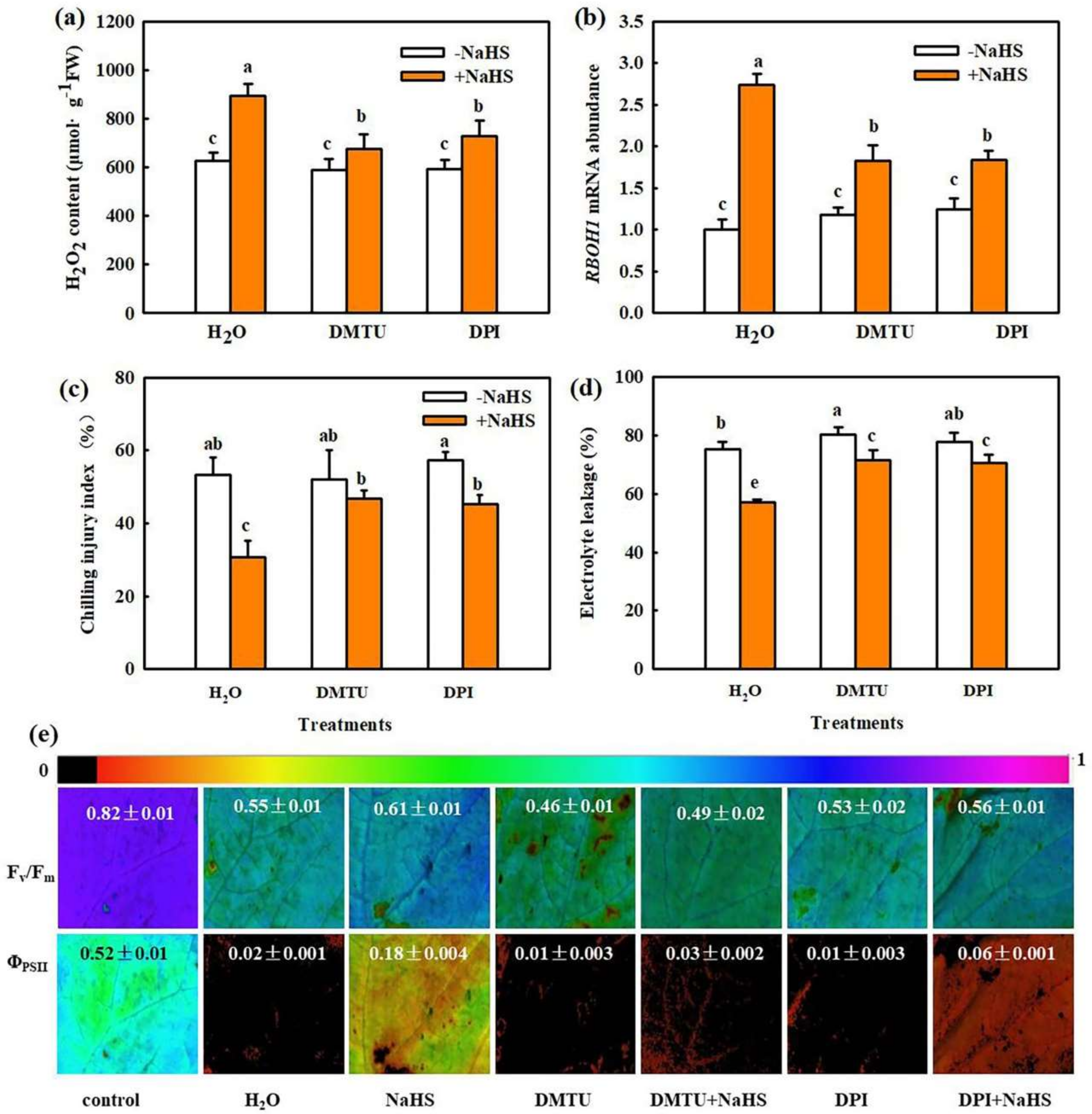
2.1. H2O2 Is Involved in H2S-Induced Chilling Tolerance in Cucumber
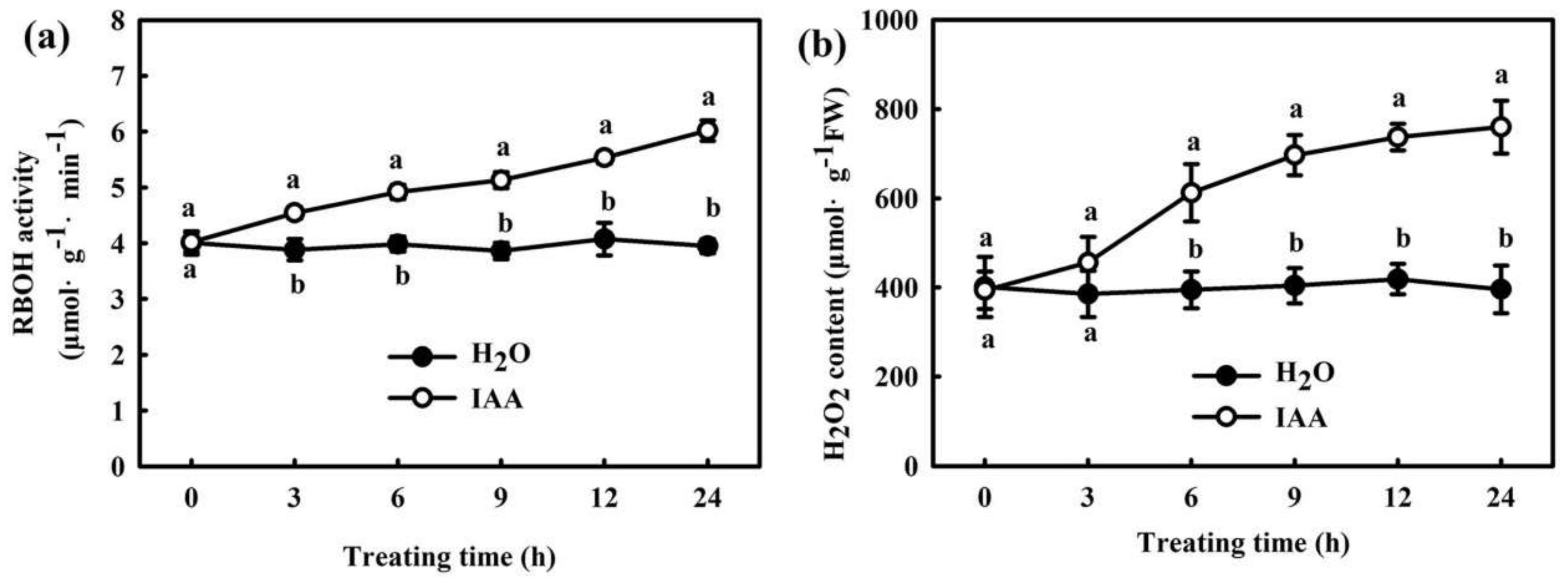
2.2. H2O2 Participates in IAA-Induced Chilling Tolerance in Cucumber
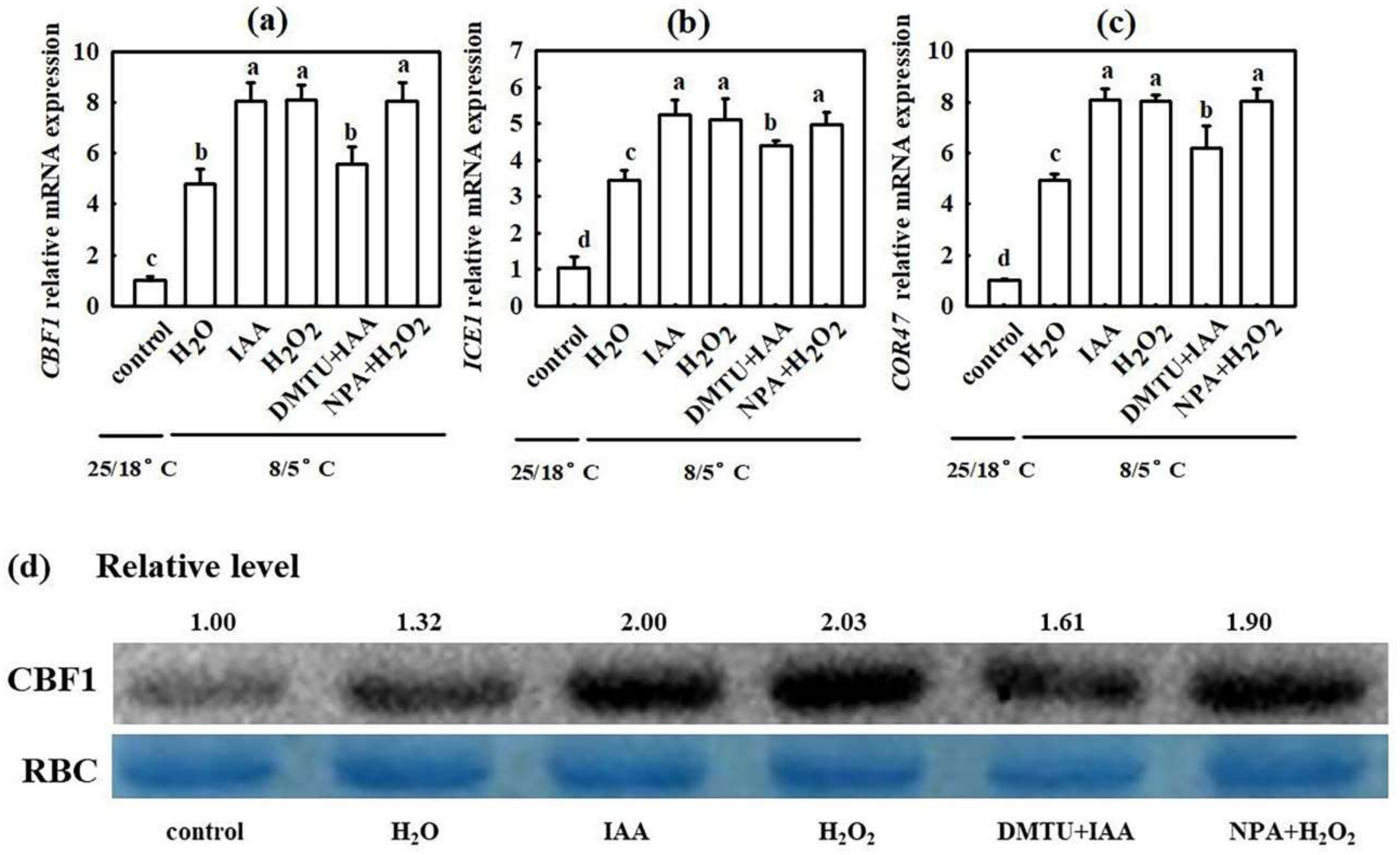
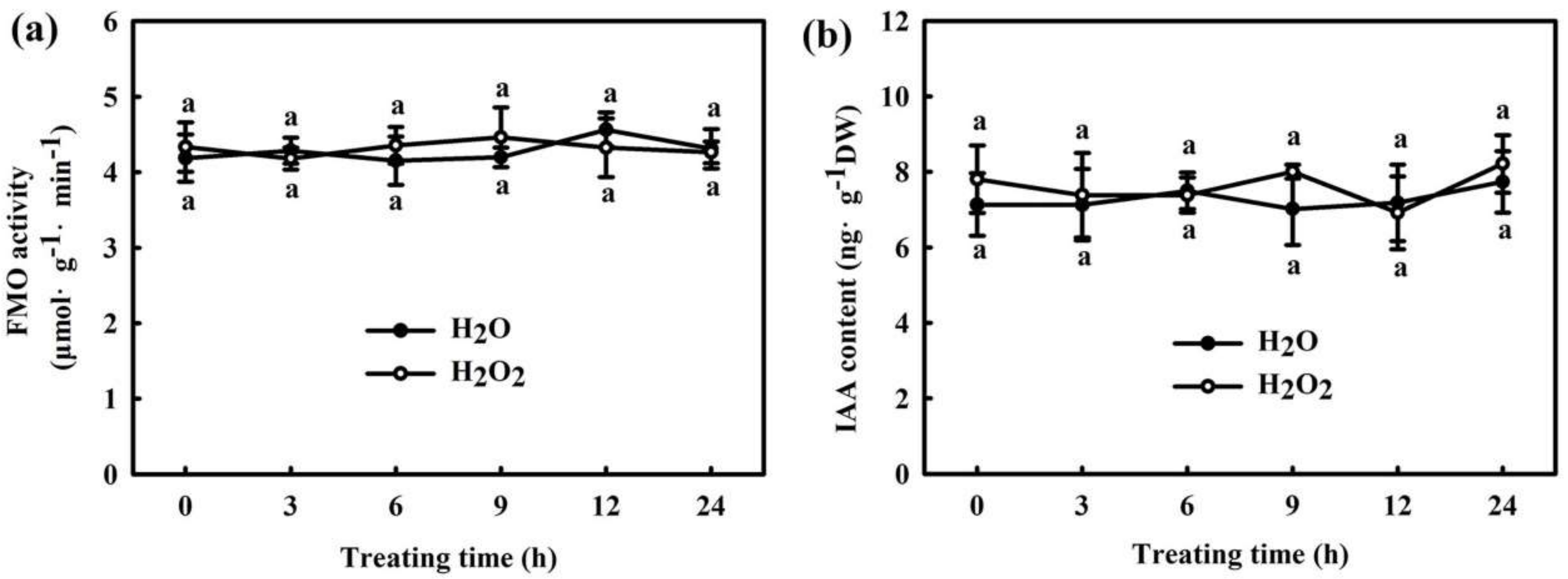
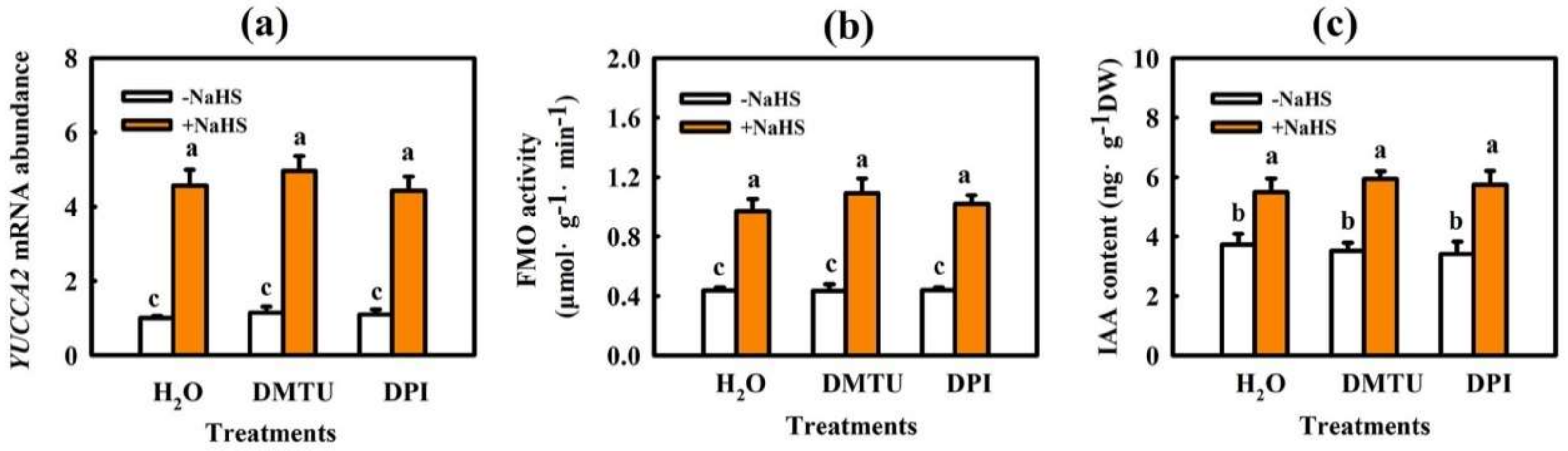
2.3. Interaction of IAA and H2O2 in H2S-Induced Chilling Tolerance in Cucumber
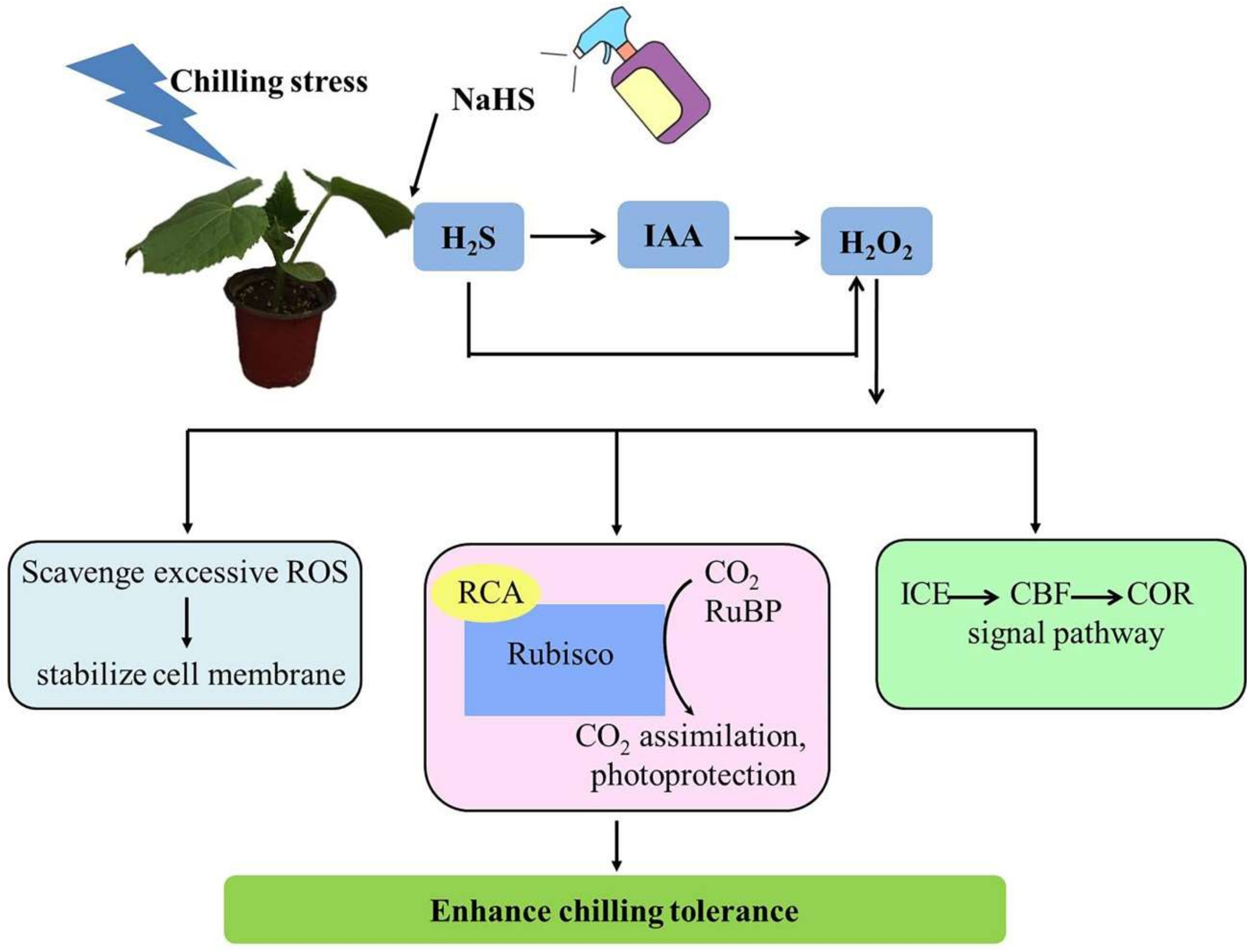
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Condition
4.2. Experimental Design
4.2.1. Effect of H2O2 on the Chilling Tolerance of Cucumber Seedlings
4.2.2. Effect of H2O2 Scavenger or Inhibitor on H2S-Induced H2O2 Biosynthesis and Chilling Tolerance
4.2.3. Interaction between IAA and H2O2 in Response to Chilling Stress
4.2.4. Effect of IAA Inhibitor on H2S-Induced H2O2 Biosynthesis in Cucumber Seedlings
4.2.5. Effect of Scavengers and Synthetic Inhibitors of H2O2 on H2S-Induced IAA Biosynthesis in Cucumber Seedlings
4.3. CI, EL, and MDA Measurements
4.4. Detection of Pn and Chlorophyll Fluorescence
4.5. Detection of H2O2 Content and RBOH Activity
4.6. IAA Content and FMO Activity Assay
4.7. Quantitative Real-Time PCR Analysis
4.8. SDS-PAGE and Immunoblot Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldstein, I.; Chastre, J.; Rouby, J.-J. Novel and Innovative Strategies to Treat Ventilator-Associated Pneumonia: Optimizing the Duration of Therapy and Nebulizing Antimicrobial Agents. Semin. Respir. Crit. Care Med. 2006, 27, 082–091. [Google Scholar] [CrossRef]
- Du, X.; Jin, Z.; Liu, D.; Yang, G.; Pei, Y. Hydrogen sulfide alleviates the cold stress through MPK4 in Arabidopsis thaliana. Plant Physiol. Biochem. 2017, 120, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Tripathi, D.K.; Roychoudhury, A. Hydrogen sulphide trapeze: Environmental stress amelioration and phytohormone crosstalk. Plant Physiol. Biochem. 2018, 132, 46–53. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Liu, F.-J.; Zhai, J.; Li, F.-D.; Bi, H.-G.; Ai, X.-Z. Auxin acts as a downstream signaling molecule involved in hydrogen sulfide-induced chilling tolerance in cucumber. Planta 2020, 251, 69. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.X.; Cai, B.B.; Zhou, C.F.; Li, D.D.; Bi, H.G.; Ai, X.Z. Hydrogen sulfide-induced chilling tolerance of cucumber and involvement of nitric oxide. J. Plant Biol. Res. 2016, 5, 58–69. [Google Scholar]
- Wu, G.X.; Li, D.D.; Sun, C.C.; Sun, S.N.; Liu, F.J.; Bi, H.G.; Ai, X.Z. Hydrogen sulfide interacts with Ca2+ to enhance chilling tolerance of cucumber seedlings. Chin. J. Biochem. Mol. Biol. 2017, 33, 1037–1046. [Google Scholar] [CrossRef]
- Li, D.D.; Zhang, X.W.; Liu, F.J.; Pan, D.Y.; Ai, X.Z. Hydrogen sulfide interacting with abscisic acid counteracts oxidative damages against chilling stress in cucumber seedlings. Acta Hortic. Sin. 2018, 45, 2395–2406. [Google Scholar] [CrossRef]
- Sun, M.; Jiang, F.; Zhou, R.; Wen, J.; Cui, S.; Wang, W.; Wu, Z. Respiratory burst oxidase homologue-dependent H2O2 is essential during heat stress memory in heat sensitive tomato. Sci. Hortic. 2019, 258, 108777. [Google Scholar] [CrossRef]
- Nobakht, P.; Ebadi, A.; Parmoon, G.; Bahrami, R.N. Study role H2O2 in Photosynthetic pigments Peppermint (Mentha piperita L) on Water stress conditions. J. Plant Proc. Func. 2019, 7, 19–30. [Google Scholar]
- Nazir, F.; Fariduddin, Q.; Alam Khan, T. Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 2020, 252, 126486. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, T.; Potters, G.; Caubergs, R.; Jansen, M. Complementary interactions between oxidative stress and auxins control plant growth responses at plant, organ, and cellular level. J. Exp. Bot. 2005, 56, 1991–2001. [Google Scholar] [CrossRef]
- Takáč, T.; Obert, B.; Rolčík, J.; Šamaj, J. Improvement of adventitious root formation in flax using hydrogen peroxide. New Biotechnol. 2016, 33, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Deng, X.; Zhou, X.; Zhu, L.; Zou, L.; Li, P.; Zhang, D.; Lin, H. Ethylene and hydrogen peroxide are involved in brassinosteroid-induced salt tolerance in tomato. Sci. Rep. 2016, 6, 35392. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Fu, X.; Wu, G.; Feng, Y.; Li, F.; Bi, H.; Ai, X. Hydrogen peroxide is involved in hydrogen sulfide-induced carbon assimilation and photoprotection in cucumber seedlings. Environ. Exp. Bot. 2020, 175, 104052. [Google Scholar] [CrossRef]
- Rahman, A. Auxin: A regulator of cold stress response. Physiol. Plant 2013, 147, 28–35. [Google Scholar] [CrossRef]
- Ivanchenko, M.G.; Os, D.D.; Monshausen, G.B.; Dubrovsky, J.G.; Bednarova, A.; Krishnan, N. Auxin increases the hydrogen peroxide (H2O2) concentration in tomato (Solanum lycopersicum) root tips while inhibiting root growth. Ann. Bot. 2013, 112, 1107–1116. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Zhang, X.; Cai, B.; Pan, D.; Fu, X.; Bi, H.; Ai, X. Physiological response and transcription profiling analysis reveal the role of glutathione in H2S-induced chilling stress tolerance of cucumber seedlings. Plant Sci. 2020, 291, 110363. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Shi, K.; Xia, X.J.; Zhou, Y.H.; Yu, J.Q. Hydrogen peroxide is involved in the cold acclimation-induced chilling tolerance of tomato plants. Plant Physiol. Biochem. 2012, 60, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, C.; Yang, M.; Dong, X.; Lv, W.; Meng, Q. Cold-regulated protein (SlCOR413IM1) confers chilling stress tolerance in tomato plants. Plant Physiol. Biochem. 2018, 124, 29–39. [Google Scholar] [CrossRef]
- Fan, J.; Hu, Z.; Xie, Y.; Chan, Z.; Chen, K.; Amombo, E.; Chen, L.; Fu, J. Alleviation of cold damage to photosystem II and metabolisms by melatonin in Bermudagrass. Front. Plant Sci. 2015, 6, 925. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Huo, J.; Liao, W. Hydrogen sulfide: Roles in plant abiotic stress response and crosstalk with other signals. Plant Sci. 2021, 302, 110733. [Google Scholar] [CrossRef]
- Arif, Y.; Hayat, S.; Yusuf, M.; Bajguz, A. Hydrogen sulfide: A versatile gaseous molecule in plants. Plant Physiol. Biochem. 2021, 158, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Mahmoudi, R.; Razavi, F.; Rabiei, V.; Soleimani, A. Hydrogen sulfide treatment confers chilling tolerance in hawthorn fruit during cold storage by triggering endogenous H 2 S accumulation, enhancing antioxidant enzymes activity and promoting phenols accumulation. Sci. Hortic. 2018, 238, 264–271. [Google Scholar] [CrossRef]
- Khan, M.N.; Siddiqui, M.H.; AlSolami, M.A.; Alamri, S.; Hu, Y.; Ali, H.M.; Al-Amri, A.A.; Alsubaie, Q.D.; Al-Munqedhi, B.M.; Al-Ghamdi, A. Crosstalk of hydrogen sulfide and nitric oxide requires calcium to mitigate impaired photosynthesis under cadmium stress by activating defense mechanisms in Vigna radiata. Plant Physiol. Biochem. 2020, 156, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Kour, J.; Khanna, K.; Sharma, P.; Singh, A.D.; Sharma, I.; Arora, P.; Kumar, P.; Devi, K.; Ibrahim, M.; Ohri, P.; et al. Hydrogen sulfide and phytohormones crosstalk in plant defense against abiotic stress. In Hydrogen Sulfide in Plant Biology; Singh, S., Singh, V.P., Tripathi, D.K., Prasad, S.M., Chauhan, D.K., Dubey, N.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 267–302. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, H.; Xie, Y. SnRK2.6 phosphorylation/persulfidation: Where ABA and H2S signaling meet. Trends Plant Sci. 2021, 26, 1207–1209. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.-Y.; Fu, X.; Zhang, X.-W.; Liu, F.-J.; Bi, H.-G.; Ai, X.-Z. Hydrogen sulfide is required for salicylic acid–induced chilling tolerance of cucumber seedlings. Protoplasma 2020, 257, 1543–1557. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, D.; Shi, X.; Deng, B.; Ren, Y.; Lin, W.; Miao, Y. H2O2 as a Feedback Signal on Dual-Located WHIRLY1 Associates with Leaf Senescence in Arabidopsis. Cells 2019, 8, 1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, F.; Yao, Z.; Liu, Y.; Sun, M.; Peng, X. H2O2 response gene 1/2 are novel sensors or responders of H2O2 and involve in maintaining embryonic root meristem activity in Arabidopsis thaliana. Plant Sci. 2021, 310, 110981. [Google Scholar] [CrossRef]
- Islam, M.; Ye, W.; Matsushima, D.; Rhaman, M.S.; Munemasa, S.; Okuma, E.; Nakamura, Y.; Biswas, S.; Mano, J.; Murata, Y. Reactive Carbonyl Species Function as Signal Mediators Downstream of H2O2 Production and Regulate [Ca2+]cyt Elevation in ABA Signal Pathway in Arabidopsis Guard Cells. Plant Cell Physiol. 2019, 60, 1146–1159. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, Y.; Lan, Z.; Xu, K.; Chang, J.; Ahammed, G.J.; Ma, J.; Wei, C.; Zhang, X. Methyl jasmonate mediates melatonin-induced cold tolerance of grafted watermelon plants. Hortic. Res. 2021, 8, 57. [Google Scholar] [CrossRef]
- Brumos, J.; Robles, L.M.; Yun, J.; Vu, T.C.; Jackson, S.; Alonso, J.; Stepanova, A.N. Local Auxin Biosynthesis Is a Key Regulator of Plant Development. Dev. Cell 2018, 47, 306–318.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, B.; Yan, Z.; Tian, H.; Zhang, X.; Ding, Z. Local Auxin Biosynthesis Mediates Plant Growth and Development. Trends Plant Sci. 2019, 24, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, K.; Uemura, M.; Tsurumi, S.; Rahman, A. Auxin Response inArabidopsisunder Cold Stress: Underlying Molecular Mechanisms. Plant Cell 2009, 21, 3823–3838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popko, J.; Hänsch, R.; Mendel, R.-R.; Polle, A.; Teichmann, T. The role of abscisic acid and auxin in the response of poplar to abiotic stress. Plant Biol. 2010, 12, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, H.; Xiong, L. Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front. Plant Sci. 2013, 4, 397. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Wu, J. Coupling and Decoupling between Range and Angle. Synth. Impulse Aperture Radar (SIAR) 2014, 111, 241–257. [Google Scholar] [CrossRef]
- Dong, X.; Bi, H.; Wu, G.; Ai, X. Drought-induced chilling tolerance in cucumber involves membrane stabilisation improved by antioxidant system. Int. J. Plant Prod. 2013, 7, 67–80. [Google Scholar]
- Heath, R.L.; Packer, L.J. Photoperoxidation in isolated chloroplasts. i. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Xanthophyll cycle and light stress in nature: Uniform response to excess direct sunlight among higher plant species. Planta 1996, 198, 460–470. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Tian, Y.; Ungerer, P.; Zhang, H.; Ruban, A.V. Direct impact of the sustained decline in the photosystem II efficiency upon plant productivity at different developmental stages. J. Plant Physiol. 2017, 212, 45–53. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, Y.; Xu, C.; Liu, K.; Bi, H.; Ai, X. H2O2 Functions as a Downstream Signal of IAA to Mediate H2S-Induced Chilling Tolerance in Cucumber. Int. J. Mol. Sci. 2021, 22, 12910. https://doi.org/10.3390/ijms222312910
Zhang X, Zhang Y, Xu C, Liu K, Bi H, Ai X. H2O2 Functions as a Downstream Signal of IAA to Mediate H2S-Induced Chilling Tolerance in Cucumber. International Journal of Molecular Sciences. 2021; 22(23):12910. https://doi.org/10.3390/ijms222312910
Chicago/Turabian StyleZhang, Xiaowei, Yanyan Zhang, Chenxiao Xu, Kun Liu, Huangai Bi, and Xizhen Ai. 2021. "H2O2 Functions as a Downstream Signal of IAA to Mediate H2S-Induced Chilling Tolerance in Cucumber" International Journal of Molecular Sciences 22, no. 23: 12910. https://doi.org/10.3390/ijms222312910
APA StyleZhang, X., Zhang, Y., Xu, C., Liu, K., Bi, H., & Ai, X. (2021). H2O2 Functions as a Downstream Signal of IAA to Mediate H2S-Induced Chilling Tolerance in Cucumber. International Journal of Molecular Sciences, 22(23), 12910. https://doi.org/10.3390/ijms222312910




