Multimodal Spectroscopic Imaging of Pea Root Nodules to Assess the Nitrogen Fixation in the Presence of Biofertilizer Based on Nod-Factors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Purification of the Biofertilizer Containing Nod Factors
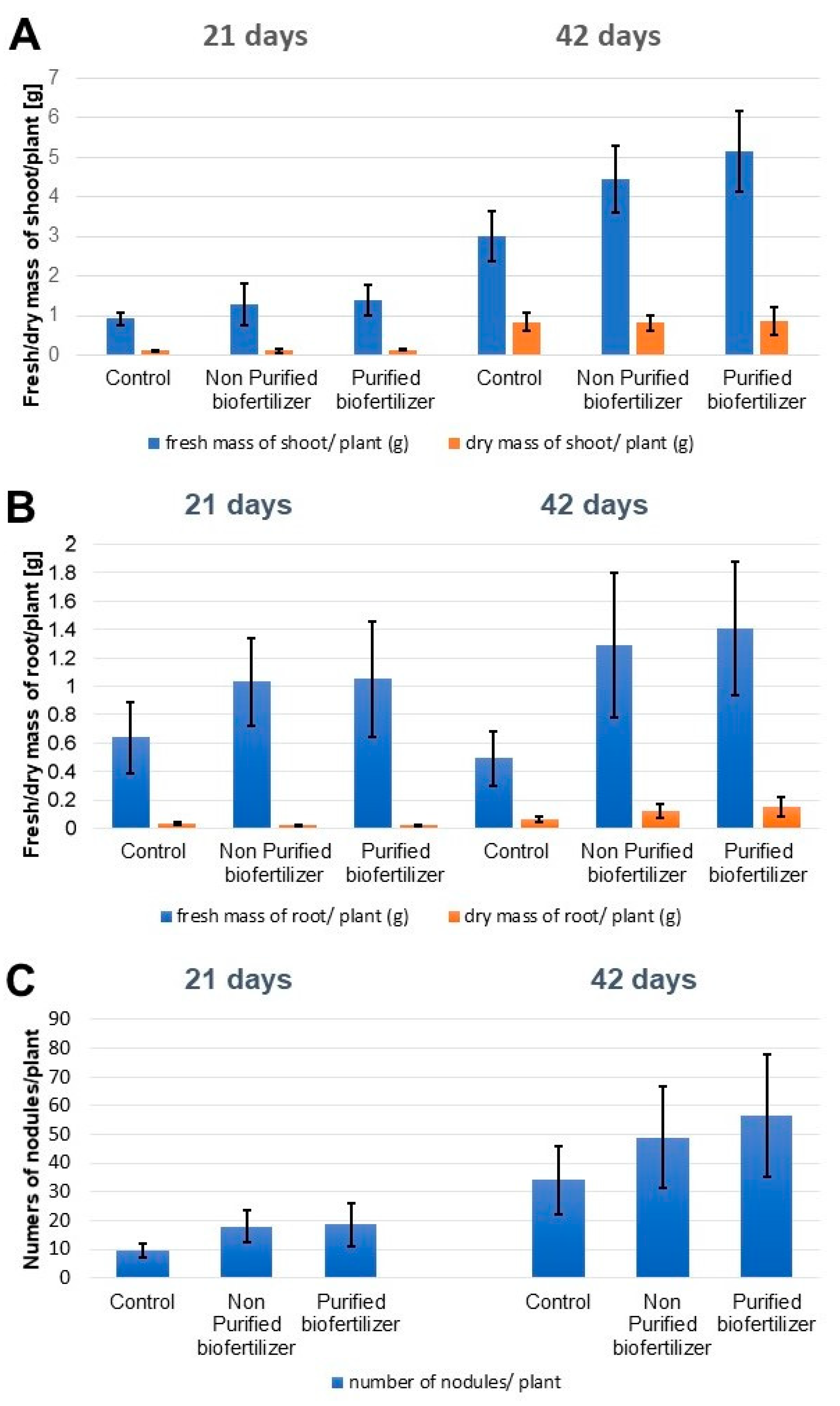
2.2. Influence of the Biofertilizer on the Plant Development
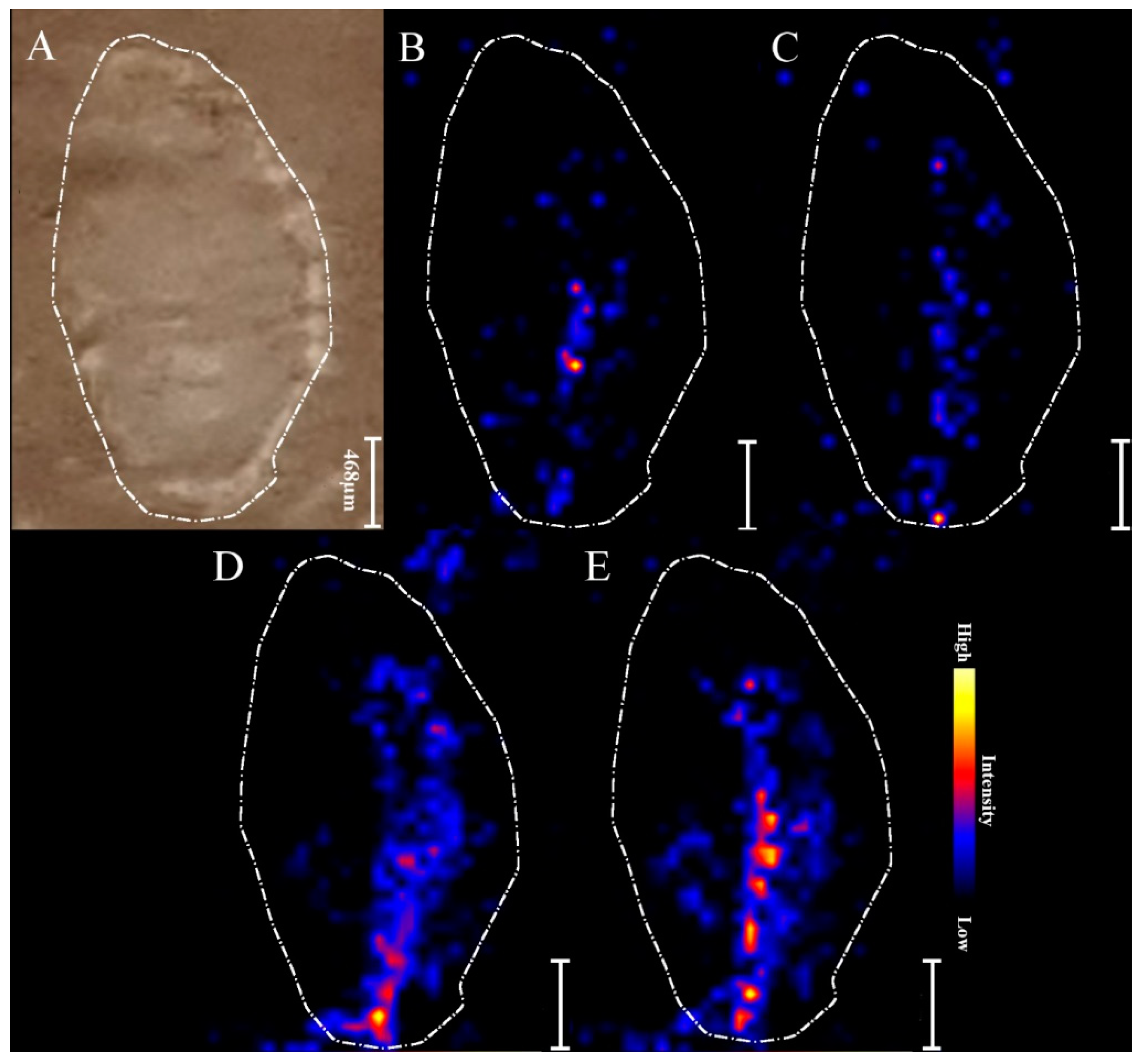
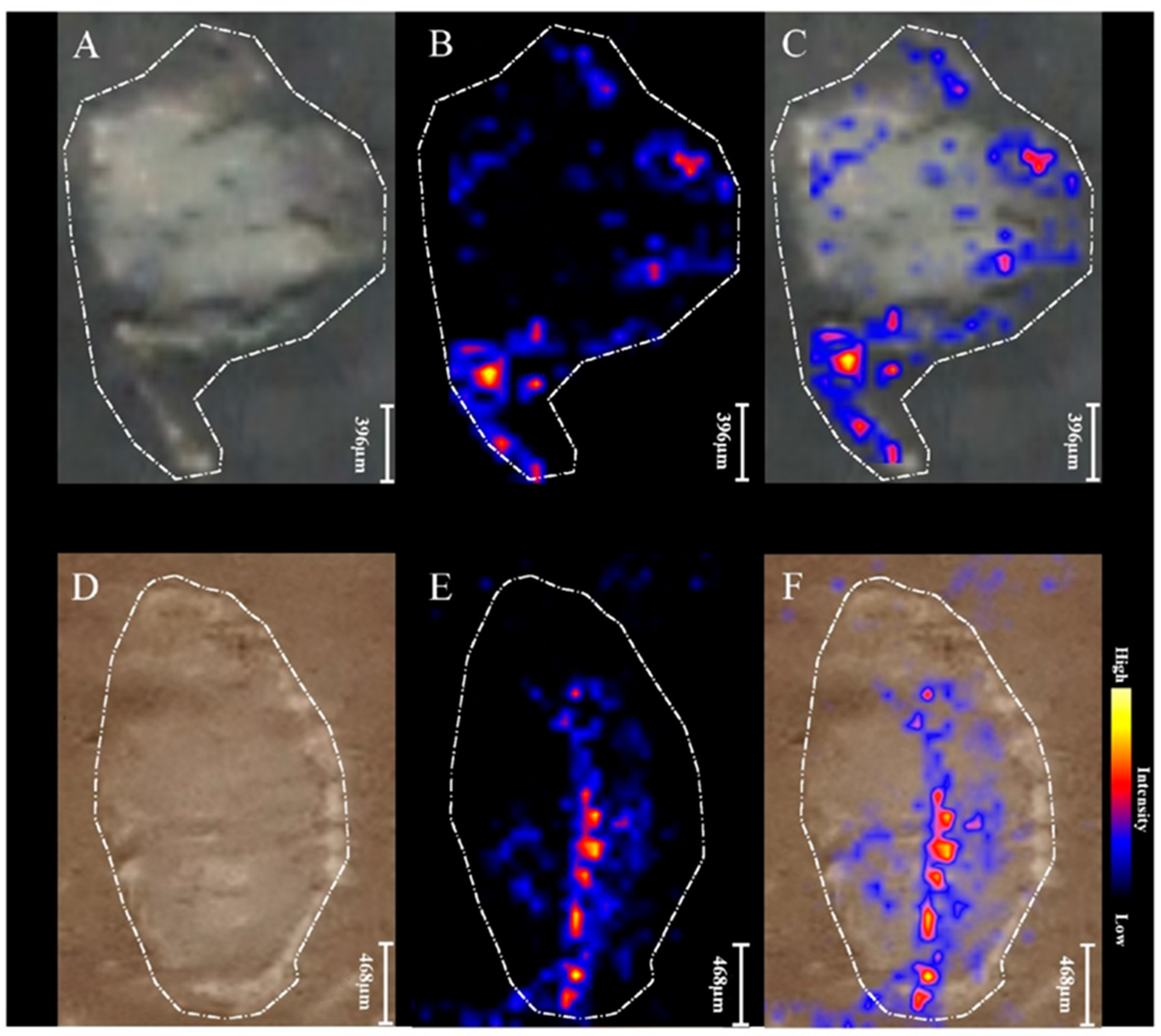
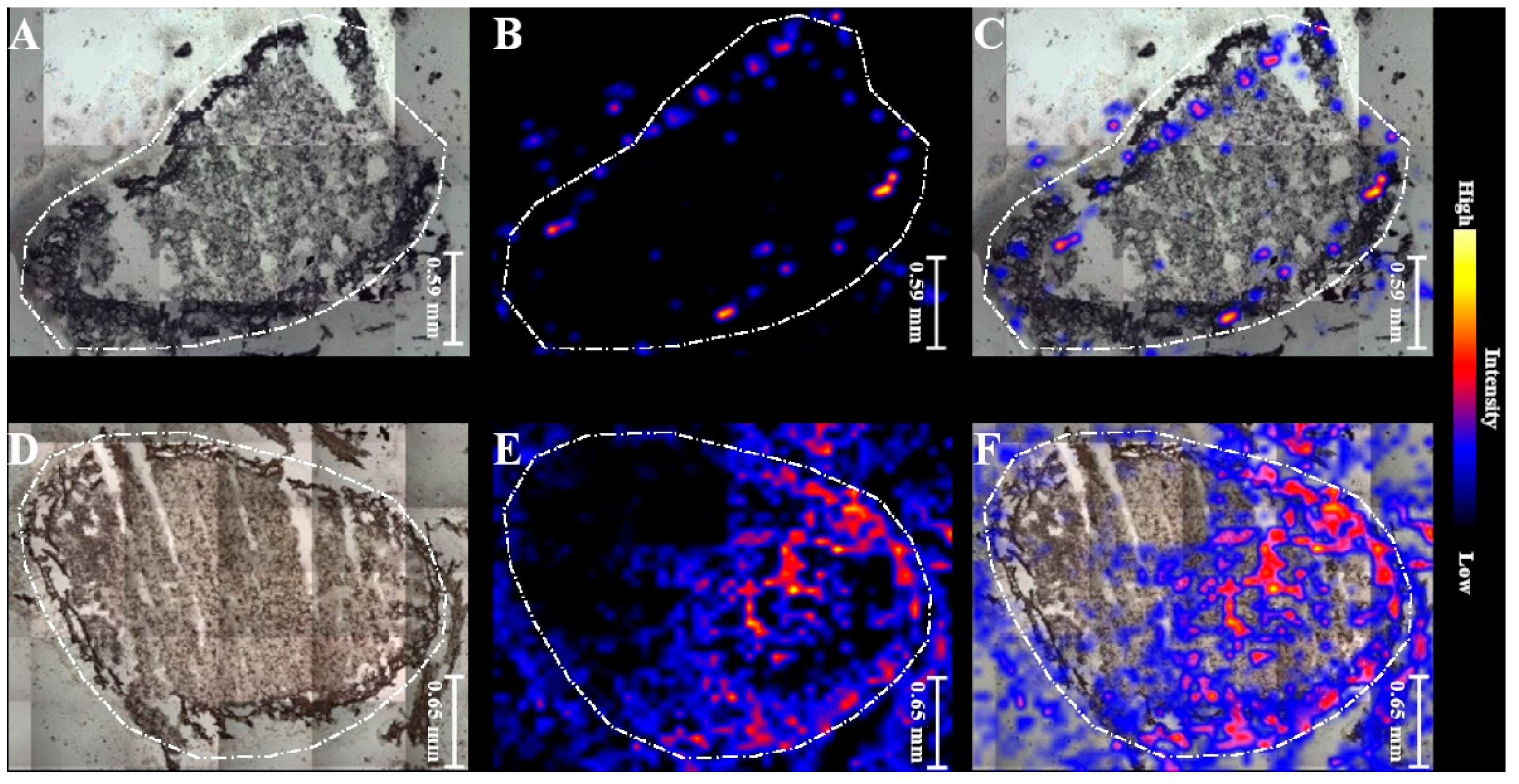
2.3. MALDI MSI
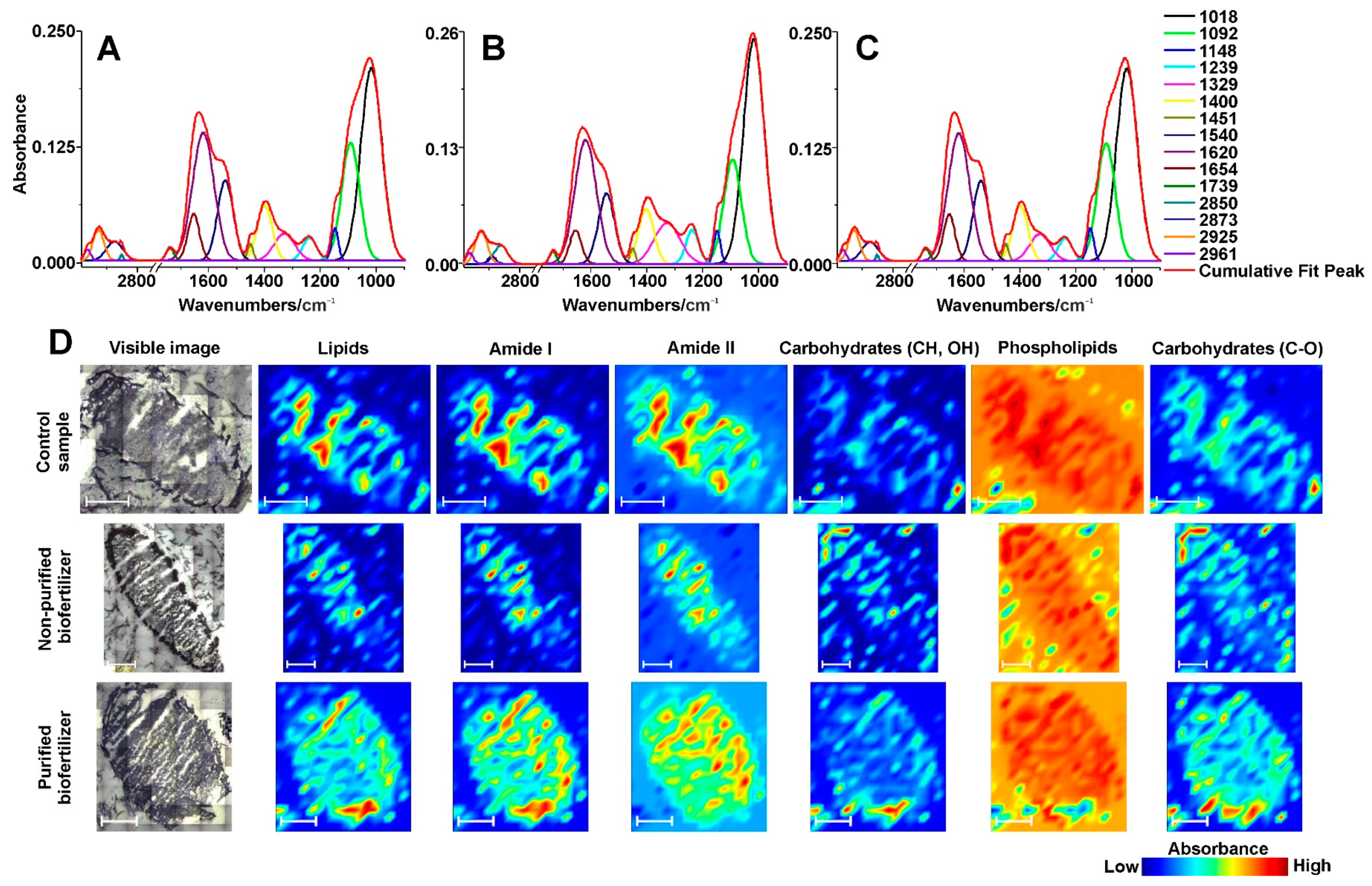
2.4. Infrared Spectra with Statistical Analysis
2.5. FT-IR Spectroscopic Imaging
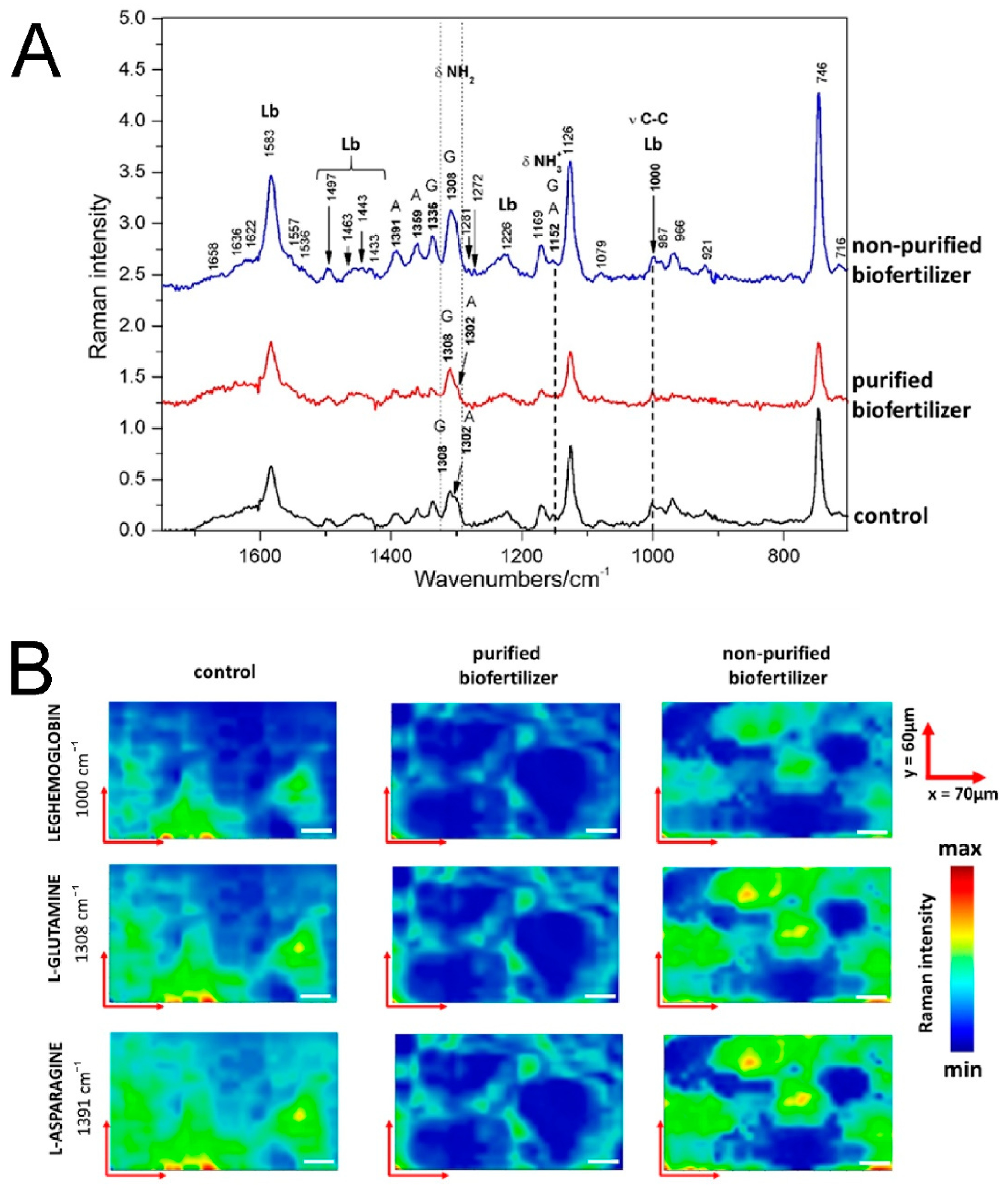
2.6. Raman Spectroscopic Imaging
3. Materials and Methods
3.1. Reagents and Materials
3.2. Isolation of Nod Factors
3.3. Fractionation of the Butanolic Extract Containing Nod Factors by SPE
3.4. UPLC and ESI-MS Analyses
3.5. Plant Experiments
3.6. MALDI-MSI Sample Preparation
3.7. MALDI MSI Data Acquisition
3.8. Sample Preparation for FTIR and Raman Imaging
3.9. FT-IR ATR Spectra Acquisition
3.10. FT-IR Spectroscopic Imaging Data Acquisition
3.11. Raman Spectroscopic Imaging Data Acquisition
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lodwig, E.; Poole, P. Metabolism of Rhizobium Bacteroids. CRC Crit. Rev. Plant Sci. 2003, 22, 37–78. [Google Scholar] [CrossRef]
- Sprent, J.I.; Ardley, J.; James, E.K. Biogeography of Nodulated Legumes and Their Nitrogen-Fixing Symbionts. New Phytol. 2017, 215, 40–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, K.M.; Kobayashi, H.; Davies, B.W.; Taga, M.E.; Graham, C. How Rhizobial Symbionts Invade Plants. Nat. Rev. Microbiol. 2007, 5, 619–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perret, X.; Staehelin, C.; Broughton, W.J. Molecular Basis of Symbiotic Promiscuity. Microbiol. Mol. Biol. Rev. 2000, 64, 180–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaink, H.P. Root Nodulation and Infection Factors Produced by Rhizobial Bacteria. Annu. Rev. Microbiol. 2000, 54, 257–288. [Google Scholar] [CrossRef] [Green Version]
- Oldroyd, G.E.D.; Downie, J.A. Coordinating Nodule Morphogenesis with Rhizobial Infection in Legumes. Annu. Rev. Plant Biol. 2008, 59, 519–546. [Google Scholar] [CrossRef]
- Pacios-Bras, C.; Schlaman, H.R.M.; Boot, K.; Admiraal, P.; Langerak, J.M.; Stougaard, J.; Spaink, H.P. Auxin Distribution in Lotus japonicus during Root Nodule Development. Plant Mol. Biol. 2003, 52, 1169–1180. [Google Scholar] [CrossRef]
- Tanaka, K.; Cho, S.H.; Lee, H.; Pham, A.Q.; Batek, J.M.; Cui, S.; Qiu, J.; Khan, S.M.; Joshi, T.; Zhang, Z.J.; et al. Effect of Lipo-Chitooligosaccharide on Early Growth of C4 Grass Seedlings. J. Exp. Bot. 2015, 66, 5727–5738. [Google Scholar] [CrossRef]
- Kidaj, D.; Wielbo, J.; Skorupska, A. Nod Factors Stimulate Seed Germination and Promote Growth and Nodulation of Pea and Vetch under Competitive Conditions. Microbiol. Res. 2012, 167, 144–150. [Google Scholar] [CrossRef]
- Macchiavelli, R.E.; Brelles-Mariño, G. Nod Factor-Treated Medicago truncatula Roots and Seeds Show an Increased Number of Nodules When Inoculated with a Limiting Population of Sinorhizobium Meliloti. J. Exp. Bot. 2004, 55, 2635–2640. [Google Scholar] [CrossRef] [Green Version]
- Maj, D.; Wielbo, J.; Marek-Kozaczuk, M.; Martyniuk, S.; Skorupska, A. Pretreatment of Clover Seeds with Nod Factors Improves Growth and Nodulation of Trifolium Pratense. J. Chem. Ecol. 2009, 35, 479–487. [Google Scholar] [CrossRef]
- Prithiviraj, B.; Zhou, X.; Souleimanov, A.; Kahn, W.M.; Smith, D.L. A Host-Specific Bacteria-to-Plant Signal Molecule (Nod factor) Enhances Germination and Early Growth of Diverse Crop Plants. Planta 2003, 216, 437–445. [Google Scholar] [CrossRef]
- Souleimanov, A.; Prithiviraj, B.; Smith, L. The Major Nod Factor of Bradyrhizobium japonicum Promotes Early Growth of Soybean and Corn. J. Exp. Bot. 2002, 53, 1929–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appleby, C.A. Leghemoglobin and Rhizobium Respiration. Annu. Rev. Plant Physiol. 1984, 35, 443–478. [Google Scholar] [CrossRef]
- Gemperline, E.; Keller, C.; Li, L. Mass Spectrometry in Plant-Omics. Anal. Chem. 2016, 88, 3422–3434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shariatgorji, M.; Nilsson, A.; Goodwin, R.J.A.; Källback, P.; Schintu, N.; Zhang, X.; Crossman, A.R.; Bezard, E.; Svenningsson, P.; Andren, P.E. Direct Targeted Quantitative Molecular Imaging of Neurotransmitters in Brain Tissue Sections. Neuron 2014, 84, 697–707. [Google Scholar] [CrossRef] [Green Version]
- Baranska, M.; Roman, M.; Cz Dobrowolski, J.; Schulz, H.; Baranski, R. Recent Advances in Raman Analysis of Plants: Alkaloids, Carotenoids, and Polyacetylenes. Curr. Anal. Chem. 2012, 9, 108–127. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and Quantification of Valuable Plant Substances by IR and Raman Spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Gierlinger, N. New Insights into Plant Cell Walls by Vibrational Microspectroscopy. Appl. Spectrosc. Rev. 2018, 53, 517–551. [Google Scholar] [CrossRef] [Green Version]
- Szymańska-Chargot, M.; Chylińska, M.; Pieczywek, P.M.; Rösch, P.; Schmitt, M.; Popp, J.; Zdunek, A. Raman Imaging of Changes in the Polysaccharides Distribution in the Cell Wall during Apple Fruit Development and Senescence. Planta 2016, 243, 935–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, F.J.; Perston, B.B.; Galindez-Najera, S.P.; Edwards, C.H.; Powell, P.O.; Mandalari, G.; Campbell, G.M.; Butterworth, P.J.; Ellis, P.R. Infrared Microspectroscopic Imaging of Plant Tissues: Spectral Visualization of Triticum aestivum Kernel and Arabidopsis Leaf Microstructure. Plant J. 2015, 84, 634–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, T.T.; Pu, H.; Sun, D.W. Insights into the Changes in Chemical Compositions of the Cell Wall of Pear Fruit Infected by Alternaria alternata with Confocal Raman Microspectroscopy. Postharvest. Biol. Technol. 2017, 132, 119–129. [Google Scholar] [CrossRef]
- Philip-Hollingsworth, S.; Orgambide, G.G.; Bradford, J.J.; Smith, D.K.; Hollingsworth, R.I.; Dazzo, F.B. Mutation or Increased Copy Number of nodE Has No Effect on the Spectrum of Chitolipooligosaccharide Nod Factors Made by Rhizobium leguminosarum bv. trifolii. J. Biol. Chem. 1995, 270, 20968–20977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies; J. Wiley & Sons: Hoboken, NJ, USA, 2001; pp. 1–326. [Google Scholar]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Zhang, J.; Dehoog, R.J.; Pennathur, S.; Anderton, C.R.; Venkatachalam, M.A.; Alexandrov, T.; Eberlin, L.S.; Sharma, K. DESI-MSI and METASPACE Indicates Lipid Abnormalities and Altered Mitochondrial Membrane Components in Diabetic Renal Proximal Tubules. Metabolomics 2020, 16, 11. [Google Scholar] [CrossRef]
- Oberg, K.A.; Ruysschaert, J.M.; Goormaghtigh, E. The Optimization of Protein Secondary Structure Determination with Infrared and Circular Dichroism Spectra. Eur. J. Biochem. 2004, 271, 2937–2948. [Google Scholar] [CrossRef] [Green Version]
- Escuredo, P.R.; Minchin, F.R.; Gogorcena, Y.; Iturbe-Ormaetxe, I.; Klucas, R.V.; Becana, M. Involvement of Activated Oxygen in Nitrate-Induced Senescence of Pea Root Nodules. Plant Physiol. 1996, 110, 1187–1195. [Google Scholar] [CrossRef] [Green Version]
- Ott, T.; Van Dongen, J.T.; Günther, C.; Krusell, L.; Desbrosses, G.; Vigeolas, H.; Bock, V.; Czechowski, T.; Geigenberger, P.; Udvardi, M.K. Symbiotic Leghemoglobins Are Crucial for Nitrogen Fixation in Legume Root Nodules But Not for General Plant Growth and Development. Curr. Biol. 2005, 15, 531–535. [Google Scholar] [CrossRef] [Green Version]
- Nash, D.T.; Schulman, H.M. Leghemoglobins and Nitrogenase Activity during Soybean Root Nodule Development. Can. J. Bot. 1976, 54, 2790–2797. [Google Scholar] [CrossRef]
- Silva, L.; Carvalho, H. Possible Role of Glutamine Synthetase in the NO Signaling Response in Root Nodules by Contributing to the Antioxidant Defenses. Front. Plant Sci. 2013, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, D.L.; Ondrias, M.R.; LaMar, G.N.; Kong, S.B.; Smith, K.M. Resonance Raman Spectra of the Heme in Leghemoglobin. Evidence for the Absence of Ruffling and the Influence of the Vinyl Groups. J. Biol. Chem. 1983, 258, 1740–1746. [Google Scholar] [CrossRef]
- Navascués, J.; Pérez-Rontomé, C.; Gay, M.; Marcos, M.; Yang, F.; Walker, F.A.; Desbois, A.; Abián, J.; Becana, M. Leghemoglobin Green Derivatives with Nitrated Hemes Evidence Production of Highly Reactive Nitrogen Species during Aging of Legume Nodules. Proc. Natl. Acad. Sci. USA 2012, 109, 2660–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golichenko, B.O.; Naseka, V.M.; Strelchuk, V.V.; Kolomys, O.F. Raman Study of L-Asparagine and L-Glutamine Molecules Adsorbed on Aluminum Films in a Wide Frequency Range. Semicond. Phys. Quantum Electron. Optoelectron. 2017, 20, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Garneau, M.G.; Tan, Q.; Tegeder, M. Function of Pea Amino Acid Permease AAP6 in Nodule Nitrogen Metabolism and Export, and Plant Nutrition. J. Exp. Bot. 2018, 69, 5205–5219. [Google Scholar] [CrossRef] [PubMed]
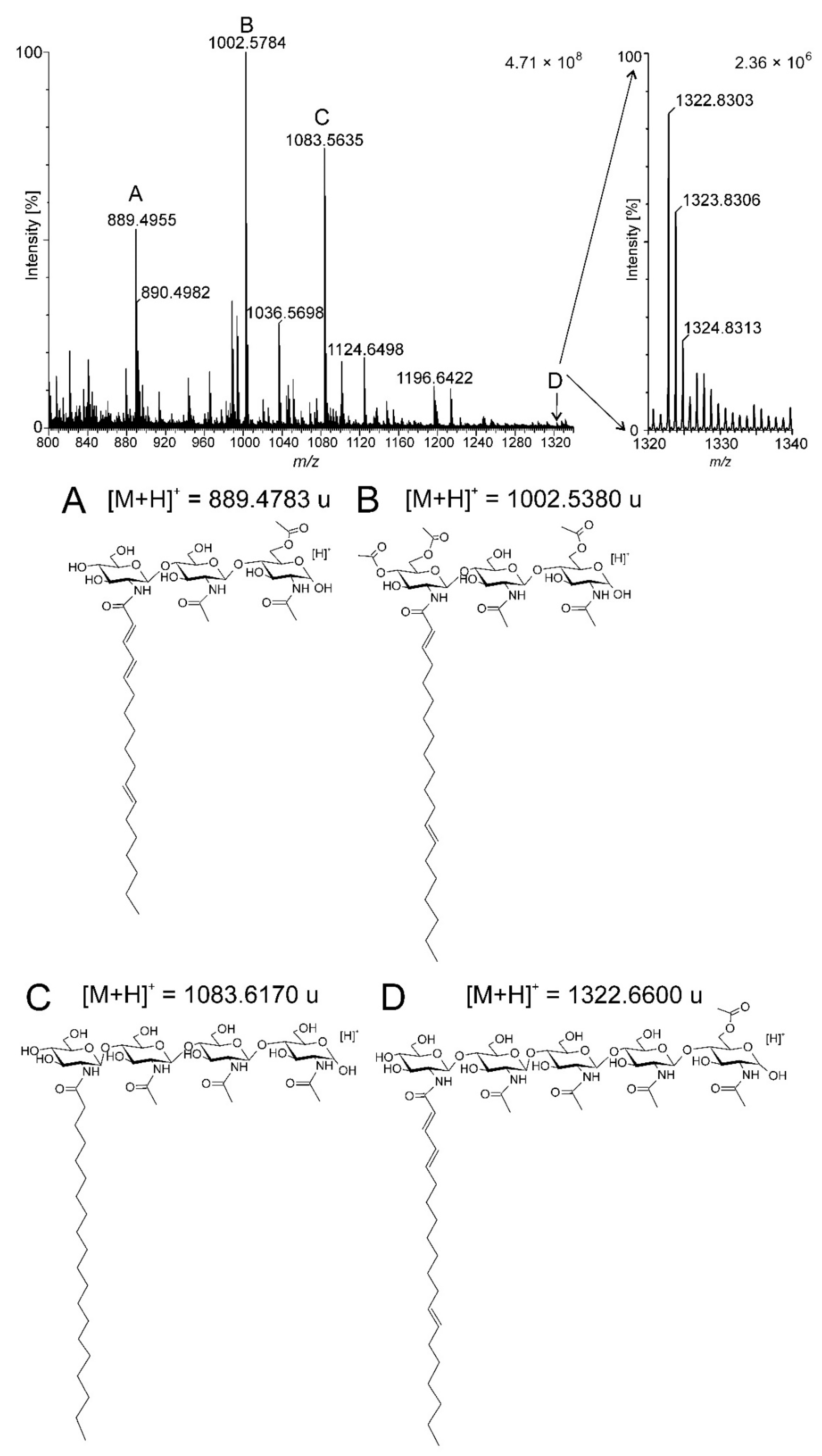
| Signal | Observed Signal [M + H]+ | Calculated Molecular Mas [M + H]+ | Assignment |
|---|---|---|---|
| A | 889.4956 | 889.4783 | 3 × GlcN; 18:3; Ac |
| B | 1002.5784 | 1002.5380 | 3 × GlcN; 20:2; 3 × Ac |
| C | 1083.5635 | 1083.6170 | 4 × GlcN; 20:0 |
| D | 1322.7389 | 1322.6600 | 5 × GlcN; 20:3; Ac |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susniak, K.; Krysa, M.; Kidaj, D.; Szymanska-Chargot, M.; Komaniecka, I.; Zamlynska, K.; Choma, A.; Wielbo, J.; Ilag, L.L.; Sroka-Bartnicka, A. Multimodal Spectroscopic Imaging of Pea Root Nodules to Assess the Nitrogen Fixation in the Presence of Biofertilizer Based on Nod-Factors. Int. J. Mol. Sci. 2021, 22, 12991. https://doi.org/10.3390/ijms222312991
Susniak K, Krysa M, Kidaj D, Szymanska-Chargot M, Komaniecka I, Zamlynska K, Choma A, Wielbo J, Ilag LL, Sroka-Bartnicka A. Multimodal Spectroscopic Imaging of Pea Root Nodules to Assess the Nitrogen Fixation in the Presence of Biofertilizer Based on Nod-Factors. International Journal of Molecular Sciences. 2021; 22(23):12991. https://doi.org/10.3390/ijms222312991
Chicago/Turabian StyleSusniak, Katarzyna, Mikolaj Krysa, Dominika Kidaj, Monika Szymanska-Chargot, Iwona Komaniecka, Katarzyna Zamlynska, Adam Choma, Jerzy Wielbo, Leopold L. Ilag, and Anna Sroka-Bartnicka. 2021. "Multimodal Spectroscopic Imaging of Pea Root Nodules to Assess the Nitrogen Fixation in the Presence of Biofertilizer Based on Nod-Factors" International Journal of Molecular Sciences 22, no. 23: 12991. https://doi.org/10.3390/ijms222312991
APA StyleSusniak, K., Krysa, M., Kidaj, D., Szymanska-Chargot, M., Komaniecka, I., Zamlynska, K., Choma, A., Wielbo, J., Ilag, L. L., & Sroka-Bartnicka, A. (2021). Multimodal Spectroscopic Imaging of Pea Root Nodules to Assess the Nitrogen Fixation in the Presence of Biofertilizer Based on Nod-Factors. International Journal of Molecular Sciences, 22(23), 12991. https://doi.org/10.3390/ijms222312991








