Rapidly Growing Mycobacterium Species: The Long and Winding Road from Tuberculosis Vaccines to Potent Stress-Resilience Agents
Abstract
1. Introduction
1.1. The “Old Friends” Hypothesis: A Biological Concept to Explain the Increasing Prevalence Rates of Stress-Associated Inflammatory Disorders in Modern Urban Societies
1.2. Mycobacterium vaccae NCTC 11659: General Information
2. History of Mycobacterium vaccae NCTC 11659 Research
2.1. Observational Studies on the Protective Effects of M. vaccae NCTC 11659 and M. vaccae ATCC 15483T: Chronological Evidence
2.1.1. M. vaccae NCTC 11659 and TB
Single Intradermal M. vaccae NCTC 11659 Administration as an Adjunct Therapy for First-Line Drug Therapy for Treatment of TB
Repeated Intradermal M. vaccae NCTC 11659 Administrations as an Adjunct Therapy for First-Line Drug Therapy for Treatment of TB
Repeated Oral M. vaccae NCTC 11659 Administrations Promote Treatment of TB
Repeated Intradermal M. vaccae NCTC 11659 Administration Prevents TB in Persons with HIV Infection
2.1.2. M. vaccae NCTC 11659 and Leprosy
2.1.3. M. vaccae NCTC 11659 and Psoriasis
2.1.4. M. vaccae NCTC 11659 and Atopic Dermatitis
2.1.5. M. vaccae NCTC 11659 and Asthma
2.1.6. M. vaccae NCTC 11659 and Cancer
2.2. Mechanistic Studies on the Protective Effects of M. vaccae NCTC 11659 and M. vaccae ATCC 15483T
2.2.1. M. vaccae NCTC 11659 Effects on DCs and Th1/Th2 Immune Profile
2.2.2. M. vaccae NCTC 11659 Effects on γδ T Cells
2.2.3. M. vaccae NCTC 11659 Effects on CD11b+ Myeloid Cells
2.2.4. M. vaccae ATCC 15483T Effects on CD8+ CTL
2.2.5. M. vaccae NCTC 11659 Effects on Tregs
2.2.6. M. vaccae NCTC 11659 Effects on Brain Microglia
2.2.7. M. vaccae ATCC 15483T Effects on Gene Expression in the Context of TB Infection
3. The Route of M. vaccae NCTC 11659 Administration Affects Its Immunoregulatory Effects
3.1. Invasive Route: s.c. Administration of M. vaccae NCTC 11659 and M. vaccae ATCC 15483T
3.2. Non-Invasive Routes of Administration of M. vaccae NCTC 11659
3.2.1. i.n. Administration of M. vaccae NCTC 11659
3.2.2. i.g./p.o. Administration of M. vaccae NCTC 11659
4. Summary and Conclusions
- Preparations of M. vaccae NCTC 11659 have been shown, regardless of their administration route, to have immunomodulatory properties (for summary see Figure 1).
- Preparations of M. vaccae NCTC 11659 have been shown to be beneficial in a plethora of conditions such as TB, leprosy, psoriasis, dermatitis, allergy, asthma, and several cancers as well as inescapable and chronic psychosocial stress.
- While invasive s.c. and non-invasive i.g. administration of M. vaccae NCTC 11659 mediate their protective effects at least in part via induction of Tregs, the non-invasive i.n. administration of M. vaccae NCTC 11659 protects against the negative pro-inflammatory consequences of chronic psychosocial stress without affecting splenic and mesLN Treg counts.
5. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 10(Z)-HDA | 10(Z)-hexadecenoic acid |
| ACTH | adrenocorticotropic hormone |
| ART | antiretroviral therapy |
| ATCC | American Type Culture Collection, Manassas, VA, USA |
| AUC | area under the curve |
| BAL | bronchoalveolar lavage |
| BBS | borate-buffered saline |
| BCG | Bacillus Calmette Guérin |
| CCL2 | C–C motif chemokine ligand 2, also referred to as monocyte chemoattractant protein-1 (MCP-1) |
| CD | cluster of differentiation |
| CECT | Colección Española de Cultivos Tipo |
| CFU | colony-forming units |
| CCUG | Culture Collection |
| University of Goteborg | Sweden |
| CLR | C-type lectin receptor |
| COVID-19 | coronavirus disease 19 |
| CREB | cAMP-response element binding protein |
| CSC | chronic subordinate colony housing |
| CTL | cytotoxic T lymphocyte |
| CXCL2 | C-X-C motif chemokine ligand 2 |
| DC | dendritic cell |
| DSM | DSMZ-Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH |
| Braunschweig | Germany |
| DSS | dextran sulfate sodium |
| ESR | erythrocyte sedimentation rate |
| EZM | elevated zero-maze |
| FoxP3 | forkhead box protein P3 |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| GYM | Glucose, yeast, and malt agar |
| HIV | human immunodeficiency virus |
| HPA | hypothalamic-pituitary-adrenal |
| hsp | heat-shock protein |
| i.d. | intradermal |
| IFNγ | interferon gamma |
| i.g. | intragastric |
| Ig | immunoglobulin |
| i.m. | intramuscular |
| i.n. | intranasal |
| KCTC | Korean Collection of Type Cultures |
| IL | interleukin |
| INH | isonicotinic acid hydrazide (isoniazid) |
| IPT | INH preventative therapy |
| i.t. | intratracheal |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MB7H10 | middlebrook 7H10 agar |
| M cells | microfold cells |
| MCP-1 | monocyte chemoattractant protein-1, also referred to as C-C motif chemokine ligand 2 (CCL2) |
| MDD | major depressive disorder |
| mesLN | mesenteric lymph nodes |
| mesLNC | mesenteric lymph node cells |
| MHC | major histocompatibility complex |
| M. kyogaense | Mycobacterium kyogaense |
| M. vaccae | Mycobacterium vaccae |
| MyD88 | MYD88 innate immune signal transduction adaptor |
| NCIB | National Collection of Industrial Bacteria |
| NCTC | National Collection of Type Cultures, Central Public Laboratory Service, London, UK |
| NF-κB | nuclear factor-κB |
| NK | natural killer cell |
| NKG2D | natural killer group 2D |
| NLR | nucleotide-binding oligomerization domain (NOD)-like receptors |
| NOD | nucleotide-binding oligomerization domain |
| OVA | ovalbumin |
| PASI | Psoriasis Area Severity Index |
| PBMC | peripheral blood mononuclear cells |
| PBS | phosphate-buffered saline |
| PEA | palmitoylethanolamide |
| PMG | proteose peptone-meat extract-glycerol agar |
| p.o. | per os (i.e., orally) |
| PPARα | peroxisome proliferator-activated receptor alpha |
| PRR | pattern recognition receptor |
| PTSD | posttraumatic stress disorder |
| RA | retinoic acid |
| RGMs | rapidly growing mycobacteria |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus type 2 |
| s.c. | subcutaneous |
| SHC | single-housed control |
| SN | Australian Mycological Panel |
| SPF | specific pathogen-free |
| TB | tuberculosis |
| TCR | T cell receptor |
| TGFβ1 | transforming growth factor beta 1 |
| Th | T helper cell |
| TLR | Toll-like receptor |
| TMC | Trudeau Mycobacterial Culture Collection |
| TNF | tumor necrosis factor, also referred to as tumor necrosis factor alpha |
| Treg | regulatory T cells |
| Veh | vehicle |
References
- Eder, W.; Ege, M.J.; von Mutius, E. The asthma epidemic. N. Engl. J. Med. 2006, 355, 2226–2235. [Google Scholar] [CrossRef]
- Bach, J.F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 2002, 347, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A. Review series on helminths, immune modulation and the hygiene hypothesis: The broader implications of the hygiene hypothesis. Immunology 2009, 126, 3–11. [Google Scholar] [CrossRef]
- Rook, G.A.; Stanford, J.L. Give us this day our daily germs. Immunol. Today 1998, 19, 113–116. [Google Scholar] [CrossRef]
- Stene, L.C.; Nafstad, P. Relation between occurrence of type 1 diabetes and asthma. Lancet 2001, 357, 607–608. [Google Scholar] [CrossRef]
- Langgartner, D.; Lowry, C.A.; Reber, S.O. Old Friends, immunoregulation, and stress resilience. Pflug. Arch. 2019, 471, 237–269. [Google Scholar] [CrossRef]
- Rook, G.A.; Lowry, C.A. The hygiene hypothesis and psychiatric disorders. Trends Immunol. 2008, 29, 150–158. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, B.; Qiu, W.; Yang, L.; Hu, B.; Tian, X.; Yang, H. Altered expression of CD4+ CD25+ regulatory T cells and its 5-HT(1a) receptor in patients with major depression disorder. J. Affect. Disord. 2010, 124, 68–75. [Google Scholar] [CrossRef]
- Sommershof, A.; Aichinger, H.; Engler, H.; Adenauer, H.; Catani, C.; Boneberg, E.M.; Elbert, T.; Groettrup, M.; Kolassa, I.T. Substantial reduction of naive and regulatory T cells following traumatic stress. Brain Behav. Immun. 2009, 23, 1117–1124. [Google Scholar] [CrossRef]
- Jergović, M.; Bendelja, K.; Vidović, A.; Savić, A.; Vojvoda, V.; Aberle, N.; Rabatić, S.; Jovanovic, T.; Sabioncello, A. Patients with posttraumatic stress disorder exhibit an altered phenotype of regulatory T cells. Allergy Asthma Clin. Immunol. 2014, 10, 43. [Google Scholar] [CrossRef]
- Rook, G.A. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: Darwinian medicine and the ‘hygiene’ or ‘old friends’ hypothesis. Clin. Exp. Immunol. 2010, 160, 70–79. [Google Scholar] [CrossRef]
- Comas, I.; Coscolla, M.; Luo, T.; Borrell, S.; Holt, K.E.; Kato-Maeda, M.; Parkhill, J.; Malla, B.; Berg, S.; Thwaites, G.; et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat. Genet. 2013, 45, 1176–1182. [Google Scholar] [CrossRef]
- Wolfe, N.D.; Dunavan, C.P.; Diamond, J. Origins of major human infectious diseases. Nature 2007, 447, 279–283. [Google Scholar] [CrossRef]
- Hanski, I.; von Hertzen, L.; Fyhrquist, N.; Koskinen, K.; Torppa, K.; Laatikainen, T.; Karisola, P.; Auvinen, P.; Paulin, L.; Makela, M.J.; et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc. Natl. Acad. Sci. USA 2012, 109, 8334–8339. [Google Scholar] [CrossRef]
- Hoffmann, C.; Dollive, S.; Grunberg, S.; Chen, J.; Li, H.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. Archaea and fungi of the human gut microbiome: Correlations with diet and bacterial residents. PLoS ONE 2013, 8, e66019. [Google Scholar] [CrossRef]
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S.; et al. Compartmentalized control of skin immunity by resident commensals. Science 2012, 337, 1115–1119. [Google Scholar] [CrossRef]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.; Lowry, C.A.; Raison, C.L. Microbial ‘Old Friends’, immunoregulation and stress resilience. Evol. Med. Public Health 2013, 2013, 46–64. [Google Scholar] [CrossRef]
- Rook, G.A.; Raison, C.L.; Lowry, C.A. Microbial ‘old friends’, immunoregulation and socioeconomic status. Clin. Exp. Immunol. 2014, 177, 1–12. [Google Scholar] [CrossRef]
- Amoroso, M.; Bottcher, A.; Lowry, C.A.; Langgartner, D.; Reber, S.O. Subcutaneous Mycobacterium vaccae promotes resilience in a mouse model of chronic psychosocial stress when administered prior to or during psychosocial stress. Brain Behav. Immun. 2020, 87, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Lowry, C.A.; Hollis, J.H.; de Vries, A.; Pan, B.; Brunet, L.R.; Hunt, J.R.; Paton, J.F.; van Kampen, E.; Knight, D.M.; Evans, A.K.; et al. Identification of an immune-responsive mesolimbocortical serotonergic system: Potential role in regulation of emotional behavior. Neuroscience 2007, 146, 756–772. [Google Scholar] [CrossRef]
- Fonken, L.K.; Frank, M.G.; D’Angelo, H.M.; Heinze, J.D.; Watkins, L.R.; Lowry, C.A.; Maier, S.F. Mycobacterium vaccae immunization protects aged rats from surgery-elicited neuroinflammation and cognitive dysfunction. Neurobiol. Aging 2018, 71, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Bowers, S.J.; Lambert, S.; He, S.; Lowry, C.A.; Fleshner, M.; Wright, K.P.; Turek, F.W.; Vitaterna, M.H. Immunization with a heat-killed bacterium, Mycobacterium vaccae NCTC 11659, prevents the development of cortical hyperarousal and a PTSD-like sleep phenotype after sleep disruption and acute stress in mice. Sleep 2021, 44, zsaa271. [Google Scholar] [CrossRef]
- Frank, M.G.; Fonken, L.K.; Dolzani, S.D.; Annis, J.L.; Siebler, P.H.; Schmidt, D.; Watkins, L.R.; Maier, S.F.; Lowry, C.A. Immunization with Mycobacterium vaccae induces an anti-inflammatory milieu in the CNS: Attenuation of stress-induced microglial priming, alarmins and anxiety-like behavior. Brain Behav. Immun. 2018, 73, 352–363. [Google Scholar] [CrossRef]
- Fox, J.H.; Hassell, J.E., Jr.; Siebler, P.H.; Arnold, M.R.; Lamb, A.K.; Smith, D.G.; Day, H.E.W.; Smith, T.M.; Simmerman, E.M.; Outzen, A.A.; et al. Preimmunization with a heat-killed preparation of Mycobacterium vaccae enhances fear extinction in the fear-potentiated startle paradigm. Brain Behav. Immun. 2017, 66, 70–84. [Google Scholar] [CrossRef]
- Foxx, C.L.; Heinze, J.D.; González, A.; Vargas, F.D.; Baratta, M.V.; Elsayed, A.I.; Stewart, J.R.; Loupy, K.M.; Arnold, M.R.; Flux, M.C.; et al. Effects of immunization with the soil-derived bacterium Mycobacterium vaccae on stress coping behaviors and cognitive performance in a” two hit” stressor model. Front. Physiol. 2020, 11, 1602. [Google Scholar] [CrossRef]
- Hassell, J.E., Jr.; Fox, J.H.; Arnold, M.R.; Siebler, P.H.; Lieb, M.W.; Schmidt, D.; Spratt, E.J.; Smith, T.M.; Nguyen, K.T.; Gates, C.A.; et al. Treatment with a heat-killed preparation of Mycobacterium vaccae after fear conditioning enhances fear extinction in the fear-potentiated startle paradigm. Brain Behav. Immun. 2019, 81, 151–160. [Google Scholar] [CrossRef]
- Loupy, K.M.; Arnold, M.R.; Hassell, J.E., Jr.; Lieb, M.W.; Milton, L.N.; Cler, K.E.; Fox, J.H.; Siebler, P.H.; Schmidt, D.; Noronha, S.; et al. Evidence that preimmunization with a heat-killed preparation of Mycobacterium vaccae reduces corticotropin-releasing hormone mRNA expression in the extended amygdala in a fear-potentiated startle paradigm. Brain Behav. Immun. 2019, 77, 127–140. [Google Scholar] [CrossRef]
- Loupy, K.M.; Cler, K.E.; Marquart, B.M.; Yifru, T.W.; D’Angelo, H.M.; Arnold, M.R.; Elsayed, A.I.; Gebert, M.J.; Fierer, N.; Fonken, L.K.; et al. Comparing the effects of two different strains of mycobacteria, Mycobacterium vaccae NCTC 11659 and M. vaccae ATCC 15483, on stress-resilient behaviors and lipid-immune signaling in rats. Brain Behav. Immun. 2021, 91, 212–229. [Google Scholar] [CrossRef]
- Siebler, P.H.; Heinze, J.D.; Kienzle, D.M.; Hale, M.W.; Lukkes, J.L.; Donner, N.C.; Kopelman, J.M.; Rodriguez, O.A.; Lowry, C.A. Acute administration of the nonpathogenic, saprophytic bacterium, Mycobacterium vaccae, induces activation of serotonergic neurons in the dorsal raphe nucleus and antidepressant-like behavior in association with mild hypothermia. Cell. Mol. Neurobiol. 2018, 38, 289–304. [Google Scholar] [CrossRef]
- Smith, Z.Z.; Kubiak, R.A.; Arnold, M.R.; Loupy, K.M.; Taylor, J.A.; Crist, T.G.; Bernier, A.E.; D’Angelo, H.M.; Heinze, J.D.; Lowry, C.A.; et al. Effects of immunization with heat-killed Mycobacterium vaccae on autism spectrum disorder-like behavior and epileptogenesis in a rat model of comorbid autism and epilepsy. Brain Behav. Immun. 2020, 88, 763–780. [Google Scholar] [CrossRef]
- Amoroso, M.; Kempter, E.; Eleslambouly, T.; Lowry, C.A.; Langgartner, D.; Reber, S.O. Intranasal Mycobacterium vaccae administration prevents stress-induced aggravation of dextran sulfate sodium (DSS) colitis. Brain Behav. Immun. 2019, 80, 595–604. [Google Scholar] [CrossRef]
- Reber, S.O.; Siebler, P.H.; Donner, N.C.; Morton, J.T.; Smith, D.G.; Kopelman, J.M.; Lowe, K.R.; Wheeler, K.J.; Fox, J.H.; Hassell, J.E., Jr.; et al. Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E3130–E3139. [Google Scholar] [CrossRef]
- Nouioui, I.; Brunet, L.R.; Simpson, D.; Klenk, H.P.; Goodfellow, M. Description of a novel species of fast growing mycobacterium: Mycobacterium kyogaense sp. nov. a scotochromogenic strain received as Mycobacterium vaccae. Int. J. Syst. Evol. Microbiol. 2018, 68, 3726–3734. [Google Scholar] [CrossRef]
- Gupta, R.S.; Lo, B.; Son, J. Phylogenomics and comparative genomic studies robustly support division of the genus Mycobacterium into an emended genus Mycobacterium and four novel genera. Front. Microbiol. 2018, 9, 67. [Google Scholar] [CrossRef]
- Breivik, T.; Rook, G.A. Oral treatment with SRP299 (killed Mycobacterium vaccae) inhibits experimental periodontal disease in Wistar rats. J. Clin. Periodontol. 2003, 30, 931–936. [Google Scholar] [CrossRef]
- Berth-Jones, J.; Arkwright, P.D.; Marasovic, D.; Savani, N.; Aldridge, C.R.; Leech, S.N.; Morgan, C.; Clark, S.M.; Ogilvie, S.; Chopra, S.; et al. Killed Mycobacterium vaccae suspension in children with moderate-to-severe atopic dermatitis: A randomized, double-blind, placebo-controlled trial. Clin. Exp. Allergy 2006, 36, 1115–1121. [Google Scholar] [CrossRef]
- Arkwright, P.D.; David, T.J. Effect of Mycobacterium vaccae on atopic dermatitis in children of different ages. Br. J. Derm. 2003, 149, 1029–1034. [Google Scholar] [CrossRef]
- Adams, V.C.; Hunt, J.R.F.; Martinelli, R.; Palmer, R.; Rook, G.A.W.; Brunet, L.R. Mycobacterium vaccae induces a population of pulmonary CD11c+ cells with regulatory potential in allergic mice. Eur. J. Immunol. 2004, 34, 631–638. [Google Scholar] [CrossRef]
- Zuany-Amorim, C.; Sawicka, E.; Manlius, C.; Le Moine, A.; Brunet, L.R.; Kemeny, D.M.; Bowen, G.; Rook, G.; Walker, C. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat. Med. 2002, 8, 625–629. [Google Scholar] [CrossRef]
- Dlugovitzky, D.; Fiorenza, G.; Farroni, M.; Bogue, C.; Stanford, C.; Stanford, J. Immunological consequences of three doses of heat-killed Mycobacterium vaccae in the immunotherapy of tuberculosis. Respir. Med. 2006, 100, 1079–1087. [Google Scholar] [CrossRef]
- von Reyn, C.F.; Mtei, L.; Arbeit, R.D.; Waddell, R.; Cole, B.; Mackenzie, T.; Matee, M.; Bakari, M.; Tvaroha, S.; Adams, L.V.; et al. Prevention of tuberculosis in Bacille Calmette-Guerin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. Aids 2010, 24, 675–685. [Google Scholar] [CrossRef]
- Bourinbaiar, A.S.; Batbold, U.; Efremenko, Y.; Sanjagdorj, M.; Butov, D.; Damdinpurev, N.; Grinishina, E.; Mijiddorj, O.; Kovolev, M.; Baasanjav, K.; et al. Phase III, placebo-controlled, randomized, double-blind trial of tableted, therapeutic TB vaccine (V7) containing heat-killed M. vaccae administered daily for one month. J. Clin. Tuberc. Other Mycobact. Dis. 2020, 18, 100141. [Google Scholar] [CrossRef]
- Butov, D.A.; Efremenko, Y.V.; Prihoda, N.D.; Zaitzeva, S.I.; Yurchenko, L.V.; Sokolenko, N.I.; Butova, T.S.; Stepanenko, A.L.; Kutsyna, G.A.; Jirathitikal, V.; et al. Randomized, placebo-controlled Phase II trial of heat-killed Mycobacterium vaccae (Immodulon batch) formulated as an oral pill (V7). Immunotherapy 2013, 5, 1047–1054. [Google Scholar] [CrossRef]
- Lahey, T.; Arbeit, R.D.; Bakari, M.; Horsburgh, C.R.; Matee, M.; Waddell, R.; Mtei, L.; Vuola, J.M.; Pallangyo, K.; von Reyn, C.F. Immunogenicity of a protective whole cell mycobacterial vaccine in HIV-infected adults: A phase III study in Tanzania. Vaccine 2010, 28, 7652–7658. [Google Scholar] [CrossRef]
- Le Bert, N.; Chain, B.M.; Rook, G.; Noursadeghi, M. DC priming by M. vaccae inhibits Th2 responses in contrast to specific TLR2 priming and is associated with selective activation of the CREB pathway. PLoS ONE 2011, 6, e18346. [Google Scholar] [CrossRef][Green Version]
- Johnson, D.; Waddell, R.D.; Pelton, S.I.; Jaeger, A.S.; Modlin, J.F.; Yogev, R.; Morin, P.; Arbeit, R.D.; von Reyn, C.F. Randomised trial of intradermal Mycobacterium vaccae or intradermal hepatitis B immunisation in children with HIV infection. Vaccine 1999, 17, 2583–2587. [Google Scholar] [CrossRef]
- Vuola, J.M.; Ristola, M.A.; Cole, B.; Jarviluoma, A.; Tvaroha, S.; Ronkko, T.; Rautio, O.; Arbeit, R.D.; von Reyn, C.F. Immunogenicity of an inactivated mycobacterial vaccine for the prevention of HIV-associated tuberculosis: A randomized, controlled trial. Aids 2003, 17, 2351–2355. [Google Scholar] [CrossRef]
- Stanford, J.L.; Bahr, G.M.; Rook, G.A.; Shaaban, M.A.; Chugh, T.D.; Gabriel, M.; al-Shimali, B.; Siddiqui, Z.; Ghardani, F.; Shahin, A.; et al. Immunotherapy with Mycobacterium vaccae as an adjunct to chemotherapy in the treatment of pulmonary tuberculosis. Tubercle 1990, 71, 87–93. [Google Scholar] [CrossRef]
- Boenickse, R.; Juhasz, E. [Description of the new species Mycobacterium vaccae N. Sp]. Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. 1. Abt. Medizinisch-hygienische Bakteriologie, Virusforschung und Parasitologie. Originale 1964, 192, 133–135. [Google Scholar]
- Efremenko, Y.V.; Butov, D.A.; Prihoda, N.D.; Zaitzeva, S.I.; Yurchenko, L.V.; Sokolenko, N.I.; Butova, T.S.; Stepanenko, A.L.; Kutsyna, G.A.; Jirathitikal, V.; et al. Randomized, placebo-controlled phase II trial of heat-killed Mycobacterium vaccae (Longcom batch) formulated as an oral pill (V7). Hum. Vaccines Immunother. 2013, 9, 1852–1856. [Google Scholar] [CrossRef]
- Gong, W.P.; Liang, Y.; Ling, Y.B.; Zhang, J.X.; Yang, Y.R.; Wang, L.; Wang, J.; Shi, Y.C.; Wu, X.Q. Effects of Mycobacterium vaccae vaccine in a mouse model of tuberculosis: Protective action and differentially expressed genes. Mil. Med. Res. 2020, 7, 25. [Google Scholar] [CrossRef]
- Rodriguez-Guell, E.; Agusti, G.; Corominas, M.; Cardona, P.J.; Casals, I.; Parella, T.; Sempere, M.A.; Luquin, M.; Julian, E. The production of a new extracellular putative long-chain saturated polyester by smooth variants of Mycobacterium vaccae interferes with Th1-cytokine production. Antonie Van Leeuwenhoek 2006, 90, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Corlan, E.; Marica, C.; Macavei, C.; Stanford, J.L.; Stanford, C.A. Immunotherapy with Mycobacterium vaccae in the treatment of tuberculosis in Romania. 2. Chronic or relapsed disease. Respir. Med. 1997, 91, 21–29. [Google Scholar] [CrossRef]
- Dlugovitzky, D.; Bottasso, O.; Dominino, J.C.; Valentini, E.; Hartopp, R.; Singh, M.; Stanford, C.; Stanford, J. Clinical and serological studies of tuberculosis patients in Argentina receiving immunotherapy with Mycobacterium vaccae (SRL 172). Respir. Med. 1999, 93, 557–562. [Google Scholar] [CrossRef]
- Johnson, J.L.; Kamya, R.M.; Okwera, A.; Loughlin, A.M.; Nyole, S.; Hom, D.L.; Wallis, R.S.; Hirsch, C.S.; Wolski, K.; Foulds, J.; et al. Randomized controlled trial of Mycobacterium vaccae immunotherapy in non-human immunodeficiency virus-infected ugandan adults with newly diagnosed pulmonary tuberculosis. The Uganda-Case Western Reserve University Research Collaboration. J. Infect. Dis. 2000, 181, 1304–1312. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Onyebujoh, P.C.; Abdulmumini, T.; Robinson, S.; Rook, G.A.; Stanford, J.L. Immunotherapy with Mycobacterium vaccae as an addition to chemotherapy for the treatment of pulmonary tuberculosis under difficult conditions in Africa. Respir. Med. 1995, 89, 199–207. [Google Scholar] [CrossRef][Green Version]
- Dlugovitzky, D.; Notario, R.; Martinel-Lamas, D.; Fiorenza, G.; Farroni, M.; Bogue, C.; Stanford, C.; Stanford, J. Immunotherapy with oral, heat-killed, Mycobacterium vaccae in patients with moderate to advanced pulmonary tuberculosis. Immunotherapy 2010, 2, 159–169. [Google Scholar] [CrossRef]
- Bottasso, O.; Merlin, V.; Cannon, L.; Cannon, H.; Ingledew, N.; Keni, M.; Hartopp, R.; Stanford, C.; Stanford, J. Studies of vaccination of persons in close contact with leprosy patients in Argentina. Vaccine 1998, 16, 1166–1171. [Google Scholar] [CrossRef]
- Truoc, L.V.; Ly, H.M.; Thuy, N.K.; Trach, D.D.; Stanford, C.A.; Stanford, J.L. Vaccination against leprosy at Ben San Leprosy Centre, Ho Chi Minh City, Vietnam. Vaccine 2001, 19, 3451–3458. [Google Scholar] [CrossRef]
- Abbot, N.C.; Beck, J.S.; Feval, F.; Weiss, F.; Mobayen, M.H.; Ghazi-Saidi, K.; Dowlati, Y.; Velayati, A.A.; Stanford, J.L. Immunotherapy with Mycobacterium vaccae and peripheral blood flow in long-treated leprosy patients, a randomised, placebo-controlled trial. Eur. J. Vasc. Endovasc. Surg. 2002, 24, 202–208. [Google Scholar] [CrossRef]
- Stanford, J.; de las Aguas, J.T.; Torres, P.; Gervasioni, B.; Ravioli, R.J.H.C.P. Studies on the effects of a potential immunotherapeutic agent in leprosy patients. Health Coop. Pap. 1987, 7, 201–206. [Google Scholar]
- Lehrer, A.; Bressanelli, A.; Wachsmann, V.; Bottasso, O.; Bay, M.L.; Singh, M.; Stanford, C.; Stanford, J. Immunotherapy with Mycobacterium vaccae in the treatment of psoriasis. Fems Immunol. Med. Microbiol. 1998, 21, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Arkwright, P.D.; David, T.J. Intradermal administration of a killed Mycobacterium vaccae suspension (SRL 172) is associated with improvement in atopic dermatitis in children with moderate-to-severe disease. J. Allergy Clin. Immunol. 2001, 107, 531–534. [Google Scholar] [CrossRef]
- Camporota, L.; Corkhill, A.; Long, H.; Lordan, J.; Stanciu, L.; Tuckwell, N.; Cross, A.; Stanford, J.L.; Rook, G.A.; Holgate, S.T.; et al. The effects of Mycobacterium vaccae on allergen-induced airway responses in atopic asthma. Eur. Respir. J. 2003, 21, 287–293. [Google Scholar] [CrossRef]
- Cananzi, F.C.; Mudan, S.; Dunne, M.; Belonwu, N.; Dalgleish, A.G. Long-term survival and outcome of patients originally given Mycobacterium vaccae for metastatic malignant melanoma. Hum. Vaccines Immunother. 2013, 9, 2427–2433. [Google Scholar] [CrossRef][Green Version]
- Maraveyas, A.; Baban, B.; Kennard, D.; Rook, G.A.; Westby, M.; Grange, J.M.; Lydyard, P.; Stanford, J.L.; Jones, M.; Selby, P.; et al. Possible improved survival of patients with stage IV AJCC melanoma receiving SRL 172 immunotherapy: Correlation with induction of increased levels of intracellular interleukin-2 in peripheral blood lymphocytes. Ann. Oncol. 1999, 10, 817–824. [Google Scholar] [CrossRef]
- O’Brien, M.E.; Anderson, H.; Kaukel, E.; O’Byrne, K.; Pawlicki, M.; Von Pawel, J.; Reck, M.; Group, S.-O.-S. SRL172 (killed Mycobacterium vaccae) in addition to standard chemotherapy improves quality of life without affecting survival, in patients with advanced non-small-cell lung cancer: Phase III results. Ann. Oncol. 2004, 15, 906–914. [Google Scholar] [CrossRef]
- Smit, J.J.; Van Loveren, H.; Hoekstra, M.O.; Schijf, M.A.; Folkerts, G.; Nijkamp, F.P. Mycobacterium vaccae administration during allergen sensitization or challenge suppresses asthmatic features. Clin. Exp. Allergy 2003, 33, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Rook, G.A. Inhibition of an established allergic response to ovalbumin in BALB/c mice by killed Mycobacterium vaccae. Immunology 1998, 93, 307–313. [Google Scholar] [CrossRef]
- Fowler, D.W.; Copier, J.; Wilson, N.; Dalgleish, A.G.; Bodman-Smith, M.D. Mycobacteria activate gammadelta T-cell anti-tumour responses via cytokines from type 1 myeloid dendritic cells: A mechanism of action for cancer immunotherapy. Cancer Immunol. Immunother. 2012, 61, 535–547. [Google Scholar] [CrossRef]
- Bazzi, S.; Modjtahedi, H.; Mudan, S.; Akle, C.; Bahr, G.M. Analysis of the immunomodulatory properties of two heat-killed mycobacterial preparations in a human whole blood model. Immunobiology 2015, 220, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Baran, J.; Baj-Krzyworzeka, M.; Weglarczyk, K.; Ruggiero, I.; Zembala, M. Modulation of monocyte-tumour cell interactions by Mycobacterium vaccae. Cancer Immunol. Immunother. 2004, 53, 1127–1134. [Google Scholar] [CrossRef]
- Skinner, M.A.; Yuan, S.; Prestidge, R.; Chuk, D.; Watson, J.D.; Tan, P.L. Immunization with heat-killed Mycobacterium vaccae stimulates CD8+ cytotoxic T cells specific for macrophages infected with Mycobacterium tuberculosis. Infect. Immun. 1997, 65, 4525–4530. [Google Scholar] [CrossRef]
- Hunt, J.R.; Martinelli, R.; Adams, V.C.; Rook, G.A.; Brunet, L.R. Intragastric administration of Mycobacterium vaccae inhibits severe pulmonary allergic inflammation in a mouse model. Clin. Exp. Allergy 2005, 35, 685–690. [Google Scholar] [CrossRef]
- Smith, D.G.; Martinelli, R.; Besra, G.S.; Illarionov, P.A.; Szatmari, I.; Brazda, P.; Allen, M.A.; Xu, W.; Wang, X.; Nagy, L.; et al. Identification and characterization of a novel anti-inflammatory lipid isolated from Mycobacterium vaccae, a soil-derived bacterium with immunoregulatory and stress resilience properties. Psychopharmacology 2019, 236, 1653–1670. [Google Scholar] [CrossRef]
- de Carvalho, C.; Teixeira, R.; Fernandes, P. Mycobacterium vaccae adaptation to disinfectants and hand sanitisers, and evaluation of cross-tolerance with antimicrobials. Antibiotics 2020, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Hoisington, A.J.; Brenner, L.A.; Kinney, K.A.; Postolache, T.T.; Lowry, C.A. The microbiome of the built environment and mental health. Microbiome 2015, 3, 60. [Google Scholar] [CrossRef]
- Gebert, M.J.; Delgado-Baquerizo, M.; Oliverio, A.M.; Webster, T.M.; Nichols, L.M.; Honda, J.R.; Chan, E.D.; Adjemian, J.; Dunn, R.R.; Fierer, N. Ecological analyses of mycobacteria in showerhead biofilms and their relevance to human health. mBio 2018, 9, e01614-18. [Google Scholar] [CrossRef] [PubMed]
- Macovei, L.; McCafferty, J.; Chen, T.; Teles, F.; Hasturk, H.; Paster, B.J.; Campos-Neto, A. The hidden ‘mycobacteriome’ of the human healthy oral cavity and upper respiratory tract. J. Oral Microbiol. 2015, 7, 26094. [Google Scholar] [CrossRef]
- Lehtimaki, J.; Thorsen, J.; Rasmussen, M.A.; Hjelmso, M.; Shah, S.; Mortensen, M.S.; Trivedi, U.; Vestergaard, G.; Bonnelykke, K.; Chawes, B.L.; et al. Urbanized microbiota in infants, immune constitution, and later risk of atopic diseases. J. Allergy Clin. Immunol. 2021, 148, 234–243. [Google Scholar] [CrossRef]
- Hachem, R.; Raad, I.; Rolston, K.V.; Whimbey, E.; Katz, R.; Tarrand, J.; Libshitz, H. Cutaneous and pulmonary infections caused by Mycobacterium vaccae. Clin. Infect. Dis. 1996, 23, 173–175. [Google Scholar] [CrossRef][Green Version]
- Congedo, P.; Gardellini, A.; Corich, L.; Papa, A.; Turrini, M. The first case of Mycobacterium vaccae sepsis in a non-Hodgkin lymphoma patient: Biological understandings and clinical consequencies. Access Microbiol. 2020, 2, acmi000161. [Google Scholar] [CrossRef]
- Publich Health England Culture Collections Bacteria Collection: Mycobacterium vaccae. Available online: https://www.phe-culturecollections.org.uk/products/bacteria/detail.jsp?refId=NCTC+10916&collection=nctc (accessed on 28 July 2021).
- Stanford, J.L.; Paul, R.C. A preliminary report on some studies of environmental mycobacteria. Ann. Soc. Belg. Med. Trop. 1973, 53, 389–393. [Google Scholar]
- BacDive Mycobacterium kyogaense CECT 9646 Is an Aerobe, Mesophilic, Gram-Positive Bacterium That Was Isolated from Mud. Available online: https://bacdive.dsmz.de/search?search=NCTC+11659&submit (accessed on 28 July 2021).
- Stanford, J.L.; Rook, G.A.; Bahr, G.M.; Dowlati, Y.; Ganapati, R.; Ghazi Saidi, K.; Lucas, S.; Ramu, G.; Torres, P.; Minh Ly, H.; et al. Mycobacterium vaccae in immunoprophylaxis and immunotherapy of leprosy and tuberculosis. Vaccine 1990, 8, 525–530. [Google Scholar] [CrossRef]
- Stanford, J.; Stanford, C.; Stansby, G.; Bottasso, O.; Bahr, G.; Grange, J. The common mycobacterial antigens and their importance in the treatment of disease. Curr. Pharm. Des. 2009, 15, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Stanford, J.L.; Stanford, C.A. Immunotherapy of tuberculosis with Mycobacterium vaccae NCTC 11659. Immunobiology 1994, 191, 555–563. [Google Scholar] [CrossRef]
- Smit, J.J.; Van Loveren, H.; Hoekstra, M.O.; Van der Kant, P.A.; Folkerts, G.; Nijkamp, F.P. Therapeutic treatment with heat-killed Mycobacterium vaccae (SRL172) in a mild and severe mouse model for allergic asthma. Eur. J. Pharmacol. 2003, 470, 193–199. [Google Scholar] [CrossRef]
- Capece, D.; Verzella, D.; Fischietti, M.; Zazzeroni, F.; Alesse, E. Targeting costimulatory molecules to improve antitumor immunity. J. Biomed. Biotechnol. 2012, 2012, 926321. [Google Scholar] [CrossRef] [PubMed]
- Eaton, J.D.; Perry, M.J.; Nicholson, S.; Guckian, M.; Russell, N.; Whelan, M.; Kirby, R.S. Allogeneic whole-cell vaccine: A phase I/II study in men with hormone-refractory prostate cancer. Bju Int. 2002, 89, 19–26. [Google Scholar] [CrossRef]
- O’Brien, M.E.; Saini, A.; Smith, I.E.; Webb, A.; Gregory, K.; Mendes, R.; Ryan, C.; Priest, K.; Bromelow, K.V.; Palmer, R.D.; et al. A randomized phase II study of SRL172 (Mycobacterium vaccae) combined with chemotherapy in patients with advanced inoperable non-small-cell lung cancer and mesothelioma. Br. J. Cancer 2000, 83, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.M.; Sim, S.; O’Donnell, D.O.; Protheroe, A.; Beirne, D.; Stanley, A.; Tourani, J.M.; Khayat, D.; Hancock, B.; Vasey, P.; et al. An evaluation of a preparation of Mycobacterium vaccae (SRL172) as an immunotherapeutic agent in renal cancer. Eur. J. Cancer 2008, 44, 216–223. [Google Scholar] [CrossRef]
- Stanford, J.L.; Stanford, C.A.; O’Brien, M.E.; Grange, J.M. Successful immunotherapy with Mycobacterium vaccae in the treatment of adenocarcinoma of the lung. Eur. J. Cancer 2008, 44, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Batt, S.M.; Minnikin, D.E.; Besra, G.S. The thick waxy coat of mycobacteria, a protective layer against antibiotics and the host’s immune system. Biochem J. 2020, 477, 1983–2006. [Google Scholar] [CrossRef]
- Dulberger, C.L.; Rubin, E.J.; Boutte, C.C. The mycobacterial cell envelope—A moving target. Nat. Rev. Microbiol. 2020, 18, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, E.; Mori, D.; Yamasaki, S. Recognition of Mycobacterial Lipids by Immune Receptors. Trends Immunol. 2017, 38, 66–76. [Google Scholar] [CrossRef]
- Brennan, P.J.; Nikaido, H. The envelope of mycobacteria. Annu. Rev. Biochem. 1995, 64, 29–63. [Google Scholar] [CrossRef]
- Zuber, B.; Chami, M.; Houssin, C.; Dubochet, J.; Griffiths, G.; Daffé, M. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J. Bacterial. 2008, 190, 5672–5680. [Google Scholar] [CrossRef]
- Christensen, H.; Garton, N.J.; Horobin, R.W.; Minnikin, D.E.; Barer, M.R. Lipid domains of mycobacteria studied with fluorescent molecular probes. Mol. Microbiol. 1999, 31, 1561–1572. [Google Scholar] [CrossRef]
- Kleen, T.O.; Galdon, A.A.; MacDonald, A.S.; Dalgleish, A.G. Mitigating coronavirus induced dysfunctional immunity for at-risk populations in COVID-19: Trained immunity, BCG and “New Old Friends”. Front. Immunol. 2020, 11, 2059. [Google Scholar] [CrossRef]
- Stanford, J.; Stanford, C. Mycobacteria and their world. Int. J. Mycobacteriol. 2012, 1, 3–12. [Google Scholar] [CrossRef]
- Stanford, J.L.; Grange, J.M. The meaning and structure of species as applied to mycobacteria. Tubercle 1974, 55, 143–152. [Google Scholar] [CrossRef]
- Burdon, R.H. Heat shock and the heat shock proteins. Biochem. J. 1986, 240, 313–324. [Google Scholar] [CrossRef]
- Dudani, A.K.; Gupta, R.S. Immunological characterization of a human homolog of the 65-kilodalton mycobacterial antigen. Infect. Immun. 1989, 57, 2786–2793. [Google Scholar] [CrossRef]
- Deocaris, C.C.; Kaul, S.C.; Wadhwa, R. On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones 2006, 11, 116–128. [Google Scholar] [CrossRef]
- Garsia, R.J.; Hellqvist, L.; Booth, R.J.; Radford, A.J.; Britton, W.J.; Astbury, L.; Trent, R.J.; Basten, A. Homology of the 70-kilodalton antigens from Mycobacterium leprae and Mycobacterium bovis with the Mycobacterium tuberculosis 71-kilodalton antigen and with the conserved heat shock protein 70 of eucaryotes. Infect. Immun. 1989, 57, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Bahr, G.M.; Shaaban, M.A.; Gabriel, M.; al-Shimali, B.; Siddiqui, Z.; Chugh, T.D.; Denath, F.M.; Shahin, A.; Behbehani, K.; Chedid, L.; et al. Improved immunotherapy for pulmonary tuberculosis with Mycobacterium vaccae. Tubercle 1990, 71, 259–266. [Google Scholar] [CrossRef]
- Andersen, P.; Doherty, T.M. The success and failure of BCG—Implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 2005, 3, 656–662. [Google Scholar] [CrossRef]
- Grange, J.M.; Gibson, J.; Osborn, T.W.; Collins, C.H.; Yates, M.D. What is BCG? Tubercle 1983, 64, 129–139. [Google Scholar] [CrossRef]
- Goter-Robinson, C.; Derrick, S.C.; Yang, A.L.; Jeon, B.Y.; Morris, S.L. Protection against an aerogenic Mycobacterium tuberculosis infection in BCG-immunized and DNA-vaccinated mice is associated with early type I cytokine responses. Vaccine 2006, 24, 3522–3529. [Google Scholar] [CrossRef]
- Irwin, S.M.; Izzo, A.A.; Dow, S.W.; Skeiky, Y.A.; Reed, S.G.; Alderson, M.R.; Orme, I.M. Tracking antigen-specific CD8 T lymphocytes in the lungs of mice vaccinated with the Mtb72F polyprotein. Infect. Immun. 2005, 73, 5809–5816. [Google Scholar] [CrossRef]
- Kawai, K.; Miyazaki, J.; Joraku, A.; Nishiyama, H.; Akaza, H. Bacillus Calmette-Guerin (BCG) immunotherapy for bladder cancer: Current understanding and perspectives on engineered BCG vaccine. Cancer Sci. 2013, 104, 22–27. [Google Scholar] [CrossRef]
- Kumar, P.; John, V.; Gupta, A.; Bhaskar, S. Enhanced survival of BCG-stimulated dendritic cells: Involvement of anti-apoptotic proteins and NF-kappaB. Biol. Open 2018, 7, bio032045. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.E. BCG vaccination against tuberculosis and leprosy. Br. Med. Bull. 1988, 44, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Setia, M.S.; Steinmaus, C.; Ho, C.S.; Rutherford, G.W. The role of BCG in prevention of leprosy: A meta-analysis. Lancet. Infect. Dis. 2006, 6, 162–170. [Google Scholar] [CrossRef]
- Trial, T.P. Trial of BCG vaccines in south India for tuberculosis prevention: First report. J. Bull. World Health Organ. 1979, 57, 819–827. [Google Scholar]
- Stanford, J.L.; Shield, M.J.; Rook, G.A. How environmental mycobacteria may predetermine the protective efficacy of BCG. Tubercle 1981, 62, 55–62. [Google Scholar] [CrossRef]
- Lord, R.; Naish, C.; Taylor, C.; Stanford, C.A.; Stanford, C.J.; Chacko, J.G.; Debanbu, V.; Samson, P.D.; Berchmans, J.; Surendran, D.; et al. Skin test studies on close contacts of leprosy patients in India. Int. J. Lepr. Other Mycobact. Dis. 1989, 57, 801–809. [Google Scholar]
- Paul, R.C.; Stanford, J.L.; Misljenovic, O.; Lefering, J. Multiple skin testing of Kenyan schoolchildren with a series of new tuberculins. J. Hyg. 1975, 75, 303–313. [Google Scholar] [CrossRef][Green Version]
- Rook, G.A.; Bahr, G.M.; Stanford, J.L. The effect of two distinct forms of cell-mediated response to mycobacteria on the protective efficacy of BCG. Tubercle 1981, 62, 63–68. [Google Scholar] [CrossRef]
- Koch, R. An address on bacteriological research. Br. Med. J. 1890, 2, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Koch, R. A further communication on a remedy for tuberculosis. Br. Med. J. 1891, 1, 125. [Google Scholar] [CrossRef]
- Rook, G.A.; Hernandez-Pando, R. Immunological and endocrinological characteristics of tuberculosis that provide opportunities for immunotherapeutic intervention. Novartis Found. Symp. 1998, 217, 73–87; discussion 87–98. [Google Scholar] [PubMed]
- Yong, A.J.; Grange, J.M.; Tee, R.D.; Beck, J.S.; Bothamley, G.H.; Kemeny, D.M.; Kardjito, T. Total and anti-mycobacterial IgE levels in serum from patients with tuberculosis and leprosy. Tubercle 1989, 70, 273–279. [Google Scholar] [CrossRef]
- Debré, R.; Bonnet, H. Surinfection du cobaye tuberculoux avant et après l’établissement de l’état allergique. C. R. Séances Société Biol. 1922, 87, 449. [Google Scholar]
- Huebner, R.E. BCG vaccination in the control of tuberculosis. Curr. Top. Microbiol. Immunol. 1996, 215, 263–282. [Google Scholar]
- Mackaness, G.B. The Immunology of Antituberculous Immunity; American Lung Association: Chicago, IL, USA, 1968. [Google Scholar]
- Patel, P.J.; Lefford, M.J. Induction of cell-mediated immunity to Mycobacterium leprae in mice. Infect. Immun. 1978, 19, 87–93. [Google Scholar] [CrossRef]
- Stanford, J.L.; Shield, M.J.; Rook, G.A.W. Mycobacterium leprae, other mycobacteria and a possible vaccine. Int. J. Lepr. 1979, 47, 357. [Google Scholar]
- Stanford, J.L.; Paul, R.C. A preliminary study of the effect of contact with environmental mycobacteria on the pattern of sensitivity to a range of new tuberculins amongst Ugandan adults. Epidemiol. Infect. 1976, 76, 205–214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shepard, C.C.; Draper, P.; Rees, R.J.; Lowe, C. Effect of purification steps on the immunogenicity of Mycobacterium leprae. Br. J. Exp. Pathol. 1980, 61, 376–379. [Google Scholar]
- Stanford, J.L.; Rook, G.A.; Samuel, N.; Madlener, F.; Khamenei, A.A.; Nemati, T.; Modabber, F.; Rees, R.J. Preliminary immunological studies in search of correlates of protective immunity carried out on some Iranian leprosy patients and their families. Lepr. Rev. 1980, 51, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, C.; Gares, M.P.; Inwald, J.; Janssen, R.; Zhang, Y.; Young, D.B.; Hetzel, C.; Lamb, J.R.; Baldwin, S.L.; Orme, I.M.; et al. Induction of a type 1 immune response to a recombinant antigen from Mycobacterium tuberculosis expressed in Mycobacterium vaccae. Infect. Immun. 1997, 65, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Grange, J.M.; Stanford, J.L.; Rook, G.A. Tuberculosis and cancer: Parallels in host responses and therapeutic approaches? Lancet 1995, 345, 1350–1352. [Google Scholar] [CrossRef]
- Hrouda, D.; Baban, B.; Dunsmuir, W.; Kirby, R.; Dalgleish, A.G. Immunotherapy of advanced prostate cancer: A phase I/II trial using Mycobacterium vaccae (SRL172). Brit. J. Urol. 1998, 82, 568–573. [Google Scholar] [CrossRef]
- Hernandez-Pando, R.; Pavon, L.; Arriaga, K.; Orozco, H.; Madrid-Marina, V.; Rook, G. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte before infection. Infect. Immun. 1997, 65, 3317–3327. [Google Scholar] [CrossRef]
- Hernandez-Pando, R.; Orozcoe, H.; Sampieri, A.; Pavon, L.; Velasquillo, C.; Larriva-Sahd, J.; Alcocer, J.M.; Madrid, M.V. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology 1996, 89, 26–33. [Google Scholar]
- Hernandez-Pando, R.; Rook, G.A. The role of TNF-alpha in T-cell-mediated inflammation depends on the Th1/Th2 cytokine balance. Immunology 1994, 82, 591–595. [Google Scholar]
- Bechelli, L.M.; Lwin, K.; Gallego Garbajosa, P.; Mg Mg, G.; Uemura, K.; Sundaresan, T.; Tamondong, C.; Matejka, M.; Sansarricq, H.; Walter, J. BCG vaccination of children against leprosy: Nine-year findings of the controlled WHO trial in Burma. Bull. World Health Organ. 1974, 51, 93–99. [Google Scholar] [PubMed]
- Bloom, B.R.; Atun, R.; Cohen, T.; Dye, C.; Fraser, H.; Gomez, G.B.; Knight, G.; Murray, M.; Nardell, E.; Rubin, E.; et al. Tuberculosis. In Major Infectious Diseases; Holmes, K.K., Bertozzi, S., Bloom, B.R., Jha, P., Eds.; The International Bank for Reconstruction and Development/The World Bank © 2021; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017. [Google Scholar]
- Nunn, A.J.; Phillips, P.P.J.; Meredith, S.K.; Chiang, C.Y.; Conradie, F.; Dalai, D.; van Deun, A.; Dat, P.T.; Lan, N.; Master, I.; et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N. Engl. J. Med. 2019, 380, 1201–1213. [Google Scholar] [CrossRef]
- Almeida Da Silva, P.E.; Palomino, J.C. Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis: Classical and new drugs. J. Antimicrob. Chemother. 2011, 66, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Roca, F.J.; Whitworth, L.J.; Redmond, S.; Jones, A.A.; Ramakrishnan, L. TNF Induces pathogenic programmed macrophage necrosis in tuberculosis through a mitochondrial-lysosomal-endoplasmic reticulum circuit. Cell 2019, 178, 1344–1361. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Isenberg, D.A. Mycobacteria and autoimmunity. Immunol. Today 1988, 9, 178–182. [Google Scholar] [CrossRef]
- Filley, E.A.; Bull, H.A.; Dowd, P.M.; Rook, G.A. The effect of Mycobacterium tuberculosis on the susceptibility of human cells to the stimulatory and toxic effects of tumour necrosis factor. Immunology 1992, 77, 505–509. [Google Scholar]
- Filley, E.A.; Rook, G.A. Effect of mycobacteria on sensitivity to the cytotoxic effects of tumor necrosis factor. Infect. Immun. 1991, 59, 2567–2572. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.; Stanford, J. The Koch phenomenon and the immunopathology of tuberculosis. In Tuberculosis; Springer: Berlin/Heidelberg, Germany, 1996; pp. 239–262. [Google Scholar]
- Karopoulos, C.; Rowley, M.J.; Handley, C.J.; Strugnell, R.A. Antibody reactivity to mycobacterial 65 kDa heat shock protein: Relevance to autoimmunity. J. Autoimmun. 1995, 8, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Stanford, J.; Stanford, C.; Grange, J. Immunotherapy with Mycobacterium vaccae in the treatment of tuberculosis. Front. Biosci. 2004, 9, 1701–1719. [Google Scholar] [CrossRef][Green Version]
- Hernandez-Pando, R.; Pavon, L.; Orozco, E.; Rangel, J.; Rook, G. Interactions between hormone-mediated and vaccine-mediated immunotherapy for pulmonary tuberculosis in BALB/c mice. Immunology 2000, 100, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.G.; Chambers, M.A.; Wangoo, A.; Shaw, R.J.; Young, D.B. Production of tumor necrosis factor and nitric oxide by macrophages infected with live and dead mycobacteria and their suppression by an interleukin-10-secreting recombinant. Infect. Immun. 1997, 65, 1931–1935. [Google Scholar] [CrossRef]
- Corlan, E.; Marica, C.; Macavei, C.; Stanford, J.L.; Stanford, C.A. Immunotherapy with Mycobacterium vaccae in the treatment of tuberculosis in Romania. 1. Newly-diagnosed pulmonary disease. Respir. Med. 1997, 91, 13–19. [Google Scholar] [CrossRef][Green Version]
- Gebert, A.; Rothkotter, H.J.; Pabst, R. M cells in Peyer’s patches of the intestine. Int. Rev. Cytol. 1996, 167, 91–159. [Google Scholar]
- Fujimura, Y. Functional morphology of microfold cells (M cells) in Peyer’s patches--phagocytosis and transport of BCG by M cells into rabbit Peyer’s patches. Gastroenterol. Jpn. 1986, 21, 325–335. [Google Scholar] [CrossRef]
- Golub, J.E.; Saraceni, V.; Cavalcante, S.C.; Pacheco, A.G.; Moulton, L.H.; King, B.S.; Efron, A.; Moore, R.D.; Chaisson, R.E.; Durovni, B. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. Aids 2007, 21, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Havlir, D.V.; Getahun, H.; Sanne, I.; Nunn, P. Opportunities and challenges for HIV care in overlapping HIV and TB epidemics. JAMA 2008, 300, 423–430. [Google Scholar] [PubMed]
- Lawn, S.D.; Myer, L.; Bekker, L.G.; Wood, R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: Impact on treatment outcomes and implications for tuberculosis control. Aids 2006, 20, 1605–1612. [Google Scholar] [CrossRef]
- Whalen, C.C.; Johnson, J.L.; Okwera, A.; Hom, D.L.; Huebner, R.; Mugyenyi, P.; Mugerwa, R.D.; Ellner, J.J. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. Uganda-Case Western Reserve University Research Collaboration. N. Engl. J. Med. 1997, 337, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Ansari, N.A.; Kombe, A.H.; Kenyon, T.A.; Hone, N.M.; Tappero, J.W.; Nyirenda, S.T.; Binkin, N.J.; Lucas, S.B. Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997–1998. Int. J. Tuberc. Lung Dis. 2002, 6, 55–63. [Google Scholar]
- Immunotherapy with Mycobacterium vaccae in patients with newly diagnosed pulmonary tuberculosis: A randomised controlled trial. Durban Immunotherapy Trial Group. Lancet 1999, 354, 116–119. [CrossRef]
- Waddell, R.D.; Chintu, C.; Lein, A.D.; Zumla, A.; Karagas, M.R.; Baboo, K.S.; Habbema, J.D.; Tosteson, A.N.; Morin, P.; Tvaroha, S.; et al. Safety and immunogenicity of a five-dose series of inactivated Mycobacterium vaccae vaccination for the prevention of HIV-associated tuberculosis. Clin. Infect. Dis. 2000, 30 (Suppl. 3), S309–S315. [Google Scholar] [CrossRef][Green Version]
- von Reyn, C.F.; Arbeit, R.D.; Yeaman, G.; Waddell, R.D.; Marsh, B.J.; Morin, P.; Modlin, J.F.; Remold, H.G. Immunization of healthy adult subjects in the United States with inactivated Mycobacterium vaccae administered in a three-dose series. Clin. Infect. Dis. 1997, 24, 843–848. [Google Scholar] [CrossRef][Green Version]
- von Reyn, C.F.; Marsh, B.J.; Waddell, R.; Lein, A.D.; Tvaroha, S.; Morin, P.; Modlin, J.F. Cellular immune responses to mycobacteria in healthy and human immunodeficiency virus-positive subjects in the United States after a five-dose schedule of Mycobacterium vaccae vaccine. Clin. Infect. Dis. 1998, 27, 1517–1520. [Google Scholar] [CrossRef][Green Version]
- Floyd, S.; Ponnighaus, J.M.; Bliss, L.; Warndorff, D.K.; Kasunga, A.; Mogha, P.; Fine, P.E. BCG scars in northern Malawi: Sensitivity and repeatability of scar reading, and factors affecting scar size. Int. J. Tuberc. Lung Dis. 2000, 4, 1133–1142. [Google Scholar]
- Nigou, J.; Gilleron, M.; Puzo, G. Lipoarabinomannans: From structure to biosynthesis. Biochimie 2003, 85, 153–166. [Google Scholar] [CrossRef]
- Lahey, T.; Laddy, D.; Hill, K.; Schaeffer, J.; Hogg, A.; Keeble, J.; Dagg, B.; Ho, M.M.; Arbeit, R.D.; von Reyn, C.F. Immunogenicity and Protective Efficacy of the DAR-901 Booster Vaccine in a Murine Model of Tuberculosis. PLoS ONE 2016, 11, e0168521. [Google Scholar] [CrossRef]
- Rook, G.A.; Stanford, J.L. The relevance to protection of three forms of delayed skin-test response evoked by m. leprae and other mycobacteria in mice. Correlation with the classical work in the guinea-pig. Parasite Immunol. 1979, 1, 111–123. [Google Scholar] [CrossRef]
- Stanford, J.; Cordess, G.; Rook, G.; Barnass, S.; Lucas, S. Immunotherapy of tuberculosis in mice and guinea pigs. Bull. Int. Union Tuberc. Lung Dis. 1988, 62, 10–11. [Google Scholar]
- Bhandari, J.; Awais, M.; Robbins, B.A.; Gupta, V. Leprosy. In StatPearls; StatPearls Publishing Copyright © 2021; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Mungroo, M.R.; Khan, N.A.; Siddiqui, R. Mycobacterium leprae: Pathogenesis, diagnosis, and treatment options. Microb. Pathog. 2020, 149, 104475. [Google Scholar] [CrossRef] [PubMed]
- Britton, W.J.; Lockwood, D.N. Leprosy. Lancet 2004, 363, 1209–1219. [Google Scholar] [CrossRef]
- Britton, W.J. Immunology of leprosy. Trans. R Soc. Trop Med. Hyg. 1993, 87, 508–514. [Google Scholar] [CrossRef]
- Yamamura, M.; Wang, X.H.; Ohmen, J.D.; Uyemura, K.; Rea, T.H.; Bloom, B.R.; Modlin, R.L. Cytokine patterns of immunologically mediated tissue damage. J. Immunol. 1992, 149, 1470–1475. [Google Scholar] [PubMed]
- Jullien, D.; Sieling, P.A.; Uyemura, K.; Mar, N.D.; Rea, T.H.; Modlin, R.L. IL-15, an immunomodulator of T cell responses in intracellular infection. J. Immunol. 1997, 158, 800–806. [Google Scholar] [PubMed]
- Little, D.; Khanolkar-Young, S.; Coulthart, A.; Suneetha, S.; Lockwood, D.N. Immunohistochemical analysis of cellular infiltrate and gamma interferon, interleukin-12, and inducible nitric oxide synthase expression in leprosy type 1 (reversal) reactions before and during prednisolone treatment. Infect. Immun. 2001, 69, 3413–3417. [Google Scholar] [CrossRef]
- García, V.E.; Uyemura, K.; Sieling, P.A.; Ochoa, M.T.; Morita, C.T.; Okamura, H.; Kurimoto, M.; Rea, T.H.; Modlin, R.L. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J. Immunol. 1999, 162, 6114–6121. [Google Scholar]
- Hernandez-Pando, R.; Aguilar, D.; Orozco, H.; Cortez, Y.; Brunet, L.R.; Rook, G.A. Orally administered Mycobacterium vaccae modulates expression of immunoregulatory molecules in BALB/c mice with pulmonary tuberculosis. Clin. Vaccine Immunol. 2008, 15, 1730–1736. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brennan, P.J. Skin test development in leprosy: Progress with first-generation skin test antigens, and an approach to the second generation. Lepr. Rev. 2000, 71, S50–S54. [Google Scholar] [CrossRef]
- Stanford, J.L. The history and future of vaccination and immunotherapy for leprosy. Trop. Geogr. Med. 1994, 46, 93–107. [Google Scholar] [PubMed]
- Fine, P.E.; Sterne, J.A.; Ponnighaus, J.M.; Rees, R.J. Delayed-type hypersensitivity, mycobacterial vaccines and protective immunity. Lancet 1994, 344, 1245–1249. [Google Scholar] [CrossRef]
- Brown, J.A.; Stone, M.M.B.C.G. vaccination of children against leprosy: First results of a trial in Uganda. Br. Med. J. 1966, 1, 7–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fine, P.E. Variation in protection by BCG: Implications of and for heterologous immunity. Lancet 1995, 346, 1339–1345. [Google Scholar] [CrossRef]
- Ganapati, R.; Revankar, C.R.; Lockwood, D.N.; Wilson, R.C.; Price, J.E.; Ashton, P.; Ashton, L.A.; Holmes, R.M.; Bennett, C.; Stanford, J.L.; et al. A pilot study of three potential vaccines for leprosy in Bombay. Int. J. Lepr. Other Mycobact. Dis. 1989, 57, 33–37. [Google Scholar] [PubMed]
- Ghazi Saidi, K.; Stanford, J.L.; Stanford, C.A.; Dowlati, Y. containing Mycobacterium vaccae and their use in the children of leprosy patients in Iran. Med. J. Islam. Repub. Iran 1994, 8, 87–91. [Google Scholar]
- Ghazi Saidi, K.; Stanford, J.L.; Stanford, C.A.; Dowlati, Y.; Farshchi, Y.; Rook, G.A.; Rees, R.J. Vaccination and skin test studies on children living in villages with differing endemicity for leprosy and tuberculosis. Int. J. Lepr. Mycobact. Dis. Off. Organ. Int. Lepr. Assoc. 1989, 57, 45–53. [Google Scholar]
- Stanford, J.L.; Stanford, C.A.; Ghazi Saidi, K.; Dowlati, Y.; Weiss, S.F.; Farshchi, Y.; Madlener, F.; Rees, R.J. Vaccination and skin test studies on the children of leprosy patients. Int. J. Lepr. Mycobact. Dis. Off. Organ. Int. Lepr. Assoc. 1989, 57, 38–44. [Google Scholar]
- Bahr, G.M.; Stanford, J.L.; Rook, G.A.; Rees, R.J.; Abdelnoor, A.M.; Frayha, G.J. Two potential improvements to BCG and their effect on skin test reactivity in the Lebanon. Tubercle 1986, 67, 205–218. [Google Scholar] [CrossRef]
- Jonk, A.M.; Houben, A.J.; Schaper, N.C.; de Leeuw, P.W.; Serne, E.H.; Smulders, Y.M.; Stehouwer, C.D. Meal-related increases in microvascular vasomotion are impaired in obese individuals: A potential mechanism in the pathogenesis of obesity-related insulin resistance. Diabetes Care 2011, 34, S342–S348. [Google Scholar] [CrossRef]
- Kraemer-Aguiar, L.G.; Laflor, C.M.; Bouskela, E. Skin microcirculatory dysfunction is already present in normoglycemic subjects with metabolic syndrome. Metab. Clin. Exp. 2008, 57, 1740–1746. [Google Scholar] [CrossRef]
- Shepard, C.C. Temperature optimum of Mycobacterium leprae in mice. J. Bacteriol. 1965, 90, 1271–1275. [Google Scholar] [CrossRef]
- Gottlieb, A.B. Immunologic mechanisms in psoriasis. J. Investig. Dermatol. 1990, 95, 18S–19S. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, clinical presentation, and Treatment of psoriasis: A review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Bata-Csorgo, Z.; Hammerberg, C.; Voorhees, J.J.; Cooper, K.D. Intralesional T-lymphocyte activation as a mediator of psoriatic epidermal hyperplasia. J. Investig. Dermatol. 1995, 105, 89S–94S. [Google Scholar] [CrossRef]
- Wong, R.L.; Winslow, C.M.; Cooper, K.D. The mechanisms of action of cyclosporin A in the treatment of psoriasis. Immunol. Today 1993, 14, 69–74. [Google Scholar] [CrossRef]
- Ramu, G.; Prema, G.; Balakrishnan, S.; Shanker Narayan, N.; Stanford, J.J.I.M.G. A preliminary report on the immunotherapy of psoriasis. Indian Med. Gaz. 1990, 124, 381–382. [Google Scholar]
- Fredriksson, T.; Pettersson, U.J.D. Severe psoriasis–oral therapy with a new retinoid. Dermatology 1978, 157, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Ettehadi, P.; Greaves, M.W.; Wallach, D.; Aderka, D.; Camp, R.D. Elevated tumour necrosis factor-alpha (TNF-alpha) biological activity in psoriatic skin lesions. Clin. Exp. Immunol. 1994, 96, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, B.J.; Karabin, G.D.; Barker, J.N.; Griffiths, C.E.; Sarma, V.; Mitra, R.S.; Elder, J.T.; Kunkel, S.L.; Dixit, V.M. Cellular localization of interleukin-8 and its inducer, tumor necrosis factor-alpha in psoriasis. Am. J. Pathol. 1991, 138, 129–140. [Google Scholar]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Williams, H.C. Atopic Dermatitis: The Epidemiology, Causes and Prevention of Atopic Eczema; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Von Mutius, E. The environmental predictors of allergic disease. J. Allergy Clin. Immunol. 2000, 105, 9–19. [Google Scholar] [CrossRef]
- Romagnani, S. The role of lymphocytes in allergic disease. J. Allergy Clin. Immunol. 2000, 105, 399–408. [Google Scholar] [CrossRef]
- Stead, W.W.J.C.i.c.m. The origin and erratic global spread of tuberculosis: How the past explains the present and is the key to the future. Clin. Chest Med. 1997, 18, 65–77. [Google Scholar] [CrossRef]
- Tocque, K.; Bellis, M.A.; Tam, C.M.; Chan, S.L.; Syed, Q.; Remmington, T.; Davies, P.D. Long-term trends in tuberculosis: Comparison of age-cohort data between Hong Kong and England and Wales. Am. J. Respir. Crit. Care Med. 1998, 158, 484–488. [Google Scholar] [CrossRef]
- Shirakawa, T.; Enomoto, T.; Shimazu, S.-I.; Hopkin, J.M. The inverse association between tuberculin responses and atopic disorder. Science 1997, 275, 77–79. [Google Scholar] [CrossRef]
- Berth-Jones, J. Six area, six sign atopic dermatitis (SASSAD) severity score: A simple system for monitoring disease activity in atopic dermatitis. Br. J. Derm. 1996, 135, 25–30. [Google Scholar] [CrossRef]
- Schaible, U.E.; Hagens, K.; Fischer, K.; Collins, H.L.; Kaufmann, S.H. Intersection of group I CD1 molecules and mycobacteria in different intracellular compartments of dendritic cells. J. Immunol. 2000, 164, 4843–4852. [Google Scholar] [CrossRef]
- Strannegard, I.L.; Larsson, L.O.; Wennergren, G.; Strannegard, O. Prevalence of allergy in children in relation to prior BCG vaccination and infection with atypical mycobacteria. Allergy 1998, 53, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.S.; Hamid, Q.; Ying, S.; Tsicopoulos, A.; Barkans, J.; Bentley, A.M.; Corrigan, C.; Durham, S.R.; Kay, A.B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 1992, 326, 298–304. [Google Scholar] [CrossRef]
- Romagnani, S. Regulation of the development of type 2 T-helper cells in allergy. Curr. Opin. Immunol. 1994, 6, 838–846. [Google Scholar] [CrossRef]
- To, T.; Stanojevic, S.; Moores, G.; Gershon, A.S.; Bateman, E.D.; Cruz, A.A.; Boulet, L.P. Global asthma prevalence in adults: Findings from the cross-sectional world health survey. Bmc Public Health 2012, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Cabieses, B.; Uphoff, E.; Pinart, M.; Anto, J.M.; Wright, J. A systematic review on the development of asthma and allergic diseases in relation to international immigration: The leading role of the environment confirmed. PLoS ONE 2014, 9, e105347. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Björkstén, B. Risk factors in early childhood for the development of atopic diseases. Allergy 1994, 49, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.G. Environmental factors and primary T-cell sensitisation to inhalant allergens in infancy: Reappraisal of the role of infections and air pollution. Pediatric Allergy Immunol. 1995, 6, 1–10. [Google Scholar] [CrossRef]
- Holt, P.G. Infections and the development of allergy. Toxicol. Lett. 1996, 86, 205–210. [Google Scholar] [CrossRef]
- Matricardi, P.M.; Rosmini, F.; Riondino, S.; Fortini, M.; Ferrigno, L.; Rapicetta, M.; Bonini, S. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: Epidemiological study. BMJ 2000, 320, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Twentyman, O.P.; Finnerty, J.P.; Harris, A.; Palmer, J.; Holgate, S.T. Protection against allergen-induced asthma by salmeterol. Lancet 1990, 336, 1338–1342. [Google Scholar] [CrossRef]
- Carlson, H.; Bell, E.T. A statistical study of the occurrence of cancer and tuberculosis in 11,195 postmortem examinations. J. Cancer Res. 1929, 13, 126–135. [Google Scholar]
- Pearl, R. Cancer and tuberculosis. Am. J. Hyg. 1929, 9, 97–159. [Google Scholar] [CrossRef]
- Grange, J.M.; Stanford, J.L. BCG vaccination and cancer. Tubercle 1990, 71, 61–64. [Google Scholar] [CrossRef]
- Alexandroff, A.B.; Jackson, A.M.; O’Donnell, M.A.; James, K. BCG immunotherapy of bladder cancer: 20 years on. Lancet 1999, 353, 1689–1694. [Google Scholar] [CrossRef]
- Krone, B.; Kölmel, K.F.; Henz, B.M.; Grange, J.M. Protection against melanoma by vaccination with Bacille Calmette-Guerin (BCG) and/or vaccinia: An epidemiology-based hypothesis on the nature of a melanoma risk factor and its immunological control. Eur. J. Cancer 2005, 41, 104–117. [Google Scholar] [CrossRef]
- Kölmel, K.; Grange, J.; Krone, B.; Mastrangelo, G.; Rossi, C.; Henz, B.; Seebacher, C.; Botev, I.; Niin, M.; Lambert, D. Prior immunisation of patients with malignant melanoma with vaccinia or BCG is associated with better survival. An European Organization for Research and Treatment of Cancer cohort study on 542 patients. Eur. J. Cancer 2005, 41, 118–125. [Google Scholar] [CrossRef]
- Assersohn, L.; Souberbielle, B.E.; O’Brien, M.E.; Archer, C.D.; Mendes, R.; Bass, R.; Bromelow, K.V.; Palmer, R.D.; Bouilloux, E.; Kennard, D.A.; et al. A randomized pilot study of SRL172 (Mycobacterium vaccae) in patients with small cell lung cancer (SCLC) treated with chemotherapy. Clin. Oncol. 2002, 14, 23–27. [Google Scholar] [CrossRef]
- Dalgleish, A.G.; Stebbing, J.; Adamson, D.J.; Arif, S.S.; Bidoli, P.; Chang, D.; Cheeseman, S.; Diaz-Beveridge, R.; Fernandez-Martos, C.; Glynne-Jones, R.; et al. Randomised, open-label, phase II study of gemcitabine with and without IMM-101 for advanced pancreatic cancer. Br. J. Cancer 2016, 115, 789–796. [Google Scholar] [CrossRef]
- Michopoulos, V.; Powers, A.; Gillespie, C.F.; Ressler, K.J.; Jovanovic, T. Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 2017, 42, 254–270. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Raison, C.L.; Rook, G.W.; Miller, A.H.; Begay, T.K. Role of inflammation in psychiatric disease. In Neurobiology of Brain Disorders; Zigmond, M.J., Rowland, L.P., Coyle, J.T., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 396–421. [Google Scholar]
- Schultebraucks, K.; Qian, M.; Abu-Amara, D.; Dean, K.; Laska, E.; Siegel, C.; Gautam, A.; Guffanti, G.; Hammamieh, R.; Misganaw, B.; et al. Pre-deployment risk factors for PTSD in active-duty personnel deployed to Afghanistan: A machine-learning approach for analyzing multivariate predictors. Mol. Psychiatry 2021, 26, 5011–5022. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.D.; Georgiou, P.; Brenner, L.A.; Brundin, L.; Can, A.; Courtet, P.; Donaldson, Z.R.; Dwivedi, Y.; Guillaume, S.; Gottesman, I.I.; et al. Animal models to improve our understanding and treatment of suicidal behavior. Transl. Psychiatry 2017, 7, e1092. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.; Teli, S.; Rijal, J.; Bhat, H.; Raza, M.; Khoueiry, G.; Meghani, M.; Akhtar, M.; Costantino, T. Neutrophil to lymphocyte ratio and cardiovascular diseases: A review. Expert Rev. Cardiovasc. Ther. 2013, 11, 55–59. [Google Scholar] [CrossRef]
- Everds, N.E.; Snyder, P.W.; Bailey, K.L.; Bolon, B.; Creasy, D.M.; Foley, G.L.; Rosol, T.J.; Sellers, T. Interpreting stress responses during routine toxicity studies: A review of the biology, impact, and assessment. Toxicol. Pathol. 2013, 41, 560–614. [Google Scholar] [CrossRef]
- Liang, M.; Du, B.; Zhang, H.; Lu, X.; Chen, C.; Fan, C.; Bi, X. NLR Is associated with geriatric depression in chinese women: A community-based cross-sectional study in eastern China. Front. Psychol. 2019, 10, 2941. [Google Scholar] [CrossRef]
- Lynall, M.E.; Turner, L.; Bhatti, J.; Cavanagh, J.; de Boer, P.; Mondelli, V.; Jones, D.; Drevets, W.C.; Cowen, P.; Harrison, N.A.; et al. Peripheral blood cell-stratified subgroups of inflamed depression. Biol. Psychiatry 2020, 88, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Sutin, A.R.; Milaneschi, Y.; Cannas, A.; Ferrucci, L.; Uda, M.; Schlessinger, D.; Zonderman, A.B.; Terracciano, A. Impulsivity-related traits are associated with higher white blood cell counts. J. Behav. Med. 2012, 35, 616–623. [Google Scholar] [CrossRef]
- Ekinci, O.; Ekinci, A. The connections among suicidal behavior, lipid profile and low-grade inflammation in patients with major depressive disorder: A specific relationship with the neutrophil-to-lymphocyte ratio. Nord. J. Psychiatry 2017, 71, 574–580. [Google Scholar] [CrossRef]
- Ivkovic, M.; Pantovic-Stefanovic, M.; Dunjic-Kostic, B.; Jurisic, V.; Lackovic, M.; Totic-Poznanovic, S.; Jovanovic, A.A.; Damjanovic, A. Neutrophil-to-lymphocyte ratio predicting suicide risk in euthymic patients with bipolar disorder: Moderatory effect of family history. Compr. Psychiatry 2016, 66, 87–95. [Google Scholar] [CrossRef]
- Orum, M.H.; Kara, M.Z.; Egilmez, O.B. Relationship between immune cells and alcohol dependents and controls: What about the lymphocyte-related ratios? J. Immunoass. Immunochem. 2018, 39, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.; Rodriguez-Revuelta, J.; Olie, E.; Abad, I.; Fernandez-Pelaez, A.; Cazals, A.; Guillaume, S.; de la Fuente-Tomas, L.; Jimenez-Trevino, L.; Gutierrez, L.; et al. Neutrophil-to-lymphocyte ratio: A potential new peripheral biomarker of suicidal behavior. Eur. Psychiatry 2020, 63, e14. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, Y.J.; Park, B. Higher monocyte count with normal white blood cell count is positively associated with 10-year cardiovascular disease risk determined by Framingham risk score among community-dwelling Korean individuals. Medicine 2019, 98, e15340. [Google Scholar] [CrossRef] [PubMed]
- Cherfane, C.E.; Gessel, L.; Cirillo, D.; Zimmerman, M.B.; Polyak, S. Monocytosis and a low lymphocyte to monocyte ratio are effective biomarkers of ulcerative colitis disease activity. Inflamm. Bowel Dis. 2015, 21, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Nowak, W.; Grendas, L.N.; Sanmarco, L.M.; Estecho, I.G.; Arena, A.R.; Eberhardt, N.; Rodante, D.E.; Aoki, M.P.; Daray, F.M.; Carrera Silva, E.A.; et al. Pro-inflammatory monocyte profile in patients with major depressive disorder and suicide behaviour and how ketamine induces anti-inflammatory M2 macrophages by NMDAR and mTOR. EBioMedicine 2019, 50, 290–305. [Google Scholar] [CrossRef]
- Schiweck, C.; Claes, S.; Van Oudenhove, L.; Lafit, G.; Vaessen, T.; de Beeck, G.O.; Berghmans, R.; Wijkhuijs, A.; Muller, N.; Arolt, V.; et al. Childhood trauma, suicide risk and inflammatory phenotypes of depression: Insights from monocyte gene expression. Transl. Psychiatry 2020, 10, 296. [Google Scholar] [CrossRef]
- Serafini, G.; Parisi, V.M.; Aguglia, A.; Amerio, A.; Sampogna, G.; Fiorillo, A.; Pompili, M.; Amore, M. A specific inflammatory profile underlying suicide risk? Systematic review of the main literature findings. Int. J. Environ. Res. Public Health 2020, 17, 2393. [Google Scholar] [CrossRef]
- Foertsch, S.; Reber, S.O. The role of physical trauma in social stress-induced immune activation. Neurosci. Biobehav. Rev. 2020, 113, 169–178. [Google Scholar] [CrossRef]
- Hodes, G.E.; Pfau, M.L.; Leboeuf, M.; Golden, S.A.; Christoffel, D.J.; Bregman, D.; Rebusi, N.; Heshmati, M.; Aleyasin, H.; Warren, B.L.; et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. USA 2014, 111, 16136–16141. [Google Scholar] [CrossRef]
- McKim, D.B.; Patterson, J.M.; Wohleb, E.S.; Jarrett, B.L.; Reader, B.F.; Godbout, J.P.; Sheridan, J.F. Sympathetic release of splenic monocytes promotes recurring anxiety following repeated social defeat. Biol. Psychiatry 2016, 79, 803–813. [Google Scholar] [CrossRef]
- Niraula, A.; Witcher, K.G.; Sheridan, J.F.; Godbout, J.P. Interleukin-6 induced by social stress promotes a unique transcriptional signature in the monocytes that facilitate anxiety. Biol. Psychiatry 2019, 85, 679–689. [Google Scholar] [CrossRef]
- Wohleb, E.S.; McKim, D.B.; Shea, D.T.; Powell, N.D.; Tarr, A.J.; Sheridan, J.F.; Godbout, J.P. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol. Psychiatry 2014, 75, 970–981. [Google Scholar] [CrossRef] [PubMed]
- Wohleb, E.S.; Patterson, J.M.; Sharma, V.; Quan, N.; Godbout, J.P.; Sheridan, J.F. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J. Neurosci. 2014, 34, 2583–2591. [Google Scholar] [CrossRef]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, K.; Salgame, P. Host innate immune response to Mycobacterium tuberculosis. J. Clin. Immunol. 2007, 27, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.L.; Chan, J. Immunology of tuberculosis. Annu. Rev. Immunol. 2001, 19, 93–129. [Google Scholar] [CrossRef]
- Drage, M.G.; Pecora, N.D.; Hise, A.G.; Febbraio, M.; Silverstein, R.L.; Golenbock, D.T.; Boom, W.H.; Harding, C.V. TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell. Immunol. 2009, 258, 29–37. [Google Scholar] [CrossRef]
- Krutzik, S.R.; Ochoa, M.T.; Sieling, P.A.; Uematsu, S.; Ng, Y.W.; Legaspi, A.; Liu, P.T.; Cole, S.T.; Godowski, P.J.; Maeda, Y.; et al. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat. Med. 2003, 9, 525–532. [Google Scholar] [CrossRef]
- Sweet, L.; Schorey, J.S. Glycopeptidolipids from Mycobacterium avium promote macrophage activation in a TLR2- and MyD88-dependent manner. J. Leukoc. Biol. 2006, 80, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, Y.; Municio, C.; Alonso, S.; Sanchez Crespo, M.; Fernandez, N. The induction of IL-10 by zymosan in dendritic cells depends on CREB activation by the coactivators CREB-binding protein and TORC2 and autocrine PGE2. J. Immunol. 2009, 183, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Lamichhane, G.; Gupta, R.; Nolan, S.; Bishai, W.R. Cyclic AMP intoxication of macrophages by a Mycobacterium tuberculosis adenylate cyclase. Nature 2009, 460, 98–102. [Google Scholar] [CrossRef]
- Roach, S.K.; Lee, S.B.; Schorey, J.S. Differential activation of the transcription factor cyclic AMP response element binding protein (CREB) in macrophages following infection with pathogenic and nonpathogenic mycobacteria and role for CREB in tumor necrosis factor alpha production. Infect. Immun. 2005, 73, 514–522. [Google Scholar] [CrossRef]
- Samten, B.; Howard, S.T.; Weis, S.E.; Wu, S.; Shams, H.; Townsend, J.C.; Safi, H.; Barnes, P.F. Cyclic AMP response element-binding protein positively regulates production of IFN-gamma by T cells in response to a microbial pathogen. J. Immunol. 2005, 174, 6357–6363. [Google Scholar] [CrossRef]
- Strygin, A.V.; Nesmiyanov, P.P.; Petrov, V.I.; Tolkachev, B.E.; Morkovin, E.I.; Gutov, M.V.; Strygina, A.O. Mycobacterium vaccae lysate induces anti-allergic immune response in vitro. Bull. Exp. Biol. Med. 2020, 170, 226–229. [Google Scholar] [CrossRef]
- Li, H.; Lebedeva, M.I.; Llera, A.S.; Fields, B.A.; Brenner, M.B.; Mariuzza, R.A. Structure of the Vdelta domain of a human gammadelta T-cell antigen receptor. Nature 1998, 391, 502–506. [Google Scholar] [CrossRef]
- Lawand, M.; Dechanet-Merville, J.; Dieu-Nosjean, M.C. Key features of gamma-delta T-cell subsets in human diseases and their immunotherapeutic implications. Front. Immunol. 2017, 8, 761. [Google Scholar] [CrossRef] [PubMed]
- Girardi, M.; Oppenheim, D.E.; Steele, C.R.; Lewis, J.M.; Glusac, E.; Filler, R.; Hobby, P.; Sutton, B.; Tigelaar, R.E.; Hayday, A.C. Regulation of cutaneous malignancy by gammadelta T cells. Science 2001, 294, 605–609. [Google Scholar] [CrossRef]
- Liu, Z.; Eltoum, I.E.; Guo, B.; Beck, B.H.; Cloud, G.A.; Lopez, R.D. Protective immunosurveillance and therapeutic antitumor activity of gammadelta T cells demonstrated in a mouse model of prostate cancer. J. Immunol. 2008, 180, 6044–6053. [Google Scholar] [CrossRef]
- Garrido, F.; Algarra, I.; García-Lora, A.M. The escape of cancer from T lymphocytes: Immunoselection of MHC class I loss variants harboring structural-irreversible “hard” lesions. Cancer Immunol. Immunother. 2010, 59, 1601–1606. [Google Scholar] [CrossRef]
- Morita, C.T.; Jin, C.; Sarikonda, G.; Wang, H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: Discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol. Rev. 2007, 215, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Chen, Y.Q.; Gercken, J.; Ernst, M.; Bohle, A.; Flad, H.D.; Ulmer, A.J. Specific activation of human peripheral blood gamma/delta + lymphocytes by sonicated antigens of Mycobacterium tuberculosis: Role in vitro in killing human bladder carcinoma cell lines. Scand. J. Immunol. 1993, 38, 239–246. [Google Scholar] [CrossRef]
- Hallermalm, K.; Seki, K.; Wei, C.; Castelli, C.; Rivoltini, L.; Kiessling, R.; Levitskaya, J. Tumor necrosis factor-alpha induces coordinated changes in major histocompatibility class I presentation pathway, resulting in increased stability of class I complexes at the cell surface. Blood 2001, 98, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, J.M.; Bixby, D.L.; Borowski, C.; Yannelli, J.R. Characterization of human non-small cell lung cancer (NSCLC) cell lines for expression of MHC, co-stimulatory molecules and tumor-associated antigens. Lung Cancer 2001, 33, 181–194. [Google Scholar] [CrossRef]
- Dormond, O.; Lejeune, F.J.; Ruegg, C. Modulation of cdk2, cyclin D1, p16INK4a, p21WAF and p27Kip1 expression in endothelial cells by TNF/IFN gamma. Anticancer Res. 2002, 22, 3159–3163. [Google Scholar]
- Egwuagu, C.E.; Li, W.; Yu, C.R.; Che Mei Lin, M.; Chan, C.C.; Nakamura, T.; Chepelinsky, A.B. Interferon-gamma induces regression of epithelial cell carcinoma: Critical roles of IRF-1 and ICSBP transcription factors. Oncogene 2006, 25, 3670–3679. [Google Scholar] [CrossRef]
- Bradley, L.M.; Dalton, D.K.; Croft, M. A direct role for IFN-gamma in regulation of Th1 cell development. J. Immunol. 1996, 157, 1350–1358. [Google Scholar] [PubMed]
- Prevost-Blondel, A.; Roth, E.; Rosenthal, F.M.; Pircher, H. Crucial role of TNF-alpha in CD8 T cell-mediated elimination of 3LL-A9 Lewis lung carcinoma cells in vivo. J. Immunol. 2000, 164, 3645–3651. [Google Scholar] [CrossRef]
- Alexander, A.A.; Maniar, A.; Cummings, J.S.; Hebbeler, A.M.; Schulze, D.H.; Gastman, B.R.; Pauza, C.D.; Strome, S.E.; Chapoval, A.I. Isopentenyl pyrophosphate-activated CD56+ {gamma}{delta} T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin. Cancer Res. 2008, 14, 4232–4240. [Google Scholar] [CrossRef]
- Gramaglia, I.; Cooper, D.; Miner, K.T.; Kwon, B.S.; Croft, M. Co-stimulation of antigen-specific CD4 T cells by 4-1BB ligand. Eur. J. Immunol. 2000, 30, 392–402. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Sundseth, S.S.; Jones, S.A.; Brown, P.J.; Wisely, G.B.; Koble, C.S.; Devchand, P.; Wahli, W.; Willson, T.M.; Lenhard, J.M.; et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc. Natl. Acad. Sci. USA 1997, 94, 4318–4323. [Google Scholar] [CrossRef]
- Kota, B.P.; Huang, T.H.; Roufogalis, B.D. An overview on biological mechanisms of PPARs. Pharm. Res. 2005, 51, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Luongo, L.; Boccella, S.; Giordano, M.E.; Romano, R.; Bellini, G.; Manzo, I.; Furiano, A.; Rizzo, A.; Imperatore, R.; et al. Palmitoylethanolamide induces microglia changes associated with increased migration and phagocytic activity: Involvement of the CB2 receptor. Sci Rep. 2017, 7, 375. [Google Scholar] [CrossRef]
- Lo Verme, J.; Fu, J.; Astarita, G.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharm. 2005, 67, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Wuchty, S.; Myers, A.J.; Ramirez-Restrepo, M.; Huentelman, M.; Richolt, R.; Gould, F.; Harvey, P.D.; Michopolous, V.; Steven, J.S.; Wingo, A.P.; et al. Integration of peripheral transcriptomics, genomics, and interactomics following trauma identifies causal genes for symptoms of post-traumatic stress and major depression. Mol. Psychiatry 2021, 26, 3077–3092. [Google Scholar] [CrossRef]
- Flesch, I.E.; Hess, J.H.; Huang, S.; Aguet, M.; Rothe, J.; Bluethmann, H.; Kaufmann, S.H. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon gamma and tumor necrosis factor alpha. J. Exp. Med. 1995, 181, 1615–1621. [Google Scholar] [CrossRef]
- Zuany-Amorim, C.; Manlius, C.; Trifilieff, A.; Brunet, L.R.; Rook, G.; Bowen, G.; Pay, G.; Walker, C. Long-term protective and antigen-specific effect of heat-killed Mycobacterium vaccae in a murine model of allergic pulmonary inflammation. J. Immunol. 2002, 169, 1492–1499. [Google Scholar] [CrossRef]
- Akbari, O.; DeKruyff, R.H.; Umetsu, D.T. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2001, 2, 725–731. [Google Scholar] [CrossRef]
- Akbari, O.; Freeman, G.J.; Meyer, E.H.; Greenfield, E.A.; Chang, T.T.; Sharpe, A.H.; Berry, G.; DeKruyff, R.H.; Umetsu, D.T. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 2002, 8, 1024–1032. [Google Scholar] [CrossRef]
- Reber, S.O.; Langgartner, D.; Foertsch, S.; Postolache, T.T.; Brenner, L.A.; Guendel, H.; Lowry, C.A. Chronic subordinate colony housing paradigm: A mouse model for mechanisms of PTSD vulnerability, targeted prevention, and treatment-2016 Curt Richter Award Paper. Psychoneuroendocrinology 2016, 74, 221–230. [Google Scholar] [CrossRef]
- Park, H.J.; Shim, H.S.; An, K.; Starkweather, A.; Kim, K.S.; Shim, I. IL-4 Inhibits IL-1beta-induced depressive-like behavior and central neurotransmitter alterations. Mediat. Inflamm. 2015, 2015, 941413. [Google Scholar] [CrossRef] [PubMed]
- Wachholz, S.; Knorr, A.; Mengert, L.; Plumper, J.; Sommer, R.; Juckel, G.; Friebe, A. Interleukin-4 is a participant in the regulation of depressive-like behavior. Behav. Brain Res. 2017, 326, 165–172. [Google Scholar] [CrossRef]
- Doenni, V.M.; Song, C.M.; Hill, M.N.; Pittman, Q.J. Early-life inflammation with LPS delays fear extinction in adult rodents. Brain Behav. Immun. 2017, 63, 176–185. [Google Scholar] [CrossRef]
- Jones, M.E.; Lebonville, C.L.; Barrus, D.; Lysle, D.T. The role of brain interleukin-1 in stress-enhanced fear learning. Neuropsychopharmacology 2015, 40, 1289–1296. [Google Scholar] [CrossRef]
- Parekh, S.V.; Paniccia, J.E.; Lebonville, C.L.; Lysle, D.T. Dorsal hippocampal interleukin-1 signaling mediates heroin withdrawal-enhanced fear learning. Psychopharmacology 2020, 237, 3653–3664. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.M.; Maldonado, L.; Velazquez, B.; Porter, J.T.J.B. Candesartan ameliorates impaired fear extinction induced by innate immune activation. Brain Behav. Immun. 2016, 52, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Young, M.B.; Howell, L.L.; Hopkins, L.; Moshfegh, C.; Yu, Z.; Clubb, L.; Seidenberg, J.; Park, J.; Swiercz, A.P.; Marvar, P.J. A peripheral immune response to remembering trauma contributes to the maintenance of fear memory in mice. Psychoneuroendocrinology 2018, 94, 143–151. [Google Scholar] [CrossRef]
- Huang, C.Y.; Hsieh, W.Y. Efficacy of Mycobacterium vaccae immunotherapy for patients with tuberculosis: A systematic review and meta-analysis. Hum. Vaccines Immunother. 2017, 13, 1960–1971. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, E.; Iona, E.; Ferroni, L.; Miettinen, M.; Fattorini, L.; Orefici, G.; Julkunen, I.; Coccia, E.M. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J. Immunol. 2001, 166, 7033–7041. [Google Scholar] [CrossRef]
- Sugawara, I.; Yamada, H.; Mizuno, S.; Takeda, K.; Akira, S. Mycobacterial infection in MyD88-deficient mice. Microbiol. Immunol. 2003, 47, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.M.; Friedland, J.S. Regulation of monocyte chemokine and MMP-9 secretion by proinflammatory cytokines in tuberculous osteomyelitis. J. Leukoc. Biol. 2004, 75, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Deguine, J.; Barton, G.M. MyD88: A central player in innate immune signaling. F1000prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef]
- Cervantes, J.L. MyD88 in Mycobacterium tuberculosis infection. Med. Microbiol. Immunol. 2017, 206, 187–193. [Google Scholar] [CrossRef]
- Gu, X.; Gao, Y.; Mu, D.G.; Fu, E.Q. MiR-23a-5p modulates mycobacterial survival and autophagy during mycobacterium tuberculosis infection through TLR2/MyD88/NF-kappaB pathway by targeting TLR2. Exp. Cell Res. 2017, 354, 71–77. [Google Scholar] [CrossRef]
- Coombes, J.L.; Powrie, F. Dendritic cells in intestinal immune regulation. Nat. Rev. Immunol. 2008, 8, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Sato, A.; Fukuyama, S.; Sagara, H.; Nagatake, T.; Kong, I.G.; Goda, K.; Nochi, T.; Kunisawa, J.; Sato, S.; et al. The airway antigen sampling system: Respiratory M cells as an alternative gateway for inhaled antigens. J. Immunol. 2011, 186, 4253–4262. [Google Scholar] [CrossRef] [PubMed]
- Ruane, D.; Brane, L.; Reis, B.S.; Cheong, C.; Poles, J.; Do, Y.; Zhu, H.; Velinzon, K.; Choi, J.H.; Studt, N.; et al. Lung dendritic cells induce migration of protective T cells to the gastrointestinal tract. J. Exp. Med. 2013, 210, 1871–1888. [Google Scholar] [CrossRef]
- Unger, W.W.; Hauet-Broere, F.; Jansen, W.; van Berkel, L.A.; Kraal, G.; Samsom, J.N. Early events in peripheral regulatory T cell induction via the nasal mucosa. J. Immunol. 2003, 171, 4592–4603. [Google Scholar] [CrossRef]
- Huang, C.T.; Workman, C.J.; Flies, D.; Pan, X.; Marson, A.L.; Zhou, G.; Hipkiss, E.L.; Ravi, S.; Kowalski, J.; Levitsky, H.I.; et al. Role of LAG-3 in regulatory T cells. Immunity 2004, 21, 503–513. [Google Scholar] [CrossRef]
- McMenamin, C.; Schon-Hegrad, M.; Oliver, J.; Girn, B.; Holt, P.G. Regulation of IgE responses to inhaled antigens: Cellular mechanisms underlying allergic sensitization versus tolerance induction. Int. Arch. Allergy Appl. Immunol. 1991, 94, 78–82. [Google Scholar] [CrossRef]
- Perdomo, C.; Zedler, U.; Kuhl, A.A.; Lozza, L.; Saikali, P.; Sander, L.E.; Vogelzang, A.; Kaufmann, S.H.; Kupz, A. Mucosal BCG vaccination induces protective lung-resident memory T cell populations against tuberculosis. MBio 2016, 7, e01686-16. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Kelsall, B.L. Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 1999, 190, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Polanski, M.; Melican, N.S.; Zhang, J.; Weiner, H.L. Oral administration of the immunodominant B-chain of insulin reduces diabetes in a co-transfer model of diabetes in the NOD mouse and is associated with a switch from Th1 to Th2 cytokines. J. Autoimmun. 1997, 10, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Groux, H.; O’Garra, A.; Bigler, M.; Rouleau, M.; Antonenko, S.; de Vries, J.E.; Roncarolo, M.G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997, 389, 737–742. [Google Scholar] [CrossRef]
- Chen, Y.; Kuchroo, V.K.; Inobe, J.; Hafler, D.A.; Weiner, H.L. Regulatory T cell clones induced by oral tolerance: Suppression of autoimmune encephalomyelitis. Science 1994, 265, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.M.; Jenks, S.M. Ingestion of Mycobacterium vaccae decreases anxiety-related behavior and improves learning in mice. Behav. Process. 2013, 96, 27–35. [Google Scholar] [CrossRef]
- Cervantes-Barragan, L.; Chai, J.N.; Tianero, M.D.; Di Luccia, B.; Ahern, P.P.; Merriman, J.; Cortez, V.S.; Caparon, M.G.; Donia, M.S.; Gilfillan, S.; et al. Lactobacillus reuteri induces gut intraepithelial CD4+ CD8alphaalpha+ T cells. Science 2017, 357, 806–810. [Google Scholar] [CrossRef]
- Sun, C.M.; Hall, J.A.; Blank, R.B.; Bouladoux, N.; Oukka, M.; Mora, J.R.; Belkaid, Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007, 204, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.L.; Siddiqui, K.R.; Arancibia-Carcamo, C.V.; Hall, J.; Sun, C.M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef]
- Mucida, D.; Cheroutre, H. TGFbeta and retinoic acid intersect in immune-regulation. Cell Adhes. Migr. 2007, 1, 142–144. [Google Scholar] [CrossRef]
- Mucida, D.; Park, Y.; Kim, G.; Turovskaya, O.; Scott, I.; Kronenberg, M.; Cheroutre, H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 2007, 317, 256–260. [Google Scholar] [CrossRef]
- Crooks, J.; Brown, S.; Gauthier, A. The effects of combination treatment of IMM-101, a heat-killed whole cell preparation of Mycobacterium obuense (NCTC 13365) with checkpoint inhibitors in pre-clinical models. In Proceedings of the Annual Meeting of the SITC 2016, National Harbor, MD, USA, 11–13 November 2016; pp. 9–13. [Google Scholar]
- Dominguez-Andres, J.; Joosten, L.A.; Netea, M.G. Induction of innate immune memory: The role of cellular metabolism. Curr. Opin. Immunol. 2019, 56, 10–16. [Google Scholar] [CrossRef]
- Netea, M.G. Training innate immunity: The changing concept of immunological memory in innate host defence. Eur. J. Clin. Investig. 2013, 43, 881–884. [Google Scholar] [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Dalgleish, A.G.; Mudan, S.; Fusi, A. Enhanced effect of checkpoint inhibitors when given after or together with IMM-101: Significant responses in four advanced melanoma patients with no additional major toxicity. J. Transl. Med. 2018, 16, 227. [Google Scholar] [CrossRef]
- Bazzi, S.; Modjtahedi, H.; Mudan, S.; Achkar, M.; Akle, C.; Bahr, G.M. Immunomodulatory effects of heat-killed Mycobacterium obuense on human blood dendritic cells. Innate Immun. 2017, 23, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Crooks, J.; Brown, S.; Forss, C.; Phythian-Adams, A.; Cook, P.; Brunet, L.R.; MacDonald, A. The impact of Mycobacterium obuense on innate and adaptive immunity. Eur. J. Cancer 2016, 1, S72. [Google Scholar] [CrossRef]
- Galdon, A.; Crooks, J.; Brown, S.L.; Kampinga, J.; Brunet, L.R.; MacDonald, A.S. Defining the immunomodulatory effects of IMM-101: A promising, novel co-therapy for cancer. In Proceedings of the CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference, Paris, France, 25–28 September 2019. [Google Scholar]
- ClinicalTrials.gov Immunization with IMM-101 vs. Observation for Prevention of Respiratory and Severe COVID-19 Related Infections in Cancer Patients at Increased Risk of Exposure (COV-IMMUNO): ClinicalTrials.gov Identifier: NCT04442048. Available online: https://clinicaltrials.gov (accessed on 28 July 2021).
- Tsukamura, M.; Mizuno, S. Mycobacterium obuense, a rapidly growing scotochromogenic mycobacterium capable of forming a black product from p-aminosalicylate and salicylate. J. Gen. Microbiol. 1971, 68, 129–134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stone, B.B.; Nietupski, R.M.; Breton, G.L.; Weisburg, W.G. Comparison of Mycobacterium 23S rRNA sequences by high-temperature reverse transcription and PCR. Int. J. Syst. Bacteriol. 1995, 45, 811–819. [Google Scholar] [CrossRef][Green Version]
- Postolache, T.T.; Benros, M.E.; Brenner, L.A. Targetable Biological Mechanisms Implicated in Emergent Psychiatric Conditions Associated with SARS-CoV-2 Infection. JAMA Psychiatry 2020. Available online: https://www.amedeolucente.it/public/Targetable%20Biological%20Mechanisms%20Implicated%20in%20Emergent%20Psychiatric%20Conditions%20Associated%20With%20SARS-CoV-2%20Infection.pdf (accessed on 28 July 2021).
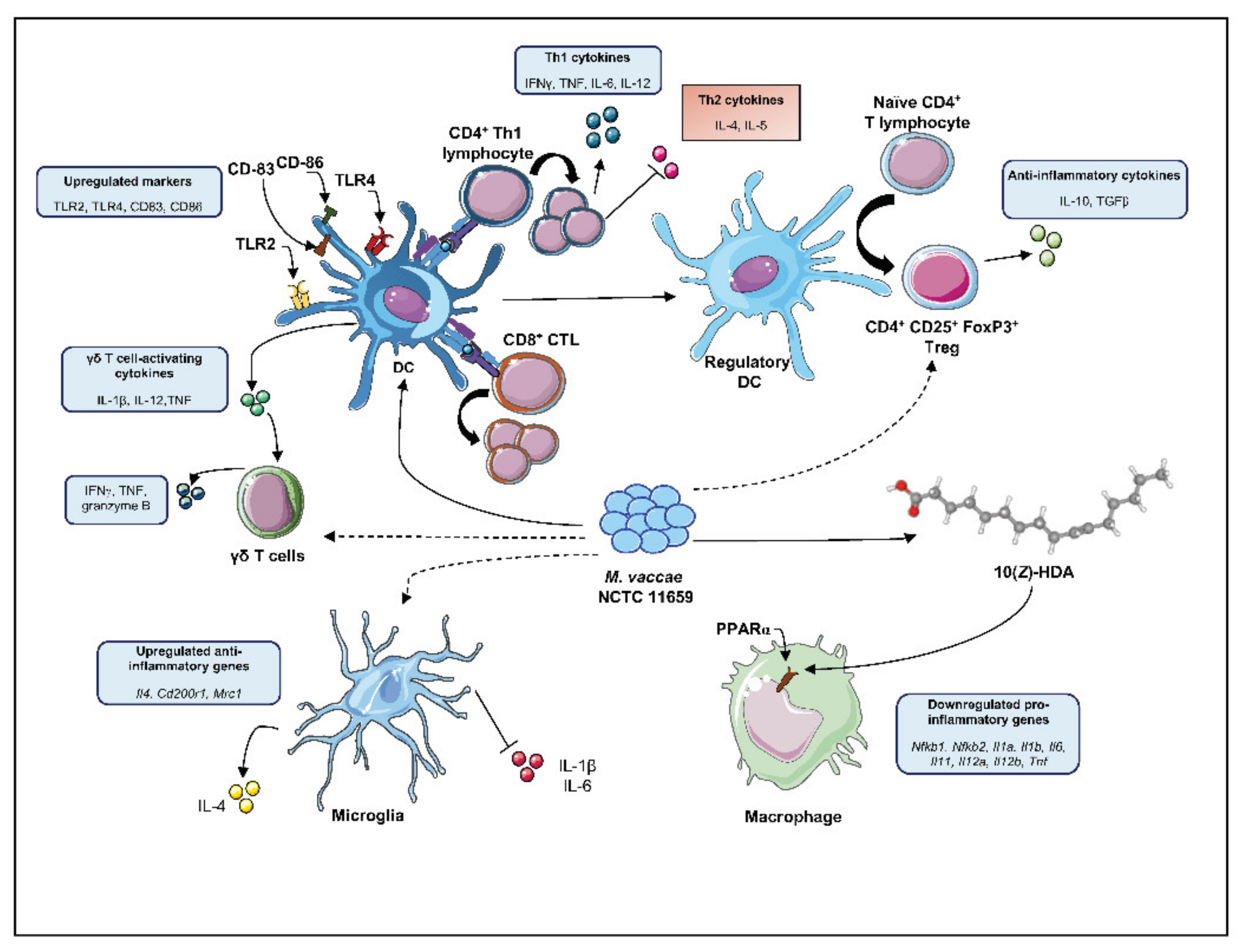
| Reference Strain | Original Source and Year of Isolation | Alternative Collection Numbers | Culture Medium | Batch Name | Inactivation Method | Administration Vehicle | Reference |
|---|---|---|---|---|---|---|---|
| Mycobacterium vaccae NCTC 11659 [34] | Mud (lake Kyoga, Uganda), 1973 | - DSM 107316 - CECT 9646 | - Peptone meat extract glycerol agar - Middlebrook medium - Löwenstein-Jensen medium - Tryptic Soy Agar - MB7H10 agar - PMG agar - GYM agar | SRP 299 | Heat-killed 2 | PBS | [36,37,38] |
| Saline | [39,40] | ||||||
| SRL172 | Heat-killed 2 | BBS | [41] | ||||
| Irradiation-killed 3,* | BBS | [42] | |||||
| V7 | Hydrolyzed and heat-killed 2 | Oral pill excipients | [43,44] | ||||
| Dar-901 (SRL 172) | Heat-killed 2 | Citrate buffer | [45] | ||||
| MV07 | Heat-killed 2 | Culture media | [46] | ||||
| MV001 | Heat-killed 2 | BBS * | [47] | ||||
| MV007 | Heat-killed 2 | BBS | [48] | ||||
| ENG1 | Heat-killed 2 | BBS | [20,22,24,29,32] | ||||
| R877R | Irradiation-killed 3 | BBS | [49] | ||||
| Mycobacterium vaccae ATCC 15483T [50] | Bovine milk, 1964 | - ATCC 23004 - CCUG 21003T - DSM 43292 - KCTC 19087 - NCIB 9937 - NCTC 10916 - SN 920 (Bonicke SN920) - TMC 1526 | - Middlebrook medium - Medium 55 for Mycobacterium | V7 | Hydrolyzed and heat-killed 2 | Oral pill excipients | [51] |
| M. vaccaeTM | Heat-killed 2 | Distilled water | [52] | ||||
| ATCC® 15483 | Heat-killed 2 | PBS | [53] | ||||
| BBS | [29] |
| Disease Investigated | M. vaccae Strain | Dosage | Vehicle | Effects | Underlying Mechanisms | Reference |
|---|---|---|---|---|---|---|
| Tuberculosis | NCTC 11659 | 1 dose (i.d.; 1 mg in 0.1 mL) | BBS | - improved clearance of TB bacilli - normalized ESR - improved sputum conversion - increased survival | - reduced serum IL-4, IL-10, TNF - increased serum IFNγ | [54,55,56,57] |
| Tuberculosis | NCTC 11659 (SRL172) | 3 doses (i.d.; 1 mg in 0.1 mL) | BBS | - improved clearance of TB bacilli - normalized ESR - improved sputum conversion | increased serum IL-4 & TNF | [41] |
| Tuberculosis | NCTC 11659 (SRL172) | 10 doses (p.o; day 0,7,14,21,28 and then monthly; 1 mg/dose) | Gelatine tablet | - normalized ESR - improved sputum conversion and body weight gain | - increased Th1 parameters - decreased Th2 parameters | [58] |
| Tuberculosis | NCTC 11659 (SRL172) | 5 doses (i.d.; 1 mg in 0.1 mL) | BBS | proliferation of PBMCs | increased IFNγ in PBMCs | [42] |
| Tuberculosis | NCTC 11659 (V7) | 30 doses (p.o.; 10 μg/tablet) | V7 tablet (Immunitor®) | improved clearance of TB bacilli | -reduced blood leukocyte number | [43,44] |
| Tuberculosis | Longcom batch (ATCC 15483T) | 30 doses (p.o.; 10 μg/tablet) | V7 tablet (Immunitor®) | improved clearance of TB bacilli | not investigated | [51] |
| Leprosy | NCTC 11659 | 1 dose (i.d.; 0.1 mg in 0.1 mL) | BBS | positive Leprosin A response | not investigated | [59,60] |
| Leprosy | NCTC 11659 | 1 dose (i.d.; 1 mg in 0.1 mL) | BBS | improved skin capillary flow | not investigated | [61] |
| Leprosy | NCTC 11659 | 3 doses (i.d.; 0.01, 0.1, 1 mg in 0.1 mL) | BBS | positive Leprosin A response | not investigated | [62] |
| Psoriasis | NCTC 11659 | 1 dose (i.d.; 1 mg in 0.1 mL) | BBS | reduced PASI | reduced lymphocyte proliferation | [63] |
| Dermatitis | NCTC 11659 (SRL172) | 1 dose (i.d.; 3 mg in 0.3 mL) | BBS | reduced dermatitis lesion area | not investigated | [64] |
| Asthma | NCTC 11659 (SRL172) | 1 dose (i.d.; 1 mg in 0.1 mL) | BBS | trend towards improved airway response to allergen | reduced IL-5 and IgE in PBMCs | [65] |
| Cancer 2 | NCTC 11659 (SRL172) | - 1 dose (i.d.; 0.5 mg in 0.1 mL); - 3 doses (i.d.; 1 mg in 0.1 mL) | BBS | increased survival | not investigated | [66] |
| Cancer 2 | NCTC 11659 (SRL172) | - 1 dose (i.d.; 50 µL (5 × 108 heat-killed bacilli)); - 4 doses (i.d.; 100 µL (109 heat-killed bacilli)) | BBS | improved survival | intracellular IL-2 induction | [67] |
| Cancer 3 | NCTC 11659 (SRL172) | 5 doses (i.d.; 1 mg in 0.1 mL) | BBS | - improved quality of life (cognitive function and vitality); - reduced body pain, nausea and dyspnea | not investigated | [68] |
| Disease Investigated | M. vaccae Strain | Dosage | Vehicle | Effects | Underlying Mechanisms | Reference |
|---|---|---|---|---|---|---|
| Negative consequences of stress | NCTC 11659 | 3 doses (s.c.; 0.1 mg in 0.1 mL) | BBS | reduced stress-induced anxiety and colitis | - increased number of Treg (CD4+ CD25+ FoxP3+) - increased IL-10 | [20,33] |
| Negative consequences of stress | NCTC 11659 | 3 doses (i.n.; 0.1 mg in 0.012 mL) | BBS | reduced stress-induced colitis | not investigated | [32] |
| Negative consequences of stress | NCTC 11659 | 3 doses (s.c.; 0.1 mg in 0.1 mL) | BBS | enhanced between- and within-session extinction in fear-potentiated startle paradigm | alteration in serotonergic gene expression | [25,27,28] |
| Negative consequences of stress | NCTC 11659 | 3 doses (s.c.; 0.1 mg in 0.1 mL) | BBS | prevention of stress-induced exaggeration of anxiety and microglial priming | - upregulated hippocampal IL4, Cd200r1 and Mrc1 - downregulated hippocampal Nlrp3 and Nfkbia | [24] |
| Negative consequences of stress | NCTC 11659 | 3 doses (s.c.; 0.1 mg in 0.1 mL) | BBS | prevention of post-operative cognitive dysfunction | - upregulated hippocampal IL4, Arg1 and Foxp3 - downregulated hippocampal NfκBbia and IL1β | [22] |
| Negative consequences of stress | NCTC 11659 | 3 doses (s.c.; 0.1 mg in 0.1 mL) | BBS | prevention of negative outcomes of a two-hit stress models | - prevention of stress-induced decreased Tph2 and Slc6a4 expression - prevention of stress-induced REM sleep disturbances | [23,26] |
| Cellular/ Molecular Target Investigated | M. vaccae Strain | Dosage | Vehicle | Species | Underlying Mechanisms | Reference |
|---|---|---|---|---|---|---|
| Th1/Th2 balance | NCTC 11659 (MV07) | 1, 10, 100 µg/mL (in vitro) | BBS | human (DCs) | - reduced IL-4 - upregulated CD83 and CD86 | [46] |
| Th1/Th2 balance | NCTC 11659 (SRL172) | 3 doses (s.c.; 106,107,108 bacteria in 0.1 mL) | BBS | mouse | - reduced serum IgE, IL-4 and IL-5 - reduced eosinophil count in BAL | [69] |
| Th1/Th2 balance | NCTC 11659 | 1 dose (s.c.; 107, 108 or 109 bacteria in 0.1 mL) | BBS | mouse | - reduced serum IgE and IL-4 - increased IL-2 in splenocytes | [70] |
| γδ T cells | NCTC 11659 (SRL172) | 100 μg/mL (in vitro) | BBS | human (PBMCs) | upregulated IFNγ, TNF and granzyme B | [71] |
| CD11b+ myeloid cells | NCTC 11659 | 300 μg/mL (in vitro) | BBS | human (PBMCs) | - downregulated CD62L - upregulated TLR2, TLR4, CD18, CD11a, CD14, CD36, CD44, CD45, CD54 CD58k, CD80, CD86, CD137L, CD206 | [72] |
| CD11c+ APC | NCTC 11659 (SRP299) | 1 dose (s.c.; 0.1 mg in 0.1 mL) | NaCl | mouse | - decreased cell number in BAL; - increased IL-10+ and TGFβ in lung DCs | [39] |
| CD14+ monocytes | ATCC 15483T (SN920) | in vitro incubation with 1:10 ratio cells:mycobacteria | Medium | human (PBMCs) | increased secretion of TNF and IL-12 | [73] |
| CD8+ CTL | ATCC 15483T | 1 dose (i.p.; 1 mg in 0.1 mL) | PBS | mouse | - increased expression of IFNγ in TB-infected macrophages - increased cytotoxic activity of CTL against TB-infected macrophages | [74] |
| Tregs | NCTC 11659 (SRP299) | 1 dose (s.c.; 0.1 mg in 0.2 mL) | NaCl | mouse | - increased number of Treg (CD4+ CD45RBLo) - suppressed airway inflammation upon Treg transfer | [40] |
| Tregs | NCTC 11659 (SRP299) | - 1 dose (i.g.; 0.1 mg in 0.1 mL) - 100, 200 or 400 μg/mL (in vitro) | -H2O-NaCl | mouse | - reduced cellular infiltrate in lungs - increased IL-10 in mesLNC | [75] |
| Tregs | NCTC 11659 | 3 doses (s.c.; 0.1 mg in 0.1 mL) | BBS | mouse | - increased number of Treg (CD4+ CD25+ FoxP3+) - increased IL-10 - reduced stress-induced anxiety and colitis | [33] |
| PPARα | 10(Z)-HDA from NCTC 11659 | 200 µM (in vitro) | DMEM/F-12 | mouse | PPARα-dependent downregulation of pro-inflammatory transcription factors, cytokines and chemokines | [76] |
| Serotonergic neurons | NCTC 11659 | 3 doses (s.c.; 0.1 mg in 0.1 mL) | BBS | mouse | activation of serotonergic neurons in interfascicular part of dorsal raphe nucleus | [21] |
| Microglia | NCTC 11659 | 3 doses (s.c.; 0.1 mg in 0.1 mL) | BBS | rat | - increased expression of Il4 mRNA and IL-4-responsive genes (Cd200r1, Mrc1) - reduced IL-1β secretion from freshly isolated and LPS-stimulated hippocampal microglia | [24] |
| Microglia | NCTC 11659, ATCC 15483T | 3 doses (s.c.; 0.1 mg in 0.1 mL) | BBS | rat | prevention of stress-induced upregulation of hippocampal Il6 mRNA expression | [29] |
| Microglia | NCTC 11659 | 3 doses (s.c.; 0.1 mg in 0.1 mL) | BBS | rat | increased hippocampal Il4, Foxp3, Arg1, decreased Il1β, Il6 and Nfκbia mRNA expression | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoroso, M.; Langgartner, D.; Lowry, C.A.; Reber, S.O. Rapidly Growing Mycobacterium Species: The Long and Winding Road from Tuberculosis Vaccines to Potent Stress-Resilience Agents. Int. J. Mol. Sci. 2021, 22, 12938. https://doi.org/10.3390/ijms222312938
Amoroso M, Langgartner D, Lowry CA, Reber SO. Rapidly Growing Mycobacterium Species: The Long and Winding Road from Tuberculosis Vaccines to Potent Stress-Resilience Agents. International Journal of Molecular Sciences. 2021; 22(23):12938. https://doi.org/10.3390/ijms222312938
Chicago/Turabian StyleAmoroso, Mattia, Dominik Langgartner, Christopher A. Lowry, and Stefan O. Reber. 2021. "Rapidly Growing Mycobacterium Species: The Long and Winding Road from Tuberculosis Vaccines to Potent Stress-Resilience Agents" International Journal of Molecular Sciences 22, no. 23: 12938. https://doi.org/10.3390/ijms222312938
APA StyleAmoroso, M., Langgartner, D., Lowry, C. A., & Reber, S. O. (2021). Rapidly Growing Mycobacterium Species: The Long and Winding Road from Tuberculosis Vaccines to Potent Stress-Resilience Agents. International Journal of Molecular Sciences, 22(23), 12938. https://doi.org/10.3390/ijms222312938







