Salivary Biomarkers in Patients with Sjögren’s Syndrome—A Systematic Review
Abstract
:1. Introduction
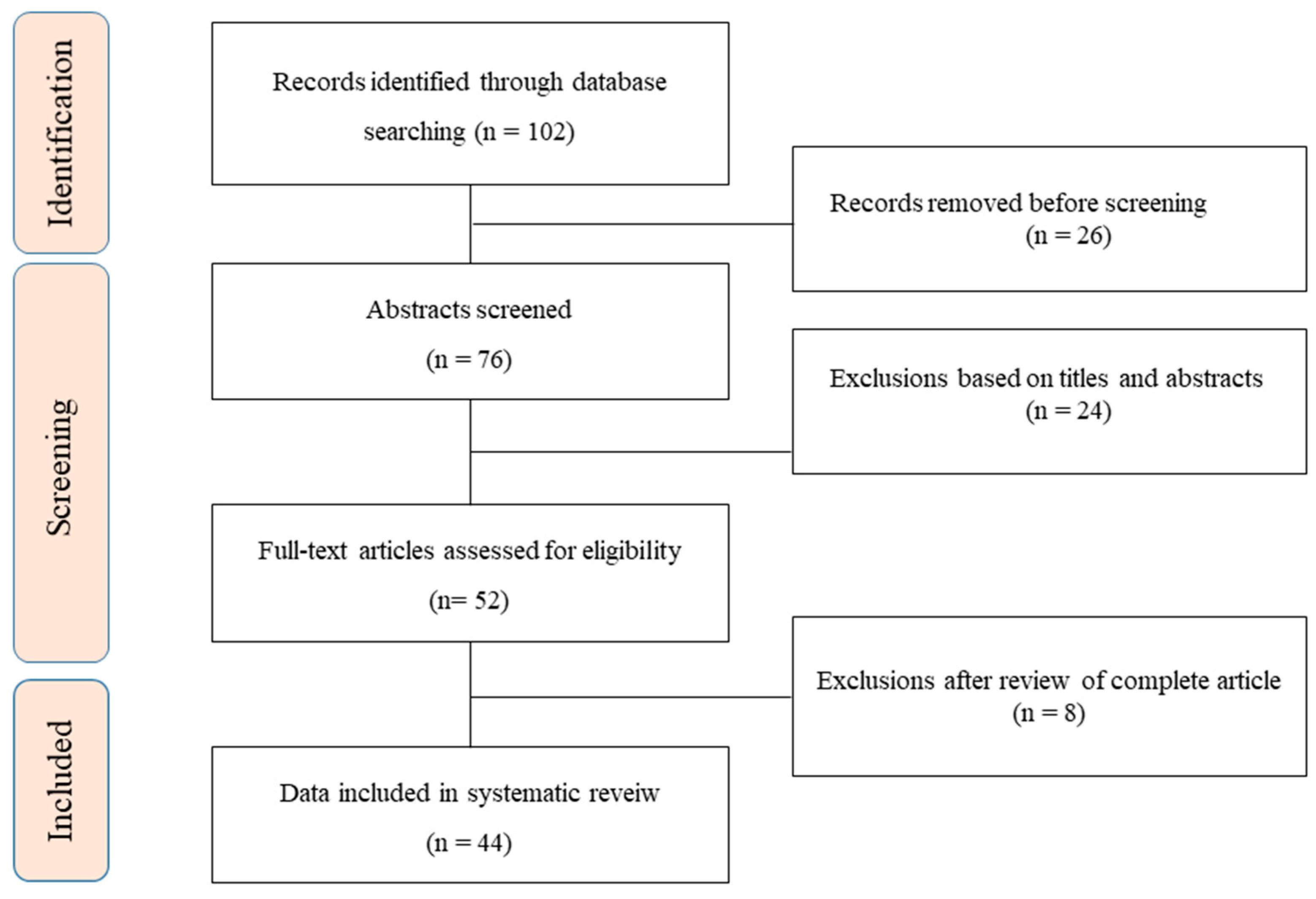
2. Methods
3. Proteomic Analysis
4. Salivary Biomarkers of Sjogren’s Syndrome
4.1. Salivary β2-Microglobulin (β2m)
4.2. Salivary Lactroferrin
4.3. Salivary Neutrophil Gelatinase-Associated Lipocalin (NGAL)
4.4. Salivary Soluble Sialic Acid-Binding Immunoglobulin-like Lectin (Siglec)-5
4.5. Salivary Cytokines
4.6. Salivary Autoantibody
4.7. Salivary Calprotectin
4.8. Salivary Carbonic Anhydrase VI
4.9. Salivary Adiponectin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Brito-Zerón, P.; Baldini, C.; Bootsma, H.; Bowman, S.J.; Jonsson, R.; Mariette, X.; Sivils, K.; Theander, E.; Tzioufas, A.; Ramos-Casals, M. Sjögren syndrome. Nat. Rev. Dis. Primers 2016, 2, 16047. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League against Rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann. Rheum. Dis. 2017, 76, 9–16. [Google Scholar] [CrossRef]
- Kyriakidis, N.C.; Kapsogeorgou, E.K.; Tzioufas, A.G. A comprehensive review of autoantibodies in primary Sjögren’s syndrome: Clinical phenotypes and regulatory mechanisms. J. Autoimmun. 2014, 51, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Skopouli, F.N.; Fox, P.C.; Galanopoulou, V.; Atkinson, J.C.; Jaffe, E.S.; Moutsopoulos, H.M. T cell subpopulations in the labial minor salivary gland histopathologic lesion of Sjögren’s syndrome. J. Rheumatol. 1991, 18, 210–214. [Google Scholar]
- Lee, Y.-H.; Wong, D.T. Saliva: An emerging biofluid for early detection of diseases. Am. J. Dent. 2009, 22, 241. [Google Scholar]
- Ferrari, E.; Pezzi, M.; Cassi, D.; Pertinhez, T.; Spisni, A.; Meleti, M. Salivary Cytokines as Biomarkers for Oral Squamous Cell Carcinoma: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 6795. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, V.; Lacout, C.; Lozac’h, P.; Ghali, A.; Gury, A.; Lavigne, C.; Urbanski, G. Unstimulated whole saliva flow for diagnosis of primary Sjögren’s syndrome: Time to revisit the threshold? Arthritis Res. 2020, 22, 38. [Google Scholar] [CrossRef] [Green Version]
- Alvariño, C.; Bagan, L.; Murillo-Cortes, J.; Calvo, J.; Bagan, J. Stimulated whole salivary flow rate: The most appropriate technique for assessing salivary flow in Sjögren syndrome. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e404–e407. [Google Scholar] [CrossRef]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjögren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef] [Green Version]
- Shiboski, S.C.; Shiboski, C.H.; Criswell, L.; Baer, A.; Challacombe, S.; Lanfranchi, H.; Schiødt, M.; Umehara, H.; Vivino, F.; Zhao, Y.; et al. American College of Rheumatology classification criteria for Sjögren’s syndrome: A data-driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res. 2012, 64, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [Green Version]
- Ryu, O.H.; Atkinson, J.C.; Hoehn, G.T.; Illei, G.G.; Hart, T.C. Identification of parotid salivary biomarkers in Sjogren’s syndrome by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry and two-dimensional difference gel electrophoresis. Rheumatology 2006, 45, 1077–1086. [Google Scholar] [CrossRef] [Green Version]
- Giusti, L.; Baldini, C.; Bazzichi, L.; Ciregia, F.; Tonazzini, I.; Mascia, G.; Bombardieri, S.; Lucacchini, A. Proteome analysis of whole saliva: A new tool for rheumatic diseases--the example of Sjögren’s syndrome. Proteomics 2007, 7, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, J.; Meijer, J.; Ieong, S.; Xie, Y.; Yu, T.; Zhou, H.; Henry, S.; Vissink, A.; Pijpe, J.; et al. Salivary proteomic and genomic biomarkers for primary Sjögren’s syndrome. Arthritis Rheum. 2007, 56, 3588–3600. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Gao, K.; Pollard, R.; Arellano-Garcia, M.; Zhou, H.; Zhang, L.; Elashoff, D.; Kallenberg, C.G.; Vissink, A.; Wong, D.T. Preclinical validation of salivary biomarkers for primary Sjögren’s syndrome. Arthritis Care Res. 2010, 62, 1633–1638. [Google Scholar] [CrossRef] [Green Version]
- Baldini, C.; Giusti, L.; Ciregia, F.; Da Valle, Y.; Giacomelli, C.; Donadio, E.; Sernissi, F.; Bazzichi, L.; Giannaccini, G.; Bombardieri, S.; et al. Proteomic analysis of saliva: A unique tool to distinguish primary Sjögren’s syndrome from secondary Sjögren’s syndrome and other sicca syndromes. Arthritis Res. Ther. 2011, 13, R194. [Google Scholar] [CrossRef] [Green Version]
- Ambatipudi, K.S.; Swatkoski, S.; Moresco, J.J.; Tu, P.G.; Coca, A.; Anolik, J.H.; Gucek, M.; Sanz, I.; Yates III, J.R.; Melvin, J.E. Quantitative proteomics of parotid saliva in primary Sjögren’s syndrome. Proteomics 2012, 12, 3113–3120. [Google Scholar] [CrossRef] [Green Version]
- Delaleu, N.; Mydel, P.; Kwee, I.; Brun, J.G.; Jonsson, M.V.; Jonsson, R. High fidelity between saliva proteomics and the biologic state of salivary glands defines biomarker signatures for primary Sjögren’s syndrome. Arthritis Rheumatol. 2015, 67, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, O.; Krief, G.; Konttinen, Y.T.; Zaks, B.; Wong, D.T.; Aframian, D.J.; Palmon, A. Identification of Sjögren’s syndrome oral fluid biomarker candidates following high-abundance protein depletion. Rheumatology 2014, 54, 884–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, S.C.; Hassis, M.E.; Williams, K.E.; Albertolle, M.E.; Prakobphol, A.; Dykstra, A.B.; Laurance, M.; Ona, K.; Niles, R.K.; Prasad, N.; et al. Alterations in the Salivary Proteome and N-Glycome of Sjögren’s Syndrome Patients. J. Proteome Res. 2017, 16, 1693–1705. [Google Scholar] [CrossRef]
- Aqrawi, L.A.; Galtung, H.K.; Vestad, B.; Øvstebø, R.; Thiede, B.; Rusthen, S.; Young, A.; Guerreiro, E.M.; Utheim, T.P.; Chen, X.; et al. Identification of potential saliva and tear biomarkers in primary Sjögren’s syndrome, utilising the extraction of extracellular vesicles and proteomics analysis. Arthritis Res. Ther. 2017, 19, 14. [Google Scholar] [CrossRef] [Green Version]
- Aqrawi, L.A.; Galtung, H.K.; Guerreiro, E.M.; Øvstebø, R.; Thiede, B.; Utheim, T.P.; Chen, X.; Utheim, Ø.A.; Palm, Ø.; Skarstein, K.; et al. Proteomic and histopathological characterisation of sicca subjects and primary Sjögren’s syndrome patients reveals promising tear, saliva and extracellular vesicle disease biomarkers. Arthritis Res. Ther. 2019, 21, 181. [Google Scholar] [CrossRef] [Green Version]
- Sembler-Møller, M.L.; Belstrøm, D.; Locht, H.; Pedersen, A.M.L. Proteomics of saliva, plasma, and salivary gland tissue in Sjögren’s syndrome and non-Sjögren patients identify novel biomarker candidates. J. Proteom. 2020, 225, 103877. [Google Scholar] [CrossRef]
- Sembler-Møller, M.L.; Belstrøm, D.; Locht, H.; Pedersen, A.M.L. Combined serum anti-SSA/Ro and salivary TRIM29 reveals promising high diagnostic accuracy in patients with primary Sjögren’s syndrome. PLoS ONE 2021, 16, e0258428. [Google Scholar] [CrossRef]
- Ozato, K.; Shin, D.-M.; Chang, T.-H.; Morse, H.C., III. TRIM family proteins and their emerging roles in innate immunity. Nat. Rev. Immunol. 2008, 8, 849–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Qi, Y.; Wang, G.; Bu, S.; Chen, M.; Yu, J.; Luo, T.; Meng, L.; Dai, A.; Zhou, Y.; et al. Proteomic profiling of saliva reveals association of complement system with primary Sjögren’s syndrome. Immun. Inflamm. Dis. 2021, 9, 1724–1739. [Google Scholar] [CrossRef] [PubMed]
- Berko, D.; Carmi, Y.; Cafri, G.; Ben-Zaken, S.; Sheikhet, H.M.; Tzehoval, E.; Eisenbach, L.; Margalit, A.; Gross, G. Membrane-anchored beta 2-microglobulin stabilizes a highly receptive state of MHC class I molecules. J. Immunol. 2005, 174, 2116–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markusse, H.M.; Otten, H.G.; Vroom, T.M.; Smeets, T.J.; Fokkens, N.; Breedveld, F.C. The diagnostic value of salivary fluid levels of beta 2-microglobulin, lysozyme and lactoferrin for primary Sjögren’s syndrome. Clin. Rheumatol. 1992, 11, 521–525. [Google Scholar] [CrossRef]
- van der Geest, S.A.; Markusse, H.M.; Swaak, A.J. Beta 2 microglobulin measurements in saliva of patients with primary Sjögren’s syndrome: Influence of flow. Ann. Rheum. Dis. 1993, 52, 461–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swaak, A.J.; Visch, L.L.; Zonneveld, A. Diagnostic significance of salivary levels of beta 2-microglobulin in Sjögren’s syndrome. Clin. Rheumatol. 1988, 7, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Mogi, M.; Kage, T.; Chino, T.; Yoshitake, K.; Harada, M. Increased beta 2-microglobulin in both parotid and submandibular/sublingual saliva from patients with Sjögren’s syndrome. Arch. Oral Biol. 1994, 39, 913–915. [Google Scholar] [CrossRef]
- Asashima, H.; Inokuma, S.; Onoda, M.; Oritsu, M. Cut-off levels of salivary beta2-microglobulin and sodium differentiating patients with Sjögren’s syndrome from those without it and healthy controls. Clin. Exp. Rheumatol. 2013, 31, 699–703. [Google Scholar] [PubMed]
- Garza-García, F.; Delgado-García, G.; Garza-Elizondo, M.; Ceceñas-Falcón, L.; Galarza-Delgado, D.; Riega-Torres, J. Salivary β2-microglobulin positively correlates with ESSPRI in patients with primary Sjögren’s syndrome. Rev. Bras. Reumatol. Engl. Ed. 2017, 57, 182–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Interactions of lactoferrin with cells involved in immune functionThis paper is one of a selection of papers published in this Special Issue, entitled 7th International Conference on Lactoferrin: Structure, Function, and Applications, and has undergone the Journal’s usual peer review process. Biochem. Cell Biol. 2006, 84, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Konttinen, Y.T.; Kulomaa, M.; Malmström, M.; Kilpi, A.; Reitamo, S. Lactoferrin in Sjögren’s syndrome. Arthritis Rheum. 1984, 27, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Jezequel, N.; Depasse, F.; Jouquan, J.; Lelong, A.; Roncin, S.; Pare, G.; Pennec, Y.L.; Youinou, P. Salivary lactoferrin in primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 1989, 7, 123–125. [Google Scholar] [PubMed]
- Haghighat, N.; al-Hashimi, I. The status of lactoferrin and total iron binding capacity of human parotid saliva in Sjögren’s syndrome. Clin. Exp. Rheumatol. 2003, 21, 485–488. [Google Scholar]
- Ramenzoni, L.; Lehner, M.; Kaufmann, M.; Wiedemeier, D.; Attin, T.; Schmidlin, P. Oral Diagnostic Methods for the Detection of Periodontal Disease. Diagnostics 2021, 11, 571. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Nam, J.-Y.; Ryu, K.-S.; Son, I.-O.; Shin, J.-H.; Baek, W.-Y.; Kim, H.-A.; Suh, C.-H. Salivary Immunoglobulin Gamma-3 Chain C Is a Promising Noninvasive Biomarker for Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2021, 22, 1374. [Google Scholar] [CrossRef]
- Pawar, R.D.; Goilav, B.; Xia, Y.; Zhuang, H.; Herlitz, L.; Reeves, W.H.; Putterman, C. Serum autoantibodies in pristane induced lupus are regulated by neutrophil gelatinase associated lipocalin. Clin. Immunol. 2014, 154, 49–65. [Google Scholar] [CrossRef] [Green Version]
- Katano, M.; Okamoto, K.; Arito, M.; Kawakami, Y.; Kurokawa, M.S.; Suematsu, N.; Shimada, S.; Nakamura, H.; Xiang, Y.; Masuko, K.; et al. Implication of granulocyte-macrophage colony-stimulating factor induced neutrophil gelatinase-associated lipocalin in pathogenesis of rheumatoid arthritis revealed by proteome analysis. Arthritis Res. Ther. 2009, 11, R3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aqrawi, L.A.; Jensen, J.L.; Fromreide, S.; Galtung, H.K.; Skarstein, K. Expression of NGAL-specific cells and mRNA levels correlate with inflammation in the salivary gland, and its overexpression in the saliva, of patients with primary Sjögren’s syndrome. Autoimmunity 2020, 53, 333–343. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Kwok, S.K.; Baek, S.; Jang, S.G.; Hong, S.M.; Min, J.W.; Choi, S.S.; Lee, J.; Cho, M.L.; et al. JAK-1 Inhibition Suppresses Interferon-Induced BAFF Production in Human Salivary Gland: Potential Therapeutic Strategy for Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2018, 70, 2057–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianchecchi, E.; Arena, A.; Fierabracci, A. Sialic Acid-Siglec Axis in Human Immune Regulation, Involvement in Autoimmunity and Cancer and Potential Therapeutic Treatments. Int. J. Mol. Sci. 2021, 22, 5774. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.; Baek, S.; Koh, J.H.; Kim, J.W.; Kim, S.Y.; Chung, S.H.; Choi, S.S.; Cho, M.L.; Kwok, S.K.; et al. Soluble siglec-5 is a novel salivary biomarker for primary Sjogren’s syndrome. J. Autoimmun. 2019, 100, 114–119. [Google Scholar] [CrossRef]
- Nguyen, C.Q.; Hu, M.H.; Li, Y.; Stewart, C.; Peck, A.B. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren’s syndrome: Findings in humans and mice. Arthritis Rheum. 2008, 58, 734–743. [Google Scholar] [CrossRef] [Green Version]
- Benchabane, S.; Boudjelida, A.; Toumi, R.; Belguendouz, H.; Youinou, P.; Touil-Boukoffa, C. A case for IL-6, IL-17A, and nitric oxide in the pathophysiology of Sjögren’s syndrome. Int. J. Immunopathol. Pharmacol. 2016, 29, 386–397. [Google Scholar] [CrossRef] [Green Version]
- Kabeerdoss, J.; Sandhya, P.; Mandal, S.K.; Gowri, M.; Danda, D. High salivary soluble L-selectin and interleukin-7 levels in Asian Indian patients with primary Sjögren’s syndrome. Clin. Rheumatol. 2016, 35, 3063–3067. [Google Scholar] [CrossRef]
- Hung, Y.H.; Lee, Y.H.; Chen, P.P.; Lin, Y.Z.; Lin, C.H.; Yen, J.H. Role of Salivary Immune Parameters in Patients with Primary Sjogren’s Syndrome. Ann. Lab Med. 2019, 39, 76–80. [Google Scholar] [CrossRef]
- Hu, S.; Vissink, A.; Arellano, M.; Roozendaal, C.; Zhou, H.; Kallenberg, C.G.; Wong, D.T. Identification of autoantibody biomarkers for primary Sjögren’s syndrome using protein microarrays. Proteomics 2011, 11, 1499–1507. [Google Scholar] [CrossRef] [Green Version]
- Sumida, T.; Iizuka, M.; Asashima, H.; Tsuboi, H.; Matsumoto, I. Pathogenic role of anti-M3 muscarinic acetylcholine receptor immune response in Sjögren’s syndrome. Presse Med. 2012, 41, e461–e466. [Google Scholar] [CrossRef]
- Jayakanthan, K.; Ramya, J.; Mandal, S.K.; Sandhya, P.; Gowri, M.; Danda, D. Younger patients with primary Sjögren’s syndrome are more likely to have salivary IgG anti-muscarinic acetylcholine receptor type 3 antibodies. Clin. Rheumatol. 2016, 35, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Mona, M.; Mondello, S.; Hyon, J.Y.; Saleh, W.; Han, K.; Lee, H.J.; Ha, Y.J.; Kang, E.H.; Lee, Y.J.; Cha, S. Clinical usefulness of anti-muscarinic type 3 receptor autoantibodies in patients with primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2021, 39, 795–803. [Google Scholar] [PubMed]
- Jin, Y.; Li, J.; Chen, J.; Shao, M.; Zhang, R.; Liang, Y.; Zhang, X.; Zhang, X.; Zhang, Q.; Li, F.; et al. Tissue-Specific Autoantibodies Improve Diagnosis of Primary Sjögren’s Syndrome in the Early Stage and Indicate Localized Salivary Injury. J. Immunol. Res. 2019, 2019, 3642937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foell, D.; Roth, J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 2004, 50, 3762–3771. [Google Scholar] [CrossRef]
- Nordal, H.H.; Brun, J.G.; Halse, A.K.; Madland, T.M.; Fagerhol, M.K.; Jonsson, R. Calprotectin (S100A8/A9), S100A12, and EDTA-resistant S100A12 complexes (ERAC) in primary Sjögren’s syndrome. Scand. J. Rheumatol. 2014, 43, 76–78. [Google Scholar] [CrossRef]
- Nicaise, C.; Weichselbaum, L.; Schandene, L.; Gangji, V.; Dehavay, F.; Bouchat, J.; Balau, B.; Vogl, T.; Soyfoo, M.S. Phagocyte-specific S100A8/A9 is upregulated in primary Sjögren’s syndrome and triggers the secretion of pro-inflammatory cytokines in vitro. Clin. Exp. Rheumatol. 2017, 35, 129–136. [Google Scholar] [PubMed]
- Cuida, M.; Halse, A.K.; Johannessen, A.C.; Tynning, T.; Jonsson, R. Indicators of salivary gland inflammation in primary Sjogren’s syndrome. Eur. J. Oral Sci. 1997, 105, 228–233. [Google Scholar] [CrossRef]
- Jazzar, A.A.; Shirlaw, P.J.; Carpenter, G.H.; Challacombe, S.J.; Proctor, G.B. Salivary S100A8/A9 in Sjögren’s syndrome accompanied by lymphoma. J. Oral Pathol. Med. 2018, 47, 900–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tvarijonaviciute, A.; Zamora, C.; Martinez-Subiela, S.; Tecles, F.; Pina, F.; Lopez-Jornet, P. Salivary adiponectin, but not adenosine deaminase, correlates with clinical signs in women with Sjögren’s syndrome: A pilot study. Clin. Oral Investig. 2018, 23, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
| 2002 American-European Consensus Group (AECG) [9] | 2012 Sjögren’s International Collaborative Clinical Alliance (SICCA) [10] | 2016 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) [2] | |
|---|---|---|---|
| Item | I. Ocular symptoms: positive response ≥ 1 of the following questions: 1. Have you had daily, persistent, troublesome dry eyes for more than 3 months? 2. Do you have a recurrent sensation of sand or gravel in the eyes? 3. Do you use tear substitutes more than three times a day? II. Oral symptoms: positive response ≥ 1 of the following questions: 1. Have you had a daily feeling of dry mouth for more than 3 months? 2. Have you had recurrently or persistently swollen salivary glands as an adult? 3. Do you frequently drink liquids to aid in swallowing dry food? III. Ocular signs (objective evidence of ocular involvement): positive result ≥ 1 of the following two tests: 1. Schirmer’s I test, performed without anesthesia (<5 mm in 5 min) 2. Rose bengal score or other ocular dye scores (>4 according to van Bijsterveld’s scoring system) IV. Histopathology: In minor salivary glands, focal lymphocytic sialoadenitis, evaluated by an expert histopathologist, with a focus score >1, defined as a number of lymphocytic foci (which are adjacent to normal-appearing mucous acini and contain >50 lymphocytes) per 4 mm2 of glandular tissue V. Salivary gland involvement (objective evidence of salivary gland involvement): positive result ≥ 1 of the following diagnostic tests: 1. Unstimulated whole salivary flow (<1.5 mL in 15 min) 2. Parotid sialography showing the presence of diffuse sialectasias (punctate, cavitary, or destructive pattern), without evidence ofobstruction in the major ducts 3. Salivary scintigraphy showing delayed uptake, reduced concentration and/or delayed excretion of tracer VI. Autoantibodies: presence in the serum of antibodies to Ro(SSA) or La(SSB) antigens, or both |
| The classification of SS applies to any individual who meets the inclusion criteria, does not have any condition listed as exclusion criteria, and who has a score ≥ 4 when summing the weights from the following items:
|
| Inclusion criteria | For primary SS a. The presence of any 4 of the 6 items is indicative of primary SS, as long as either item IV or VI is positive b. The presence of any 3 of the 4 objective criteria items (that is, items III, IV, V, VI) c. The classification tree procedure represents a valid alternative method for classification, although it should be more properly used in a clinical-epidemiological survey For secondary SS In patients with a potentially associated disease (for instance, another well-defined connective tissue disease), the presence of item I or item II plus any 2 from among items III, IV, and V | ≥1 symptom of ocular or oral dryness (defined as a positive response to at least one of the following questions: (1) Have you had daily, persistent, troublesome dry eyes for more than 3 months? (2) Do you have a recurrent sensation of sand or gravel in the eyes? (3) Do you use tear substitutes more than 3 times a day? (4) Have you had a daily feeling of dry mouth for more than 3 months? (5) Do you frequently drink liquids to aid in swallowing dry food?), or suspicion of SS from the ESSDAI questionnaire (at least one domain with positive item) | |
| Exclusion criteria | Past head and neck radiation treatment Hepatitis C infection Acquired immunodeficiency disease (AIDS) Pre-existing lymphoma Sarcoidosis Graft versus host disease Use of anticholinergic drugs (since a time shorter than 4-fold the half-life of the drug) | History of head and neck radiation treatment Hepatitis C infection AIDS Sarcoidosis Amyloidosis Graft versus host disease IgG4-related disease | History of head and neck radiation treatment Active Hepatitis C infection (with positive PCR) AIDS Sarcoidosis Amyloidosis Graft versus host disease IgG4-related disease |
| Salivary Biomarker | Authors [Ref.] | Subjects | Sample | Used Criteria | Analytical Methods | Findings |
|---|---|---|---|---|---|---|
| β2-microglobulin | Markusse et al. [28] | 39 pSS, 42 non-SS, 41 HC | Stimulated parotid saliva | - | ELISA | 58% of pSS higher levels of mean + 2SD of levels of HC (7%) |
| van der Geest et al. [29] | 29 pSS, 30 HC | Unstimulated and stimulated parotid saliva | - | Radioimmunoassay | Higher in pSS (p < 0.001) | |
| Mogi et al. [31] | pSS, HC, sialoadenitis, diabetes mellitus | Unstimulated whole saliva | - | ELISA | Higher in pSS (p < 0.001) | |
| Ryu et al. [12] | 41 pSS, 15 non-SS sicca, 20 HCs | Stimulated parotid saliva | 2002 AECG | ELISA | Higher 4.3- and 3.7-fold for the low/medium and for the medium/high focus | |
| Hu et al. [15] | 34 pSS, 34 SLE, 34 HC | Stimulated whole saliva | 2002 AECG | ELISA | Higher in pSS (p = 1.25 × 10−10), AUC 0.87 | |
| Baldini et al. [16] | 19 pSS, 10 non-SS sicca SD, 25 sSS, 10 HC | Unstimulated whole saliva | 2002 AECG | ELISA | Higher in pSS than HC (p < 0.001) and RA-sSS (p < 0.05) | |
| Asashima et al. [32] | 71 pSS, 50 sSS, 54 non-SS-CTD, 75 HC | Unstimulated whole saliva | 2002 AECG | ELISA | Higher in pSS (5.3 ± 4.6 mg/L) than non-SS-CTD (2.5 ± 2.1) and HC (1.2 ± 0.7) | |
| Garza-García et al. [33] | 71 pSS | Unstimulated whole saliva | 2012 SICCA | ELISA | Positive correlation with ESSPRI (Kendall’s tau 0.759, 95% CI 0.656–0.837, p < 0.0001) | |
| Lactoferrin | Ryu et al. [12] | 41 pSS, 15 non-SS sicca, 20 HCs | Stimulated parotid saliva | 2002 AECG | ELISA | Higher 3.7- and 3.6-fold in pSS patients with low/medium focus and medium/high focus |
| Markusse et al. [28] | 39 pSS, 42 non SS sicca SD, 41 HC | Stimulated parotid saliva | - | ELISA | 26% of pSS higher levels of mean + 2 SD of levels of HC (0%) | |
| Konttinen et al. [35] | 3 pSS, 5 sSS, 8 HCs | Unstimulated whole saliva | - | radioimmunoassay | Higher in SS (48.92 ± 14.21 μg/mL) than HC (4.03 ± 1.48) | |
| NGAL | Aqrawi et al. [42] | 11 pSS, 11 HCs | Stimulated whole saliva | 2002 AECG | LC-MS | Detected in 8/11 pSS and 2/11 HCs |
| Siglec-5 | Lee et al. [45] | 170 pSS, 43 SLE, 25 HCs | Unstimulated whole saliva | 2012 SICCA | WB, ELISA | Higher in pSS than non-SS (p < 0.001) Negative correlation with salivary flow rate, positive correlation with ocular surface score and serum immunoglobulin G, AUC 0.774 |
| TRIM29 | Sembler-Møller et al. [23] | 24 pSS, 16 non-SS | Unstimulated and stimulated whole saliva | 2016 ACR/EULAR | nano-scale LC-MS | AUC 0.881 for pSS |
| Proinflammatory cytokines | Nguyen et al. [46] | 21 pSS, 19 HCs | Unstimulated whole saliva | 2002 AECG | ELISA | No difference in levels of IL-17 Higher levels of IL-6 in pSS (p < 0.05) |
| Benchabane et al. [47] | 44 pSS, 15 HCs | Stimulated whole saliva | 2002 AECG | ELISA | Higher IL-17A, IL-6, TNF-α, IL-10 in pSS | |
| Kabeerdoss et al. [48] | 43 pSS, 31 HCs | Unstimulated whole saliva | 2002 AECG or 2012 SICCA | ELISA | Higher median levels of sL-selectin, IL-7 in pSS No association between ESSDAI and levels of sL-selectin, IL7 | |
| Hung et al. [49] | 138 pSS, 100 HCs | Unstimulated whole saliva | 2002 AECG | ELISA | Higher IL-6 (p = 0.012), but not TNF-α, IL-17A, RF-IgA IL-6 correlated with ESR (r = 0.252), IgG (0.248) | |
| Tvarijonaviciute et al. [60] | 17 SS, 13 HCs, 19 non-SS sicca | Unstimulated whole saliva | 2002 AECG | ELISA | Higher IL-1β vs. HC or non-SS sicca (both p < 0.001) Higher IL-8 vs. HC or non-SS sicca (both p < 0.001) | |
| Anti-histone, anti-transglutaminase, anti-SSA, and anti-SSB Ab | Hu et al. [50] | 34 pSS, 34 SLE, 34 HCs | Unstimulated whole saliva | 2002 AECG | ELISA | AUC of anti-histone, anti-transglutaminase, anti-SSA, anti-SSB antibody 0.95, 0.87, 0.93, 0.94 |
| Muscarinic type 3 receptor (M3R) | Jayakanthan et al. [52] | 43 pSS, 34 SLE, 42 HCs | Unstimulated whole saliva | 2002 AECG or 2012 SICCA | ELISA | Positive in 55.81, 17.64, 7% of pSS, SLE, HCs Positivity was associated with lower age, shorter disease duration, higher globulin levels |
| Mona et al. [53] | 37 pSS, 26 non SS-sicca | Unstimulated and stimulated whole saliva | 2002 AECG | On-Cell-Western assay | Higher (3.59) in pSS, AUC 0.84 | |
| Tissue-specific antibodies (anti-CA6, SP1 and PSP) | Jin et al. [54] | 137 pSS, 32 SLE-sSS, 127 HCs | Unstimulated whole saliva | 2002 AECG or 2012 SICCA | ELISA | Higher anti-CA6 IgG (p < 0.01), anti-SP1 IgG (p < 0.01), anti-PSP2 IgG (p < 0.05) in pSS than HCs |
| Calprotectin | Jazzar et al. [59] | 51 SS, 14 SS with MALT lymphoma, 18 HCs | Unstimulated whole and parotid saliva | 2002 AECG and 2012 SICCA | ELISA | Higher S100A8/A9 in parotid saliva of SS (743.1 ng/mL) than HC (31.9, p = 0.001) and than SNOX (208.9, p = 0.031) |
| Adiponectin | Tvarijonaviciute et al. [60] | 17 SS, 13 HCs, 19 non-SS sicca | Unstimulated whole saliva | 2002 AECG | ELISA | Higher adiponectin in SS than HC (p = 0.034) and non-SS sicca (p = 0.007) Higher ADA in SS than HC (p = 0.034) and non-SS sicca (p = 0.033) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.-Y.; Kim, J.-W.; Kim, H.-A.; Suh, C.-H. Salivary Biomarkers in Patients with Sjögren’s Syndrome—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 12903. https://doi.org/10.3390/ijms222312903
Jung J-Y, Kim J-W, Kim H-A, Suh C-H. Salivary Biomarkers in Patients with Sjögren’s Syndrome—A Systematic Review. International Journal of Molecular Sciences. 2021; 22(23):12903. https://doi.org/10.3390/ijms222312903
Chicago/Turabian StyleJung, Ju-Yang, Ji-Won Kim, Hyoun-Ah Kim, and Chang-Hee Suh. 2021. "Salivary Biomarkers in Patients with Sjögren’s Syndrome—A Systematic Review" International Journal of Molecular Sciences 22, no. 23: 12903. https://doi.org/10.3390/ijms222312903
APA StyleJung, J.-Y., Kim, J.-W., Kim, H.-A., & Suh, C.-H. (2021). Salivary Biomarkers in Patients with Sjögren’s Syndrome—A Systematic Review. International Journal of Molecular Sciences, 22(23), 12903. https://doi.org/10.3390/ijms222312903







