Role of Salivary MicroRNA and Cytokines in the Diagnosis and Prognosis of Oral Squamous Cell Carcinoma
Abstract
1. Introduction
2. Salivary MicroRNA (miRNA) in OSCC Diagnosis and Prognosis
2.1. Exosomal miRNAs with Diagnostic Capacity
2.2. MiRNAs with Tumor-Suppressor Role
2.3. MiRNAs with Tumor-Activator Role
2.4. Circular RNA in OSCC Diagnosis
3. Salivary Cytokines in OSCC Diagnosis and Prognosis
3.1. IL-6
3.2. IL-8
3.3. TNF-α
3.4. MMP-9
3.5. IL-1-β
3.6. IL-1-Ra
3.7. IL-10
3.8. 8-OHdG (8-Oxo-dG)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OSCC | Oral squamous cell carcinoma |
| EVs | Extracellular vesicles |
| EZH2 | Enhancer of zeste homolog 2 |
| EGFR | Epidermal growth factor receptor |
| PKCε | Protein kinase Cε |
| TGF | Transforming growth factor |
| circRNAs | Circular RNAs |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor alpha |
| IL-8 | Interleukin-8 |
| MMP-9 | Matrix metallopeptidase 9 |
| IL-1-β | Interleukin-1-β |
| IL-1-Ra | IL-1 receptor antagonist |
| IL-10 | Interleukin-10 |
| 8-OHdG (8-oxo-dG) | 8-Oxo-2′-deoxyguanosine |
References
- Cristaldi, M.; Mauceri, R.; Di Fede, O.; Giuliana, G.; Campisi, G.; Panzarella, V. Salivary Biomarkers for Oral Squamous Cell Carcinoma Diagnosis and Follow-Up: Current Status and Perspectives. Front. Physiol. 2019, 10, 1476. [Google Scholar] [CrossRef]
- Guha, N.; Warnakulasuriya, S.; Vlaanderen, J.; Straif, K. Betel quid chewing and the risk of oral and oropharyngeal cancers: A meta-analysis with implications for cancer control. Int. J. Cancer 2014, 135, 1433–1443. [Google Scholar] [CrossRef]
- Jeng, J.H.; Chang, M.C.; Hahn, L.J. Role of areca nut in betel quid-associated chemical carcinogenesis: Current awareness and future perspectives. Oral Oncol. 2001, 37, 477–492. [Google Scholar] [CrossRef]
- Bellairs, J.A.; Hasina, R.; Agrawal, N. Tumor DNA: An emerging biomarker in head and neck cancer. Cancer Metastasis Rev. 2017, 36, 515–523. [Google Scholar] [CrossRef]
- Woolgar, J.A. Histopathological prognosticators in oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2006, 42, 229–239. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Perron, M.P.; Provost, P. Protein interactions and complexes in human microRNA biogenesis and function. Front. Biosci. 2008, 13, 2537–2547. [Google Scholar] [CrossRef] [PubMed]
- Faur, C.I.; Rotaru, H.; Osan, C.; Jurj, A.; Roman, R.C.; Moldovan, M.; Chirila, M.; Hedesiu, M. Salivary exosomal microRNAs as biomarkers for head and neck cancer detection-a literature review. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 19. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ping, F.; Fan, Z.; Zhang, C.; Deng, M.; Cheng, B.; Xia, J. Salivary exosomal miR-24-3p serves as a potential detective biomarker for oral squamous cell carcinoma screening. Biomed. Pharmacother. 2020, 121, 109553. [Google Scholar] [CrossRef] [PubMed]
- Langevin, S.; Kuhnell, D.; Parry, T.; Biesiada, J.; Huang, S.; Wise-Draper, T.; Casper, K.; Zhang, X.; Medvedovic, M.; Kasper, S. Comprehensive microRNA-sequencing of exosomes derived from head and neck carcinoma cells in vitro reveals common secretion profiles and potential utility as salivary biomarkers. Oncotarget 2017, 8, 82459–82474. [Google Scholar] [CrossRef]
- Byun, J.-S.; Hong, S.-H.; Choi, J.-K.; Jung, J.-K.; Lee, H.-J. Diagnostic profiling of salivary exosomal microRNAs in oral lichen planus patients. Oral Dis. 2015, 21, 987–993. [Google Scholar] [CrossRef]
- Coon, J.; Kingsley, K.; Howard, K.M. miR-365 (microRNA): Potential Biomarker in Oral Squamous Cell Carcinoma Exosomes and Extracellular Vesicles. Int. J. Mol. Sci. 2020, 21, 5317. [Google Scholar] [CrossRef] [PubMed]
- Al Rawi, N.; Elmabrouk, N.; Abu Kou, R.; Mkadmi, S.; Rizvi, Z.; Hamdoon, Z. The role of differentially expressed salivary microRNA in oral squamous cell carcinoma. A systematic review. Arch. Oral Biol. 2021, 125, 105108. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Bala, S. Emerging role of non-coding RNA in oral cancer. Cell. Signal. 2018, 42, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.; Jimenez, L.; Kawachi, N.; Fan, J.-B.; Chen, J.; Belbin, T.; Ramnauth, A.; Loudig, O.; Keller, C.E.; Smith, R.; et al. Low-level expression of miR-375 correlates with poor outcome and metastasis while altering the invasive properties of head and neck squamous cell carcinomas. Am. J. Pathol. 2012, 180, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, F.C.; Giles, K.M.; Candy, P.A.; Ali, A.; Ganda, C.; Epis, M.R.; Webster, R.J.; Leedman, P.J. Regulation of epidermal growth factor receptor signaling and erlotinib sensitivity in head and neck cancer cells by miR-7. PLoS ONE 2012, 7, e47067. [Google Scholar] [CrossRef]
- Lu, Z.-M.; Lin, Y.-F.; Jiang, L.; Chen, L.-S.; Luo, X.-N.; Song, X.-H.; Chen, S.-H.; Zhang, S.-Y. Micro-ribonucleic acid expression profiling and bioinformatic target gene analyses in laryngeal carcinoma. OncoTargets Ther. 2014, 7, 525–533. [Google Scholar] [CrossRef]
- Datta, J.; Smith, A.; Lang, J.C.; Islam, M.; Dutt, D.; Teknos, T.N.; Pan, Q. microRNA-107 functions as a candidate tumor-suppressor gene in head and neck squamous cell carcinoma by downregulation of protein kinase Cɛ. Oncogene 2012, 31, 4045–4053. [Google Scholar] [CrossRef]
- Kinoshita, T.; Nohata, N.; Hanazawa, T.; Kikkawa, N.; Yamamoto, N.; Yoshino, H.; Itesako, T.; Enokida, H.; Nakagawa, M.; Okamoto, Y.; et al. Tumour-suppressive microRNA-29s inhibit cancer cell migration and invasion by targeting laminin-integrin signalling in head and neck squamous cell carcinoma. Br. J. Cancer 2013, 109, 2636–2645. [Google Scholar] [CrossRef]
- Alajez, N.M.; Shi, W.; Wong, D.; Lenarduzzi, M.; Waldron, J.; Weinreb, I.; Liu, F.-F. Lin28b Promotes Head and Neck Cancer Progression via Modulation of the Insulin-Like Growth Factor Survival Pathway. Oncotarget 2012, 3, 1641–1652. [Google Scholar] [CrossRef]
- Wiklund, E.D.; Gao, S.; Hulf, T.; Sibbritt, T.; Nair, S.; Costea, D.E.; Villadsen, S.B.; Bakholdt, V.; Bramsen, J.B.; Sørensen, J.A.; et al. MicroRNA alterations and associated aberrant DNA methylation patterns across multiple sample types in oral squamous cell carcinoma. PLoS ONE 2011, 6, e27840. [Google Scholar] [CrossRef]
- Zahran, F.; Ghalwash, D.; Shaker, O.; Al-Johani, K.; Scully, C. Salivary microRNAs in oral cancer. Oral Dis. 2015, 21, 739–747. [Google Scholar] [CrossRef]
- Greither, T.; Vorwerk, F.; Kappler, M.; Bache, M.; Taubert, H.; Kuhnt, T.; Hey, J.; Eckert, A.W. Salivary miR-93 and miR-200a as post-radiotherapy biomarkers in head and neck squamous cell carcinoma. Oncol. Rep. 2017, 38, 1268–1275. [Google Scholar] [CrossRef]
- Maheswari, T.N.U.; Venugopal, A.; Sureshbabu, N.M.; Ramani, P. Salivary micro RNA as a potential biomarker in oral potentially malignant disorders: A systematic review. Tzu-Chi Med. J. 2018, 30, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xin, Z.; Guo, S.; Li, S.; Cheng, J.; Jiang, H. Blood and Salivary MicroRNAs for Diagnosis of Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2021, 79, 1082.e1–1082.e13. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.-S.; Liu, C.-J.; Chou, C.-S.; Kao, S.-Y.; Yang, C.-C.; Chang, K.-W.; Chiu, T.-H.; Lin, S.-C. miR-146a Enhances the Oncogenicity of Oral Carcinoma by Concomitant Targeting of the IRAK1, TRAF6 and NUMB Genes. PLoS ONE 2013, 8, e79926. [Google Scholar] [CrossRef]
- Larrea, E.; Sole, C.; Manterola, L.; Goicoechea, I.; Armesto, M.; Arestin, M.; Caffarel, M.M.; Araujo, A.M.; Araiz, M.; Fernandez-Mercado, M.; et al. New Concepts in Cancer Biomarkers: Circulating miRNAs in Liquid Biopsies. Int. J. Mol. Sci. 2016, 17, 627. [Google Scholar] [CrossRef] [PubMed]
- Giner, M.; Montoya, M.J.; Vázquez, M.A.; Miranda, C.; Miranda, M.J.; Pérez-Cano, R. ¿Qué son los microARNs?: Posibles biomarcadores y dianas terapéuticas en la enfermedad osteoporótica. Rev. Osteoporos. Metab. Min. 2016, 8, 40–44. [Google Scholar] [CrossRef]
- Wu, B.; Xiong, X.; Jia, J.; Zhang, W. MicroRNAs: New actors in the oral cancer scene. Oral Oncol. 2011, 47, 314–319. [Google Scholar] [CrossRef]
- Sannigrahi, M.K.; Sharma, R.; Singh, V.; Panda, N.K.; Rattan, V.; Khullar, M. Role of Host miRNA Hsa-miR-139-3p in HPV-16-Induced Carcinomas. Clin. Cancer Res. 2017, 23, 3884–3895. [Google Scholar] [CrossRef]
- Gai, C.; Camussi, F.; Broccoletti, R.; Gambino, A.; Cabras, M.; Molinaro, L.; Carossa, S.; Camussi, G.; Arduino, P.G. Salivary extracellular vesicle-associated miRNAs as potential biomarkers in oral squamous cell carcinoma. BMC Cancer 2018, 18, 439. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.F.; Sabry, D.; Hassabou, N.F.; Alaa EL-Din, Y. MicroRNA-134/MicroRNA-200a Derived Salivary Exosomes are Novel Diagnostic Biomarkers of Oral Squamous Cell Carcinoma. Egypt. Dent. J. 2021, 67, 367–377. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, Z.; Alaouna, M.; Mbatha, S.; Bhayat, A.; Mabongo, M.; Chatziioannou, A.; Hull, R. Genetic Drivers of Head and Neck Squamous Cell Carcinoma: Aberrant Splicing Events, Mutational Burden, HPV Infection and Future Targets. Genes 2021, 12, 422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-Y.; Wang, J.; Ouyang, S.-B.; Huang, Z.-K.; Liao, L. Salivary Circular RNAs Hsa_Circ_0001874 and Hsa_Circ_0001971 as Novel Biomarkers for the Diagnosis of Oral Squamous Cell Carcinoma. Cell. Physiol. Biochem. 2018, 47, 2511–2521. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef]
- Loo, S.W.; Pui, T.-S. Cytokine and Cancer Biomarkers Detection: The Dawn of Electrochemical Paper-Based Biosensor. Sensors 2020, 20, 1854. [Google Scholar] [CrossRef]
- Dikova, V.; Jantus-Lewintre, E.; Bagan, J. Potential Non-Invasive Biomarkers for Early Diagnosis of Oral Squamous Cell Carcinoma. J. Clin. Med. 2021, 10, 1658. [Google Scholar] [CrossRef]
- Shin, Y.-J.; Vu, H.; Lee, J.-H.; Kim, H.-D. Diagnostic and prognostic ability of salivary MMP-9 for oral squamous cell carcinoma: A pre-/post-surgery case and matched control study. PLoS ONE 2021, 16, e0248167. [Google Scholar] [CrossRef]
- Lee, L.T.; Wong, Y.K.; Hsiao, H.Y.; Wang, Y.W.; Chan, M.Y.; Chang, K.W. Evaluation of saliva and plasma cytokine biomarkers in patients with oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2018, 47, 699–707. [Google Scholar] [CrossRef]
- Vesty, A.; Gear, K.; Biswas, K.; Radcliff, F.J.; Taylor, M.W.; Douglas, R.G. Microbial and inflammatory-based salivary biomarkers of head and neck squamous cell carcinoma. Clin. Exp. Dent. Res. 2018, 4, 255–262. [Google Scholar] [CrossRef]
- Khyani, I.A.M.; Qureshi, M.A.; Mirza, T.; Farooq, M.U. Detection of interleukins-6 and 8 in saliva as potential biomarkers of oral pre-malignant lesion and oral carcinoma: A breakthrough in salivary diagnostics in Pakistan. Pak. J. Pharm. Sci. 2017, 30, 817–823. [Google Scholar]
- Csősz, É.; Lábiscsák, P.; Kalló, G.; Márkus, B.; Emri, M.; Szabó, A.; Tar, I.; Tőzsér, J.; Kiss, C.; Márton, I. Proteomics investigation of OSCC-specific salivary biomarkers in a Hungarian population highlights the importance of identification of population-tailored biomarkers. PLoS ONE 2017, 12, e0177282. [Google Scholar] [CrossRef]
- Ghallab, N.A.; Shaker, O.G. Serum and salivary levels of chemerin and MMP-9 in oral squamous cell carcinoma and oral premalignant lesions. Clin. Oral Investig. 2017, 21, 937–947. [Google Scholar] [CrossRef]
- Peisker, A.; Raschke, G.-F.; Fahmy, M.-D.; Guentsch, A.; Roshanghias, K.; Hennings, J.; Schultze-Mosgau, S. Salivary MMP-9 in the detection of oral squamous cell carcinoma. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e270–e275. [Google Scholar] [CrossRef] [PubMed]
- Gleber-Netto, F.O.; Yakob, M.; Li, F.; Feng, Z.; Dai, J.; Kao, H.-K.; Chang, Y.-L.; Chang, K.-P.; Wong, D.T.W. Salivary Biomarkers for Detection of Oral Squamous Cell Carcinoma in a Taiwanese Population. Clin. Cancer Res. 2016, 22, 3340–3347. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Jacobs, R. Proinflammatory cytokine levels in oral lichen planus, oral leukoplakia, and oral submucous fibrosis. J. Korean Assoc. Oral Maxillofac. Surg. 2015, 41, 171–175. [Google Scholar] [CrossRef]
- Arduino, P.G.; Menegatti, E.; Cappello, N.; Martina, E.; Gardino, N.; Tanteri, C.; Cavallo, F.; Scully, C.; Broccoletti, R. Possible role for interleukins as biomarkers for mortality and recurrence in oral cancer. Int. J. Biol. Markers 2015, 30, e262–e266. [Google Scholar] [CrossRef] [PubMed]
- Lisa Cheng, Y.-S.; Jordan, L.; Gorugantula, L.M.; Schneiderman, E.; Chen, H.-S.; Rees, T. Salivary interleukin-6 and -8 in patients with oral cancer and patients with chronic oral inflammatory diseases. J. Periodontol. 2014, 85, 956–965. [Google Scholar] [CrossRef]
- Krishnan, R.; Thayalan, D.K.; Padmanaban, R.; Ramadas, R.; Annasamy, R.K.; Anandan, N. Association of serum and salivary tumor necrosis factor-α with histological grading in oral cancer and its role in differentiating premalignant and malignant oral disease. Asian Pac. J. Cancer Prev. 2014, 15, 7141–7148. [Google Scholar] [CrossRef] [PubMed]
- Brailo, V.; Vucicevic-Boras, V.; Lukac, J.; Biocina-Lukenda, D.; Zilic-Alajbeg, I.; Milenovic, A.; Balija, M. Salivary and serum interleukin 1 beta, interleukin 6 and tumor necrosis factor alpha in patients with leukoplakia and oral cancer. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e10–e15. [Google Scholar] [CrossRef]
- Korostoff, A.; Reder, L.; Masood, R.; Sinha, U.K. The role of salivary cytokine biomarkers in tongue cancer invasion and mortality. Oral Oncol. 2011, 47, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Katakura, A.; Kamiyama, I.; Takano, N.; Shibahara, T.; Muramatsu, T.; Ishihara, K.; Takagi, R.; Shouno, T. Comparison of salivary cytokine levels in oral cancer patients and healthy subjects. Bull. Tokyo Dent. Coll. 2007, 48, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Rhodus, N.L.; Ho, V.; Miller, C.S.; Myers, S.; Ondrey, F. NF-kappaB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect. Prev. 2005, 29, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; John, M.A.R.S.; Zhou, X.; Kim, Y.; Sinha, U.; Jordan, R.C.K.; Eisele, D.; Abemayor, E.; Elashoff, D.; Park, N.-H.; et al. Salivary Transcriptome Diagnostics for Oral Cancer Detection. Clin. Cancer Res. 2004, 10, 8442–8450. [Google Scholar] [CrossRef]
- Brinkmann, O.; Kastratovic, D.A.; Dimitrijevic, M.V.; Konstantinovic, V.S.; Jelovac, D.B.; Antic, J.; Nesic, V.S.; Markovic, S.Z.; Martinovic, Z.R.; Akin, D.; et al. Oral squamous cell carcinoma detection by salivary biomarkers in a Serbian population. Oral Oncol. 2011, 47, 51–55. [Google Scholar] [CrossRef]
- Kamatani, T.; Shiogama, S.; Yoshihama, Y.; Kondo, S.; Shirota, T.; Shintani, S. Interleukin-1 beta in unstimulated whole saliva is a potential biomarker for oral squamous cell carcinoma. Cytokine 2013, 64, 497–502. [Google Scholar] [CrossRef]
- Niklander, S.E. Inflammatory Mediators in Oral Cancer: Pathogenic Mechanisms and Diagnostic Potential. Front. Oral Health 2021, 2, 2. [Google Scholar] [CrossRef]
- Shiiba, M.; Saito, K.; Yamagami, H.; Nakashima, D.; Higo, M.; Kasamatsu, A.; Sakamoto, Y.; Ogawara, K.; Uzawa, K.; Takiguchi, Y.; et al. Interleukin-1 receptor antagonist (IL1RN) is associated with suppression of early carcinogenic events in human oral malignancies. Int. J. Oncol. 2015, 46, 1978–1984. [Google Scholar] [CrossRef]
- Aziz, S.; Ahmed, S.S.; Ali, A.; Khan, F.A.; Zulfiqar, G.; Iqbal, J.; Khan, A.A.; Shoaib, M. Salivary Immunosuppressive Cytokines IL-10 and IL-13 Are Significantly Elevated in Oral Squamous Cell Carcinoma Patients. Cancer Investig. 2015, 33, 318–328. [Google Scholar] [CrossRef]
- Arantes, L.M.R.B.; De Carvalho, A.C.; Melendez, M.E.; Lopes Carvalho, A. Serum, plasma and saliva biomarkers for head and neck cancer. Expert Rev. Mol. Diagn. 2018, 18, 85–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Sung, W.-W.; Su, T.-C.; Chen, M.-K.; Wu, P.-R.; Yeh, K.-T.; Ko, J.-L.; Lee, H. High expression of interleukin 10 might predict poor prognosis in early stage oral squamous cell carcinoma patients. Clin. Chim. Acta 2013, 415, 25–30. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Guan, X.; Wu, H.; Sun, Z.; Zeng, H. Immunosuppression Induced by Chronic Inflammation and the Progression to Oral Squamous Cell Carcinoma. Mediat. Inflamm. 2016, 2016, 5715719. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pant, M.C.; Singh, H.S.; Khandelwal, S. Determinants of oxidative stress and DNA damage (8-OhdG) in squamous cell carcinoma of head and neck. Indian J. Cancer 2012, 49, 309. [Google Scholar] [CrossRef]
- Nandakumar, A.; Nataraj, P.; James, A.; Krishnan, R.; K M, M. Estimation of Salivary 8-Hydroxydeoxyguanosine (8-OHdG) as a Potential Biomarker in Assessing Progression towards Malignancy: A Case-Control Study. Asian Pac. J. Cancer Prev. 2020, 21, 2325–2329. [Google Scholar] [CrossRef]
- Anderson, P. Post-transcriptional regulons coordinate the initiation and resolution of inflammation. Nat. Rev. Immunol. 2010, 10, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Hop, H.T.; Huy, T.X.N.; Reyes, A.W.B.; Arayan, L.T.; Vu, S.H.; Min, W.; Lee, H.J.; Kang, C.K.; Kim, D.H.; Tark, D.S.; et al. Interleukin 6 Promotes Brucella abortus Clearance by Controlling Bactericidal Activity of Macrophages and CD8+ T Cell Differentiation. Infect. Immun. 2019, 87, e00431-19. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef]
- Mauer, J.; Denson, J.L.; Brüning, J.C. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 2015, 36, 92–101. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Sasaki, M.; Kodama, Y.; Shimoyama, Y.; Ishikawa, T.; Kimura, S. Aciduricity and acid tolerance mechanisms of Streptococcus anginosus. J. Gen. Appl. Microbiol. 2018, 64, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Roi, A.; Roi, C.I.; Negruțiu, M.L.; Riviș, M.; Sinescu, C.; Rusu, L.-C. The Challenges of OSCC Diagnosis: Salivary Cytokines as Potential Biomarkers. J. Clin. Med. 2020, 9, 2866. [Google Scholar] [CrossRef]
- Duffy, S.A.; Taylor, J.M.G.; Terrell, J.E.; Islam, M.; Li, Y.; Fowler, K.E.; Wolf, G.T.; Teknos, T.N. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer 2008, 113, 750–757. [Google Scholar] [CrossRef]
- Ferrari, E.; Pezzi, M.E.; Cassi, D.; Pertinhez, T.A.; Spisni, A.; Meleti, M. Salivary Cytokines as Biomarkers for Oral Squamous Cell Carcinoma: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 6795. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H. Interleukin-8 in the Tumor Immune Niche: Lessons from Comparative Oncology. In Tumor Microenvironment: The Role of Interleukins—Part A; Birbrair, A., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; pp. 25–33. ISBN 978-3-030-38315-2. [Google Scholar]
- Baggiolini, M.; Walz, A.; Kunkel, S.L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Investig. 1989, 84, 1045–1049. [Google Scholar] [CrossRef]
- Waugh, D.J.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef]
- Ben-Baruch, A. Inflammation-associated immune suppression in cancer: The roles played by cytokines, chemokines and additional mediators. Semin. Cancer Biol. 2006, 16, 38–52. [Google Scholar] [CrossRef]
- Cavaillon, J.M. Pro- versus anti-inflammatory cytokines: Myth or reality. Cell. Mol. Biol. 2001, 47, 695–702. [Google Scholar]
- Sahibzada, H.A.; Khurshid, Z.; Sannam Khan, R.; Naseem, M.; Mahmood Siddique, K.; Mali, M.; Zafar, M.S. Salivary IL-8, IL-6 and TNF-α as Potential Diagnostic Biomarkers for Oral Cancer. Diagnostics 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
- Aderka, D.; Englemann, H.; Hornik, V.; Skornick, Y.; Levo, Y.; Wallach, D.; Kushtai, G. Increased serum levels of soluble receptors for tumor necrosis factor in cancer patients. Cancer Res. 1991, 51, 5602–5607. [Google Scholar]
- Selinsky, C.L.; Boroughs, K.L.; Halsey, W.A.; Howell, M.D. Multifaceted inhibition of anti-tumour immune mechanisms by soluble tumour necrosis factor receptor type I. Immunology 1998, 94, 88–93. [Google Scholar] [CrossRef]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Ren, K.; Torres, R. Role of interleukin-1β during pain and inflammation. Brain Res. Rev. 2009, 60, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Abderrazak, A.; Syrovets, T.; Couchie, D.; El Hadri, K.; Friguet, B.; Simmet, T.; Rouis, M. NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015, 4, 296–307. [Google Scholar] [CrossRef]
- Lin, C.-C.; Edelson, B.T. New Insights into the Role of IL-1β in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. J. Immunol. 2017, 198, 4553–4560. [Google Scholar] [CrossRef]
- Kanneganti, T.-D. Intracellular innate immune receptors: Life inside the cell. Immunol. Rev. 2020, 297, 5–12. [Google Scholar] [CrossRef]
- Radhika, T.; Jeddy, N.; Nithya, S.; Muthumeenakshi, R.M. Salivary biomarkers in oral squamous cell carcinoma—An insight. J. Oral Biol. Craniofac. Res. 2016, 6, S51–S54. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Tayaar, A.S. Analysis of clinical and histopathological profiles of oral squamous cell carcinoma in young Indian adults: A retrospective study. J. Dent. Sci. 2012, 7, 224–230. [Google Scholar] [CrossRef][Green Version]
- Liu, K.Y.P.; Lu, X.J.D.; Zhu, Y.S.; Le, N.; Kim, H.; Poh, C.F. Plasma-Derived Inflammatory Proteins Predict Oral Squamous Cell Carcinoma. Front. Oncol. 2018, 8, 585. [Google Scholar] [CrossRef]
- Sabat, R.; Grütz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Alhamarneh, O.; Agada, F.; Madden, L.; Stafford, N.; Greenman, J. Serum IL10 and circulating CD4(+) CD25(high) regulatory T cell numbers as predictors of clinical outcome and survival in patients with head and neck squamous cell carcinoma. Head Neck 2011, 33, 415–423. [Google Scholar] [CrossRef]
- Arantes, D.A.C.; Costa, N.L.; Mendonça, E.F.; Silva, T.A.; Batista, A.C. Overexpression of immunosuppressive cytokines is associated with poorer clinical stage of oral squamous cell carcinoma. Arch. Oral Biol. 2016, 61, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary Oxidative Stress, Inflammation and Cancer: Respirable Particulate Matter, Fibrous Dusts and Ozone as Major Causes of Lung Carcinogenesis through Reactive Oxygen Species Mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef]
- Roszkowski, K.; Jozwicki, W.; Blaszczyk, P.; Mucha-Malecka, A.; Siomek, A. Oxidative damage DNA: 8-oxoGua and 8-oxodG as molecular markers of cancer. Med. Sci. Monit. 2011, 17, CR329–CR333. [Google Scholar] [CrossRef] [PubMed]
- Agha-Hosseini, F.; Mirzaii-Dizgah, I.; Farmanbar, N.; Abdollahi, M. Oxidative stress status and DNA damage in saliva of human subjects with oral lichen planus and oral squamous cell carcinoma. J. Oral Pathol. Med. 2012, 41, 736–740. [Google Scholar] [CrossRef]
- Korkmaz, K.S.; Butuner, B.D.; Roggenbuck, D. Detection of 8-OHdG as a diagnostic biomarker. J. Lab. Precis. Med. 2018, 3, 95. [Google Scholar] [CrossRef]
- Paredes-Sánchez, E.; Montiel-Company, J.M.; Iranzo-Cortés, J.E.; Almerich-Torres, T.; Bellot-Arcís, C.; Almerich-Silla, J.M. Meta-Analysis of the Use of 8-OHdG in Saliva as a Marker of Periodontal Disease. Dis. Markers 2018, 2018, 7916578. [Google Scholar] [CrossRef]
- Mascitti, M.; Togni, L.; Rubini, C.; Troiano, G.; Lo Muzio, L.; Santarelli, A. Tumour-associated tissue eosinophilia (TATE) in oral squamous cell carcinoma: A comprehensive review. Histol. Histopathol. 2021, 36, 113–122. [Google Scholar] [CrossRef]
- Troiano, G.; Rubini, C.; Togni, L.; Caponio, V.C.A.; Zhurakivska, K.; Santarelli, A.; Cirillo, N.; Lo Muzio, L.; Mascitti, M. The immune phenotype of tongue squamous cell carcinoma predicts early relapse and poor prognosis. Cancer Med. 2020, 9, 8333–8344. [Google Scholar] [CrossRef] [PubMed]
| Reference | Biomarker | Findings | Clinical Relevance |
|---|---|---|---|
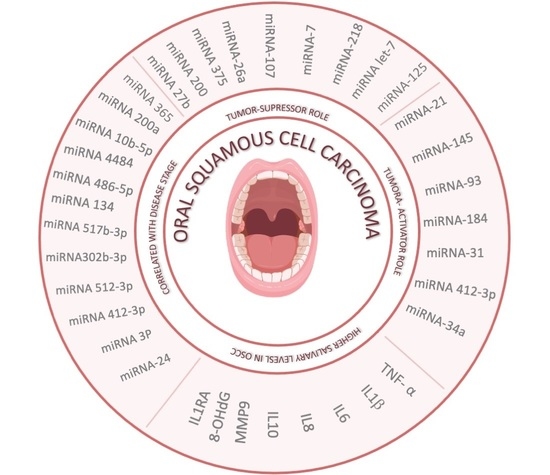
| [8,9,10,11,12] | miRNA-24- miRNA 3P miRNA412-3p miRNA 512-3p miRNA302b-3p miRNA 517b-3p miRNA 134 miRNA 486-5p miRNA 4484 miRNA 10b-5p miRNA 200a miRNA 365 | Correlated with disease stage, histopathological type, and/or grade of OSCC | Possible tool for the early diagnosis of OSCC; in general, the expression of these miRNAs indicates poor prognosis and higher risk of malignant transformation and oral cancer |
| [13,14,15,16,17,18,19,20] | miRNA-27b miRNA-200 miRNA-375 miRNA-26a miRNA-7 miRNA-107 miRNA-218 miRNA let-7 miRNA-125 | Tumor-suppressor role | Increased levels of these biomarkers can be useful for the diagnosis, staging, and prognosis of OSCC; the expression of these miRNAs reduce the progession of OSCC |
| [1,21,22,23,24,25,26] | miRNA-21 miRNA-145 miRNA-93 miRNA-184 miRNA-31 miRNA-412-3p miRNA-34a | Tumor-activator role; these miRNAs acts as an oncogenes, promoting OSCC development and progression | Elevated concentrations in the saliva offer a reliable method to detect OSCC and potentially malignant oral lesions |
| Reference | Biomarker | Findings | Clinical Relevance |
|---|---|---|---|
| [38] | IL-6, IL-8 & TNF-α | Notably higher levels of these cytokines in advanced stages of OSCC compared to early stages of the disease; presence of neck metastases associated with increased levels of these molecules. | Possible tool to indicate OSCC progression |
| [39] | MMP-9 | Elevated salivary levels of MMP-9 were associated with OSCC Levels of the biomarker decreased dramatically after tumor surgery | MMP-9 as a critical diagnostic and prognostic biomarker for OSCC |
| [40] | IL-6, IL-8, IL-1β & TNF-α | Significant differences in levels of IL-6, IL-8, IL-1β, and TNF-α between OSCC patients and to controls | Useful complementary tool for the early detection of OSCC |
| [41] | IL-8 | Significantly increased levels of the cytokine in patients with head and neck squamous cell carcinoma; IL-8 levels were positively correlated with the abundance of C. albicans | A salivary microbial and inflammatory biomarker of head and neck squamous cell carcinoma that is influenced by oral health |
| [42] | IL-6 & IL-8 | Correlation of qualitative salivary detection of IL-6 and IL-8 between control and disease groups | Probable biomarker for detection of premalignant lesions and OSCC |
| [43] | IL-6 & TNF-α | Elevated levels of those cytokines compared to age-matched controls | IL-6 and TNF-α are potential biomarkers for the monitorization of OSCC |
| [44] | MMP-9 | MMP-9 levels significantly higher in OSCC patients than in controls or patients with premalignant lesions | Salivary diagnostic biomarker for the detection of premalignant oral lesions and early stages of OSCC |
| [45] | MMP-9 | Higher levels of MMP-9 in OSCC patients than in controls | MMP-9 is a good tool for the detection of OSCC |
| [46] | IL-8 | Protein concentration of IL-8 was significantly elevated in patients with OSCC than in those with dysplasia and controls | Important marker to discriminate between OSCC and control patients; IL-8 combined with H3F3A mRNA provides good discrimination between OSCC and potentially malignant oral disorders |
| [47] | IL-6, IL-8 & TNF-α | Increased levels of these cytokines in patients with oral leukoplakia, submucous fibrosis, and lichen planus than in healthy controls | Diagnostic tool for the detection of premalignant lesions |
| [48] | IL-6 | Higher pretreatment levels of IL-6 in patients with oral cancer, associated with better survival | Possible prognosis biomarker |
| [49] | IL-6 | Higher salivary levels of IL-6 in OSCC patients when compared with patients with chronic periodontitis, active oral lichen planus, inactive oral lichen planus, or healthy controls | Useful biomarker for the detection of OSCC |
| [50] | TNF-α | Increased serum and saliva TNF-α levels in OSCC patients compared with controls and those with premalignant disease | TNF-α as a useful biomarker for OSCC detection; increased levels are associated with histological grade and clinical stage, suggesting a role in the prognosis of OSCC |
| [51] | IL-6 | Increased levels | Monitoring of OSCC |
| [48] | TNF-α | No differences between control and OSCC group | - |
| [52] | IL-6, IL-8 & TNF-α | Higher levels in endophytic squamous cell carcinoma of the tongue than in exophytic squamous cell carcinoma of the tongue, correlated with decreased survival in the endophytic versus exophytic group; IL-6, IL-8, and TNF-α also higher in the exophytic group than in smoking and drinking controls | These biomarkers can identify the progression of squamous cell carcinoma of the tongue from high risk to neoplasm; important biomarker for cancer screening and early detection; correlation between these proteins and survival implies a prognostic benefit potentially useful for management decisions and future target treatments |
| [53] | IL-6 & IL-8 | Higher expression in patients with OSCC | Potential tool for OSCC diagnosis |
| [54] | IL-6, IL-8 & TNF-α | Increased levels in patients with OSCC and premalignant oral lesions | Proangiogenic and proinflammatory cytokines are elevated in patients with these lesions; diagnosis and prognosis significance of these markers |
| [55] | IL-8 & IL-1β | Increased levels in patients with OSCC | Potential use as a diagnostic tool for OSCC |
| [56] | IL-8 & IL-1β | Higher levels in OSCC patients, depending on the tumor stage | Increased levels of these biomarkers can be useful for OSCC diagnosis, staging, and prognosis |
| [57] | IL-1β | Levels significantly differ between before and after surgery | IL-1β levels may be useful for the detection of early stage OSCC |
| [58] | IL-1-Ra | Expression of IL-1-Ra is lower in OSCC and oral dysplasia cells than in normal cells | Possible use as a biomarker for prediction of malignant transformation |
| [59] | IL-1-Ra | Expression of IL-1-Ra decreases gradually with the progression of oral dysplasia | IL-1-Ra could be a reliable biomarker for the early diagnosis and follow-up of OSCC; it could be useful to discriminate between premalignant oral lesions and OSCC |
| [60] | IL-1-Ra & IL-10 | Salivary IL-10 levels are higher in OSCC patients; IL-1-Ra levels are lower in well-defined tumors than in immature tumors | IL-10 is an interesting tool for diagnosing OSCC, and IL-1-Ra can be helpful for cancer staging |
| [61] | IL-10 | High levels of IL-10 expression are found in OSCC, especially in advanced stage tumors and metastatic cells | Salivary IL-10 levels could be used as a biomarker for OSCC diagnosis; a high concentration appears to favor tumor proliferation and dissemination |
| [62] | IL-10 | High levels of IL-10 expression correlate with shorter survival, worse prognosis, and increased risk of death | Overexpression of IL-10 is associated with aggressive forms of OSCC, and its level can be used as a survival predictor |
| [63] | IL-10 | IL-10 levels increase with tumor progression | Useful as staging biomarker |
| [64] | 8-OHdG | 8-OHdG levels are approximately two-fold higher in patients with squamous head and neck cancer than in healthy controls | Quantification of 8-OHdG levels could be used as a diagnostic tool for OSCC |
| [65] | 8-OHdG | 8-OHdG levels are more than two-fold higher in in OSCC patients than in controls | 8-OHdG can be used as DNA damage biomarker to assess disease progression |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzano-Moreno, F.J.; Costela-Ruiz, V.J.; García-Recio, E.; Olmedo-Gaya, M.V.; Ruiz, C.; Reyes-Botella, C. Role of Salivary MicroRNA and Cytokines in the Diagnosis and Prognosis of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2021, 22, 12215. https://doi.org/10.3390/ijms222212215
Manzano-Moreno FJ, Costela-Ruiz VJ, García-Recio E, Olmedo-Gaya MV, Ruiz C, Reyes-Botella C. Role of Salivary MicroRNA and Cytokines in the Diagnosis and Prognosis of Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2021; 22(22):12215. https://doi.org/10.3390/ijms222212215
Chicago/Turabian StyleManzano-Moreno, Francisco Javier, Victor J. Costela-Ruiz, Enrique García-Recio, Maria Victoria Olmedo-Gaya, Concepción Ruiz, and Candelaria Reyes-Botella. 2021. "Role of Salivary MicroRNA and Cytokines in the Diagnosis and Prognosis of Oral Squamous Cell Carcinoma" International Journal of Molecular Sciences 22, no. 22: 12215. https://doi.org/10.3390/ijms222212215
APA StyleManzano-Moreno, F. J., Costela-Ruiz, V. J., García-Recio, E., Olmedo-Gaya, M. V., Ruiz, C., & Reyes-Botella, C. (2021). Role of Salivary MicroRNA and Cytokines in the Diagnosis and Prognosis of Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences, 22(22), 12215. https://doi.org/10.3390/ijms222212215