Novel Pituitary Actions of TAC4 Gene Products in Teleost
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Chemicals
2.2. Molecular Cloning and Tissue Distribution of Grass Carp TAC4 and NKRs
2.3. Functional Expression of Grass Carp NKRs in HEK293T Cells
2.4. Transcriptome Sequence and Bioinformatics
2.5. Real-Time Quantitative PCR (RT-qPCR) Validation
2.6. Statistical Analysis
3. Result
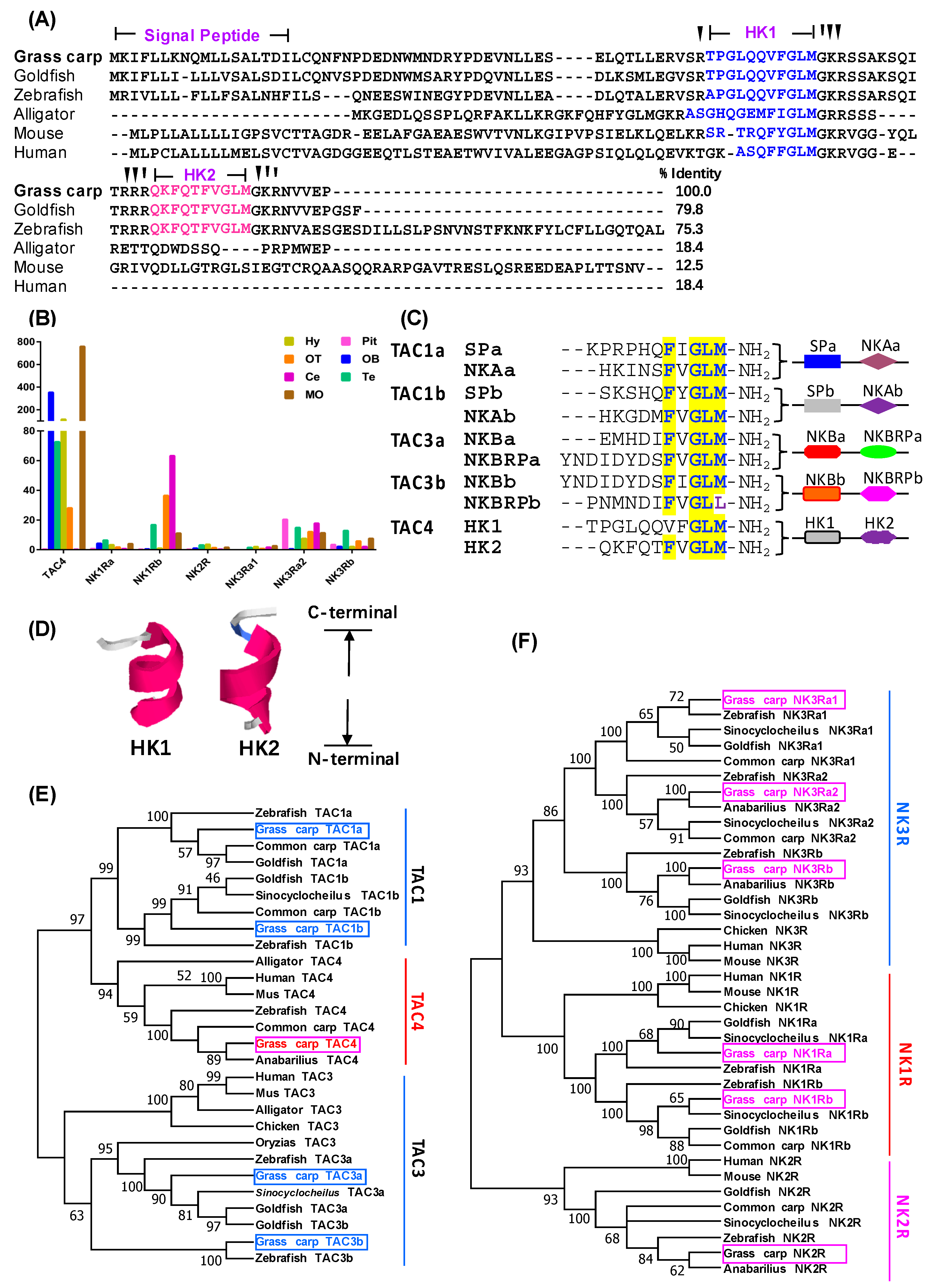
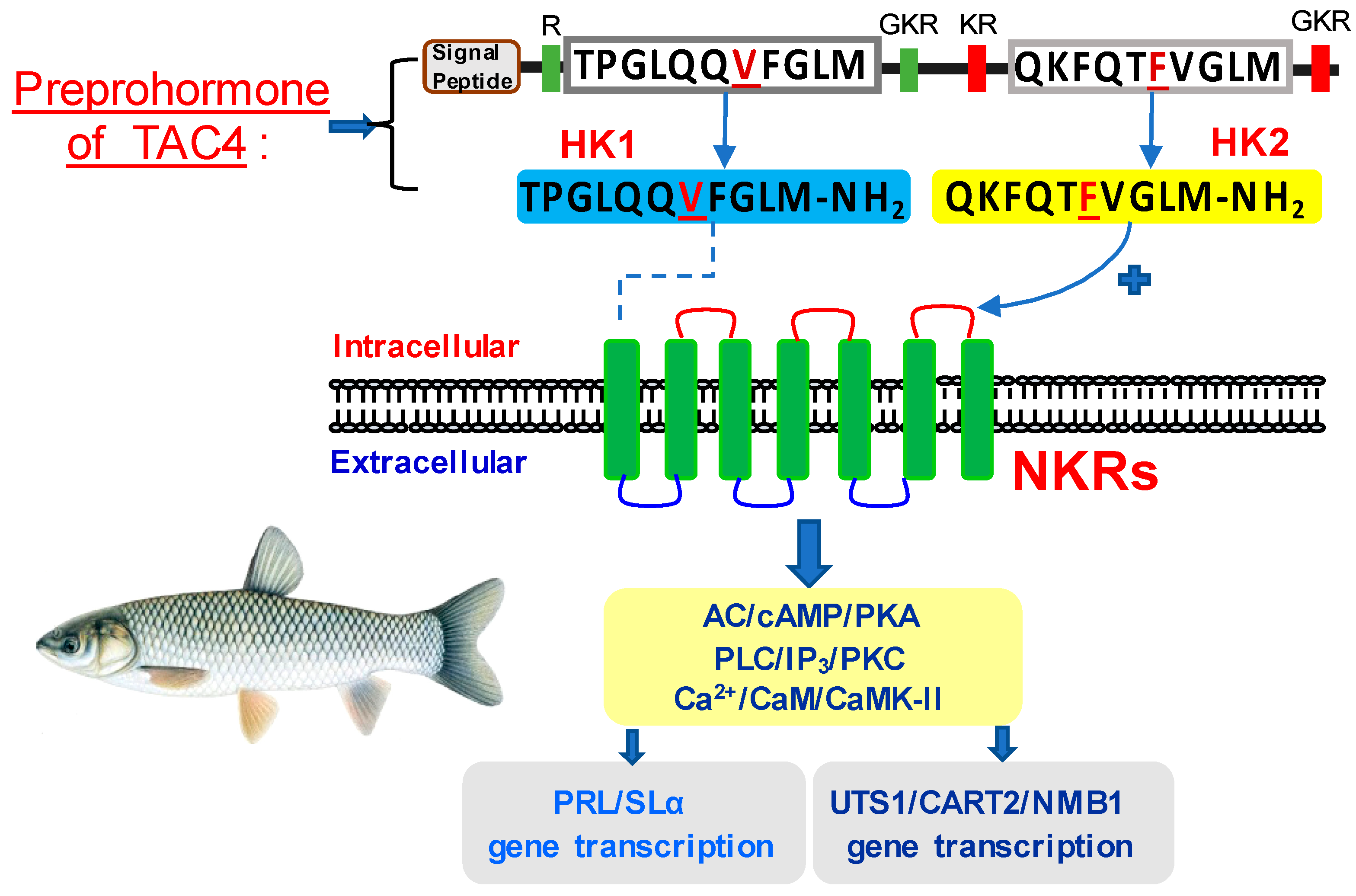
3.1. Sequence Analysis and Tissue Distribution of TAC4 and NKRs
3.2. Receptor Selectivity of TAC4 Gene Products
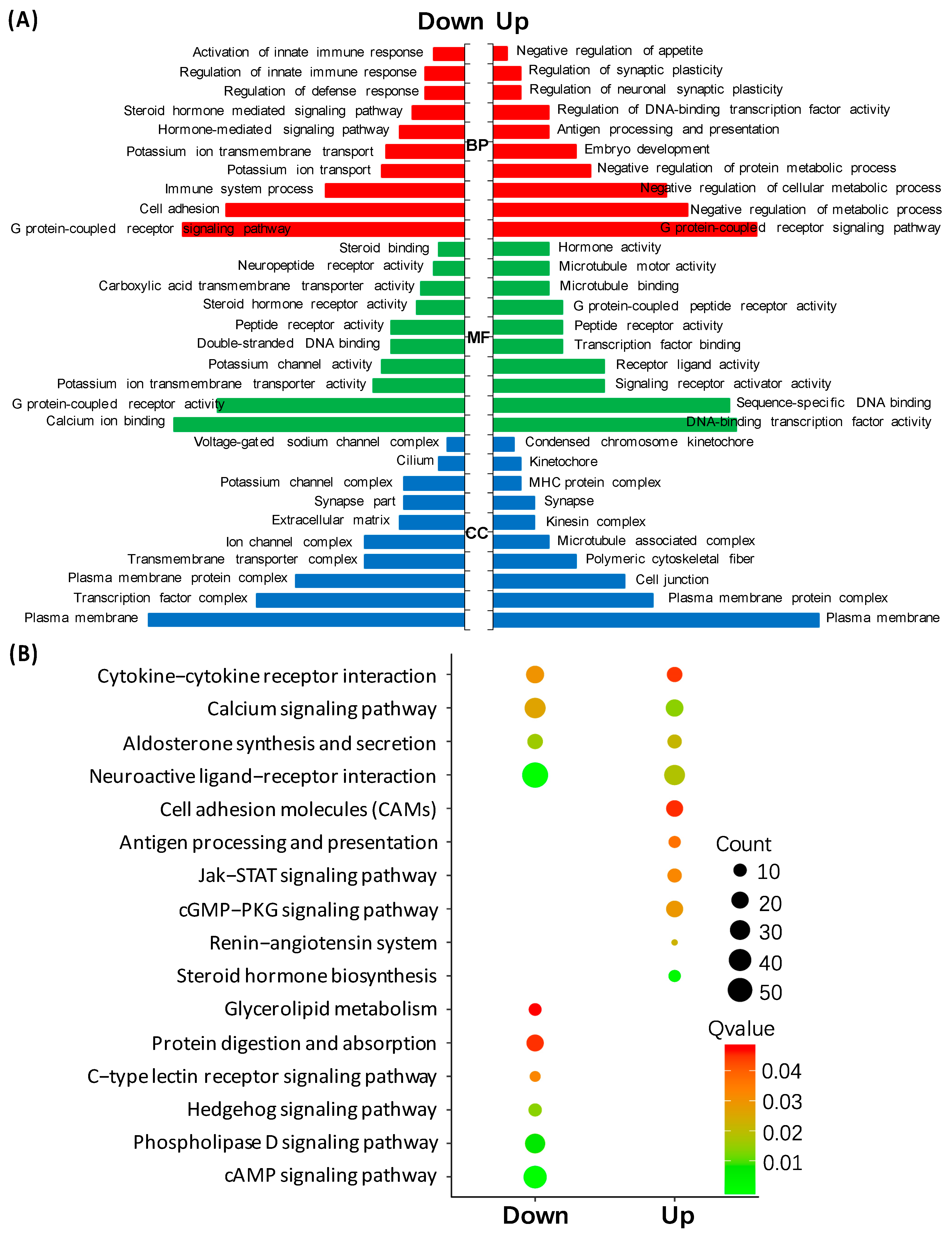
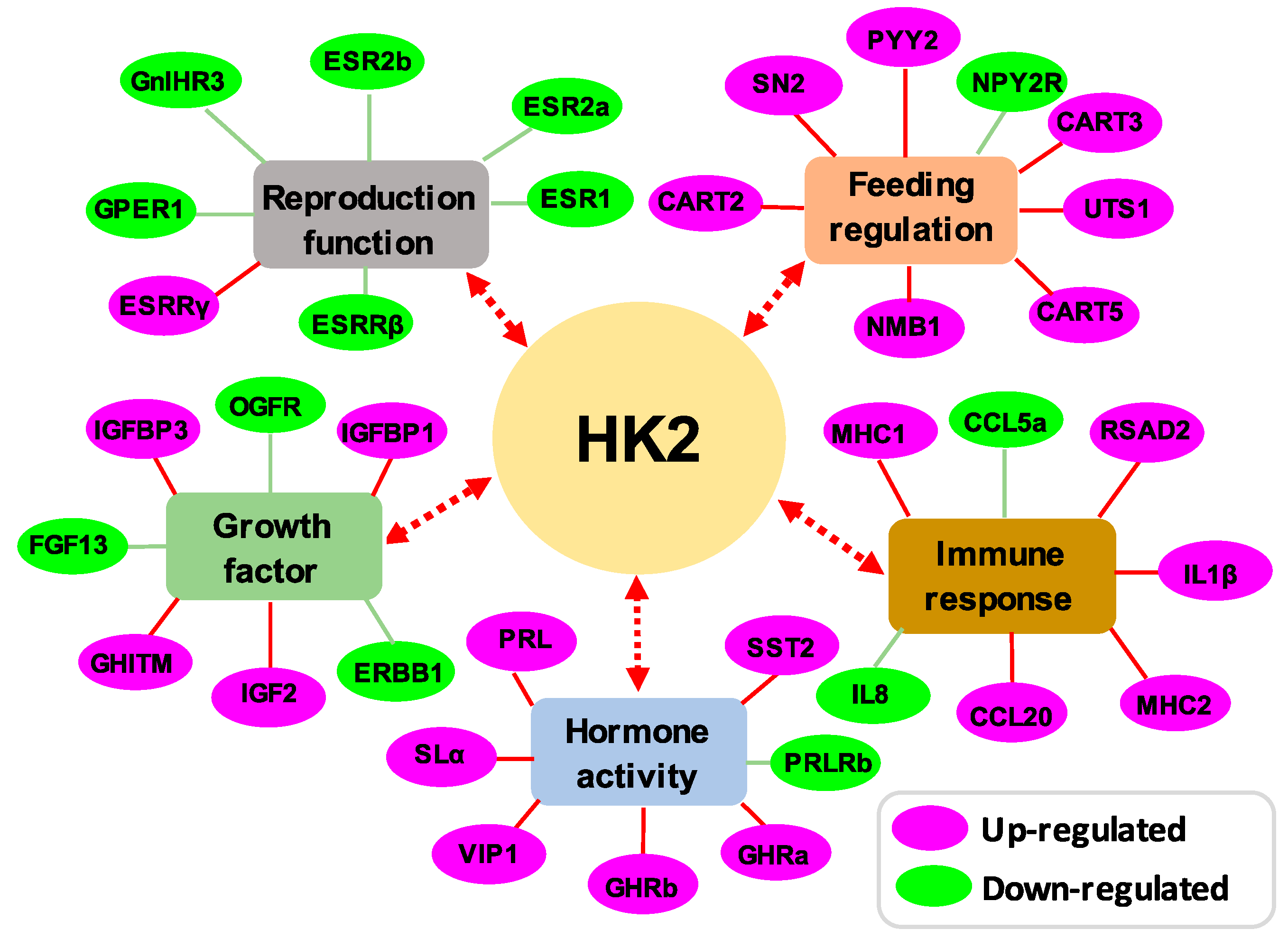
3.3. Transcriptomic Analysis of HK2 in the Pituitary
3.4. Regulation of HK1 and HK2 on the Key DEGs Expression in Grass Carp Pituitary Cells
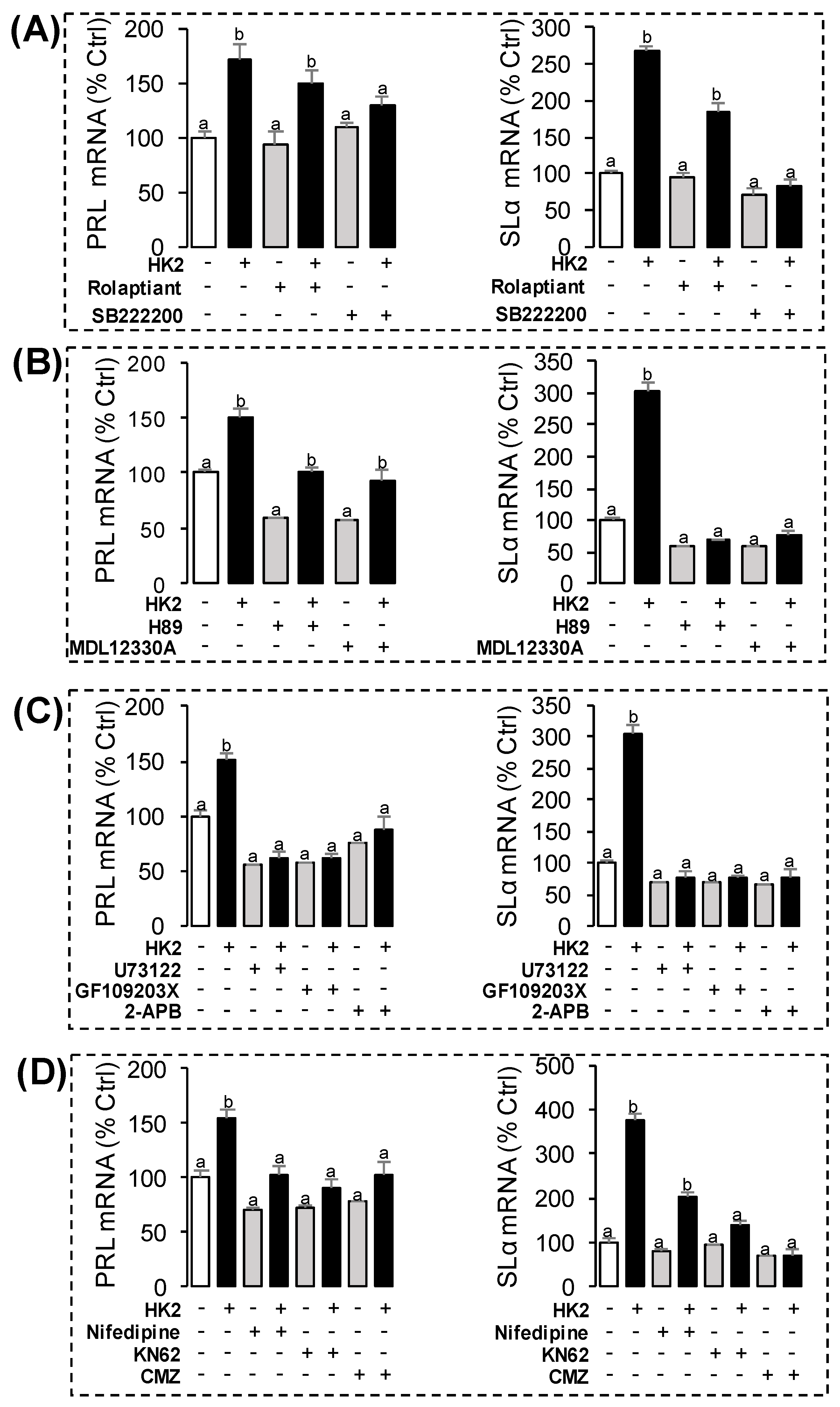
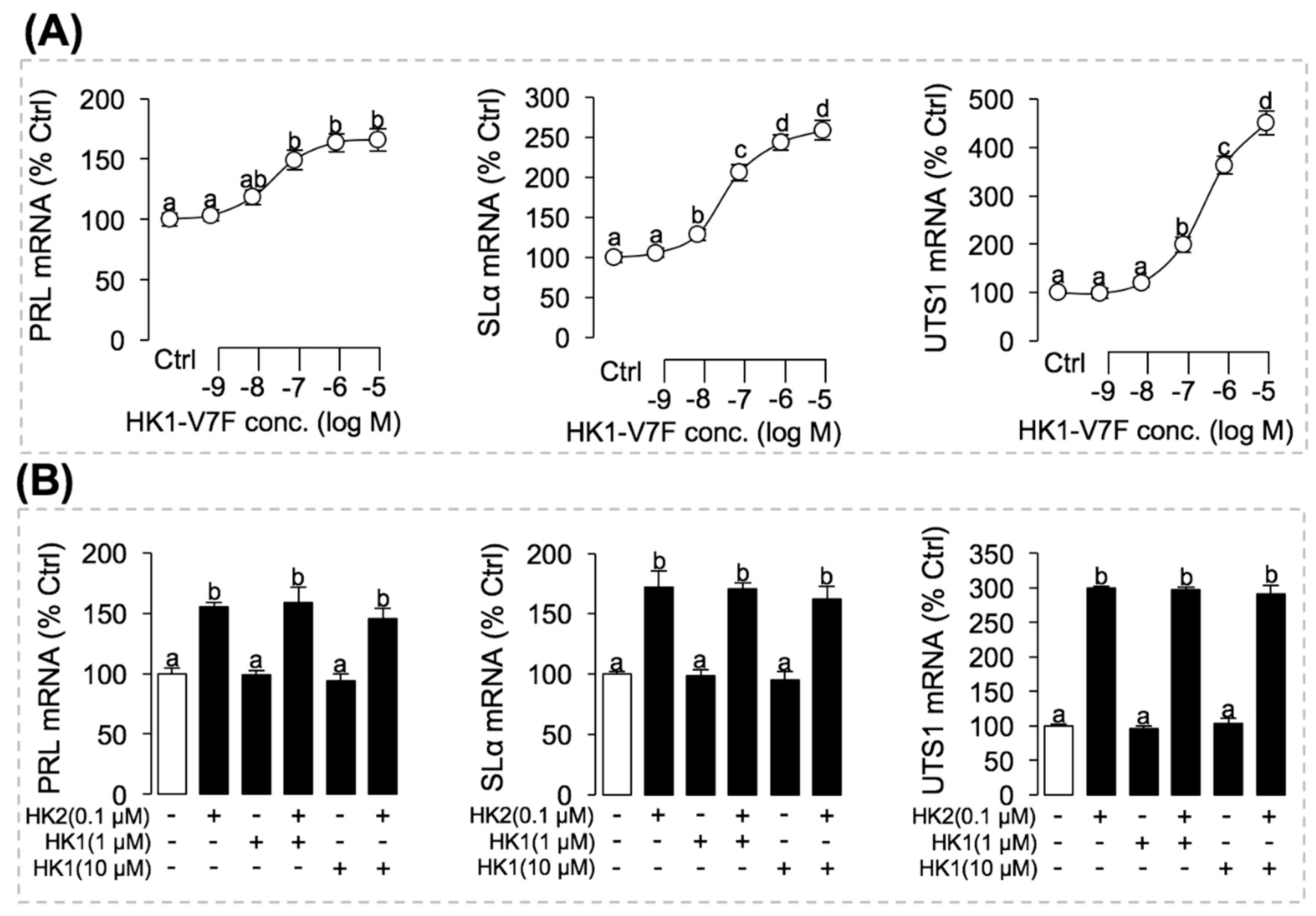
3.5. Receptor Specificity and Post-Receptor Signal Transduction for HK2-Induced SLα and PRL mRNA Expression in Grass Carp Pituitary Cells
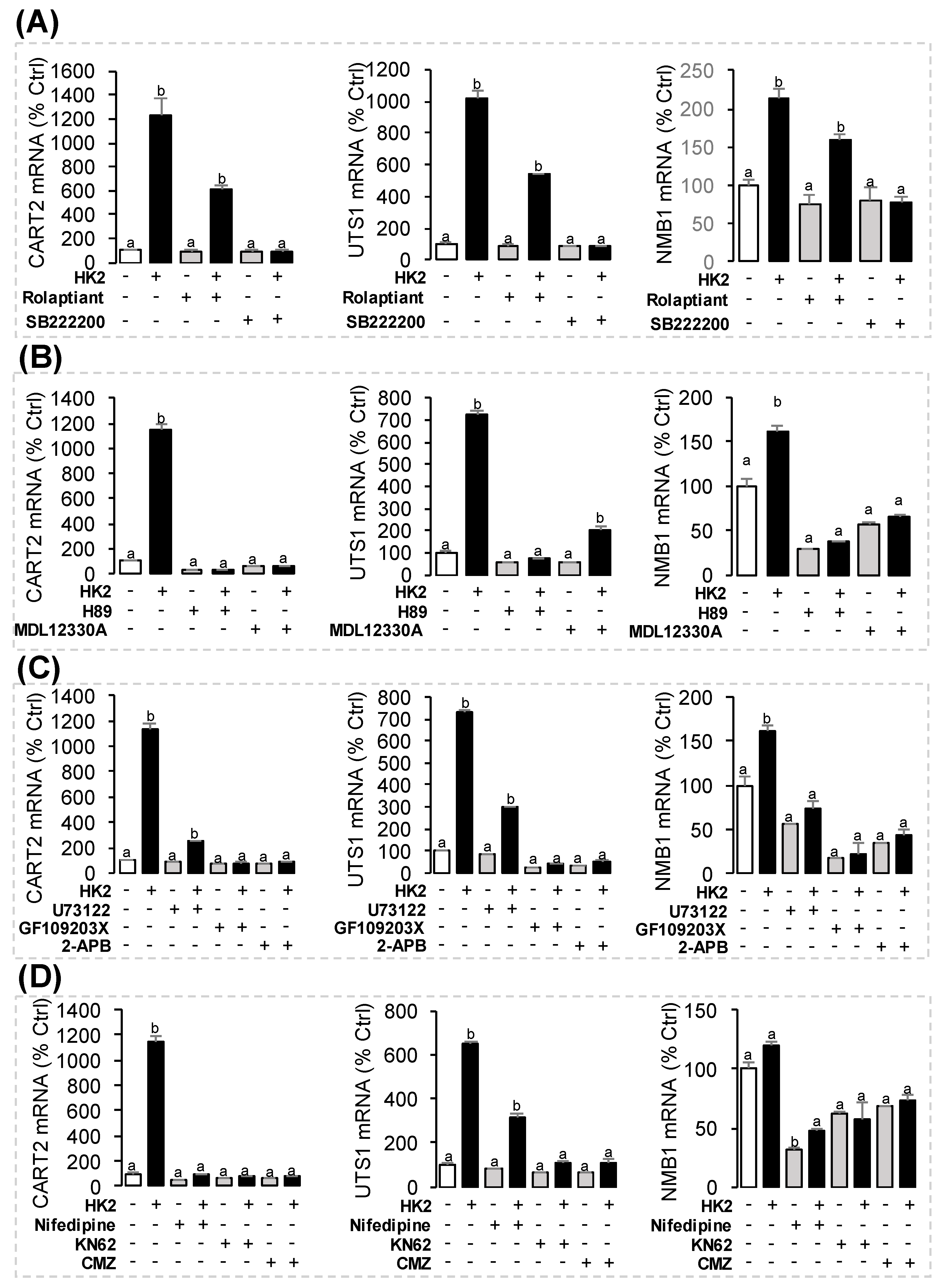
3.6. Receptor Specificity and Signal Transduction for HK2-Induced UTS1, NMB1 and CART2 mRNA Expression
3.7. Phe to Val Mutation in FXGLM Motif in UTS1, PRL and SLα Regulation by HK2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lin, C.; Chen, W.N.; Chen, C.J.; Lin, Y.W.; Zimmer, A.; Chen, C.C. An antinoci-ceptive role for substance P in acid-induced chronic muscle pain. Proc. Natl. Acad. Sci. USA 2012, 109, E76–E83. [Google Scholar] [CrossRef] [Green Version]
- Ang, S.F.; Moochhala, S.M.; Macary, P.A.; Bhatia, M. Hydrogen sulfide and neurogenic inflammation in polymicrobial sepsis: Involvement of substance P and ERK-NF-κB signaling. PLoS ONE 2017, 6, e24535. [Google Scholar] [CrossRef]
- Chitrang, T.; Shan, X.; Loraine, Y.C.; Dhiraj, K.; Jenna, H.; Sarah, A.; Kristy, H.; Henriette, K.; Yeo, G.S.H.; Diego, P.T. Tachykinin-1 in the central nervous system regulates adiposity in rodents. Endocrinology 2015, 156, 1714–1723. [Google Scholar]
- Meyer, A.; Schartl, M. Gene and genome duplications in vertebrates: The one-to-f-our (-to-eight in fish) rule and the evolution of novel gene functions. Curr. Opin. Cell Biol. 1999, 11, 699–704. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.F.; He, M.; Ko, W.; Wong, A. TAC1 GeneProducts RegulatePituitary Hormone Secretion and Gene Expression in Prepubertal Grass Carp Pituitary Cells. Endocrinology 2017, 158, 1776–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelrahman, A.M.; Pang, C. Effect of Substance P on Venous Tone in Conscious Rats. J. Cardiovac. Pharmacol. 2005, 45, 49. [Google Scholar] [CrossRef]
- Lecci, A.; Capriati, A.; Altamura, M.; Maggi, C.A. Tachykinins and tachykinin receptors in the gut, with special reference to NK2 receptors in human. Auton. Neurosci. 2006, 126, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.K.; Kani, S.; Shimizu, T.; Tanabe, K.; Nojima, H.; Kimura, Y.; Higashijima, S.I.; Hibi, M. Anatomy of zebrafish cerebellum and screen for mutations affecting its development. Dev. Biol. 2009, 330, 406–426. [Google Scholar] [CrossRef] [Green Version]
- Topaloglu, A.K.; Reimann, F.; Guclu, M.; Yalin, A.S.; Semple, R.K. Tac3 and tacr3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin b in the central control of reproduction. Nat. Genet. 2009, 41, 354–358. [Google Scholar] [CrossRef] [Green Version]
- Goodman, R.L.; Coolen, L.M.; Lehman, M.N. A role for neurokinin b in pulsatile gnrh secretion in the ewe. Neuroendocrinology 2014, 99, 18. [Google Scholar] [CrossRef]
- Hu, G.F.; Lin, C.; He, M.; Wong, A. Neurokinin B and reproductive functions: “KNDy neuron” model in mammals and the emerging story in fish. Gen. Comp. Endocrinol. 2014, 208, 94–108. [Google Scholar] [CrossRef] [Green Version]
- Biran, J.; Palevitch, O.; Ben-Dor, S.; Levavi-Sivan, B. Neurokinin Bs and neurokinin B receptors in zebrafish-potential role in controlling fish reproduction. Proc. Natl. Acad. Sci. USA 2012, 109, 10269–10274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Lu, L.; Furlonger, C.; Wu, G.; Paige, C. Hemokinin is a hematopoieticspecific tachykinin that regulates B lymphopoiesis. Nat. Immunol. 2000, 1, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, M.M.; Wang, R.; Clements, M.K.; Cascieri, M.A.; Austin, C.P.; Cunningh-am, B.R.; Chicchi, G.G.; Liu, Q. Identification, localization and receptor characterization of novel mammalian substance P-like peptides. Gene 2002, 296, 205–212. [Google Scholar] [CrossRef]
- Page, N.M.; Bell, N.J.; Gardiner, S.M.; Manyonda, I.T.; Brayley, K.J.; Strange, P.G.; Lowry, P.J. Characterization of the endokinins: Human tachykinins with cardiovascular activity. Proc. Natl. Acad. Sci. USA 2003, 100, 6245–6250. [Google Scholar] [CrossRef] [Green Version]
- Steinhoff, M.S.; Mentzer, B.V.; Geppetti, P.; Pothoulakis, C.; Bunnett, N.W. Tac-hykinins and their receptors: Contributions to physiological control and the mechanisms of disease. Physiol. Rev. 2014, 94, 265–301. [Google Scholar] [CrossRef] [Green Version]
- Zieglgänsberger, W. Substance P and pain chronicity. Cell Tissue Res. 2019, 375, 227–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, L.; Perera, D.S.; King, D.W.; Southwell, B.R.; Burcher, E.; Liu, L. Hemoki-nin-1 stimulates prostaglandin E₂ production in human colon through activationof cyclooxygenase-2 and inhibition of 15-hydroxyprostaglandin dehydrogenase. J. Pharmacol. Exp. Ther. 2012, 340, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Tsilioni, I.; Russell, J.; Stewart, R.; Gleason, T.; Theoharides, T.C. Neuropeptides CRH, SP, HK-1, and Inflammatory Cytokines IL-6 and TNF Are Increased in Serum of Patients with Fibromyalgia Syndrome, Implicating Mast Cells. J. Pharmacol. Exp. Ther. 2016, 356, 664–672. [Google Scholar] [CrossRef] [Green Version]
- Borbély, É.; Helyes, Z. Role of hemokinin-1 in health and disease. Neuropeptides 2017, 64, 9–17. [Google Scholar] [CrossRef]
- Wong, A.O.; Ng, S.; Lee, E.K.; Leung, R.C.; Ho, W.K. Somatostatin inhibits (d-Arg6, Pro9-NEt) salmon gonadotropin-releasing hormone and dopamine D1-stim-ulated growth hormone release from perifused pituitary cells of chinese grass carp, Ctenopharyngodon idellus. Gen. Comp. Endocrinol. 1998, 110, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Penn, E.F.; Ather, J.N.; Lecci, A.; Candenas, M.L.; Patak, E.; Pinto, F.M.; Maggi, C.A. Tachykinins and tachykinin receptors: A growing family. Life Sci. 2004, 74, 1445–1463. [Google Scholar]
- Hu, G.; He, M.; Ko, W.K.W.; Lin, C.; Wong, A.O.L. Novel pituitary actions of TAC3 gene products in fish model: Receptor specificity and signal transduction for prolactin and somatolactin α regulation by neurokinin B (NKB) and NKB-related peptide in carp pituitary cells. Endocrinology 2014, 155, 3582–3596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X. Rapid subfunctionalization accompanied by prolonged and substantial n-eofunctionalization in duplicate gene evolution. Genetics 2005, 169, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Khorasani, S.; Boroumand, N.; Arab, F.L.; Hashemy, S.I. The immunomodulatory effects of tachykinins and their receptors. J. Cell. Biochem. 2020, 121, 3031–3041. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, S. Effects of Endokinin A/B and Endokinin C/D on the modulation of pain in mice. Peptides 2010, 31, 94–100. [Google Scholar] [CrossRef]
- Naono, R.; Nakayama, T.; Ikeda, T.; Matsushima, O.; Nishimori, T. Effect of the carboxyl-terminal of endokinins on SP-induced pain-related behavior. Biochem. Biophys. Res. Commun. 2009, 378, 182–185. [Google Scholar] [CrossRef]
- Almeida, T.A.; Rojo, J.; Nieto, P.M.; Pinto, F.M.; Hernandez, M.; Martín, J.D.; Candenas, M.L. Tachykinins and tachykinin receptors: Structure and activity relationships. Curr. Med. Chem. 2004, 11, 2045–2081. [Google Scholar] [CrossRef]
- Duffy, R.A.; Hedrick, J.A.; Randolph, G.; Morgan, C.A.; Cohen-Williams, M.E.; Vassileva, G.; Lachowicz, J.E.; Laverty, M.; Maguire, M.; Shan, L.S. Centrally administered hemokinin-1 (HK-1), a neurokinin NK1 receptor agonist, produces substance P-like behavioral effects in mice and gerbils. Neuropharmacology 2003, 45, 242–250. [Google Scholar] [CrossRef]
- Morteau, O.; Lu, B.; Gerard, C.; Gerard, N.P. Hemokinin 1 is a full agonist at the substance p receptor. Nat. Immunol. 2002, 2, 1088. [Google Scholar] [CrossRef]
- Bellucci, F.; Carini, F.; Catalani, C.; Cucchi, P.; Lecci, A.; Meini, S.; Patacchini, R.; Quartara, L.; Ricci, R.; Tramontana, M.; et al. Pharmacological profile of the novel mammalian tachykinin, hemokinin 1. Br. J. Pharmacol. 2002, 135, 266–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aczél, T.; Kecskés, A.; Kun, J.; Szenthe, K.; Bánáti, F.; Szathmary, S.; Herczeg, R.; Urbán, P.; Gyenesei, A.; Gaszner, B.; et al. Hemokinin-1 Gene Expression Is Upregulated in Trigeminal Ganglia in an Inflammatory Orofacial Pain Model: Potential Role in Peripheral Sensitization. Int. J. Mol. Sci. 2020, 21, 2938. [Google Scholar] [CrossRef] [PubMed]
- Onuma, T.A.; Ban, M.; Makino, K.; Katsumata, H.; Hu, W.W.; Ando, H.; Fukuwaka, M.A.; Azumaya, T.; Urano, A. Changes in gene expression for GH/PRL/SL family hormones in the pituitaries of homing chum salmon during ocean migration through upstream migration. Gen. Comp. Endocrinol. 2010, 166, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Degani, G.; Yom-Din, S.; Goldberg, D.; Jackson, K. cDNA cloning of blue gour-ami (Trichogaster trichopterus) prolactin and its expression during the gonadal cycles of males and females. J. Endocrinol. Investig. 2010, 33, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Tse, M.; Wong, G.; Ping, X.; Cheng, C.; Chan, K.M. Down-regulation of goldfis-h (Carassius auratus) prolactin gene expression by dopamine and thyrotropin releasing hormone. Gen. Comp. Endocrinol. 2008, 155, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Noh, G.E.; Rho, S.; Yong, J.C.; Min, B.H. Gene encoding prolactin in cinnamon clownfish Amphiprion melanopus and its expression upon acclimation to low salinities. Aquat. Biosyst. 2013, 9, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whittington, C.M.; Wilson, A.B. Wilson. The role of prolactin in fish reproduct-ion. Gen. Comp. Endocrinol. 2013, 191, 123–136. [Google Scholar] [CrossRef] [Green Version]
- Shu, Y.Q.; Lou, Q.Y.; Dai, Z.R.; Dai, X.Y.; He, J.Y.; Hu, W.; Yin, Z. The basal function of teleost prolactin as a key regulator on ion uptake identified with zebrafish knockout models. Sci. Rep. 2016, 6, 18579. [Google Scholar] [CrossRef]
- Benedet, S.; Björnsson, B.T.; Taranger, G.L.; Andersson, E. Cloning of somatolactin alpha, beta forms and the somatolactin receptor in Atlantic salmon: Seasonalexpression profile in pituitary and ovary of maturing female broodstock. Reprod. Biol. Endocrinol. 2008, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Wan, G.H.; Chan, K.M. A study of somatolactin acitons by ectopic expression in transgenic zebrafish larvae. J. Mol. Endocinol. 2010, 45, 301–315. [Google Scholar] [CrossRef] [Green Version]
- Bertolesi, G.E.; McFarlane, S. Melanin-concentrating hormone like and somatolactin. Ateleost-specific hypothalamic-hypophyseal axis system linking physiologicaland morphological pigmentation. Pigment Cell Melanoma Res. 2021, 34, 564–574. [Google Scholar]
- Xu, S.H.; Zhou, L.L.; Chen, X.Y.; Hu, Q.Y.; Shi, X.T.; Xia, C.H.; Zhang, H.Y.; Jia, J.Y.; Yin, Z.; Hu, G.F. Novel pituitary actions of NKB for anorectic peptides regulation in grass carp. Aquaculture 2021, 531, 735857. [Google Scholar] [CrossRef]
- Lambert, R.; Couceyro, R.; Koylu, E.; Ling, N.; Kuhar, M. A role for novel CART peptide fragments in the central control of food intake. Neuropeptides 1997, 31, 620–621. [Google Scholar]
- Volkoff, H.; Peter, R.E. Effects of CART peptides on food consumption, feedingand associated behaviors in the goldfish, Carassius-auratus: Actions on neuro-peptide Y and orexin A-induced feeding. Brain Res. 2000, 887, 125–133. [Google Scholar] [CrossRef]
- Tachibana, T.; Matsuda, K.; Khan, S.I.; Ueda, H.; Cline, M.A. Feeding and drin-king response following central administrations of bombesin-like peptidesin chi-cks. Comp. Biochem. Physiol. Part A 2010, 156, 394–399. [Google Scholar] [CrossRef]
- Sayegh, A.I. The role of bombesin and bombesin-related peptides in the shortt-erm control of food intake. Prog. Mol. Biol. Transl. 2013, 114, 343–370. [Google Scholar]
- Kuperman, Y.; Chen, A. Urocortins: Emerging metabolic and energy homeostasi-s perspectives. Trends Endocrinol. Metab. 2008, 19, 122–129. [Google Scholar] [CrossRef]
- Bernier, N.J.; Peter, R.E. Appetite-suppressing e ects of urotensin I and cortico- tropin-releasing hormone in goldfish (Carassius auratus). Neuroendocrinology 2001, 73, 248. [Google Scholar] [CrossRef]
- Page, N.M. Hemokinins and endokinins. Cell. Mol. Life Sci. 2004, 61, 1652–1663. [Google Scholar] [CrossRef]
- Zhou, W.; Li, S.; Liu, Y.; Qi, X.; Chen, H.; Cheng, C.H.K.; Liu, X.; Zhang, Y.; Lin, H. The evolution of tachykinin/tachykinin receptor (TAC/TACR) in vertebrates and molecular identification of the TAC3/TACR3 system in zebrafish (Danio rerio). Mol. Cell. Endocrinol. 2012, 361, 202–212. [Google Scholar] [CrossRef]
- Grace, R.C.; Chandrashekar, I.R.; Cowsik, S.M. Solution structure of the tachyk-inin peptide eledoisin. Biophys. J. 2003, 84, 655–664. [Google Scholar] [CrossRef] [Green Version]
- Ganjiwale, A.D.; Rao, G.S.; Cowsik, S.M. Molecular modeling of neurokinin B and tachykinin NK3 receptor complex. J. Chem. Inf. Model. 2011, 51, 2932–2938. [Google Scholar] [CrossRef] [PubMed]
- Naono, R.; Nakayama, T.; Ikeda, T.; Matsushima, O.; Nishimori, T. Leucine at the carboxyl-terminal of endokinins C and D contributes to elicitation of the antagonistic effect on substance P in rat pain processing. Brain Res. 2007, 1165, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, D.; Takebuchi, F.; Nishimori, T.; Naono, R.; Ikeda, T.; Nakayama, T. Intrathecal administration of the common carboxyl-terminal decapeptide in end-okinin A and endokinin B evokes scratching behavior and thermal hyperalgesiain therat. Neurosci. Lett. 2006, 410, 193–197. [Google Scholar] [CrossRef] [PubMed]



| Gene | Description | FC | Go-Biological Process |
|---|---|---|---|
| CART2 | Cocaine and amphetamine-regulated transcript 2 precursors | 27.23 | Negative regulation of appetite |
| CART5 | Cocaine and amphetamine-regulated transcript 5 precursors | 1.23 | Negative regulation of appetite |
| CKB | Brain creatine kinase b | 1.63 | Creatine Kinase Activity |
| CRFB2 | Cytokine receptor family member b2 precursor | 1.77 | Interferon receptor activity |
| GHITM | Growth hormone-induced transmembrane protein | 1.21 | The components of the membrane |
| GPR186 | G protein-coupled receptor 186 | 1.83 | Signal sensor activity |
| IGFBP1 | Insulin-like growth factor-binding protein 1 | 1.59 | Cell growth regulation |
| MPR63 | Mitochondrial ribosomal protein 63 | 1.35 | Mitochondrial Ribosome |
| NLRP12 | Proteins containing NACHT, LRR, and PYD domains 12 | 2.73 | ATP binding |
| NMB1 | Neurotonin B1 | 1.50 | Neuropeptide signaling pathway |
| NPAS4 | Neuron contains PAS domain protein 4 | 2.69 | DNA binding |
| NSFb | N-ethylmaleimide sensitive factor b | 1.94 | ATP binding |
| PRL1 | Prolactin 1 | 1.71 | Hormonal activity |
| RBM24 | RNA binding protein 24 | 2.92 | Nucleotide binding |
| SDC2 | Heparan sulfate proteoglycan precursor | 1.62 | Gastrointestinal development |
| SERPINE2 | Glial cell-derived neuron precursor | 1.33 | Extracellular space |
| SID4 | Secreted immunoglobulin domain 4 precursor | 3.89 | Same protein binding |
| SLα | Somatostatin alpha subunit | 1.81 | Hormonal activity |
| SN2 | Secretagogue 2 precursor | 1.78 | Secretory granules |
| UTS1 | Urotensin 1 | 7.31 | Hormonal activity |
| Gene | Description | FC | Go-Biological Process |
|---|---|---|---|
| AIG1 | Androgen inducible protein 1 | 0.77 | The components of the membrane |
| CALCRLb | Calcitonin gene-related peptide-like receptor b | 0.75 | Calcitonin receptor activity |
| DHRS13 | Dehydrogenase/reductase SDR family member 13 | 0.43 | Oxidoreductase activity |
| FGF14 | Fibroblast Growth Factor 14 | 0.80 | Growth factor activity |
| FGFR2 | Fibroblast Growth Factor Receptor 2 | 0.46 | Fibroblast growth factor binding |
| GHSR1 | Growth hormone secretagogue receptor type 1 | 0.14 | Signal sensor activity |
| GnIHR3 | Gonadotropin inhibits hormone receptor 3 | 0.68 | Receptor activity |
| GPER1 | G protein-coupled estrogen receptor 1 | 0.81 | G protein-coupled receptor activity |
| GRB2a | Growth factor receptor-binding protein 2a | 0.77 | Binding activity |
| IGF1Ra | Insulin-like growth factor type 1 receptor a | 0.68 | Insulin-like growth factor binding |
| INO1 | Inositol-3-phosphate synthase 1 | 0.78 | Inositol-3-phosphate synthase activity |
| MESD | LDLR molecular chaperone MESD | 0.69 | Wnt signaling pathway |
| NRP2b | Neurocin 2b | 0.71 | Metal ion binding |
| OGFR | opioid growth factor receptor-like protein 1 | 0.36 | Receptor activity |
| OLFM2 | Olfactory protein 2 | 0.66 | Neural c cell development |
| S100A10 | S100 Calbindin A10b | 0.60 | Calcium ion binding |
| SAA | Serum amyloid A precursor | 0.72 | Response to bacteria |
| SSTR2a | Somatostatin receptor 2 | 0.54 | Somatostatin receptor activity |
| SSTR3 | Somatostatin Receptor Type 3 | 0.57 | Somatostatin receptor activity |
| ZMYND11 | Zinc finger MYND domain-containing protein 11 | 0.49 | Zinc ion binding |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Ye, C.; Qin, X.; Zhou, L.; Xia, C.; Cai, T.; Xie, Y.; Yin, Z.; Hu, G. Novel Pituitary Actions of TAC4 Gene Products in Teleost. Int. J. Mol. Sci. 2021, 22, 12893. https://doi.org/10.3390/ijms222312893
Shi X, Ye C, Qin X, Zhou L, Xia C, Cai T, Xie Y, Yin Z, Hu G. Novel Pituitary Actions of TAC4 Gene Products in Teleost. International Journal of Molecular Sciences. 2021; 22(23):12893. https://doi.org/10.3390/ijms222312893
Chicago/Turabian StyleShi, Xuetao, Cheng Ye, Xiangfeng Qin, Lingling Zhou, Chuanhui Xia, Tianyi Cai, Yunyi Xie, Zhan Yin, and Guangfu Hu. 2021. "Novel Pituitary Actions of TAC4 Gene Products in Teleost" International Journal of Molecular Sciences 22, no. 23: 12893. https://doi.org/10.3390/ijms222312893
APA StyleShi, X., Ye, C., Qin, X., Zhou, L., Xia, C., Cai, T., Xie, Y., Yin, Z., & Hu, G. (2021). Novel Pituitary Actions of TAC4 Gene Products in Teleost. International Journal of Molecular Sciences, 22(23), 12893. https://doi.org/10.3390/ijms222312893






