A New Perspective on Thyroid Hormones: Crosstalk with Reproductive Hormones in Females
Abstract
1. Introduction
2. Genomic Effect of THs
2.1. Crosstalk between THs and Estrogen
2.2. Crosstalk between THs and Progesterone
2.3. Crosstalk between THs and Androgens
2.4. Crosstalk between THs and Glucocorticoids
3. Nongenomic Effects of THs
3.1. Crosstalk between THs and FSH and LH
3.2. Crosstalk between THs and GnRH
3.3. Crosstalk between THs and Prolactin
3.4. Crosstalk between THs and Oxytocin
4. Clinical Consequences of the Crosstalk between THs and Reproductive Hormones
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beynon, M.E.; Pinneri, K. An Overview of the Thyroid Gland and Thyroid-Related Deaths for the Forensic Pathologist. Acad. Forensic Pathol. 2016, 6, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.S.; Farhana, A. Histology, Thyroid Gland; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kelly, G.S. Peripheral metabolism of thyroid hormones: A review. Altern. Med. Rev. 2000, 5, 306–333. [Google Scholar]
- Vagenakis, A.G. Pituitary-thyroid interaction: Effects of thyroid hormone, non thyroidal illness and various agents on TSH secretion. Acta Med. Austriaca 1988, 15 (Suppl. 1), 52–56. [Google Scholar] [PubMed]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef]
- Shahid, M.A.; Ashraf, M.A.; Sharma, S. Physiology, Thyroid Hormone; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Sapin, R.; Schlienger, J.L. [Thyroxine (T4) and tri-iodothyronine (T3) determinations: Techniques and value in the assessment of thyroid function]. Ann. Biol. Clin. 2003, 61, 411–420. [Google Scholar]
- Pirahanchi, Y.; Toro, F.; Jialal, I. Physiology, Thyroid Stimulating Hormone; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ortiga-Carvalho, T.M.; Chiamolera, M.I.; Pazos-Moura, C.C.; Wondisford, F.E. Hypothalamus-Pituitary-Thyroid Axis. Compr. Physiol. 2016, 6, 1387–1428. [Google Scholar] [CrossRef]
- Braun, D.; Schweizer, U. Thyroid Hormone Transport and Transporters. Vitam. Horm. 2018, 106, 19–44. [Google Scholar] [CrossRef]
- Visser, W.E.; Friesema, E.C.; Jansen, J.; Visser, T.J. Thyroid hormone transport by monocarboxylate transporters. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 223–236. [Google Scholar] [CrossRef]
- Evans, R.M. The steroid and thyroid hormone receptor superfamily. Science 1988, 240, 889–895. [Google Scholar] [CrossRef]
- Anyetei-Anum, C.S.; Roggero, V.R.; Allison, L.A. Thyroid hormone receptor localization in target tissues. J. Endocrinol. 2018, 237, R19–R34. [Google Scholar] [CrossRef]
- Zhang, J.; Roggero, V.R.; Allison, L.A. Nuclear Import and Export of the Thyroid Hormone Receptor. Vitam. Horm. 2018, 106, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.S.; Moore, D.D. Linkage of the nuclear hormone receptor genes NR1D2, THRB, and RARB: Evidence for an ancient, large-scale duplication. Genomics 1999, 57, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Casas, F.; Busson, M.; Grandemange, S.; Seyer, P.; Carazo, A.; Pessemesse, L.; Wrutniak-Cabello, C.; Cabello, G. Characterization of a novel thyroid hormone receptor alpha variant involved in the regulation of myoblast differentiation. Mol. Endocrinol. 2006, 20, 749–763. [Google Scholar] [CrossRef][Green Version]
- Koenig, R.J.; Lazar, M.A.; Hodin, R.A.; Brent, G.A.; Larsen, P.R.; Chin, W.W.; Moore, D.D. Inhibition of thyroid hormone action by a non-hormone binding c-erbA protein generated by alternative mRNA splicing. Nature 1989, 337, 659–661. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Goglia, F.; Leonard, J.L. Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 2016, 12, 111–121. [Google Scholar] [CrossRef]
- Ratman, D.; Vanden Berghe, W.; Dejager, L.; Libert, C.; Tavernier, J.; Beck, I.M.; De Bosscher, K. How glucocorticoid receptors modulate the activity of other transcription factors: A scope beyond tethering. Mol. Cell Endocrinol. 2013, 380, 41–54. [Google Scholar] [CrossRef]
- Moriyama, K.; Yamamoto, H.; Futawaka, K.; Atake, A.; Kasahara, M.; Tagami, T. Molecular characterization of human thyroid hormone receptor β isoform 4. Endocr. Res. 2016, 41, 34–42. [Google Scholar] [CrossRef]
- Chen, H.; Lin, R.J.; Schiltz, R.L.; Chakravarti, D.; Nash, A.; Nagy, L.; Privalsky, M.L.; Nakatani, Y.; Evans, R.M. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 1997, 90, 569–580. [Google Scholar] [CrossRef]
- Yen, P.M. Physiological and molecular basis of thyroid hormone action. Physiol. Rev. 2001, 81, 1097–1142. [Google Scholar] [CrossRef]
- Singh, B.K.; Sinha, R.A.; Yen, P.M. Novel Transcriptional Mechanisms for Regulating Metabolism by Thyroid Hormone. Int. J. Mol. Sci. 2018, 19, 3284. [Google Scholar] [CrossRef] [PubMed]
- Koenig, R.J. Thyroid hormone receptor coactivators and corepressors. Thyroid 1998, 8, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Graupner, G.; Zhang, X.K.; Tzukerman, M.; Wills, K.; Hermann, T.; Pfahl, M. Thyroid hormone receptors repress estrogen receptor activation of a TRE. Mol. Endocrinol. 1991, 5, 365–372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scott, R.E.; Wu-Peng, X.S.; Yen, P.M.; Chin, W.W.; Pfaff, D.W. Interactions of estrogen- and thyroid hormone receptors on a progesterone receptor estrogen response element (ERE) sequence: A comparison with the vitellogenin A2 consensus ERE. Mol. Endocrinol. 1997, 11, 1581–1592. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Yen, P.M.; Chin, W.W.; Pfaff, D.W. Estrogen and thyroid hormone interaction on regulation of gene expression. Proc. Natl. Acad. Sci. USA 1996, 93, 12587–12592. [Google Scholar] [CrossRef]
- Alexander, S.P.; Cidlowski, J.A.; Kelly, E.; Marrion, N.V.; Peters, J.A.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; Sharman, J.L.; Southan, C.; et al. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Nuclear hormone receptors. Br. J. Pharmacol. 2017, 174 (Suppl. 1), S208–S224. [Google Scholar] [CrossRef]
- De Bosscher, K.; Desmet, S.J.; Clarisse, D.; Estébanez-Perpiña, E.; Brunsveld, L. Nuclear receptor crosstalk—Defining the mechanisms for therapeutic innovation. Nat. Rev. Endocrinol. 2020, 16, 363–377. [Google Scholar] [CrossRef]
- Hernandez, A. Thyroid Hormone Deiodination and Action in the Gonads. Curr. Opin. Endocr. Metab. Res. 2018, 2, 18–23. [Google Scholar] [CrossRef]
- Piqué, D.G.; Greally, J.M.; Mar, J.C. Identification of a novel subgroup of endometrial cancer patients with loss of thyroid hormone receptor beta expression and improved survival. BMC Cancer 2020, 20, 857. [Google Scholar] [CrossRef]
- Zenri, F.; Hiroi, H.; Momoeda, M.; Tsutsumi, R.; Hosokawa, Y.; Koizumi, M.; Nakae, H.; Osuga, Y.; Yano, T.; Taketani, Y. Expression of retinoic acid-related orphan receptor alpha and its responsive genes in human endometrium regulated by cholesterol sulfate. J. Steroid Biochem. Mol. Biol. 2012, 128, 21–28. [Google Scholar] [CrossRef]
- Narayan, G.; Arias-Pulido, H.; Koul, S.; Vargas, H.; Zhang, F.F.; Villella, J.; Schneider, A.; Terry, M.B.; Mansukhani, M.; Murty, V.V. Frequent promoter methylation of CDH1, DAPK, RARB, and HIC1 genes in carcinoma of cervix uteri: Its relationship to clinical outcome. Mol. Cancer 2003, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Krezel, W.; Dupé, V.; Mark, M.; Dierich, A.; Kastner, P.; Chambon, P. RXR gamma null mice are apparently normal and compound RXR alpha +/-/RXR beta -/-/RXR gamma -/- mutant mice are viable. Proc. Natl. Acad. Sci. USA 1996, 93, 9010–9014. [Google Scholar] [CrossRef] [PubMed]
- Nickkho-Amiry, M.; McVey, R.; Holland, C. Peroxisome proliferator-activated receptors modulate proliferation and angiogenesis in human endometrial carcinoma. Mol. Cancer Res. 2012, 10, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.Z.; Teng, C.B.; Ma, H.; Ni, H.; Ma, X.H.; Xu, L.B.; Yang, Z.M. Peroxisome proliferator-activated receptor delta expression and regulation in mouse uterus during embryo implantation and decidualization. Mol. Reprod. Dev. 2003, 66, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Zelenko, Z.; Aghajanova, L.; Irwin, J.C.; Giudice, L.C. Nuclear receptor, coregulator signaling, and chromatin remodeling pathways suggest involvement of the epigenome in the steroid hormone response of endometrium and abnormalities in endometriosis. Reprod. Sci. 2012, 19, 152–162. [Google Scholar] [CrossRef]
- Burns, K.A.; Thomas, S.Y.; Hamilton, K.J.; Young, S.L.; Cook, D.N.; Korach, K.S. Early Endometriosis in Females Is Directed by Immune-Mediated Estrogen Receptor α and IL-6 Cross-Talk. Endocrinology 2018, 159, 103–118. [Google Scholar] [CrossRef]
- Hamilton, K.J.; Hewitt, S.C.; Arao, Y.; Korach, K.S. Estrogen Hormone Biology. Curr. Top. Dev. Biol. 2017, 125, 109–146. [Google Scholar] [CrossRef]
- Whirledge, S.D.; Oakley, R.H.; Myers, P.H.; Lydon, J.P.; DeMayo, F.; Cidlowski, J.A. Uterine glucocorticoid receptors are critical for fertility in mice through control of embryo implantation and decidualization. Proc. Natl. Acad. Sci. USA 2015, 112, 15166–15171. [Google Scholar] [CrossRef]
- Mukangwa, M.; Takizawa, K.; Aoki, Y.; Hamano, S.; Tetsuka, M. Expression of genes encoding mineralocorticoid biosynthetic enzymes and the mineralocorticoid receptor, and levels of mineralocorticoids in the bovine follicle and corpus luteum. J. Reprod. Dev. 2020, 66, 75–81. [Google Scholar] [CrossRef]
- Chi, R.A.; Wang, T.; Adams, N.; Wu, S.P.; Young, S.L.; Spencer, T.E.; DeMayo, F. Human Endometrial Transcriptome and Progesterone Receptor Cistrome Reveal Important Pathways and Epithelial Regulators. J. Clin. Endocrinol. Metab. 2020, 105, e1419–e1439. [Google Scholar] [CrossRef]
- Cloke, B.; Christian, M. The role of androgens and the androgen receptor in cycling endometrium. Mol. Cell Endocrinol. 2012, 358, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Ohara, M.; Sawa, T. [Current topics in the regulation of prostanoids—4. The feedback regulation by PPAR-gamma]. Masui 1999, 48, 146–151. [Google Scholar] [PubMed]
- Vicent, G.P.; Ballaré, C.; Nacht, A.S.; Clausell, J.; Subtil-Rodríguez, A.; Quiles, I.; Jordan, A.; Beato, M. Convergence on chromatin of non-genomic and genomic pathways of hormone signaling. J. Steroid Biochem. Mol. Biol. 2008, 109, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.R.; Habibi, H.R. Estrogen receptor function and regulation in fish and other vertebrates. Gen. Comp. Endocrinol. 2013, 192, 15–24. [Google Scholar] [CrossRef]
- Glass, C.K.; Holloway, J.M.; Devary, O.V.; Rosenfeld, M.G. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell 1988, 54, 313–323. [Google Scholar] [CrossRef]
- Hall, L.C.; Salazar, E.P.; Kane, S.R.; Liu, N. Effects of thyroid hormones on human breast cancer cell proliferation. J. Steroid Biochem. Mol. Biol. 2008, 109, 57–66. [Google Scholar] [CrossRef]
- Northrop, J.P.; Nguyen, D.; Piplani, S.; Olivan, S.E.; Kwan, S.T.-S.; Go, N.F.; Hart, C.P.; Schatz, P.J. Selection of Estrogen Receptor β- and Thyroid Hormone Receptor β-Specific Coactivator-Mimetic Peptides Using Recombinant Peptide Libraries. Mol. Endocrinol. 2000, 14, 605–622. [Google Scholar] [CrossRef]
- McInerney, E.M.; Tsai, M.J.; O’Malley, B.W.; Katzenellenbogen, B.S. Analysis of estrogen receptor transcriptional enhancement by a nuclear hormone receptor coactivator. Proc. Natl. Acad. Sci. USA 1996, 93, 10069–10073. [Google Scholar] [CrossRef]
- Shibata, H.; Spencer, T.E.; Oñate, S.A.; Jenster, G.; Tsai, S.Y.; Tsai, M.J.; O’Malley, B.W. Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog. Horm. Res. 1997, 52, 141–164. [Google Scholar]
- Singh, B.K.; Sinha, R.A.; Tripathi, M.; Mendoza, A.; Ohba, K.; Sy, J.A.C.; Xie, S.Y.; Zhou, J.; Ho, J.P.; Chang, C.Y.; et al. Thyroid hormone receptor and ERRα coordinately regulate mitochondrial fission, mitophagy, biogenesis, and function. Sci. Signal. 2018, 11, eaam5855. [Google Scholar] [CrossRef]
- Vasudevan, N.; Davidkova, G.; Zhu, Y.S.; Koibuchi, N.; Chin, W.W.; Pfaff, D. Differential interaction of estrogen receptor and thyroid hormone receptor isoforms on the rat oxytocin receptor promoter leads to differences in transcriptional regulation. Neuroendocrinology 2001, 74, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Faustino, L.C.; Gagnidze, K.; Ortiga-Carvalho, T.M.; Pfaff, D.W. Impact of Thyroid Hormones on Estrogen Receptor α-Dependent Transcriptional Mechanisms in Ventromedial Hypothalamus and Preoptic Area. Neuroendocrinology 2015, 101, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, N.B.; Cestari, S.H.; Conde, S.J.; Luvizotto, R.A.; De Sibio, M.T.; Perone, D.; Katayama, M.L.; Carraro, D.M.; Brentani, H.P.; Brentani, M.M.; et al. Estrogen-responsive genes overlap with triiodothyronine-responsive genes in a breast carcinoma cell line. Sci. World J. 2014, 2014, 969404. [Google Scholar] [CrossRef]
- Nogueira, C.R.; Brentani, M.M. Triiodothyronine mimics the effects of estrogen in breast cancer cell lines. J. Steroid Biochem. Mol. Biol. 1996, 59, 271–279. [Google Scholar] [CrossRef]
- Shao, Z.M.; Sheikh, M.S.; Rishi, A.K.; Dawson, M.I.; Li, X.S.; Wilber, J.F.; Feng, P.; Fontana, J.A. Thyroid hormone enhancement of estradiol stimulation of breast carcinoma proliferation. Exp. Cell Res. 1995, 218, 1–8. [Google Scholar] [CrossRef]
- Longcope, C.; Abend, S.; Braverman, L.E.; Emerson, C.H. Androstenedione and estrone dynamics in hypothyroid women. J. Clin. Endocrinol. Metab. 1990, 70, 903–907. [Google Scholar] [CrossRef]
- Ghosh, S.; Kabir, S.N.; Pakrashi, A.; Chatterjee, S.; Chakravarty, B. Subclinical hypothyroidism: A determinant of polycystic ovary syndrome. Horm. Res. 1993, 39, 61–66. [Google Scholar] [CrossRef]
- Jacobson, M.H.; Howards, P.P.; Darrow, L.A.; Meadows, J.W.; Kesner, J.S.; Spencer, J.B.; Terrell, M.L.; Marcus, M. Thyroid hormones and menstrual cycle function in a longitudinal cohort of premenopausal women. Paediatr. Perinat. Epidemiol. 2018, 32, 225–234. [Google Scholar] [CrossRef]
- Lv, P.P.; Meng, Y.; Lv, M.; Feng, C.; Liu, Y.; Li, J.Y.; Yu, D.Q.; Shen, Y.; Hu, X.L.; Gao, Q.; et al. Altered thyroid hormone profile in offspring after exposure to high estradiol environment during the first trimester of pregnancy: A cross-sectional study. BMC Med. 2014, 12, 240. [Google Scholar] [CrossRef]
- Alzahrani, A.A.; Alahmadi, A.A.; Ali, S.S.; Alahmadi, B.A.; Arab, R.A.; Wahman, L.F.; El-Shitany, N.A. Biochemical and histological evidence of thyroid gland dysfunction in estradiol valerate model of the polycystic ovary in Wistar rats. Biochem. Biophys. Res. Commun. 2019, 514, 194–199. [Google Scholar] [CrossRef]
- Sosić-Jurjević, B.; Filipović, B.; Milosević, V.; Nestorović, N.; Manojlović-Stojanoski, M.; Brkić, B.; Sekulić, M. Chronic estradiol exposure modulates thyroid structure and decreases T4 and T3 serum levels in middle-aged female rats. Horm. Res. 2005, 63, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Xiao, L.; Hu, J.; Zhang, Y.; Zhao, X.; Ge, W.; Jiang, Y.; Song, L.; Yang, S.; Luo, W. Expression of oestrogen receptor, androgen receptor and progesterone nuclear receptor in sheep uterus during the oestrous cycle. Reprod. Domest. Anim. 2019, 54, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Grimm, S.L.; Hartig, S.M.; Edwards, D.P. Progesterone Receptor Signaling Mechanisms. J. Mol. Biol. 2016, 428, 3831–3849. [Google Scholar] [CrossRef]
- Dufourny, L.; Skinner, D.C. Colocalization of progesterone receptors and thyroid hormone receptors alpha in the ovine diencephalon: No effect of estradiol. Neuroendocrinology 2003, 77, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jeyakumar, M.; Bagchi, M.K. Ligand-dependent cross-talk between steroid and thyroid hormone receptors. Evidence for common transcriptional coactivator(s). J. Biol. Chem. 1996, 271, 14825–14833. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, A.; Yen, P.M.; Ikeda, M.; Cardona, G.R.; Liu, Y.; Koibuchi, N.; Norwitz, E.R.; Chin, W.W. Thyroid hormone response elements differentially modulate the interactions of thyroid hormone receptors with two receptor binding domains in the steroid receptor coactivator-1. J. Biol. Chem. 1998, 273, 21554–21562. [Google Scholar] [CrossRef]
- Vattai, A.; Ziegelmüller, B.; Kost, B.; Kuhn, C.; Hofmann, S.; Bayer, B.; Anslinger, K.; Jeschke, U.; Ditsch, N. The expression of thyroid hormone receptors (THR) is regulated by the progesterone receptor system in first trimester placental tissue and in BeWo cells in vitro. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 195, 31–39. [Google Scholar] [CrossRef]
- Lauritzen, C. Clinical use of oestrogens and progestogens. Maturitas 1990, 12, 199–214. [Google Scholar] [CrossRef]
- Quintino-Moro, A.; Zantut-Wittmann, D.E.; Silva Dos Santos, P.N.; Melhado-Kimura, V.; da Silva, C.A.; Bahamondes, L.; Fernandes, A. Thyroid function during the first year of use of the injectable contraceptive depot medroxyprogesterone acetate. Eur. J. Contracept. Reprod. Health Care 2019, 24, 102–108. [Google Scholar] [CrossRef]
- Sathi, P.; Kalyan, S.; Hitchcock, C.L.; Pudek, M.; Prior, J.C. Progesterone therapy increases free thyroxine levels--data from a randomized placebo-controlled 12-week hot flush trial. Clin. Endocrinol. 2013, 79, 282–287. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Halasz, M.; Palkovics, T. Progesterone in pregnancy; receptor-ligand interaction and signaling pathways. J. Reprod. Immunol. 2009, 83, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Calvo, R.M.; Jauniaux, E.; Gulbis, B.; Asunción, M.; Gervy, C.; Contempré, B.; Morreale de Escobar, G. Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development. J. Clin. Endocrinol. Metab. 2002, 87, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Shiina, H.; Kawano, H.; Sato, T.; Kato, S. Androgen receptor functions in male and female physiology. J. Steroid Biochem. Mol. Biol. 2008, 109, 236–241. [Google Scholar] [CrossRef]
- Flood, D.E.; Langlois, V.S. Crosstalk between the thyroid hormone and androgen axes during reproductive development in Silurana tropicalis. Gen. Comp. Endocrinol. 2014, 203, 232–240. [Google Scholar] [CrossRef]
- Aksoy, O.; Pencik, J.; Hartenbach, M.; Moazzami, A.A.; Schlederer, M.; Balber, T.; Varady, A.; Philippe, C.; Baltzer, P.A.; Mazumder, B.; et al. Thyroid and androgen receptor signaling are antagonized by μ-Crystallin in prostate cancer. Int. J. Cancer 2021, 148, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Miyaso, H.; Nakamura, N.; Naito, M.; Hirai, S.; Matsuno, Y.; Itoh, M.; Mori, C. Early postnatal exposure to a low dose of decabromodiphenyl ether affects expression of androgen and thyroid hormone receptor-alpha and its splicing variants in mouse Sertoli cells. PLoS ONE 2014, 9, e114487. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.A.; Rittenberg, P.V.; Brown, T.J. Activation of androgen receptor-associated protein 70 (ARA70) mRNA expression in ovarian cancer. Gynecol. Oncol. 2001, 80, 132–138. [Google Scholar] [CrossRef]
- Tai, P.J.; Huang, Y.H.; Shih, C.H.; Chen, R.N.; Chen, C.D.; Chen, W.J.; Wang, C.S.; Lin, K.H. Direct regulation of androgen receptor-associated protein 70 by thyroid hormone and its receptors. Endocrinology 2007, 148, 3485–3495. [Google Scholar] [CrossRef]
- Duarte-Guterman, P.; Ryan, M.J.; Hogan, N.S.; Trudeau, V.L. Developmental profiles and thyroid hormone regulation of brain transcripts in frogs: A species comparison with emphasis on Physalaemus pustulosus. Brain Behav. Evol. 2012, 79, 98–112. [Google Scholar] [CrossRef]
- Esposito, T.; Astore, E.; Cardone, A.; Angelini, F.; Varriale, B. Regulation of androgen receptor mRNA expression in primary culture of Harderian gland cells: Cross-talk between steroid hormones. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 132, 97–105. [Google Scholar] [CrossRef]
- Duarte-Guterman, P.; Langlois, V.S.; Pauli, B.D.; Trudeau, V.L. Expression and T3 regulation of thyroid hormone- and sex steroid-related genes during Silurana (Xenopus) tropicalis early development. Gen. Comp. Endocrinol. 2010, 166, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.E.K.; Langlois, V.S. Thyroid hormones and androgens differentially regulate gene expression in testes and ovaries of sexually mature Silurana tropicalis. Gen. Comp. Endocrinol. 2018, 267, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Ahlquist, J.A.; Franklyn, J.A.; Ramsden, D.B.; Sheppard, M.C. Regulation of alpha and thyrotrophin-beta subunit mRNA levels by androgens in the female rat. J. Mol. Endocrinol. 1990, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wu, L.; Wang, Y.; Liu, L.; Yang, F.; Sun, Y.; Jiao, X.; Bao, L.; Chen, P.; Liang, Q.; et al. Dihydrotestosterone regulates oxidative stress and immunosuppressive cytokines in a female BALB/c mouse model of Graves’ disease. Autoimmunity 2019, 52, 117–125. [Google Scholar] [CrossRef]
- Muderris, I.I.; Boztosun, A.; Oner, G.; Bayram, F. Effect of thyroid hormone replacement therapy on ovarian volume and androgen hormones in patients with untreated primary hypothyroidism. Ann. Saudi Med. 2011, 31, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Arafah, B.M. Decreased levothyroxine requirement in women with hypothyroidism during androgen therapy for breast cancer. Ann. Intern. Med. 1994, 121, 247–251. [Google Scholar] [CrossRef]
- Mazer, N.A. Interaction of estrogen therapy and thyroid hormone replacement in postmenopausal women. Thyroid 2004, 14 (Suppl. 1), S27–S34. [Google Scholar] [CrossRef]
- Torre, F.; Calogero, A.E.; Condorelli, R.A.; Cannarella, R.; Aversa, A.; La Vignera, S. Effects of oral contraceptives on thyroid function and vice versa. J. Endocrinol. Investig. 2020, 43, 1181–1188. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Dalman, F.C.; Koenig, R.J.; Perdew, G.H.; Massa, E.; Pratt, W.B. In contrast to the glucocorticoid receptor, the thyroid hormone receptor is translated in the DNA binding state and is not associated with hsp90. J. Biol. Chem. 1990, 265, 3615–3618. [Google Scholar] [CrossRef]
- Emont, M.P.; Mantis, S.; Kahn, J.H.; Landeche, M.; Han, X.; Sargis, R.M.; Cohen, R.N. Silencing Mediator of Retinoid and Thyroid Hormone Receptors (SMRT) regulates glucocorticoid action in adipocytes. Mol. Cell Endocrinol. 2015, 407, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Weikum, E.R.; Knuesel, M.T.; Ortlund, E.A.; Yamamoto, K.R. Glucocorticoid receptor control of transcription: Precision and plasticity via allostery. Nat. Rev. Mol. Cell Biol. 2017, 18, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Grad, I.; Picard, D. The glucocorticoid responses are shaped by molecular chaperones. Mol. Cell Endocrinol. 2007, 275, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Leers, J.; Steiner, C.; Renkawitz, R.; Muller, M. A thyroid hormone receptor-dependent glucocorticoid induction. Mol. Endocrinol. 1994, 8, 440–447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vahrenkamp, J.M.; Yang, C.H.; Rodriguez, A.C.; Almomen, A.; Berrett, K.C.; Trujillo, A.N.; Guillen, K.P.; Welm, B.E.; Jarboe, E.A.; Janat-Amsbury, M.M.; et al. Clinical and Genomic Crosstalk between Glucocorticoid Receptor and Estrogen Receptor α In Endometrial Cancer. Cell Rep. 2018, 22, 2995–3005. [Google Scholar] [CrossRef]
- Scheller, K.; Seibel, P.; Sekeris, C.E. Glucocorticoid and thyroid hormone receptors in mitochondria of animal cells. Int. Rev. Cytol. 2003, 222, 1–61. [Google Scholar] [CrossRef]
- Geraghty, A.C.; Kaufer, D. Glucocorticoid Regulation of Reproduction. Adv. Exp. Med. Biol. 2015, 872, 253–278. [Google Scholar] [CrossRef]
- Slone-Wilcoxon, J.; Redei, E.E. Maternal-fetal glucocorticoid milieu programs hypothalamic-pituitary-thyroid function of adult offspring. Endocrinology 2004, 145, 4068–4072. [Google Scholar] [CrossRef]
- Forhead, A.J.; Jellyman, J.K.; Gardner, D.S.; Giussani, D.A.; Kaptein, E.; Visser, T.J.; Fowden, A.L. Differential effects of maternal dexamethasone treatment on circulating thyroid hormone concentrations and tissue deiodinase activity in the pregnant ewe and fetus. Endocrinology 2007, 148, 800–805. [Google Scholar] [CrossRef]
- Hammes, S.R.; Davis, P.J. Overlapping nongenomic and genomic actions of thyroid hormone and steroids. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 581–593. [Google Scholar] [CrossRef]
- Davis, P.J.; Leonard, J.L.; Lin, H.Y.; Leinung, M.; Mousa, S.A. Molecular Basis of Nongenomic Actions of Thyroid Hormone. Vitam. Horm. 2018, 106, 67–96. [Google Scholar] [CrossRef] [PubMed]
- Bergh, J.J.; Lin, H.Y.; Lansing, L.; Mohamed, S.N.; Davis, F.B.; Mousa, S.; Davis, P.J. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 2005, 146, 2864–2871. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Sun, M.; Tang, H.Y.; Lin, C.; Luidens, M.K.; Mousa, S.A.; Incerpi, S.; Drusano, G.L.; Davis, F.B.; Davis, P.J. L-Thyroxine vs. 3,5,3′-triiodo-L-thyronine and cell proliferation: Activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Am. J. Physiol. Cell Physiol. 2009, 296, C980–C991. [Google Scholar] [CrossRef] [PubMed]
- Teoh, C.M.; Tam, J.K.; Tran, T. Integrin and GPCR Crosstalk in the Regulation of ASM Contraction Signaling in Asthma. J. Allergy 2012, 2012, 341282. [Google Scholar] [CrossRef]
- Wide, L.; Eriksson, K. Molecular size and charge as dimensions to identify and characterize circulating glycoforms of human FSH, LH and TSH. Ups. J. Med. Sci. 2017, 122, 217–223. [Google Scholar] [CrossRef]
- Schubert, R.L.; Narayan, P.; Puett, D. Specificity of cognate ligand-receptor interactions: Fusion proteins of human chorionic gonadotropin and the heptahelical receptors for human luteinizing hormone, thyroid-stimulating hormone, and follicle-stimulating hormone. Endocrinology 2003, 144, 129–137. [Google Scholar] [CrossRef][Green Version]
- Williams, J.F.; Davies, T.F.; Catt, K.J.; Pierce, J.G. Receptor-binding activity of highly purified bovine luteinizing hormone and thyrotropin, and their subunits. Endocrinology 1980, 106, 1353–1359. [Google Scholar] [CrossRef]
- Bargi-Souza, P.; Romano, R.M.; Goulart-Silva, F.; Brunetto, E.L.; Nunes, M.T. T(3) rapidly regulates several steps of alpha subunit glycoprotein (CGA) synthesis and secretion in the pituitary of male rats: Potential repercussions on TSH, FSH and LH secretion. Mol. Cell Endocrinol. 2015, 409, 73–81. [Google Scholar] [CrossRef]
- Kobayashi, N.; Orisaka, M.; Cao, M.; Kotsuji, F.; Leader, A.; Sakuragi, N.; Tsang, B.K. Growth differentiation factor-9 mediates follicle-stimulating hormone-thyroid hormone interaction in the regulation of rat preantral follicular development. Endocrinology 2009, 150, 5566–5574. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, L.; Zhu, B.; Feng, Y.; Yu, S.; An, N.; Wang, X. Effects of 3, 5, 3′-triiodothyronine (t3) and follicle stimulating hormone on apoptosis and proliferation of rat ovarian granulosa cells. Chin. J. Physiol. 2013, 56, 298–305. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, G.; Tsang, B.K. Interactions of thyroid hormone and FSH in the regulation of rat granulosa cell apoptosis. Front. Biosci. 2011, 3, 1401–1413. [Google Scholar] [CrossRef]
- Manna, P.R.; Kero, J.; Tena-Sempere, M.; Pakarinen, P.; Stocco, D.M.; Huhtaniemi, I.T. Assessment of mechanisms of thyroid hormone action in mouse Leydig cells: Regulation of the steroidogenic acute regulatory protein, steroidogenesis, and luteinizing hormone receptor function. Endocrinology 2001, 142, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Zähringer, S.; Tomova, A.; von Werder, K.; Brabant, G.; Kumanov, P.; Schopohl, J. The influence of hyperthyroidism on the hypothalamic-pituitary-gonadal axis. Exp. Clin. Endocrinol. Diabetes 2000, 108, 282–289. [Google Scholar] [CrossRef]
- Unuane, D.; Velkeniers, B. Impact of thyroid disease on fertility and assisted conception. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101378. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.F.; Ocarino, N.M.; Vieira, A.L.; Nascimento, E.F.; Serakides, R. Effects of hypo- and hyperthyroidism on proliferation, angiogenesis, apoptosis and expression of COX-2 in the corpus luteum of female rats. Reprod. Domest. Anim. 2013, 48, 691–698. [Google Scholar] [CrossRef] [PubMed]
- De Vincentis, S.; Monzani, M.L.; Brigante, G. Crosstalk between gonadotropins and thyroid axis. Minerva Ginecol. 2018, 70, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Emons, G.; Gründker, C. The Role of Gonadotropin-Releasing Hormone (GnRH) in Endometrial Cancer. Cells 2021, 10, 292. [Google Scholar] [CrossRef]
- Okada, R.; Kobayashi, T.; Yamamoto, K.; Nakakura, T.; Tanaka, S.; Vaudry, H.; Kikuyama, S. Neuroendocrine regulation of thyroid-stimulating hormone secretion in amphibians. Ann. N. Y. Acad. Sci. 2009, 1163, 262–270. [Google Scholar] [CrossRef]
- Naderi, F.; Soheilirad, Z.; Haghshenas, Z. The Influence of Gonadotropin-Releasing Hormone Agonist Treatment on Thyroid Function Tests in Children with Central Idiopathic Precocious Puberty. Med. Arch. 2019, 73, 101–103. [Google Scholar] [CrossRef]
- Massart, F.; Harrell, J.C.; Federico, G.; Saggese, G. Thyroid outcome during long-term gonadotropin-releasing hormone agonist treatments for idiopathic precocious puberty. J. Adolesc. Health 2007, 40, 252–257. [Google Scholar] [CrossRef]
- Jansen, H.T.; Lubbers, L.S.; Macchia, E.; DeGroot, L.J.; Lehman, M.N. Thyroid hormone receptor (alpha) distribution in hamster and sheep brain: Colocalization in gonadotropin-releasing hormone and other identified neurons. Endocrinology 1997, 138, 5039–5047. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Ng, K.W.; Xue, X.; Ramadasan, P.N.; Sivalingam, M.; Li, S.; Levavi-Sivan, B.; Lin, H.; Liu, X.; Parhar, I.S. Thyroid Hormone Upregulates Hypothalamic kiss2 Gene in the Male Nile Tilapia, Oreochromis niloticus. Front. Endocrinol. 2013, 4, 184. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.R.; Moenter, S.M.; Barrell, G.K.; Lehman, M.N.; Karsch, F.J. Role of the thyroid gland in seasonal reproduction. III. Thyroidectomy blocks seasonal suppression of gonadotropin-releasing hormone secretion in sheep. Endocrinology 1991, 129, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Dardente, H. Melatonin-dependent timing of seasonal reproduction by the pars tuberalis: Pivotal roles for long daylengths and thyroid hormones. J. Neuroendocrinol. 2012, 24, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Diaz, N.; Huerta, I.; Marina, N.; Navarro, N.; Mena, F. Regional mechanisms within anterior pituitary of lactating rats may regulate prolactin secretion. Endocrine 2002, 18, 41–46. [Google Scholar] [CrossRef]
- Augustine, R.A.; Ladyman, S.R.; Bouwer, G.T.; Alyousif, Y.; Sapsford, T.J.; Scott, V.; Kokay, I.C.; Grattan, D.R.; Brown, C.H. Prolactin regulation of oxytocin neurone activity in pregnancy and lactation. J. Physiol. 2017, 595, 3591–3605. [Google Scholar] [CrossRef]
- Hekimsoy, Z.; Kafesçiler, S.; Güçlü, F.; Ozmen, B. The prevalence of hyperprolactinaemia in overt and subclinical hypothyroidism. Endocr. J. 2010, 57, 1011–1015. [Google Scholar] [CrossRef]
- Koyyada, A.; Orsu, P. Role of hypothyroidism and associated pathways in pregnancy and infertility: Clinical insights. Tzu Chi Med. J. 2020, 32, 312–317. [Google Scholar] [CrossRef]
- Goel, P.; KahKaSha, S.N.; GuPta, B.K.; Goel, K. Evaluation of serum prolactin level in patients of subclinical and overt hypothyroidism. J. Clin. Diagn Res. 2015, 9, BC15–BC17. [Google Scholar] [CrossRef]
- Prabhakar, V.K.; Davis, J.R. Hyperprolactinaemia. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 341–353. [Google Scholar] [CrossRef]
- Pernasetti, F.; Caccavelli, L.; Van de Weerdt, C.; Martial, J.A.; Muller, M. Thyroid hormone inhibits the human prolactin gene promoter by interfering with activating protein-1 and estrogen stimulations. Mol. Endocrinol. 1997, 11, 986–996. [Google Scholar] [CrossRef][Green Version]
- Wondisford, F.E.; Steinfelder, H.J.; Nations, M.; Radovick, S. AP-1 antagonizes thyroid hormone receptor action on the thyrotropin beta-subunit gene. J. Biol. Chem. 1993, 268, 2749–2754. [Google Scholar] [CrossRef]
- Maurer, R.A. Thyroid hormone specifically inhibits prolactin synthesis and decreases prolactin messenger ribonucleic acid levels in cultured pituitary cells. Endocrinology 1982, 110, 1507–1514. [Google Scholar] [CrossRef]
- Davis, J.R.; Lynam, T.C.; Franklyn, J.A.; Docherty, K.; Sheppard, M.C. Tri-iodothyronine and phenytoin reduce prolactin messenger RNA levels in cultured rat pituitary cells. J. Endocrinol. 1986, 109, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Tashjian, A.H., Jr.; Barowsky, N.J.; Jensen, D.K. Thyrotropin releasing hormone: Direct evidence for stimulation of prolactin production by pituitary cells in culture. Biochem. Biophys. Res. Commun. 1971, 43, 516–523. [Google Scholar] [CrossRef]
- Kang, C.W.; Han, Y.E.; Lee, M.K.; Cho, Y.H.; Kang, N.; Koo, J.; Ku, C.R.; Lee, E.J. Olfactory marker protein regulates prolactin secretion and production by modulating Ca(2+) and TRH signaling in lactotrophs. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, H.; Oride, A.; Mijiddorj, T.; Kyo, S. Role of thyrotropin-releasing hormone in prolactin-producing cell models. Neuropeptides 2015, 54, 73–77. [Google Scholar] [CrossRef]
- Bajorunas, D.R.; Rosner, W.; Kourides, I.A. Use of bromocriptine in a patient with generalized resistance to thyroid hormone. J. Clin. Endocrinol. Metab. 1984, 58, 731–735. [Google Scholar] [CrossRef]
- van Die, M.D.; Burger, H.G.; Teede, H.J.; Bone, K.M. Vitex agnus-castus extracts for female reproductive disorders: A systematic review of clinical trials. Planta Med. 2013, 79, 562–575. [Google Scholar] [CrossRef]
- Petersson, M. Oxytocin decreases plasma levels of thyroid-stimulating hormone and thyroid hormones in rats. Regul. Pept. 2002, 108, 83–87. [Google Scholar] [CrossRef]
- Carrera-González, M.P.; Ramírez-Expósito, M.J.; de Saavedra, J.M.; Sánchez-Agesta, R.; Mayas, M.D.; Martínez-Martos, J.M. Hypothalamus-pituitary-thyroid axis disruption in rats with breast cancer is related to an altered endogenous oxytocin/insulin-regulated aminopeptidase (IRAP) system. Tumour. Biol. 2011, 32, 543–549. [Google Scholar] [CrossRef]
- Adan, R.A.; Cox, J.J.; Beischlag, T.V.; Burbach, J.P. A composite hormone response element mediates the transactivation of the rat oxytocin gene by different classes of nuclear hormone receptors. Mol. Endocrinol. 1993, 7, 47–57. [Google Scholar] [CrossRef]
- Adan, R.A.; Cox, J.J.; van Kats, J.P.; Burbach, J.P. Thyroid hormone regulates the oxytocin gene. J. Biol. Chem. 1992, 267, 3771–3777. [Google Scholar] [CrossRef]
- Dellovade, T.L.; Zhu, Y.S.; Pfaff, D.W. Thyroid hormones and estrogen affect oxytocin gene expression in hypothalamic neurons. J. Neuroendocrinol. 1999, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Makarevich, A.V.; Sirotkin, A.V.; Franek, J.; Kwon, H.B.; Bulla, J. The role of oxytocin, protein kinase A, and ERK-related MAP-kinase in the control of porcine ovarian follicle functions. Exp. Clin. Endocrinol. Diabetes 2004, 112, 108–114. [Google Scholar] [CrossRef]
- Burns, P.D.; Mendes, J.O., Jr.; Yemm, R.S.; Clay, C.M.; Nelson, S.E.; Hayes, S.H.; Silvia, W.J. Cellular mechanisms by which oxytocin mediates ovine endometrial prostaglandin F2alpha synthesis: Role of G(i) proteins and mitogen-activated protein kinases. Biol. Reprod. 2001, 65, 1150–1155. [Google Scholar] [CrossRef]
- Silvia, W.J. Uterine secretion of prostaglandin F2 alpha in the ewe: Regulation at the cellular level by ovarian hormones and the conceptus. Reprod. Fertil. Dev. 1992, 4, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J.J.; Rice, G.E.; Jenkin, G.; Thorburn, G.D. Control of oxytocin secretion by ovine corpora lutea: Effects of arachidonic acid, phospholipases, and prostaglandins. Endocrinology 1988, 122, 774–781. [Google Scholar] [CrossRef]
- Silva, J.F.; Ocarino, N.M.; Serakides, R. Thyroid hormones and female reproduction. Biol. Reprod. 2018, 99, 907–921. [Google Scholar] [CrossRef]
- Sidki, Y.; Badawi, H.M.; Soliman, F.A. The influence of oestrogen and thyroid on the pituitary and blood content of FSH and LH. Experientia 1958, 14, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Redmond, G.P. Thyroid dysfunction and women’s reproductive health. Thyroid 2004, 14 (Suppl. 1), S5–S15. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Suzuki, H.; Saji, M.; Horiba, N.; Ujihara, M.; Tsushima, T.; Demura, H.; Shizume, K. High serum progesterone in hyperthyroid men with Graves’ disease. J. Clin. Endocrinol. Metab. 1988, 66, 230–232. [Google Scholar] [CrossRef]
- Kumar, A.; Mohanty, B.P.; Rani, L. Secretion of testicular steroids and gonadotrophins in hypothyroidism. Andrologia 2007, 39, 253–260. [Google Scholar] [CrossRef]
- Liu, J.; Guo, M.; Hu, X.; Weng, X.; Tian, Y.; Xu, K.; Heng, D.; Liu, W.; Ding, Y.; Yang, Y.; et al. Effects of Thyroid Dysfunction on Reproductive Hormones in Female Rats. Chin. J. Physiol. 2018, 61, 152–162. [Google Scholar] [CrossRef]
- Hatsuta, M.; Abe, K.; Tamura, K.; Ryuno, T.; Watanabe, G.; Taya, K.; Kogo, H. Effects of hypothyroidism on the estrous cycle and reproductive hormones in mature female rat. Eur. J. Pharmacol. 2004, 486, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Kakita-Kobayashi, M.; Murata, H.; Nishigaki, A.; Hashimoto, Y.; Komiya, S.; Tsubokura, H.; Kido, T.; Kida, N.; Tsuzuki-Nakao, T.; Matsuo, Y.; et al. Thyroid Hormone Facilitates in vitro Decidualization of Human Endometrial Stromal Cells via Thyroid Hormone Receptors. Endocrinology 2020, 161, bqaa049. [Google Scholar] [CrossRef]
- Kong, L.; Wei, Q.; Fedail, J.S.; Shi, F.; Nagaoka, K.; Watanabe, G. Effects of thyroid hormones on the antioxidative status in the uterus of young adult rats. J. Reprod. Dev. 2015, 61, 219–227. [Google Scholar] [CrossRef]
- Peyneau, M.; Kavian, N.; Chouzenoux, S.; Nicco, C.; Jeljeli, M.; Toullec, L.; Reboul-Marty, J.; Chenevier-Gobeaux, C.; Reis, F.M.; Santulli, P.; et al. Role of thyroid dysimmunity and thyroid hormones in endometriosis. Proc. Natl. Acad. Sci. USA 2019, 116, 11894–11899. [Google Scholar] [CrossRef]
- Di Paolo, V.; Mangialardo, C.; Zacà, C.; Barberi, M.; Sereni, E.; Borini, A.; Centanni, M.; Coticchio, G.; Verga-Falzacappa, C.; Canipari, R. Thyroid hormones T3 and T4 regulate human luteinized granulosa cells, counteracting apoptosis and promoting cell survival. J. Endocrinol. Investig. 2020, 43, 821–831. [Google Scholar] [CrossRef]
- Sayem, A.S.M.; Giribabu, N.; Karim, K.; Si, L.K.; Muniandy, S.; Salleh, N. Differential expression of the receptors for thyroid hormone, thyroid stimulating hormone, vitamin D and retinoic acid and extracellular signal-regulated kinase in uterus of rats under influence of sex-steroids. Biomed. Pharmacother. 2018, 100, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Castelán, J.; Del Moral-Morales, A.; Piña-Medina, A.G.; Zepeda-Pérez, D.; Castillo-Romano, M.; Méndez-Tepepa, M.; Espindola-Lozano, M.; Camacho-Arroyo, I.; Cuevas-Romero, E. Hypothyroidism induces uterine hyperplasia and inflammation related to sex hormone receptors expression in virgin rabbits. Life Sci. 2019, 230, 111–120. [Google Scholar] [CrossRef]
- Andreeva, P. [Thyroid gland and fertility]. Akush Ginekol. 2014, 53, 18–23. [Google Scholar]
- Canipari, R.; Mangialardo, C.; Di Paolo, V.; Alfei, F.; Ucci, S.; Russi, V.; Santaguida, M.G.; Virili, C.; Segni, M.; Misiti, S.; et al. Thyroid hormones act as mitogenic and pro survival factors in rat ovarian follicles. J. Endocrinol. Investig. 2019, 42, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Urmi, S.J.; Begum, S.R.; Fariduddin, M.; Begum, S.A.; Mahmud, T.; Banu, J.; Chowdhury, S.; Khanam, A. Hypothyroidism and its Effect on Menstrual Pattern and Fertility. Mymensingh Med. J. 2015, 24, 765–769. [Google Scholar]
- Ho, C.W.; Chen, H.H.; Hsieh, M.C.; Chen, C.C.; Hsu, S.P.; Yip, H.T.; Kao, C.H. Increased Risk of Polycystic Ovary Syndrome and It’s Comorbidities in Women with Autoimmune Thyroid Disease. Int. J. Environ. Res. Public Health 2020, 17, 2422. [Google Scholar] [CrossRef]
- Glintborg, D.; Rubin, K.H.; Nybo, M.; Abrahamsen, B.; Andersen, M. Increased risk of thyroid disease in Danish women with polycystic ovary syndrome: A cohort study. Endocr. Connect. 2019, 8, 1405–1415. [Google Scholar] [CrossRef]
- Janssen, O.E.; Mehlmauer, N.; Hahn, S.; Offner, A.H.; Gärtner, R. High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur. J. Endocrinol. 2004, 150, 363–369. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, R.; Wang, J. Relationship between Hypothyroidism and Endometrial Cancer. Aging Dis. 2019, 10, 190–196. [Google Scholar] [CrossRef]
- Seebacher, V.; Hofstetter, G.; Polterauer, S.; Reinthaller, A.; Grimm, C.; Schwameis, R.; Taucher, S.; Wagener, A.; Marth, C.; Concin, N. Does thyroid-stimulating hormone influence the prognosis of patients with endometrial cancer? A multicentre trial. Br. J. Cancer 2013, 109, 215–218. [Google Scholar] [CrossRef][Green Version]
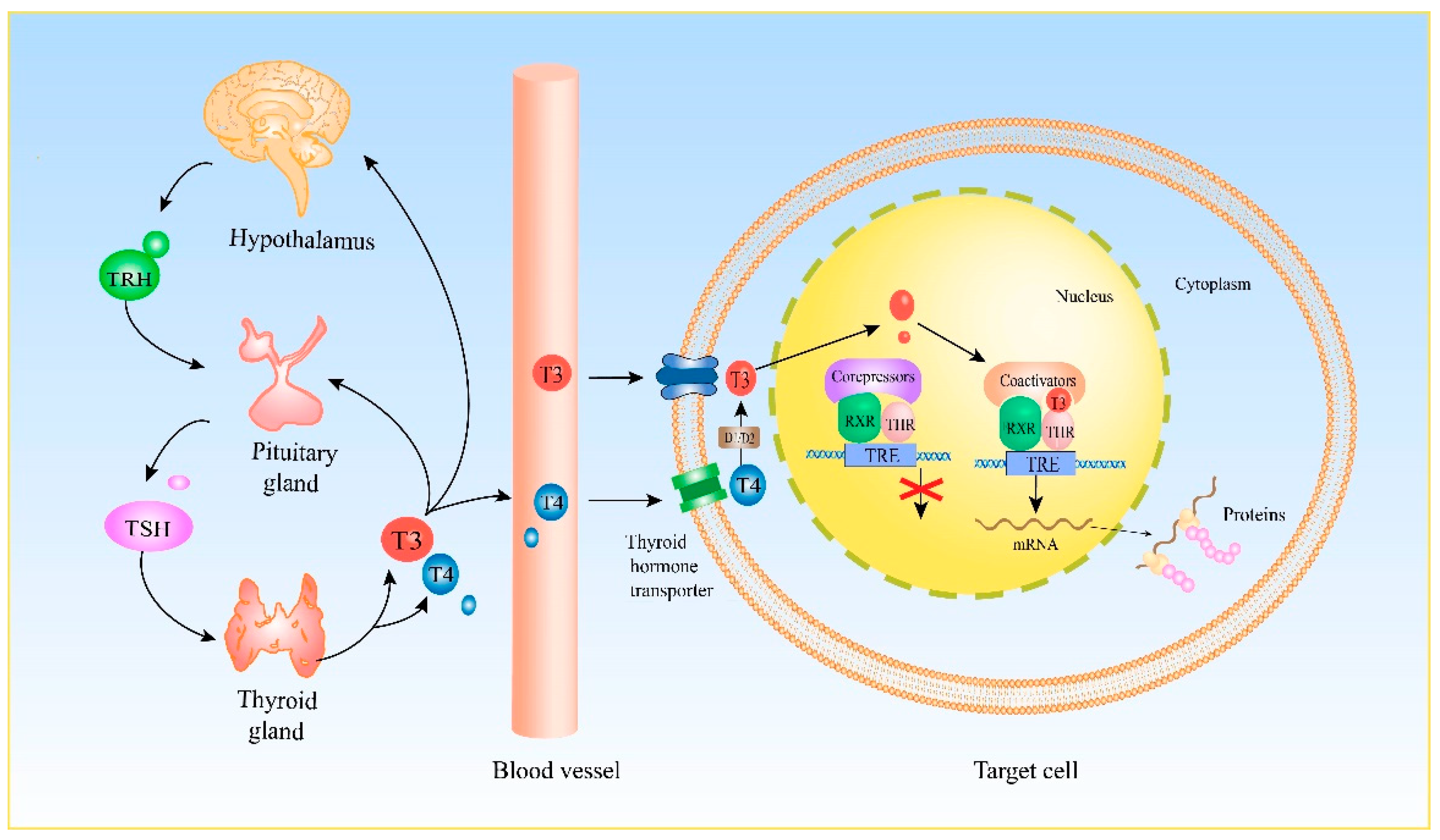

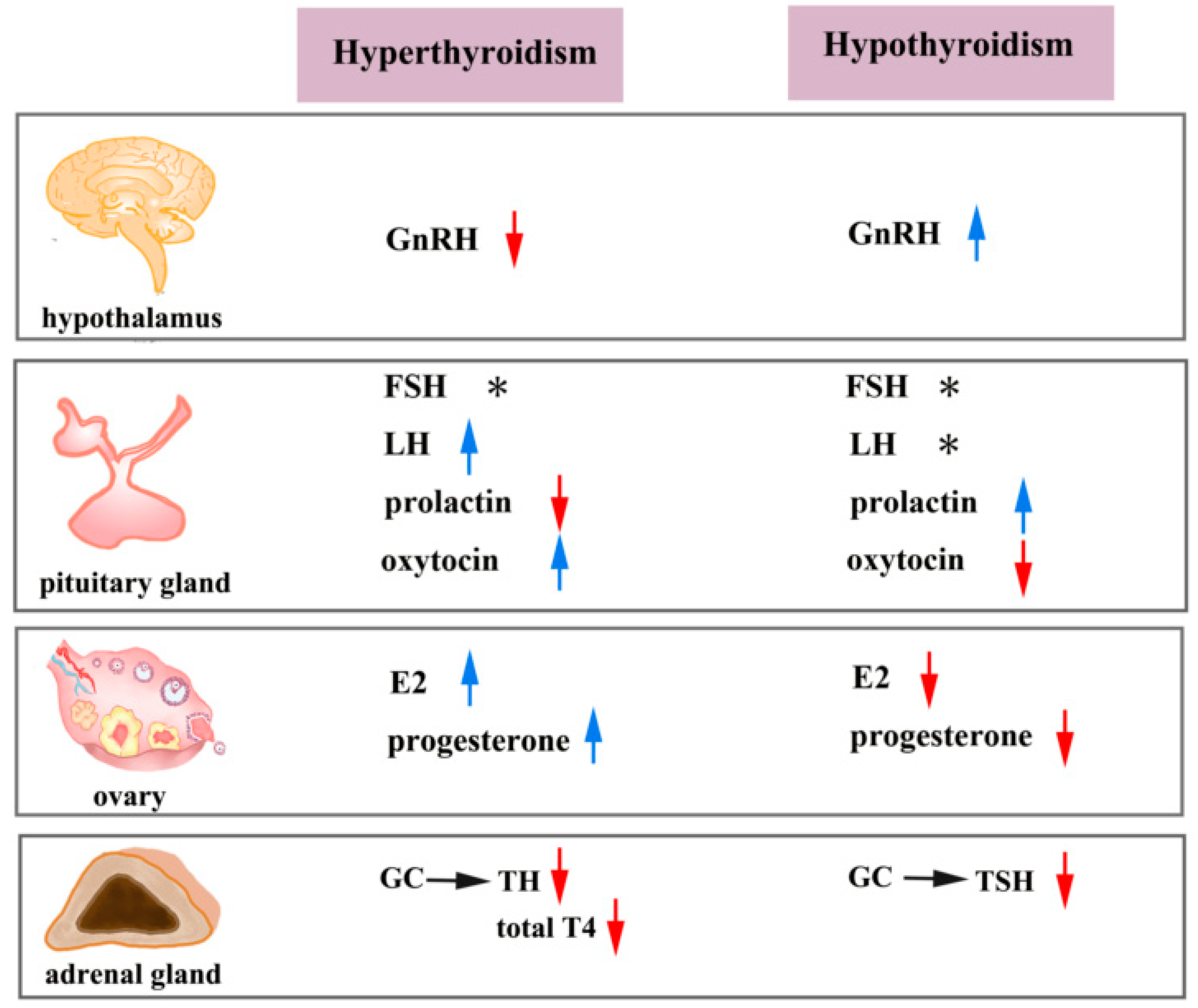
| Receptor | NR Family | The Function of Nuclear Receptors in Female Reproduction | Ligand | Half-Site Sequence |
|---|---|---|---|---|
| THRA | NR1A1 | THRA contributes to ovarian follicular development [31]. | Thyroid hormone | RGGTVA |
| THRB | NR1A2 | Loss of THRβ expression is related to the rise in the 5-year survival rate of endometrial cancer [32]. | ||
| RARA | NR1B1 | RARA might regulate vascular formation in the human endometrium [33]. | Retinoic acid | AGTTCA |
| RARB | NR1B2 | Promoter methylation of RARB and BRCA1 predicts a worse prognosis in cervical cancer [34]. | ||
| RARG | NR1B3 | Fertile: RXRB/RXRG double knockout are also fertile [35]. | ||
| PPARA | NR1C1 | PPARα activation influences endometrial cell growth and VEGF secretion [36]. | Eicosapentaenoic acid | AGGTCA |
| PPARD | NR1C2 | PPARD expression at the implantation sites requires the presence of an active blastocyst and may play an essential role for blastocyst implantation [37]. | ||
| PPARG | NR1C3 | PPARγ activation reduces proliferation of endometrial cells via regulation of PTEN [36]. | ||
| VDR | NR1I1 | VDR pathways are modulated in normal and disease endometrium by activation of vitamin-D-regulated genes [38]. | Vitamin D3 | RGKTCA |
| ER1 | NR3A1 | Early female endometriosis is mediated by immune-mediated crosstalk between ER1 and IL-6 [39]. Female mice lacking ER1 are infertile due to impaired ovarian and uterine functions [40]. | Estrogen | RGGTCA |
| ER2 | NR3A2 | Female mice lacking ERβ are sub-fertile due to ovarian defects [40]. | ||
| GR | NR3C1 | GR is required to establish the necessary cellular context for maintaining normal uterine biology and fertility through the regulation of uterine-specific actions [41]. | Cortisone | AGAACA |
| MR | NR3C2 | MR may play an endocrine/paracrine/autocrine role in the bovine ovary [42]. | Aldosterone | AGAACA |
| PR | NR3C3 | PR binds the genomic regions of genes regulating critical processes in uterine receptivity and function [43] | Progesterone | AGAACA |
| AR | NR3C4 | AR and AR signaling have a decisive role in the differentiation of human endometrial stromal cells into decidual cells [44]. | Testosterone | AGAACA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, B.; Zhu, Y. A New Perspective on Thyroid Hormones: Crosstalk with Reproductive Hormones in Females. Int. J. Mol. Sci. 2022, 23, 2708. https://doi.org/10.3390/ijms23052708
Ren B, Zhu Y. A New Perspective on Thyroid Hormones: Crosstalk with Reproductive Hormones in Females. International Journal of Molecular Sciences. 2022; 23(5):2708. https://doi.org/10.3390/ijms23052708
Chicago/Turabian StyleRen, Bingtao, and Yan Zhu. 2022. "A New Perspective on Thyroid Hormones: Crosstalk with Reproductive Hormones in Females" International Journal of Molecular Sciences 23, no. 5: 2708. https://doi.org/10.3390/ijms23052708
APA StyleRen, B., & Zhu, Y. (2022). A New Perspective on Thyroid Hormones: Crosstalk with Reproductive Hormones in Females. International Journal of Molecular Sciences, 23(5), 2708. https://doi.org/10.3390/ijms23052708






