Targeting Oncogenic Transcriptional Networks in Neuroblastoma: From N-Myc to Epigenetic Drugs
Abstract
1. Neuroblastoma: An Overview
2. Genetic Predisposition and Chromosome Instability in NB
3. Emerging Concepts of Epigenetic Dysregulation in NB
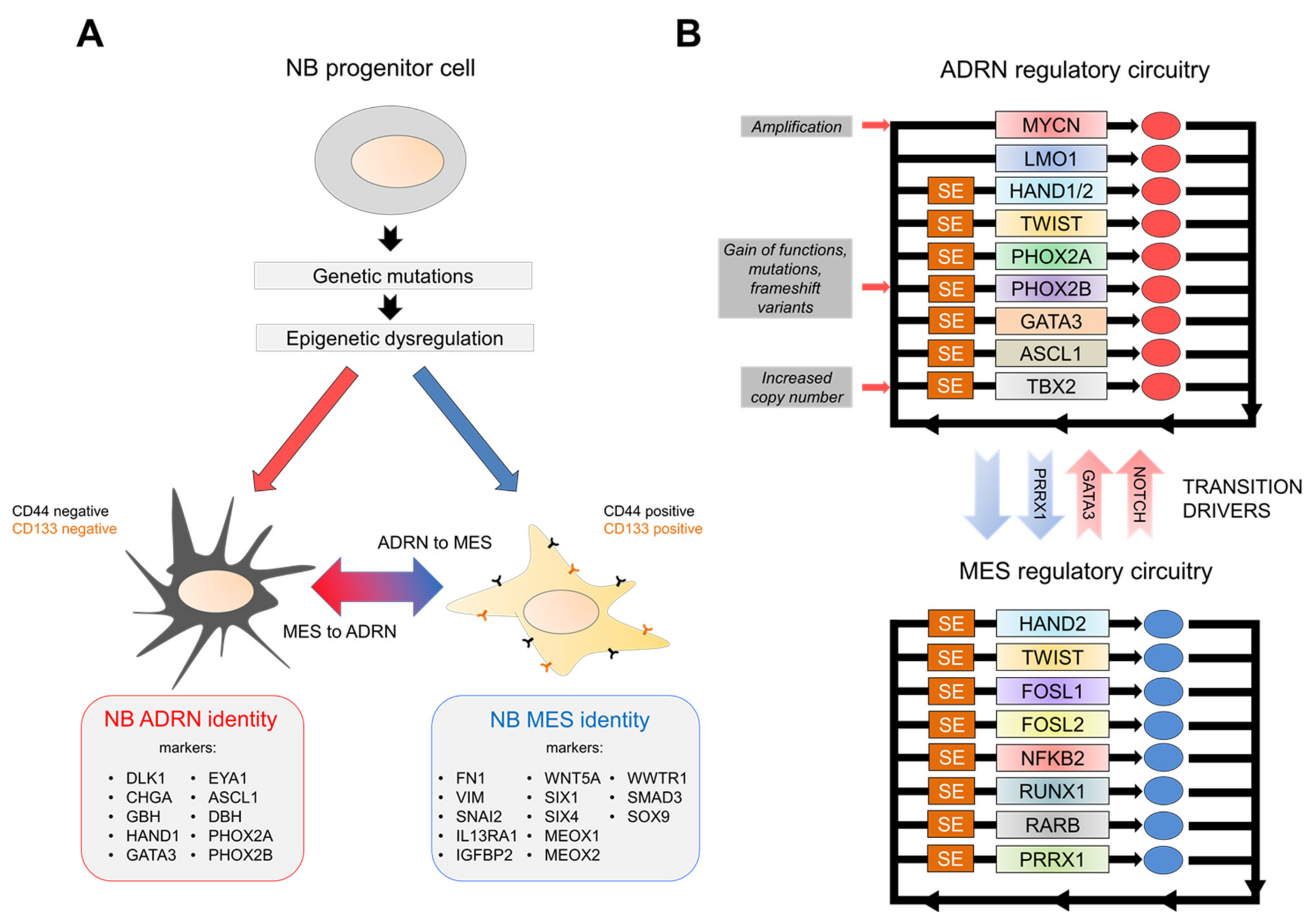
4. NB Regulatory Networks
5. N-Myc and Other Master Regulators: Oncogenic Drivers in NB Progression
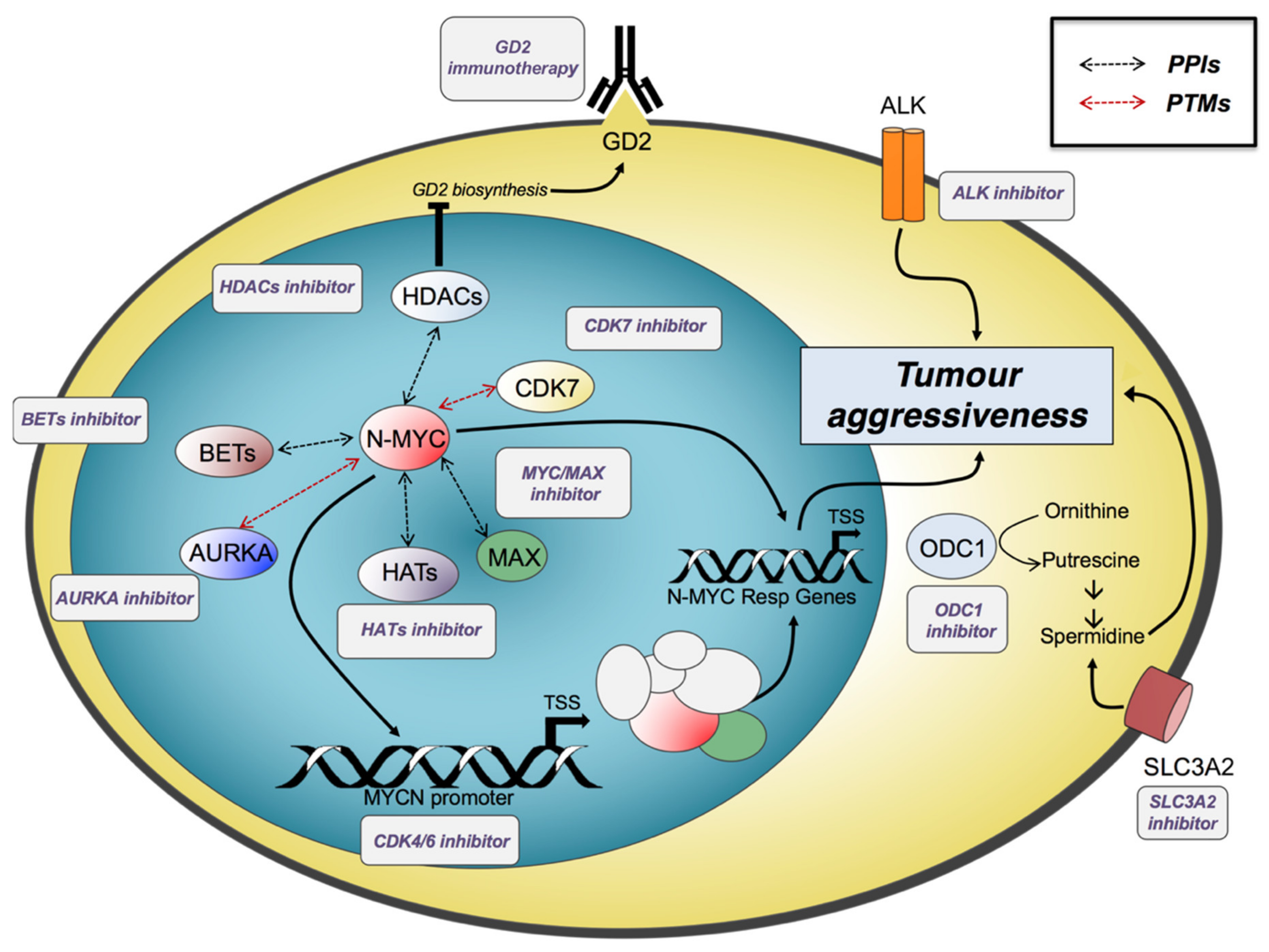
6. Transcriptional Dysregulated Programmes and Promising Therapeutic Approaches
6.1. Targeting N-Myc and Its Regulatory Networks
6.1.1. Inhibition of the N-Myc/MAX Interaction
6.1.2. Targeting N-Myc Stability
6.2. Targeting Polyamine Metabolism
6.3. Targeting CDK4/6 and PI3K/AKT/mTOR
6.4. Targeting ALK
6.5. Epigenetic Therapies
6.6. Immuno Cell Therapy: Targeting GD2
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADRN | Adrenergic |
| BET | Bromodomain and Extra Terminal |
| bHLH- | basic Heli Loop Helix |
| CDK | Cyclin Dependent Kinase |
| ChIP | Chromatin Immuno Precipitation |
| CRC | Core Regulatory Circuitry |
| EMA | European Medicines Agency |
| ESCs | Embryonic Stem Cells |
| FDA | U.S Food and Drug Administration |
| GWAS | Genome-Wide Association Studies |
| HAT | Histone Acetyl Transferase |
| HDAC | Histone DeAcetylase |
| HMT | Histone Methyltransferase |
| INSS | International NB Staging System |
| KD | Knock Down |
| KO | Knock Out |
| MES | Mesenchymal |
| NCA | Numerical Chromosomal Alterations |
| NSCs | Neuronal Stem Cells |
| NGS | Next Generation Sequencing |
| NB | Neuroblastoma |
| SE | Super Enhancer |
| RA | Retinoic Acid |
| TARGET | Therapeutically Applicable Research to generate effective Treatment |
| TF | Transcription Factor |
| WGS | Whole Genome Sequencing |
References
- Bahmad, H.F.; Chamaa, F.; Assi, S.; Chalhoub, R.M.; Abou-Antoun, T.; Abou-Kheir, W. Cancer Stem Cells in Neuroblastoma: Expanding the Therapeutic Frontier. Front. Mol. Neurosci. 2019, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.M.; Seeger, R.C.; Barrett, A.; Berthold, F.; Castleberry, R.P.; D’Angio, G.; De Bernardi, B.; Evans, A.E.; Favrot, M.; Freeman, A.I. International Criteria for Diagnosis, Staging, and Response to Treatment in Patients with Neuroblastoma. J. Clin. Oncol. 1988, 6, 1874–1881. [Google Scholar] [CrossRef]
- Jiang, M.; Stanke, J.; Lahti, J.M. The connections between Neural Crest Development and Neuroblastoma. Curr. Top. Dev. Biol. 2011, 94, 77–127. [Google Scholar] [CrossRef] [PubMed]
- Louis, C.U.; Shohet, J.M. Neuroblastoma: Molecular Pathogenesis and Therapy. Annu. Rev. Med. 2015, 66, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.-K.V.; Dyer, M.A. Neuroblastoma: Developmental Biology, Cancer Genomics and Immunotherapy. Nat. Rev. Cancer 2013, 13, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Mosse, Y.P.; Laudenslager, M.; Khazi, D.; Carlisle, A.J.; Winter, C.L.; Rappaport, E.; Maris, J.M. Germline PHOX2B Mutation in Hereditary Neuroblastoma. Am. J. Hum. Genet. 2004, 75, 727–730. [Google Scholar] [CrossRef]
- Trochet, D.; Bourdeaut, F.; Janoueix-Lerosey, I.; Deville, A.; de Pontual, L.; Schleiermacher, G.; Coze, C.; Philip, N.; Frébourg, T.; Munnich, A.; et al. Germline Mutations of the Paired-like Homeobox 2B (PHOX2B) Gene in Neuroblastoma. Am. J. Hum. Genet. 2004, 74, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, A.; Morin, X.; Cremer, H.; Goridis, C.; Brunet, J.F. The Homeobox Gene Phox2b Is Essential for the Development of Autonomic Neural Crest Derivatives. Nature 1999, 399, 366–370. [Google Scholar] [CrossRef]
- Bachetti, T.; Ceccherini, I. Causative and Common PHOX2B Variants Define a Broad Phenotypic Spectrum. Clin. Genet. 2020, 97, 103–113. [Google Scholar] [CrossRef]
- Mossé, Y.P.; Laudenslager, M.; Longo, L.; Cole, K.A.; Wood, A.; Attiyeh, E.F.; Laquaglia, M.J.; Sennett, R.; Lynch, J.E.; Perri, P.; et al. Identification of ALK as a Major Familial Neuroblastoma Predisposition Gene. Nature 2008, 455, 930–935. [Google Scholar] [CrossRef]
- Kim, E.K.; Kim, S. ALK Gene Copy Number Gain and Immunohistochemical Expression Status Using Three Antibodies in Neuroblastoma. Pediatr. Dev. Pathol. 2017, 20, 133–141. [Google Scholar] [CrossRef]
- Fransson, S.; Hansson, M.; Ruuth, K.; Djos, A.; Berbegall, A.; Javanmardi, N.; Abrahamsson, J.; Palmer, R.H.; Noguera, R.; Hallberg, B.; et al. Intragenic Anaplastic Lymphoma Kinase (ALK) Rearrangements: Translocations as a Novel Mechanism of ALK Activation in Neuroblastoma Tumors. Genes Chromosomes Cancer 2015, 54, 99–109. [Google Scholar] [CrossRef]
- Cazes, A.; Louis-Brennetot, C.; Mazot, P.; Dingli, F.; Lombard, B.; Boeva, V.; Daveau, R.; Cappo, J.; Combaret, V.; Schleiermacher, G.; et al. Characterization of Rearrangements Involving the ALK Gene Reveals a Novel Truncated Form Associated with Tumor Aggressiveness in Neuroblastoma. Cancer Res. 2013, 73, 195–204. [Google Scholar] [CrossRef]
- Brady, S.W.; Liu, Y.; Ma, X.; Gout, A.M.; Hagiwara, K.; Zhou, X.; Wang, J.; Macias, M.; Chen, X.; Easton, J.; et al. Pan-Neuroblastoma Analysis Reveals Age- and Signature-Associated Driver Alterations. Nat. Commun. 2020, 11, 5183. [Google Scholar] [CrossRef]
- Peifer, M.; Hertwig, F.; Roels, F.; Dreidax, D.; Gartlgruber, M.; Menon, R.; Krämer, A.; Roncaioli, J.L.; Sand, F.; Heuckmann, J.M.; et al. Telomerase Activation by Genomic Rearrangements in High-Risk Neuroblastoma. Nature 2015, 526, 700–704. [Google Scholar] [CrossRef]
- Molenaar, J.J.; Koster, J.; Zwijnenburg, D.A.; van Sluis, P.; Valentijn, L.J.; van der Ploeg, I.; Hamdi, M.; van Nes, J.; Westerman, B.A.; van Arkel, J.; et al. Sequencing of Neuroblastoma Identifies Chromothripsis and Defects in Neuritogenesis Genes. Nature 2012, 483, 589–593. [Google Scholar] [CrossRef]
- Maris, J.M. Recent Advances in Neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211. [Google Scholar] [CrossRef]
- Huang, M.; Weiss, W.A. Neuroblastoma and MYCN. Cold Spring Harb. Perspect. Med. 2013, 3, a014415. [Google Scholar] [CrossRef]
- Matthay, K.K.; Maris, J.M.; Schleiermacher, G.; Nakagawara, A.; Mackall, C.L.; Diller, L.; Weiss, W.A. Neuroblastoma. Nat. Rev. Dis. Primers 2016, 2, 16078. [Google Scholar] [CrossRef]
- García-López, J.; Wallace, K.; Otero, J.H.; Olsen, R.; Wang, Y.-D.; Finkelstein, D.; Gudenas, B.L.; Rehg, J.E.; Northcott, P.; Davidoff, A.M.; et al. Large 1p36 Deletions Affecting Arid1a Locus Facilitate Mycn-Driven Oncogenesis in Neuroblastoma. Cell Rep. 2020, 30, 454–464.e5. [Google Scholar] [CrossRef]
- Shi, H.; Tao, T.; Abraham, B.J.; Durbin, A.D.; Zimmerman, M.W.; Kadoch, C.; Look, A.T. ARID1A Loss in Neuroblastoma Promotes the Adrenergic-to-Mesenchymal Transition by Regulating Enhancer-Mediated Gene Expression. Sci. Adv. 2020, 6, eaaz3440. [Google Scholar] [CrossRef]
- Bown, N.; Cotterill, S.; Lastowska, M.; O’Neill, S.; Pearson, A.D.; Plantaz, D.; Meddeb, M.; Danglot, G.; Brinkschmidt, C.; Christiansen, H.; et al. Gain of Chromosome Arm 17q and Adverse Outcome in Patients with Neuroblastoma. N. Engl. J. Med. 1999, 340, 1954–1961. [Google Scholar] [CrossRef]
- Wong, M.; Sun, Y.; Xi, Z.; Milazzo, G.; Poulos, R.C.; Bartenhagen, C.; Bell, J.L.; Mayoh, C.; Ho, N.; Tee, A.E.; et al. JMJD6 Is a Tumorigenic Factor and Therapeutic Target in Neuroblastoma. Nat. Commun. 2019, 10, 3319. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, I.C.; Bei, Y.; Garcia, H.D.; Ortiz, M.V.; Toedling, J.; Klironomos, F.; Rolff, J.; Eggert, A.; Schulte, J.H.; Kentsis, A.; et al. Prohibitin Promotes De-Differentiation and Is a Potential Therapeutic Target in Neuroblastoma. JCI Insight 2019, 5, 127130. [Google Scholar] [CrossRef]
- Adam, K.; Lesperance, J.; Hunter, T.; Zage, P.E. The Potential Functional Roles of NME1 Histidine Kinase Activity in Neuroblastoma Pathogenesis. Int. J. Mol. Sci. 2020, 21, 3319. [Google Scholar] [CrossRef]
- Nagy, Z.; Seneviratne, J.A.; Kanikevich, M.; Chang, W.; Mayoh, C.; Venkat, P.; Du, Y.; Jiang, C.; Salib, A.; Koach, J.; et al. An ALYREF-MYCN Coactivator Complex Drives Neuroblastoma Tumorigenesis through Effects on USP3 and MYCN Stability. Nat. Commun. 2021, 12, 1881. [Google Scholar] [CrossRef]
- Juan Ribelles, A.; Barberá, S.; Yáñez, Y.; Gargallo, P.; Segura, V.; Juan, B.; Noguera, R.; Piqueras, M.; Fornés-Ferrer, V.; de Mora, J.F.; et al. Clinical Features of Neuroblastoma With 11q Deletion: An Increase in Relapse Probabilities In Localized And 4S Stages. Sci. Rep. 2019, 9, 13806. [Google Scholar] [CrossRef]
- Campbell, K.; Gastier-Foster, J.M.; Mann, M.; Naranjo, A.H.; Van Ryn, C.; Bagatell, R.; Matthay, K.K.; London, W.B.; Irwin, M.S.; Shimada, H.; et al. Association of MYCN Copy Number with Clinical Features, Tumor Biology, and Outcomes in Neuroblastoma: A Report from the Children’s Oncology Group. Cancer 2017, 123, 4224–4235. [Google Scholar] [CrossRef]
- Lopez, G.; Conkrite, K.L.; Doepner, M.; Rathi, K.S.; Modi, A.; Vaksman, Z.; Farra, L.M.; Hyson, E.; Noureddine, M.; Wei, J.S.; et al. Somatic Structural Variation Targets Neurodevelopmental Genes and Identifies SHANK2 as a Tumor Suppressor in Neuroblastoma. Genome Res. 2020, 30, 1228–1242. [Google Scholar] [CrossRef]
- Valentijn, L.J.; Koster, J.; Zwijnenburg, D.A.; Hasselt, N.E.; van Sluis, P.; Volckmann, R.; van Noesel, M.M.; George, R.E.; Tytgat, G.A.M.; Molenaar, J.J.; et al. TERT Rearrangements Are Frequent in Neuroblastoma and Identify Aggressive Tumors. Nat. Genet. 2015, 47, 1411–1414. [Google Scholar] [CrossRef]
- Cheung, N.-K.V.; Zhang, J.; Lu, C.; Parker, M.; Bahrami, A.; Tickoo, S.K.; Heguy, A.; Pappo, A.S.; Federico, S.; Dalton, J.; et al. Association of Age at Diagnosis and Genetic Mutations in Patients with Neuroblastoma. JAMA 2012, 307, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Zeineldin, M.; Federico, S.; Chen, X.; Fan, Y.; Xu, B.; Stewart, E.; Zhou, X.; Jeon, J.; Griffiths, L.; Nguyen, R.; et al. MYCN Amplification and ATRX Mutations Are Incompatible in Neuroblastoma. Nat. Commun. 2020, 11, 913. [Google Scholar] [CrossRef] [PubMed]
- Parodi, S.; Pistorio, A.; Erminio, G.; Ognibene, M.; Morini, M.; Garaventa, A.; Gigliotti, A.R.; Haupt, R.; Frassoni, F.; Pezzolo, A. Loss of Whole Chromosome X Predicts Prognosis of Neuroblastoma Patients with Numerical Genomic Profile. Pediatr. Blood Cancer 2019, 66, e27635. [Google Scholar] [CrossRef] [PubMed]
- Berbegall, A.P.; Villamón, E.; Tadeo, I.; Martinsson, T.; Cañete, A.; Castel, V.; Navarro, S.; Noguera, R. Neuroblastoma after Childhood: Prognostic Relevance of Segmental Chromosome Aberrations, ATRX Protein Status, and Immune Cell Infiltration. Neoplasia 2014, 16, 471–480. [Google Scholar] [CrossRef]
- Suzuki, M.; Kushner, B.H.; Kramer, K.; Basu, E.M.; Roberts, S.S.; Hammond, W.J.; LaQuaglia, M.P.; Wolden, S.L.; Cheung, N.-K.V.; Modak, S. Treatment and Outcome of Adult-Onset Neuroblastoma. Int. J. Cancer 2018, 143, 1249–1258. [Google Scholar] [CrossRef]
- Duan, K.; Dickson, B.C.; Marrano, P.; Thorner, P.S.; Chung, C.T. Adult-Onset Neuroblastoma: Report of Seven Cases with Molecular Genetic Characterization. Genes Chromosomes Cancer 2020, 59, 240–248. [Google Scholar] [CrossRef]
- Javanmardi, N.; Fransson, S.; Djos, A.; Sjöberg, R.-M.; Nilsson, S.; Truvé, K.; Kogner, P.; Martinsson, T. Low Frequency ALK Hotspots Mutations In Neuroblastoma Tumours Detected By Ultra-Deep Sequencing: Implications For ALK Inhibitor Treatment. Sci. Rep. 2019, 9, 2199. [Google Scholar] [CrossRef]
- Trigg, R.M.; Turner, S.D. ALK in Neuroblastoma: Biological and Therapeutic Implications. Cancers 2018, 10, 113. [Google Scholar] [CrossRef]
- O’Neill, S.; Ekstrom, L.; Lastowska, M.; Roberts, P.; Brodeur, G.M.; Kees, U.R.; Schwab, M.; Bown, N. MYCN Amplification and 17q in Neuroblastoma: Evidence for Structural Association. Genes Chromosomes Cancer 2001, 30, 87–90. [Google Scholar] [CrossRef]
- Chang, M.T.; Asthana, S.; Gao, S.P.; Lee, B.H.; Chapman, J.S.; Kandoth, C.; Gao, J.; Socci, N.D.; Solit, D.B.; Olshen, A.B.; et al. Identifying Recurrent Mutations in Cancer Reveals Widespread Lineage Diversity and Mutational Specificity. Nat. Biotechnol. 2016, 34, 155–163. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The CBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the CBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Pugh, T.J.; Morozova, O.; Attiyeh, E.F.; Asgharzadeh, S.; Wei, J.S.; Auclair, D.; Carter, S.L.; Cibulskis, K.; Hanna, M.; Kiezun, A.; et al. The Genetic Landscape of High-Risk Neuroblastoma. Nat. Genet. 2013, 45, 279–284. [Google Scholar] [CrossRef]
- Biswas, S.; Rao, C.M. Epigenetic Tools (The Writers, The Readers and The Erasers) and Their Implications in Cancer Therapy. Eur. J. Pharm. 2018, 837, 8–24. [Google Scholar] [CrossRef]
- Gartlgruber, M.; Sharma, A.K.; Quintero, A.; Dreidax, D.; Jansky, S.; Park, Y.-G.; Kreth, S.; Meder, J.; Doncevic, D.; Saary, P.; et al. Super Enhancers Define Regulatory Subtypes and Cell Identity in Neuroblastoma. Nat. Cancer 2021, 2, 114–128. [Google Scholar] [CrossRef]
- Burney, M.J.; Johnston, C.; Wong, K.-Y.; Teng, S.-W.; Beglopoulos, V.; Stanton, L.W.; Williams, B.P.; Bithell, A.; Buckley, N.J. An Epigenetic Signature of Developmental Potential in Neural Stem Cells and Early Neurons. Stem Cells 2013, 31, 1868–1880. [Google Scholar] [CrossRef]
- Delaval, K.; Feil, R. Epigenetic Regulation of Mammalian Genomic Imprinting. Curr. Opin. Genet. Dev. 2004, 14, 188–195. [Google Scholar] [CrossRef]
- Margueron, R.; Trojer, P.; Reinberg, D. The Key to Development: Interpreting the Histone Code? Curr. Opin. Genet. Dev. 2005, 15, 163–176. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Baylin, S.B. Cancer Epigenetics: Linking Basic Biology to Clinical Medicine. Cell Res. 2011, 21, 502–517. [Google Scholar] [CrossRef]
- Bernstein, B.E.; Mikkelsen, T.S.; Xie, X.; Kamal, M.; Huebert, D.J.; Cuff, J.; Fry, B.; Meissner, A.; Wernig, M.; Plath, K.; et al. A Bivalent Chromatin Structure Marks Key Developmental Genes in Embryonic Stem Cells. Cell 2006, 125, 315–326. [Google Scholar] [CrossRef]
- Sharma, S.V.; Lee, D.Y.; Li, B.; Quinlan, M.P.; Takahashi, F.; Maheswaran, S.; McDermott, U.; Azizian, N.; Zou, L.; Fischbach, M.A.; et al. A Chromatin-Mediated Reversible Drug-Tolerant State in Cancer Cell Subpopulations. Cell 2010, 141, 69–80. [Google Scholar] [CrossRef]
- Cohen, A.L.; Piccolo, S.R.; Cheng, L.; Soldi, R.; Han, B.; Johnson, W.E.; Bild, A.H. Genomic Pathway Analysis Reveals That EZH2 and HDAC4 Represent Mutually Exclusive Epigenetic Pathways across Human Cancers. BMC Med. Genom. 2013, 6, 35. [Google Scholar] [CrossRef]
- Corvetta, D.; Chayka, O.; Gherardi, S.; D’Acunto, C.W.; Cantilena, S.; Valli, E.; Piotrowska, I.; Perini, G.; Sala, A. Physical Interaction between MYCN Oncogene and Polycomb Repressive Complex 2 (PRC2) in Neuroblastoma: Functional and Therapeutic Implications. J. Biol. Chem. 2013, 288, 8332–8341. [Google Scholar] [CrossRef]
- Chen, L.; Alexe, G.; Dharia, N.V.; Ross, L.; Iniguez, A.B.; Conway, A.S.; Wang, E.J.; Veschi, V.; Lam, N.; Qi, J.; et al. CRISPR-Cas9 Screen Reveals a MYCN-Amplified Neuroblastoma Dependency on EZH2. J. Clin. Investig. 2018, 128, 446–462. [Google Scholar] [CrossRef]
- Tsubota, S.; Kishida, S.; Shimamura, T.; Ohira, M.; Yamashita, S.; Cao, D.; Kiyonari, S.; Ushijima, T.; Kadomatsu, K. PRC2-Mediated Transcriptomic Alterations at the Embryonic Stage Govern Tumorigenesis and Clinical Outcome in MYCN-Driven Neuroblastoma. Cancer Res. 2017, 77, 5259–5271. [Google Scholar] [CrossRef]
- Li, Z.; Takenobu, H.; Setyawati, A.N.; Akita, N.; Haruta, M.; Satoh, S.; Shinno, Y.; Chikaraishi, K.; Mukae, K.; Akter, J.; et al. EZH2 Regulates Neuroblastoma Cell Differentiation via NTRK1 Promoter Epigenetic Modifications. Oncogene 2018, 37, 2714–2727. [Google Scholar] [CrossRef]
- Schulte, J.H.; Lim, S.; Schramm, A.; Friedrichs, N.; Koster, J.; Versteeg, R.; Ora, I.; Pajtler, K.; Klein-Hitpass, L.; Kuhfittig-Kulle, S.; et al. Lysine-Specific Demethylase 1 Is Strongly Expressed in Poorly Differentiated Neuroblastoma: Implications for Therapy. Cancer Res. 2009, 69, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Walz, S.; Lorenzin, F.; Morton, J.; Wiese, K.E.; von Eyss, B.; Herold, S.; Rycak, L.; Dumay-Odelot, H.; Karim, S.; Bartkuhn, M.; et al. Activation and Repression by Oncogenic MYC Shape Tumour-Specific Gene Expression Profiles. Nature 2014, 511, 483–487. [Google Scholar] [CrossRef]
- Gartel, A.L.; Shchors, K. Mechanisms of C-Myc-Mediated Transcriptional Repression of Growth Arrest Genes. Exp. Cell Res. 2003, 283, 17–21. [Google Scholar] [CrossRef]
- Milazzo, G.; Mercatelli, D.; Di Muzio, G.; Triboli, L.; De Rosa, P.; Perini, G.; Giorgi, F.M. Histone Deacetylases (HDACs): Evolution, Specificity, Role in Transcriptional Complexes, and Pharmacological Actionability. Genes 2020, 11, 556. [Google Scholar] [CrossRef]
- Gajer, J.M.; Furdas, S.D.; Gründer, A.; Gothwal, M.; Heinicke, U.; Keller, K.; Colland, F.; Fulda, S.; Pahl, H.L.; Fichtner, I.; et al. Histone Acetyltransferase Inhibitors Block Neuroblastoma Cell Growth in Vivo. Oncogenesis 2015, 4, e137. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Q.; Zhao, W.; Yuan, D.; Zhao, H.; Zhou, Y. MiR-329 Suppresses the Growth and Motility of Neuroblastoma by Targeting KDM1A. FEBS Lett. 2014, 588, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, L.; Lin, R.Y.-T.; Müschen, M.; Koeffler, H.P. Core Transcriptional Regulatory Circuitries in Cancer. Oncogene 2020, 39, 6633–6646. [Google Scholar] [CrossRef]
- Tang, F.; Yang, Z.; Tan, Y.; Li, Y. Super-Enhancer Function and Its Application in Cancer Targeted Therapy. NPJ Precis. Oncol. 2020, 4, 2. [Google Scholar] [CrossRef]
- Whyte, W.A.; Orlando, D.A.; Hnisz, D.; Abraham, B.J.; Lin, C.Y.; Kagey, M.H.; Rahl, P.B.; Lee, T.I.; Young, R.A. Master Transcription Factors and Mediator Establish Super-Enhancers at Key Cell Identity Genes. Cell 2013, 153, 307–319. [Google Scholar] [CrossRef]
- Bradner, J.E.; Hnisz, D.; Young, R.A. Transcriptional Addiction in Cancer. Cell 2017, 168, 629–643. [Google Scholar] [CrossRef]
- Vaquerizas, J.M.; Kummerfeld, S.K.; Teichmann, S.A.; Luscombe, N.M. A Census of Human Transcription Factors: Function, Expression and Evolution. Nat. Rev. Genet. 2009, 10, 252–263. [Google Scholar] [CrossRef]
- Garraway, L.A.; Lander, E.S. Lessons from the Cancer Genome. Cell 2013, 153, 17–37. [Google Scholar] [CrossRef]
- Hnisz, D.; Abraham, B.J.; Lee, T.I.; Lau, A.; Saint-André, V.; Sigova, A.A.; Hoke, H.A.; Young, R.A. Super-Enhancers in the Control of Cell Identity and Disease. Cell 2013, 155, 934–947. [Google Scholar] [CrossRef]
- van Groningen, T.; Koster, J.; Valentijn, L.J.; Zwijnenburg, D.A.; Akogul, N.; Hasselt, N.E.; Broekmans, M.; Haneveld, F.; Nowakowska, N.E.; Bras, J.; et al. Neuroblastoma Is Composed of Two Super-Enhancer-Associated Differentiation States. Nat. Genet. 2017, 49, 1261–1266. [Google Scholar] [CrossRef]
- Boeva, V.; Louis-Brennetot, C.; Peltier, A.; Durand, S.; Pierre-Eugène, C.; Raynal, V.; Etchevers, H.C.; Thomas, S.; Lermine, A.; Daudigeos-Dubus, E.; et al. Heterogeneity of Neuroblastoma Cell Identity Defined by Transcriptional Circuitries. Nat. Genet. 2017, 49, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Durbin, A.D.; Zimmerman, M.W.; Dharia, N.V.; Abraham, B.J.; Iniguez, A.B.; Weichert-Leahey, N.; He, S.; Krill-Burger, J.M.; Root, D.E.; Vazquez, F.; et al. Selective Gene Dependencies in MYCN-Amplified Neuroblastoma Include the Core Transcriptional Regulatory Circuitry. Nat. Genet. 2018, 50, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Young, R.A. Control of the Embryonic Stem Cell State. Cell 2011, 144, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Fernando, J.; Malfettone, A.; Cepeda, E.B.; Vilarrasa-Blasi, R.; Bertran, E.; Raimondi, G.; Fabra, À.; Alvarez-Barrientos, A.; Fernández-Salguero, P.; Fernández-Rodríguez, C.M.; et al. A Mesenchymal-like Phenotype and Expression of CD44 Predict Lack of Apoptotic Response to Sorafenib in Liver Tumor Cells. Int. J. Cancer 2015, 136, E161–E172. [Google Scholar] [CrossRef]
- Interplay between Intrinsic Reprogramming Potential and Microenvironment Controls Neuroblastoma Cell Plasticity and Identity | bioRxiv. Available online: https://www.biorxiv.org/content/10.1101/2021.01.07.425710v3 (accessed on 15 October 2021).
- Van Groningen, T.; Akogul, N.; Westerhout, E.M.; Chan, A.; Hasselt, N.E.; Zwijnenburg, D.A.; Broekmans, M.; Stroeken, P.; Haneveld, F.; Hooijer, G.K.J.; et al. A NOTCH Feed-Forward Loop Drives Reprogramming from Adrenergic to Mesenchymal State in Neuroblastoma. Nat. Commun. 2019, 10, 1530. [Google Scholar] [CrossRef]
- Zeid, R.; Lawlor, M.A.; Poon, E.; Reyes, J.M.; Fulciniti, M.; Lopez, M.A.; Scott, T.G.; Nabet, B.; Erb, M.A.; Winter, G.E.; et al. Enhancer Invasion Shapes MYCN-Dependent Transcriptional Amplification in Neuroblastoma. Nat. Genet. 2018, 50, 515–523. [Google Scholar] [CrossRef]
- Capasso, M.; Lasorsa, V.A.; Cimmino, F.; Avitabile, M.; Cantalupo, S.; Montella, A.; De Angelis, B.; Morini, M.; de Torres, C.; Castellano, A.; et al. Transcription Factors Involved in Tumorigenesis Are Over-Represented in Mutated Active DNA-Binding Sites in Neuroblastoma. Cancer Res. 2020, 80, 382–393. [Google Scholar] [CrossRef]
- Zimmerman, K.A.; Yancopoulos, G.D.; Collum, R.G.; Smith, R.K.; Kohl, N.E.; Denis, K.A.; Nau, M.M.; Witte, O.N.; Toran-Allerand, D.; Gee, C.E. Differential Expression of Myc Family Genes during Murine Development. Nature 1986, 319, 780–783. [Google Scholar] [CrossRef]
- Zimmerman, M.W.; Liu, Y.; He, S.; Durbin, A.D.; Abraham, B.J.; Easton, J.; Shao, Y.; Xu, B.; Zhu, S.; Zhang, X.; et al. MYC Drives a Subset of High-Risk Pediatric Neuroblastomas and Is Activated through Mechanisms Including Enhancer Hijacking and Focal Enhancer Amplification. Cancer Discov. 2018, 8, 320–335. [Google Scholar] [CrossRef]
- Wang, L.; Tan, T.K.; Durbin, A.D.; Zimmerman, M.W.; Abraham, B.J.; Tan, S.H.; Ngoc, P.C.T.; Weichert-Leahey, N.; Akahane, K.; Lawton, L.N.; et al. ASCL1 Is a MYCN- and LMO1-Dependent Member of the Adrenergic Neuroblastoma Core Regulatory Circuitry. Nat. Commun. 2019, 10, 5622. [Google Scholar] [CrossRef]
- Otte, J.; Dyberg, C.; Pepich, A.; Johnsen, J.I. MYCN Function in Neuroblastoma Development. Front. Oncol. 2020, 10, 624079. [Google Scholar] [CrossRef]
- Otto, T.; Horn, S.; Brockmann, M.; Eilers, U.; Schüttrumpf, L.; Popov, N.; Kenney, A.M.; Schulte, J.H.; Beijersbergen, R.; Christiansen, H.; et al. Stabilization of N-Myc Is a Critical Function of Aurora A in Human Neuroblastoma. Cancer Cell 2009, 15, 67–78. [Google Scholar] [CrossRef]
- Thomas, L.R.; Wang, Q.; Grieb, B.C.; Phan, J.; Foshage, A.M.; Sun, Q.; Olejniczak, E.T.; Clark, T.; Dey, S.; Lorey, S.; et al. Interaction with WDR5 Promotes Target Gene Recognition and Tumorigenesis by MYC. Mol. Cell 2015, 58, 440–452. [Google Scholar] [CrossRef]
- Sun, Y.; Bell, J.L.; Carter, D.; Gherardi, S.; Poulos, R.C.; Milazzo, G.; Wong, J.W.H.; Al-Awar, R.; Tee, A.E.; Liu, P.Y.; et al. WDR5 Supports an N-Myc Transcriptional Complex That Drives a Protumorigenic Gene Expression Signature in Neuroblastoma. Cancer Res. 2015, 75, 5143–5154. [Google Scholar] [CrossRef]
- Cimmino, F.; Avitabile, M.; Diskin, S.J.; Vaksman, Z.; Pignataro, P.; Formicola, D.; Cardinale, A.; Testori, A.; Koster, J.; de Torres, C.; et al. Fine Mapping of 2q35 High-Risk Neuroblastoma Locus Reveals Independent Functional Risk Variants and Suggests Full-Length BARD1 as Tumor-Suppressor. Int. J. Cancer 2018, 143, 2828–2837. [Google Scholar] [CrossRef]
- Kocak, H.; Ackermann, S.; Hero, B.; Kahlert, Y.; Oberthuer, A.; Juraeva, D.; Roels, F.; Theissen, J.; Westermann, F.; Deubzer, H.; et al. Hox-C9 Activates the Intrinsic Pathway of Apoptosis and Is Associated with Spontaneous Regression in Neuroblastoma. Cell Death Dis. 2013, 4, e586. [Google Scholar] [CrossRef]
- Harenza, J.L.; Diamond, M.A.; Adams, R.N.; Song, M.M.; Davidson, H.L.; Hart, L.S.; Dent, M.H.; Fortina, P.; Reynolds, C.P.; Maris, J.M. Transcriptomic Profiling of 39 Commonly-Used Neuroblastoma Cell Lines. Sci. Data 2017, 4, 170033. [Google Scholar] [CrossRef]
- Pearson, A.D.J.; Pinkerton, C.R.; Lewis, I.J.; Imeson, J.; Ellershaw, C.; Machin, D.; European Neuroblastoma Study Group. Children’s Cancer and Leukaemia Group (CCLG formerly United Kingdom Children’s Cancer Study Group) High-Dose Rapid and Standard Induction Chemotherapy for Patients Aged over 1 Year with Stage 4 Neuroblastoma: A Randomised Trial. Lancet Oncol. 2008, 9, 247–256. [Google Scholar] [CrossRef]
- Luo, Y.-B.; Cui, X.-C.; Yang, L.; Zhang, D.; Wang, J.-X. Advances in the Surgical Treatment of Neuroblastoma. Chin. Med. J. 2018, 131, 2332–2337. [Google Scholar] [CrossRef]
- Van Golen, C.M.; Soules, M.E.; Grauman, A.R.; Feldman, E.L. N-Myc Overexpression Leads to Decreased Beta1 Integrin Expression and Increased Apoptosis in Human Neuroblastoma Cells. Oncogene 2003, 22, 2664–2673. [Google Scholar] [CrossRef]
- Tanaka, N.; Fukuzawa, M. MYCN Downregulates Integrin Alpha1 to Promote Invasion of Human Neuroblastoma Cells. Int. J. Oncol. 2008, 33, 815–821. [Google Scholar]
- Sugiura, Y.; Shimada, H.; Seeger, R.C.; Laug, W.E.; DeClerck, Y.A. Matrix Metalloproteinases-2 and -9 Are Expressed in Human Neuroblastoma: Contribution of Stromal Cells to Their Production and Correlation with Metastasis. Cancer Res. 1998, 58, 2209–2216. [Google Scholar]
- Noujaim, D.; van Golen, C.M.; van Golen, K.L.; Grauman, A.; Feldman, E.L. N-Myc and Bcl-2 Coexpression Induces MMP-2 Secretion and Activation in Human Neuroblastoma Cells. Oncogene 2002, 21, 4549–4557. [Google Scholar] [CrossRef]
- Cotterman, R.; Knoepfler, P.S. N-Myc Regulates Expression of Pluripotency Genes in Neuroblastoma Including Lif, Klf2, Klf4, and Lin28b. PLoS ONE 2009, 4, e5799. [Google Scholar] [CrossRef]
- Mugrauer, G.; Alt, F.W.; Ekblom, P. N-Myc Proto-Oncogene Expression during Organogenesis in the Developing Mouse as Revealed by in Situ Hybridization. J. Cell Biol. 1988, 107, 1325–1335. [Google Scholar] [CrossRef]
- Fletcher, S.; Prochownik, E.V. Small-Molecule Inhibitors of the Myc Oncoprotein. Biochim. Biophys. Acta 2015, 1849, 525–543. [Google Scholar] [CrossRef]
- Mujtaba, S.; Zeng, L.; Zhou, M.-M. Structure and Acetyl-Lysine Recognition of the Bromodomain. Oncogene 2007, 26, 5521–5527. [Google Scholar] [CrossRef]
- Itzen, F.; Greifenberg, A.K.; Bösken, C.A.; Geyer, M. Brd4 Activates P-TEFb for RNA Polymerase II CTD Phosphorylation. Nucleic Acids Res. 2014, 42, 7577–7590. [Google Scholar] [CrossRef]
- Puissant, A.; Frumm, S.M.; Alexe, G.; Bassil, C.F.; Qi, J.; Chanthery, Y.H.; Nekritz, E.A.; Zeid, R.; Gustafson, W.C.; Greninger, P.; et al. Targeting MYCN in Neuroblastoma by BET Bromodomain Inhibition. Cancer Discov. 2013, 3, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Henssen, A.; Althoff, K.; Odersky, A.; Beckers, A.; Koche, R.; Speleman, F.; Schäfers, S.; Bell, E.; Nortmeyer, M.; Westermann, F.; et al. Targeting MYCN-Driven Transcription By BET-Bromodomain Inhibition. Clin. Cancer Res. 2016, 22, 2470–2481. [Google Scholar] [CrossRef] [PubMed]
- Piha-Paul, S.A.; Hann, C.L.; French, C.A.; Cousin, S.; Braña, I.; Cassier, P.A.; Moreno, V.; de Bono, J.S.; Harward, S.D.; Ferron-Brady, G.; et al. Phase 1 Study of Molibresib (GSK525762), a Bromodomain and Extra-Terminal Domain Protein Inhibitor, in NUT Carcinoma and Other Solid Tumors. JNCI Cancer Spectr. 2020, 4, pkz093. [Google Scholar] [CrossRef] [PubMed]
- Sabò, A.; Kress, T.R.; Pelizzola, M.; de Pretis, S.; Gorski, M.M.; Tesi, A.; Morelli, M.J.; Bora, P.; Doni, M.; Verrecchia, A.; et al. Selective Transcriptional Regulation by Myc in Cellular Growth Control and Lymphomagenesis. Nature 2014, 511, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, C.; Resetca, D.; Redel, C.; Lin, P.; MacDonald, A.S.; Ciaccio, R.; Kenney, T.M.G.; Wei, Y.; Andrews, D.W.; Sunnerhagen, M.; et al. MYC Protein Interactors in Gene Transcription and Cancer. Nat. Rev. Cancer 2021, 21, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Chipumuro, E.; Marco, E.; Christensen, C.L.; Kwiatkowski, N.; Zhang, T.; Hatheway, C.M.; Abraham, B.J.; Sharma, B.; Yeung, C.; Altabef, A.; et al. CDK7 Inhibition Suppresses Super-Enhancer-Linked Oncogenic Transcription in MYCN-Driven Cancer. Cell 2014, 159, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Tee, A.E.; Ciampa, O.C.; Wong, M.; Fletcher, J.I.; Kamili, A.; Chen, J.; Ho, N.; Sun, Y.; Carter, D.R.; Cheung, B.B.; et al. Combination Therapy with the CDK7 Inhibitor and the Tyrosine Kinase Inhibitor Exerts Synergistic Anticancer Effects against MYCN-Amplified Neuroblastoma. Int. J. Cancer 2020, 147, 1928–1938. [Google Scholar] [CrossRef]
- Poon, E.; Liang, T.; Jamin, Y.; Walz, S.; Kwok, C.; Hakkert, A.; Barker, K.; Urban, Z.; Thway, K.; Zeid, R.; et al. Orally Bioavailable CDK9/2 Inhibitor Shows Mechanism-Based Therapeutic Potential in MYCN-Driven Neuroblastoma. J. Clin. Investig. 2020, 130, 5875–5892. [Google Scholar] [CrossRef]
- Bresler, S.C.; Weiser, D.A.; Huwe, P.J.; Park, J.H.; Krytska, K.; Ryles, H.; Laudenslager, M.; Rappaport, E.F.; Wood, A.C.; McGrady, P.W.; et al. ALK Mutations Confer Differential Oncogenic Activation and Sensitivity to ALK Inhibition Therapy in Neuroblastoma. Cancer Cell 2014, 26, 682–694. [Google Scholar] [CrossRef]
- Infarinato, N.R.; Park, J.H.; Krytska, K.; Ryles, H.T.; Sano, R.; Szigety, K.M.; Li, Y.; Zou, H.Y.; Lee, N.V.; Smeal, T.; et al. The ALK/ROS1 Inhibitor PF-06463922 Overcomes Primary Resistance to Crizotinib in ALK-Driven Neuroblastoma. Cancer Discov. 2016, 6, 96–107. [Google Scholar] [CrossRef]
- Sekimizu, M.; Osumi, T.; Fukano, R.; Koga, Y.; Kada, A.; Saito, A.M.; Mori, T. A Phase I/II Study of Crizotinib for Recurrent or Refractory Anaplastic Lymphoma Kinase-Positive Anaplastic Large Cell Lymphoma and a Phase I Study of Crizotinib for Recurrent or Refractory Neuroblastoma: Study Protocol for a Multicenter Single-Arm Open-Label Trial. Acta Med. Okayama 2018, 72, 431–436. [Google Scholar] [CrossRef]
- Krytska, K.; Ryles, H.T.; Sano, R.; Raman, P.; Infarinato, N.R.; Hansel, T.D.; Makena, M.R.; Song, M.M.; Reynolds, C.P.; Mossé, Y.P. Crizotinib Synergizes with Chemotherapy in Preclinical Models of Neuroblastoma. Clin. Cancer Res. 2016, 22, 948–960. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, B.; Baruchel, S. Oral Metronomic Topotecan Sensitizes Crizotinib Antitumor Activity in ALKF1174L Drug-Resistant Neuroblastoma Preclinical Models. Transl. Oncol. 2017, 10, 604–611. [Google Scholar] [CrossRef]
- Lu, J.; Guan, S.; Zhao, Y.; Yu, Y.; Woodfield, S.E.; Zhang, H.; Yang, K.L.; Bieerkehazhi, S.; Qi, L.; Li, X.; et al. The Second-Generation ALK Inhibitor Alectinib Effectively Induces Apoptosis in Human Neuroblastoma Cells and Inhibits Tumor Growth in a TH-MYCN Transgenic Neuroblastoma Mouse Model. Cancer Lett. 2017, 400, 61–68. [Google Scholar] [CrossRef]
- Alam, M.W.; Borenäs, M.; Lind, D.E.; Cervantes-Madrid, D.; Umapathy, G.; Palmer, R.H.; Hallberg, B. Alectinib, an Anaplastic Lymphoma Kinase Inhibitor, Abolishes ALK Activity and Growth in ALK-Positive Neuroblastoma Cells. Front. Oncol. 2019, 9, 579. [Google Scholar] [CrossRef]
- Heath, J.A.; Campbell, M.A.; Thomas, A.; Solomon, B. Good Clinical Response to Alectinib, a Second Generation ALK Inhibitor, in Refractory Neuroblastoma. Pediatr. Blood Cancer 2018, 65, e27055. [Google Scholar] [CrossRef]
- Carneiro, B.A.; Pamarthy, S.; Shah, A.N.; Sagar, V.; Unno, K.; Han, H.; Yang, X.J.; Costa, R.B.; Nagy, R.J.; Lanman, R.B.; et al. Anaplastic Lymphoma Kinase Mutation (ALK F1174C) in Small Cell Carcinoma of the Prostate and Molecular Response to Alectinib. Clin. Cancer Res. 2018, 24, 2732–2739. [Google Scholar] [CrossRef]
- Liu, T.; Merguerian, M.D.; Rowe, S.P.; Pratilas, C.A.; Chen, A.R.; Ladle, B.H. Exceptional Response to the ALK and ROS1 Inhibitor Lorlatinib and Subsequent Mechanism of Resistance in Relapsed ALK F1174L-Mutated Neuroblastoma. Cold Spring Harb. Mol. Case Stud. 2021, 7, a006064. [Google Scholar] [CrossRef]
- Redaelli, S.; Ceccon, M.; Zappa, M.; Sharma, G.G.; Mastini, C.; Mauri, M.; Nigoghossian, M.; Massimino, L.; Cordani, N.; Farina, F.; et al. Lorlatinib Treatment Elicits Multiple On- and Off-Target Mechanisms of Resistance in ALK-Driven Cancer. Cancer Res. 2018, 78, 6866–6880. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, L.; Zhou, Q.; Fen, L.; Cao, Y.; Sun, J.; Zhou, X.; Liu, A. Silencing of AURKA Augments the Antitumor Efficacy of the AURKA Inhibitor MLN8237 on Neuroblastoma Cells. Cancer Cell Int. 2020, 20, 9. [Google Scholar] [CrossRef]
- Muscal, J.A.; Scorsone, K.A.; Zhang, L.; Ecsedy, J.A.; Berg, S.L. Additive Effects of Vorinostat and MLN8237 in Pediatric Leukemia, Medulloblastoma, and Neuroblastoma Cell Lines. Investig. New Drugs 2013, 31, 39–45. [Google Scholar] [CrossRef]
- Hara, J. Development of Treatment Strategies for Advanced Neuroblastoma. Int. J. Clin. Oncol 2012, 17, 196–203. [Google Scholar] [CrossRef]
- Michaelis, M.; Selt, F.; Rothweiler, F.; Löschmann, N.; Nüsse, B.; Dirks, W.G.; Zehner, R.; Cinatl, J. Aurora Kinases as Targets in Drug-Resistant Neuroblastoma Cells. PLoS ONE 2014, 9, e108758. [Google Scholar] [CrossRef]
- Carol, H.; Boehm, I.; Reynolds, C.P.; Kang, M.H.; Maris, J.M.; Morton, C.L.; Gorlick, R.; Kolb, E.A.; Keir, S.T.; Wu, J.; et al. Efficacy and Pharmacokinetic/Pharmacodynamic Evaluation of the Aurora Kinase A Inhibitor MLN8237 against Preclinical Models of Pediatric Cancer. Cancer Chemother. Pharmacol. 2011, 68, 1291–1304. [Google Scholar] [CrossRef]
- Felgenhauer, J.; Tomino, L.; Selich-Anderson, J.; Bopp, E.; Shah, N. Dual BRD4 and AURKA Inhibition Is Synergistic against MYCN-Amplified and Nonamplified Neuroblastoma. Neoplasia 2018, 20, 965–974. [Google Scholar] [CrossRef]
- Romain, C.V.; Paul, P.; Lee, S.; Qiao, J.; Chung, D.H. Targeting Aurora Kinase A Inhibits Hypoxia-Mediated Neuroblastoma Cell Tumorigenesis. Anticancer Res. 2014, 34, 2269–2274. [Google Scholar]
- Melaiu, O.; Mina, M.; Chierici, M.; Boldrini, R.; Jurman, G.; Romania, P.; D’Alicandro, V.; Benedetti, M.C.; Castellano, A.; Liu, T.; et al. PD-L1 Is a Therapeutic Target of the Bromodomain Inhibitor JQ1 and, Combined with HLA Class I, a Promising Prognostic Biomarker in Neuroblastoma. Clin. Cancer Res. 2017, 23, 4462–4472. [Google Scholar] [CrossRef]
- Decaesteker, B.; Denecker, G.; Van Neste, C.; Dolman, E.M.; Van Loocke, W.; Gartlgruber, M.; Nunes, C.; De Vloed, F.; Depuydt, P.; Verboom, K.; et al. TBX2 Is a Neuroblastoma Core Regulatory Circuitry Component Enhancing MYCN/FOXM1 Reactivation of DREAM Targets. Nat. Commun. 2018, 9, 4866. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Rellinger, E.J.; Kim, K.W.; Craig, B.T.; Romain, C.V.; Qiao, J.; Chung, D.H. Bromodomain and Extraterminal Inhibition Blocks Tumor Progression and Promotes Differentiation in Neuroblastoma. Surgery 2015, 158, 819–826. [Google Scholar] [CrossRef]
- Slavish, P.J.; Chi, L.; Yun, M.-K.; Tsurkan, L.; Martinez, N.E.; Jonchere, B.; Chai, S.C.; Connelly, M.; Waddell, M.B.; Das, S.; et al. Bromodomain-Selective BET Inhibitors Are Potent Antitumor Agents against MYC-Driven Pediatric Cancer. Cancer Res. 2020, 80, 3507–3518. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, J.; Liu, P.Y.; Atmadibrata, B.; Bradner, J.E.; Marshall, G.M.; Lock, R.B.; Liu, T. The Bromodomain Inhibitor JQ1 and the Histone Deacetylase Inhibitor Panobinostat Synergistically Reduce N-Myc Expression and Induce Anticancer Effects. Clin. Cancer Res. 2016, 22, 2534–2544. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Nelson, C.; Wong, M.; Tee, A.E.; Liu, P.Y.; La, T.; Fletcher, J.I.; Kamili, A.; Mayoh, C.; Bartenhagen, C.; et al. Targeted Therapy of TERT-Rearranged Neuroblastoma with BET Bromodomain Inhibitor and Proteasome Inhibitor Combination Therapy. Clin. Cancer Res. 2021, 27, 1438–1451. [Google Scholar] [CrossRef] [PubMed]
- Maser, T.; Zagorski, J.; Kelly, S.; Ostrander, A.; Goodyke, A.; Nagulapally, A.; Bond, J.; Park, Y.; Saulnier Sholler, G. The MDM2 Inhibitor CGM097 Combined with the BET Inhibitor OTX015 Induces Cell Death and Inhibits Tumor Growth in Models of Neuroblastoma. Cancer Med. 2020, 9, 8144–8158. [Google Scholar] [CrossRef]
- Healy, J.R.; Hart, L.S.; Shazad, A.L.; Gagliardi, M.E.; Tsang, M.; Elias, J.; Ruden, J.; Farrel, A.; Rokita, J.L.; Li, Y.; et al. Limited Antitumor Activity of Combined BET and MEK Inhibition in Neuroblastoma. Pediatr. Blood Cancer 2020, 67, e28267. [Google Scholar] [CrossRef]
- Wyce, A.; Ganji, G.; Smitheman, K.N.; Chung, C.-W.; Korenchuk, S.; Bai, Y.; Barbash, O.; Le, B.; Craggs, P.D.; McCabe, M.T.; et al. BET Inhibition Silences Expression of MYCN and BCL2 and Induces Cytotoxicity in Neuroblastoma Tumor Models. PLoS ONE 2013, 8, e72967. [Google Scholar] [CrossRef]
- Rihani, A.; Vandesompele, J.; Speleman, F.; Van Maerken, T. Inhibition of CDK4/6 as a Novel Therapeutic Option for Neuroblastoma. Cancer Cell Int. 2015, 15, 76. [Google Scholar] [CrossRef]
- Swadi, R.R.; Sampat, K.; Herrmann, A.; Losty, P.D.; See, V.; Moss, D.J. CDK Inhibitors Reduce Cell Proliferation and Reverse Hypoxia-Induced Metastasis of Neuroblastoma Tumours in a Chick Embryo Model. Sci. Rep. 2019, 9, 9136. [Google Scholar] [CrossRef]
- Rader, J.; Russell, M.R.; Hart, L.S.; Nakazawa, M.S.; Belcastro, L.T.; Martinez, D.; Li, Y.; Carpenter, E.L.; Attiyeh, E.F.; Diskin, S.J.; et al. Dual CDK4/CDK6 Inhibition Induces Cell-Cycle Arrest and Senescence in Neuroblastoma. Clin. Cancer Res. 2013, 19, 6173–6182. [Google Scholar] [CrossRef]
- Hart, L.S.; Rader, J.; Raman, P.; Batra, V.; Russell, M.R.; Tsang, M.; Gagliardi, M.; Chen, L.; Martinez, D.; Li, Y.; et al. Preclinical Therapeutic Synergy of MEK1/2 and CDK4/6 Inhibition in Neuroblastoma. Clin. Cancer Res. 2017, 23, 1785–1796. [Google Scholar] [CrossRef]
- Geoerger, B.; Bourdeaut, F.; DuBois, S.G.; Fischer, M.; Geller, J.I.; Gottardo, N.G.; Marabelle, A.; Pearson, A.D.J.; Modak, S.; Cash, T.; et al. A Phase I Study of the CDK4/6 Inhibitor Ribociclib (LEE011) in Pediatric Patients with Malignant Rhabdoid Tumors, Neuroblastoma, and Other Solid Tumors. Clin. Cancer Res. 2017, 23, 2433–2441. [Google Scholar] [CrossRef]
- Wood, A.C.; Krytska, K.; Ryles, H.T.; Infarinato, N.R.; Sano, R.; Hansel, T.D.; Hart, L.S.; King, F.J.; Smith, T.R.; Ainscow, E.; et al. Dual ALK and CDK4/6 Inhibition Demonstrates Synergy against Neuroblastoma. Clin. Cancer Res. 2017, 23, 2856–2868. [Google Scholar] [CrossRef]
- Schubert, N.A.; Schild, L.; van Oirschot, S.; Keller, K.M.; Alles, L.K.; Vernooij, L.; Nulle, M.E.; Dolman, M.E.M.; van den Boogaard, M.L.; Molenaar, J.J. Combined Targeting of the P53 and PRb Pathway in Neuroblastoma Does Not Lead to Synergistic Responses. Eur. J. Cancer 2021, 142, 1–9. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Pearson, A.D.J.; Brock, P.; Luksch, R.; Castel, V.; Yaniv, I.; Papadakis, V.; Laureys, G.; Malis, J.; et al. Busulfan and Melphalan versus Carboplatin, Etoposide, and Melphalan as High-Dose Chemotherapy for High-Risk Neuroblastoma (HR-NBL1/SIOPEN): An International, Randomised, Multi-Arm, Open-Label, Phase 3 Trial. Lancet Oncol. 2017, 18, 500–514. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Z.; Liu, Y.; Wu, G.; Zeng, L.; Xu, J. The Inhibitory Effect of Carboplatin Injection on Human Neuroblastoma SK-N-SH. Cell Transpl. 2020, 29, 963689720920815. [Google Scholar] [CrossRef]
- Park, J.R.; Kreissman, S.G.; London, W.B.; Naranjo, A.; Cohn, S.L.; Hogarty, M.D.; Tenney, S.C.; Haas-Kogan, D.; Shaw, P.J.; Kraveka, J.M.; et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients With High-Risk Neuroblastoma: A Randomized Clinical Trial. JAMA 2019, 322, 746–755. [Google Scholar] [CrossRef]
- Baker, D.L.; Schmidt, M.L.; Cohn, S.L.; Maris, J.M.; London, W.B.; Buxton, A.; Stram, D.; Castleberry, R.P.; Shimada, H.; Sandler, A.; et al. Outcome after Reduced Chemotherapy for Intermediate-Risk Neuroblastoma. N. Engl. J. Med. 2010, 363, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.; Veal, G.J.; Errington, J.; McDonald, L.G.; Tweddle, D.A. The Use of Pharmacokinetically Guided Carboplatin Chemotherapy in a Pre-Term Infant with Neuroblastoma-Associated Spinal Cord Compression. Pediatr. Blood Cancer 2019, 66, e27825. [Google Scholar] [CrossRef] [PubMed]
- Twist, C.J.; Naranjo, A.; Schmidt, M.L.; Tenney, S.C.; Cohn, S.L.; Meany, H.J.; Mattei, P.; Adkins, E.S.; Shimada, H.; London, W.B.; et al. Defining Risk Factors for Chemotherapeutic Intervention in Infants With Stage 4S Neuroblastoma: A Report From Children’s Oncology Group Study ANBL0531. J. Clin. Oncol. 2019, 37, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, R.; Kubota, N.; Hidaka, E.; Sakashita, K.; Tanaka, M.; Nakazawa, Y.; Nakamura, T. Cisplatin-Induced Nephrotoxicity in Patients with Advanced Neuroblastoma. Pediatr. Blood Cancer 2018, 65, e27253. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-I.; Jeong, Y.J.; Yu, A.-R.; Kwak, H.J.; Cha, J.-Y.; Kang, I.; Yeo, E.-J. Carfilzomib Enhances Cisplatin-Induced Apoptosis in SK-N-BE(2)-M17 Human Neuroblastoma Cells. Sci. Rep. 2019, 9, 5039. [Google Scholar] [CrossRef]
- Yavuz, B.; Zeki, J.; Taylor, J.; Harrington, K.; Coburn, J.M.; Ikegaki, N.; Kaplan, D.L.; Chiu, B. Silk Reservoirs for Local Delivery of Cisplatin for Neuroblastoma Treatment: In Vitro and In Vivo Evaluations. J. Pharm. Sci. 2019, 108, 2748–2755. [Google Scholar] [CrossRef]
- Yogev, O.; Almeida, G.S.; Barker, K.T.; George, S.L.; Kwok, C.; Campbell, J.; Zarowiecki, M.; Kleftogiannis, D.; Smith, L.M.; Hallsworth, A.; et al. In Vivo Modeling of Chemoresistant Neuroblastoma Provides New Insights into Chemorefractory Disease and Metastasis. Cancer Res. 2019, 79, 5382–5393. [Google Scholar] [CrossRef]
- Saylors, R.L.; Stine, K.C.; Sullivan, J.; Kepner, J.L.; Wall, D.A.; Bernstein, M.L.; Harris, M.B.; Hayashi, R.; Vietti, T.J. Pediatric Oncology Group Cyclophosphamide plus Topotecan in Children with Recurrent or Refractory Solid Tumors: A Pediatric Oncology Group Phase II Study. J. Clin. Oncol. 2001, 19, 3463–3469. [Google Scholar] [CrossRef]
- Rujkijyanont, P.; Photia, A.; Traivaree, C.; Monsereenusorn, C.; Anurathapan, U.; Seksarn, P.; Sosothikul, D.; Techavichit, P.; Sanpakit, K.; Phuakpet, K.; et al. Clinical Outcomes and Prognostic Factors to Predict Treatment Response in High Risk Neuroblastoma Patients Receiving Topotecan and Cyclophosphamide Containing Induction Regimen: A Prospective Multicenter Study. BMC Cancer 2019, 19, 961. [Google Scholar] [CrossRef]
- Sagnella, S.M.; Trieu, J.; Brahmbhatt, H.; MacDiarmid, J.A.; MacMillan, A.; Whan, R.M.; Fife, C.M.; McCarroll, J.A.; Gifford, A.J.; Ziegler, D.S.; et al. Targeted Doxorubicin-Loaded Bacterially Derived Nano-Cells for the Treatment of Neuroblastoma. Mol. Cancer Ther. 2018, 17, 1012–1023. [Google Scholar] [CrossRef]
- Li, Y.; Zhuo, B.; Yin, Y.; Han, T.; Li, S.; Li, Z.; Wang, J. Anti-Cancer Effect of Oncolytic Adenovirus-Armed ShRNA Targeting MYCN Gene on Doxorubicin-Resistant Neuroblastoma Cells. Biochem. Biophys. Res. Commun. 2017, 491, 134–139. [Google Scholar] [CrossRef]
- Vittorio, O.; Le Grand, M.; Makharza, S.A.; Curcio, M.; Tucci, P.; Iemma, F.; Nicoletta, F.P.; Hampel, S.; Cirillo, G. Doxorubicin Synergism and Resistance Reversal in Human Neuroblastoma BE(2)C Cell Lines: An in Vitro Study with Dextran-Catechin Nanohybrids. Eur. J. Pharm. Biopharm. 2018, 122, 176–185. [Google Scholar] [CrossRef]
- Tan, W.-Q.; Chen, G.; Ye, M.; Jia, B. Artemether Regulates Chemosensitivity to Doxorubicin via Regulation of B7-H3 in Human Neuroblastoma Cells. Med. Sci. Monit. 2017, 23, 4252–4259. [Google Scholar] [CrossRef]
- Lebedev, T.D.; Vagapova, E.R.; Astashkova, O.O.; Spirin, P.V.; Prassolov, V.S. Inhibition of Non-Receptor Tyrosine Kinase JAK2 Reduces Neuroblastoma Cell Growth and Enhances the Action of Doxorubicin. Mol. Biol. 2020, 54, 293–299. [Google Scholar] [CrossRef]
- Tran, H.C.; Marachelian, A.; Venkatramani, R.; Jubran, R.F.; Mascarenhas, L. Oxaliplatin and Doxorubicin for Relapsed or Refractory High-Risk Neuroblastoma. Pediatr. Hematol. Oncol. 2015, 32, 26–31. [Google Scholar] [CrossRef]
- Namkaew, J.; Jaroonwitchawan, T.; Rujanapun, N.; Saelee, J.; Noisa, P. Combined Effects of Curcumin and Doxorubicin on Cell Death and Cell Migration of SH-SY5Y Human Neuroblastoma Cells. In Vitro Cell. Dev. Biol. Anim. 2018, 54, 629–639. [Google Scholar] [CrossRef]
- Hultman, I.; Haeggblom, L.; Rognmo, I.; Jansson Edqvist, J.; Blomberg, E.; Ali, R.; Phillips, L.; Sandstedt, B.; Kogner, P.; Shirazi Fard, S.; et al. Doxorubicin-Provoked Increase of Mitotic Activity and Concomitant Drain of G0-Pool in Therapy-Resistant BE(2)-C Neuroblastoma. PLoS ONE 2018, 13, e0190970. [Google Scholar] [CrossRef]
- Valter, K.; Maximchik, P.; Abdrakhmanov, A.; Senichkin, V.; Zhivotovsky, B.; Gogvadze, V. Distinct Effects of Etoposide on Glutamine-Addicted Neuroblastoma. Cell Mol. Life Sci. 2020, 77, 1197–1207. [Google Scholar] [CrossRef]
- Hiramatsu, T.; Yoshizawa, J.; Miyaguni, K.; Sugihara, T.; Harada, A.; Kaji, S.; Uchida, G.; Kanamori, D.; Baba, Y.; Ashizuka, S.; et al. Thalidomide Potentiates Etoposide-Induced Apoptosis in Murine Neuroblastoma through Suppression of NF-ΚB Activation. Pediatr. Surg. Int. 2018, 34, 443–450. [Google Scholar] [CrossRef]
- Coughlan, D.; Gianferante, M.; Lynch, C.F.; Stevens, J.L.; Harlan, L.C. Treatment and Survival of Childhood Neuroblastoma: Evidence from a Population-Based Study in the United States. Pediatr. Hematol. Oncol. 2017, 34, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Richman, S.A.; Nunez-Cruz, S.; Moghimi, B.; Li, L.Z.; Gershenson, Z.T.; Mourelatos, Z.; Barrett, D.M.; Grupp, S.A.; Milone, M.C. High-Affinity GD2-Specific CAR T Cells Induce Fatal Encephalitis in a Preclinical Neuroblastoma Model. Cancer Immunol. Res. 2018, 6, 36–46. [Google Scholar] [CrossRef]
- Moghimi, B.; Muthugounder, S.; Jambon, S.; Tibbetts, R.; Hung, L.; Bassiri, H.; Hogarty, M.D.; Barrett, D.M.; Shimada, H.; Asgharzadeh, S. Preclinical Assessment of the Efficacy and Specificity of GD2-B7H3 SynNotch CAR-T in Metastatic Neuroblastoma. Nat. Commun. 2021, 12, 511. [Google Scholar] [CrossRef] [PubMed]
- Sait, S.; Modak, S. Anti-GD2 Immunotherapy for Neuroblastoma. Expert Rev. Anticancer Ther. 2017, 17, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Voeller, J.; Sondel, P.M. Advances in Anti-GD2 Immunotherapy for Treatment of High-Risk Neuroblastoma. J. Pediatr. Hematol. Oncol. 2019, 41, 163–169. [Google Scholar] [CrossRef]
- Richards, R.M.; Sotillo, E.; Majzner, R.G. CAR T Cell Therapy for Neuroblastoma. Front. Immunol. 2018, 9, 2380. [Google Scholar] [CrossRef]
- Heczey, A.; Courtney, A.N.; Montalbano, A.; Robinson, S.; Liu, K.; Li, M.; Ghatwai, N.; Dakhova, O.; Liu, B.; Raveh-Sadka, T.; et al. Anti-GD2 CAR-NKT Cells in Patients with Relapsed or Refractory Neuroblastoma: An Interim Analysis. Nat. Med. 2020, 26, 1686–1690. [Google Scholar] [CrossRef]
- Fan, Y.-H.; Cheng, J.; Vasudevan, S.A.; Dou, J.; Zhang, H.; Patel, R.H.; Ma, I.T.; Rojas, Y.; Zhao, Y.; Yu, Y.; et al. USP7 Inhibitor P22077 Inhibits Neuroblastoma Growth via Inducing P53-Mediated Apoptosis. Cell Death Dis. 2013, 4, e867. [Google Scholar] [CrossRef]
- Zareifar, S.; Shakibazad, N.; Zekavat, O.R.; Bordbar, M.; Shahriari, M. Successful Treatment of Refractory Metastatic Neuroblastoma with Panobinostat in Combination with Chemotherapy Agents and Iodine-131-Meta-Iodobenzylguanidine Therapy. J. Oncol. Pharm. Pract. 2020, 26, 481–486. [Google Scholar] [CrossRef]
- Wang, G.; Edwards, H.; Caldwell, J.T.; Buck, S.A.; Qing, W.Y.; Taub, J.W.; Ge, Y.; Wang, Z. Panobinostat Synergistically Enhances the Cytotoxic Effects of Cisplatin, Doxorubicin or Etoposide on High-Risk Neuroblastoma Cells. PLoS ONE 2013, 8, e76662. [Google Scholar] [CrossRef]
- Jose, G.; Lu, Y.-J.; Hung, J.-T.; Yu, A.L.; Chen, J.-P. Co-Delivery of CPT-11 and Panobinostat with Anti-GD2 Antibody Conjugated Immunoliposomes for Targeted Combination Chemotherapy. Cancers 2020, 12, 3211. [Google Scholar] [CrossRef]
- Xiao, L.; Somers, K.; Murray, J.; Pandher, R.; Karsa, M.; Ronca, E.; Bongers, A.; Terry, R.; Ehteda, A.; Gamble, L.D.; et al. Dual Targeting of Chromatin Stability By The Curaxin CBL0137 and Histone Deacetylase Inhibitor Panobinostat Shows Significant Preclinical Efficacy in Neuroblastoma. Clin. Cancer Res. 2021, 27, 4338–4352. [Google Scholar] [CrossRef]
- Fang, E.; Wang, J.; Hong, M.; Zheng, L.; Tong, Q. Valproic Acid Suppresses Warburg Effect and Tumor Progression in Neuroblastoma. Biochem. Biophys. Res. Commun. 2019, 508, 9–16. [Google Scholar] [CrossRef]
- Chen, Y.; Tsai, Y.-H.; Tseng, S.-H. Combined Valproic Acid and Celecoxib Treatment Induced Synergistic Cytotoxicity and Apoptosis in Neuroblastoma Cells. Anticancer Res. 2011, 31, 2231–2239. [Google Scholar]
- Hu, T.-M.; Chung, H.-S.; Ping, L.-Y.; Hsu, S.-H.; Tsai, H.-Y.; Chen, S.-J.; Cheng, M.-C. Differential Expression of Multiple Disease-Related Protein Groups Induced by Valproic Acid in Human SH-SY5Y Neuroblastoma Cells. Brain Sci. 2020, 10, 545. [Google Scholar] [CrossRef]
- Dedoni, S.; Marras, L.; Olianas, M.C.; Ingianni, A.; Onali, P. Downregulation of TrkB Expression and Signaling by Valproic Acid and Other Histone Deacetylase Inhibitors. J. Pharm. Exp. Ther. 2019, 370, 490–503. [Google Scholar] [CrossRef]
- Khalil, M.A.; Hraběta, J.; Groh, T.; Procházka, P.; Doktorová, H.; Eckschlager, T. Valproic Acid Increases CD133 Positive Cells That Show Low Sensitivity to Cytostatics in Neuroblastoma. PLoS ONE 2016, 11, e0162916. [Google Scholar] [CrossRef]
- Blaheta, R.A.; Michaelis, M.; Natsheh, I.; Hasenberg, C.; Weich, E.; Relja, B.; Jonas, D.; Doerr, H.W.; Cinatl, J. Valproic Acid Inhibits Adhesion of Vincristine- and Cisplatin-Resistant Neuroblastoma Tumour Cells to Endothelium. Br. J. Cancer 2007, 96, 1699–1706. [Google Scholar] [CrossRef]
- DuBois, S.G.; Marachelian, A.; Fox, E.; Kudgus, R.A.; Reid, J.M.; Groshen, S.; Malvar, J.; Bagatell, R.; Wagner, L.; Maris, J.M.; et al. Phase I Study of the Aurora A Kinase Inhibitor Alisertib in Combination With Irinotecan and Temozolomide for Patients With Relapsed or Refractory Neuroblastoma: A NANT (New Approaches to Neuroblastoma Therapy) Trial. J. Clin. Oncol. 2016, 34, 1368–1375. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Chen, H.; Wang, L.; Guo, H.; Yang, L.; Xiao, D.; Qing, G.; Liu, H. FDA-Approved Drug Screen Identifies Proteasome as a Synthetic Lethal Target in MYC-Driven Neuroblastoma. Oncogene 2019, 38, 6737–6751. [Google Scholar] [CrossRef]
- Kroesen, M.; Büll, C.; Gielen, P.R.; Brok, I.C.; Armandari, I.; Wassink, M.; Looman, M.W.G.; Boon, L.; den Brok, M.H.; Hoogerbrugge, P.M.; et al. Anti-GD2 MAb and Vorinostat Synergize in the Treatment of Neuroblastoma. Oncoimmunology 2016, 5, e1164919. [Google Scholar] [CrossRef]
- Pinto, N.; DuBois, S.G.; Marachelian, A.; Diede, S.J.; Taraseviciute, A.; Glade Bender, J.L.; Tsao-Wei, D.; Groshen, S.G.; Reid, J.M.; Haas-Kogan, D.A.; et al. Phase I Study of Vorinostat in Combination with Isotretinoin in Patients with Refractory/Recurrent Neuroblastoma: A New Approaches to Neuroblastoma Therapy (NANT) Trial. Pediatr. Blood Cancer 2018, 65, e27023. [Google Scholar] [CrossRef]
- Mueller, S.; Yang, X.; Sottero, T.L.; Gragg, A.; Prasad, G.; Polley, M.-Y.; Weiss, W.A.; Matthay, K.K.; Davidoff, A.M.; DuBois, S.G.; et al. Cooperation of the HDAC Inhibitor Vorinostat and Radiation in Metastatic Neuroblastoma: Efficacy and Underlying Mechanisms. Cancer Lett. 2011, 306, 223–229. [Google Scholar] [CrossRef]
- DuBois, S.G.; Groshen, S.; Park, J.R.; Haas-Kogan, D.A.; Yang, X.; Geier, E.; Chen, E.; Giacomini, K.; Weiss, B.; Cohn, S.L.; et al. Phase I Study of Vorinostat as a Radiation Sensitizer with 131I-Metaiodobenzylguanidine (131I-MIBG) for Patients with Relapsed or Refractory Neuroblastoma. Clin. Cancer Res. 2015, 21, 2715–2721. [Google Scholar] [CrossRef]
- Cortés, C.; Kozma, S.C.; Tauler, A.; Ambrosio, S. MYCN Concurrence with SAHA-Induced Cell Death in Human Neuroblastoma Cells. Cell. Oncol. 2015, 38, 341–352. [Google Scholar] [CrossRef]
- Müller, I.; Larsson, K.; Frenzel, A.; Oliynyk, G.; Zirath, H.; Prochownik, E.V.; Westwood, N.J.; Henriksson, M.A. Targeting of the MYCN Protein with Small Molecule C-MYC Inhibitors. PLoS ONE 2014, 9, e97285. [Google Scholar] [CrossRef]
- Wang, H.; Teriete, P.; Hu, A.; Raveendra-Panickar, D.; Pendelton, K.; Lazo, J.S.; Eiseman, J.; Holien, T.; Misund, K.; Oliynyk, G.; et al. Direct Inhibition of C-Myc-Max Heterodimers by Celastrol and Celastrol-Inspired Triterpenoids. Oncotarget 2015, 6, 32380–32395. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Vogt, P.K.; Boger, D.L.; Lunec, J. Disruption of the MYC Transcriptional Function by a Small-Molecule Antagonist of MYC/MAX Dimerization. Oncol. Rep. 2008, 19, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Jain, A.D.; Truica, M.I.; Izquierdo-Ferrer, J.; Anker, J.F.; Lysy, B.; Sagar, V.; Luan, Y.; Chalmers, Z.R.; Unno, K.; et al. Small-Molecule MYC Inhibitors Suppress Tumor Growth and Enhance Immunotherapy. Cancer Cell 2019, 36, 483–497.e15. [Google Scholar] [CrossRef]
- Massó-Vallés, D.; Beaulieu, M.-E.; Soucek, L. MYC, MYCL, and MYCN as Therapeutic Targets in Lung Cancer. Expert Opin. Ther. Targets 2020, 24, 101–114. [Google Scholar] [CrossRef]
- Fiorentino, F.P.; Tokgün, E.; Solé-Sánchez, S.; Giampaolo, S.; Tokgün, O.; Jauset, T.; Kohno, T.; Perucho, M.; Soucek, L.; Yokota, J. Growth Suppression by MYC Inhibition in Small Cell Lung Cancer Cells with TP53 and RB1 Inactivation. Oncotarget 2016, 7, 31014–31028. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Yin, J.; Gan, Y.; Xu, S.; Gu, Y.; Huang, W. Alternative Approaches to Target Myc for Cancer Treatment. Signal Transduct. Target. Ther. 2021, 6, 117. [Google Scholar] [CrossRef]
- Gamble, L.D.; Purgato, S.; Murray, J.; Xiao, L.; Yu, D.M.T.; Hanssen, K.M.; Giorgi, F.M.; Carter, D.R.; Gifford, A.J.; Valli, E.; et al. Inhibition of Polyamine Synthesis and Uptake Reduces Tumor Progression and Prolongs Survival in Mouse Models of Neuroblastoma. Sci. Transl. Med. 2019, 11, eaau1099. [Google Scholar] [CrossRef]
- Sholler, G.L.S.; Ferguson, W.; Bergendahl, G.; Bond, J.P.; Neville, K.; Eslin, D.; Brown, V.; Roberts, W.; Wada, R.K.; Oesterheld, J.; et al. Maintenance DFMO Increases Survival in High Risk Neuroblastoma. Sci. Rep. 2018, 8, 14445. [Google Scholar] [CrossRef]
- Bassiri, H.; Benavides, A.; Haber, M.; Gilmour, S.K.; Norris, M.D.; Hogarty, M.D. Translational Development of Difluoromethylornithine (DFMO) for the Treatment of Neuroblastoma. Transl. Pediatr. 2015, 4, 226–238. [Google Scholar] [CrossRef]
- Evageliou, N.F.; Haber, M.; Vu, A.; Laetsch, T.W.; Murray, J.; Gamble, L.D.; Cheng, N.C.; Liu, K.; Reese, M.; Corrigan, K.A.; et al. Polyamine Antagonist Therapies Inhibit Neuroblastoma Initiation and Progression. Clin. Cancer Res. 2016, 22, 4391–4404. [Google Scholar] [CrossRef]
- Schultz, C.R.; Geerts, D.; Mooney, M.; El-Khawaja, R.; Koster, J.; Bachmann, A.S. Synergistic Drug Combination GC7/DFMO Suppresses Hypusine/Spermidine-Dependent EIF5A Activation and Induces Apoptotic Cell Death in Neuroblastoma. Biochem. J. 2018, 475, 531–545. [Google Scholar] [CrossRef]
- Rounbehler, R.J.; Li, W.; Hall, M.A.; Yang, C.; Fallahi, M.; Cleveland, J.L. Targeting Ornithine Decarboxylase Impairs Development of MYCN-Amplified Neuroblastoma. Cancer Res. 2009, 69, 547–553. [Google Scholar] [CrossRef]
- Samal, K.; Zhao, P.; Kendzicky, A.; Yco, L.P.; McClung, H.; Gerner, E.; Burns, M.; Bachmann, A.S.; Sholler, G. AMXT-1501, a Novel Polyamine Transport Inhibitor, Synergizes with DFMO in Inhibiting Neuroblastoma Cell Proliferation by Targeting Both Ornithine Decarboxylase and Polyamine Transport. Int. J. Cancer 2013, 133, 1323–1333. [Google Scholar] [CrossRef]
- Koach, J.; Holien, J.K.; Massudi, H.; Carter, D.R.; Ciampa, O.C.; Herath, M.; Lim, T.; Seneviratne, J.A.; Milazzo, G.; Murray, J.E.; et al. Drugging MYCN Oncogenic Signaling through the MYCN-PA2G4 Binding Interface. Cancer Res. 2019, 79, 5652–5667. [Google Scholar] [CrossRef]
- Xu, D.-Q.; Toyoda, H.; Yuan, X.-J.; Qi, L.; Chelakkot, V.S.; Morimoto, M.; Hanaki, R.; Kihira, K.; Hori, H.; Komada, Y.; et al. Anti-Tumor Effect of AZD8055 against Neuroblastoma Cells in Vitro and in Vivo. Exp. Cell Res. 2018, 365, 177–184. [Google Scholar] [CrossRef]
- Xu, D.-Q.; Toyoda, H.; Qi, L.; Morimoto, M.; Hanaki, R.; Iwamoto, S.; Komada, Y.; Hirayama, M. Induction of MEK/ERK Activity by AZD8055 Confers Acquired Resistance in Neuroblastoma. Biochem. Biophys. Res. Commun. 2018, 499, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Toyoda, H.; Xu, D.-Q.; Zhou, Y.; Sakurai, N.; Amano, K.; Kihira, K.; Hori, H.; Azuma, E.; Komada, Y. PDK1-MTOR Signaling Pathway Inhibitors Reduce Cell Proliferation in MK2206 Resistant Neuroblastoma Cells. Cancer Cell Int. 2015, 15, 91. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, M.K.; Curioni-Fontecedro, A.; Samaras, P.; Lang, S.; Scharl, M.; Aguzzi, A.; Oldrige, D.A.; Maris, J.M.; Rogler, G. Targeting the MTOR Complex by Everolimus in NRAS Mutant Neuroblastoma. PLoS ONE 2016, 11, e0147682. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Hua, Z.; Dong, Y.; Zhan, Y.; Zhang, X.; Tian, W.; Liu, Z.; Thiele, C.J.; Li, Z. Proteome and Acetylome Analysis Identifies Novel Pathways and Targets Regulated by Perifosine in Neuroblastoma. Sci. Rep. 2017, 7, 42062. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Shichino, H.; Kawamoto, H.; Kosaka, Y.; Chin, M.; Kato, K.; Mugishima, H. Phase I Study of Perifosine Monotherapy in Patients with Recurrent or Refractory Neuroblastoma. Pediatr. Blood Cancer 2017, 64, e26623. [Google Scholar] [CrossRef]
- Sun, W.; Modak, S. Emerging Treatment Options for the Treatment of Neuroblastoma: Potential Role of Perifosine. Onco TargetsTher. 2012, 5, 21–29. [Google Scholar] [CrossRef][Green Version]
- Qi, L.; Toyoda, H.; Shankar, V.; Sakurai, N.; Amano, K.; Kihira, K.; Iwasa, T.; Deguchi, T.; Hori, H.; Azuma, E.; et al. Heterogeneity of Neuroblastoma Cell Lines in Insulin-like Growth Factor 1 Receptor/Akt Pathway-Mediated Cell Proliferative Responses. Cancer Sci. 2013, 104, 1162–1171. [Google Scholar] [CrossRef]
- Erdreich-Epstein, A.; Singh, A.R.; Joshi, S.; Vega, F.M.; Guo, P.; Xu, J.; Groshen, S.; Ye, W.; Millard, M.; Campan, M.; et al. Association of High Microvessel Avβ3 and Low PTEN with Poor Outcome in Stage 3 Neuroblastoma: Rationale for Using First in Class Dual PI3K/BRD4 Inhibitor, SF1126. Oncotarget 2017, 8, 52193–52210. [Google Scholar] [CrossRef][Green Version]
- Radic-Sarikas, B.; Halasz, M.; Huber, K.V.M.; Winter, G.E.; Tsafou, K.P.; Papamarkou, T.; Brunak, S.; Kolch, W.; Superti-Furga, G. Lapatinib Potentiates Cytotoxicity of YM155 in Neuroblastoma via Inhibition of the ABCB1 Efflux Transporter. Sci. Rep. 2017, 7, 3091. [Google Scholar] [CrossRef]
- Whittle, S.B.; Patel, K.; Zhang, L.; Woodfield, S.E.; Du, M.; Smith, V.; Zage, P.E. The Novel Kinase Inhibitor Ponatinib Is an Effective Anti-Angiogenic Agent against Neuroblastoma. Investig. New Drugs 2016, 34, 685–692. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Chen, Z.; Lu, J.; Pan, J.; Yu, Y.; Zhao, Y.; Zhang, H.; Hu, T.; Liu, Q.; et al. Novel Multiple Tyrosine Kinase Inhibitor Ponatinib Inhibits BFGF-Activated Signaling in Neuroblastoma Cells and Suppresses Neuroblastoma Growth in Vivo. Oncotarget 2017, 8, 5874–5884. [Google Scholar] [CrossRef]
- Sidarovich, V.; De Mariano, M.; Aveic, S.; Pancher, M.; Adami, V.; Gatto, P.; Pizzini, S.; Pasini, L.; Croce, M.; Parodi, F.; et al. A High-Content Screening of Anticancer Compounds Suggests the Multiple Tyrosine Kinase Inhibitor Ponatinib for Repurposing in Neuroblastoma Therapy. Mol. Cancer Ther. 2018, 17, 1405–1415. [Google Scholar] [CrossRef]
- Kalkat, M.; Resetca, D.; Lourenco, C.; Chan, P.-K.; Wei, Y.; Shiah, Y.-J.; Vitkin, N.; Tong, Y.; Sunnerhagen, M.; Done, S.J.; et al. MYC Protein Interactome Profiling Reveals Functionally Distinct Regions That Cooperate to Drive Tumorigenesis. Mol. Cell 2018, 72, 836–848.e7. [Google Scholar] [CrossRef]
- Baluapuri, A.; Wolf, E.; Eilers, M. Target Gene-Independent Functions of MYC Oncoproteins. Nat. Rev. Mol. Cell Biol. 2020, 21, 255–267. [Google Scholar] [CrossRef]
- Meyer, N.; Penn, L.Z. Reflecting on 25 Years with MYC. Nat. Rev. Cancer 2008, 8, 976–990. [Google Scholar] [CrossRef]
- Ferrucci, F.; Ciaccio, R.; Monticelli, S.; Pigini, P.; di Giacomo, S.; Purgato, S.; Erriquez, D.; Bernardoni, R.; Norris, M.; Haber, M.; et al. MAX to MYCN Intracellular Ratio Drives the Aggressive Phenotype and Clinical Outcome of High Risk Neuroblastoma. Biochim. Biophys. Acta Gene Regul. Mech. 2018, 1861, 235–245. [Google Scholar] [CrossRef]
- Berg, T.; Cohen, S.B.; Desharnais, J.; Sonderegger, C.; Maslyar, D.J.; Goldberg, J.; Boger, D.L.; Vogt, P.K. Small-Molecule Antagonists of Myc/Max Dimerization Inhibit Myc-Induced Transformation of Chicken Embryo Fibroblasts. Proc. Natl. Acad. Sci. USA 2002, 99, 3830–3835. [Google Scholar] [CrossRef]
- Yin, X.; Giap, C.; Lazo, J.S.; Prochownik, E.V. Low Molecular Weight Inhibitors of Myc-Max Interaction and Function. Oncogene 2003, 22, 6151–6159. [Google Scholar] [CrossRef] [PubMed]
- Heller, G.T.; Aprile, F.A.; Bonomi, M.; Camilloni, C.; De Simone, A.; Vendruscolo, M. Sequence Specificity in the Entropy-Driven Binding of a Small Molecule and a Disordered Peptide. J. Mol. Biol. 2017, 429, 2772–2779. [Google Scholar] [CrossRef]
- Massó-Vallés, D.; Soucek, L. Blocking Myc to Treat Cancer: Reflecting on Two Decades of Omomyc. Cells 2020, 9, 883. [Google Scholar] [CrossRef]
- Farrell, A.S.; Sears, R.C. MYC Degradation. Cold Spring Harb. Perspect. Med. 2014, 4, a014365. [Google Scholar] [CrossRef]
- Gustafson, W.C.; Meyerowitz, J.G.; Nekritz, E.A.; Chen, J.; Benes, C.; Charron, E.; Simonds, E.F.; Seeger, R.; Matthay, K.K.; Hertz, N.T.; et al. Drugging MYCN through an Allosteric Transition in Aurora Kinase A. Cancer Cell 2014, 26, 414–427. [Google Scholar] [CrossRef]
- Thomas, L.R.; Adams, C.M.; Fesik, S.W.; Eischen, C.M.; Tansey, W.P. Targeting MYC through WDR5. Mol. Cell. Oncol. 2020, 7, 1709388. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, S.; Goeser, F.; Schulte, J.H.; Schramm, A.; Ehemann, V.; Hero, B.; Eggert, A.; Berthold, F.; Fischer, M. Polo-like Kinase 1 Is a Therapeutic Target in High-Risk Neuroblastoma. Clin. Cancer Res. 2011, 17, 731–741. [Google Scholar] [CrossRef]
- Tavana, O.; Li, D.; Dai, C.; Lopez, G.; Banerjee, D.; Kon, N.; Chen, C.; Califano, A.; Yamashiro, D.J.; Sun, H.; et al. HAUSP Deubiquitinates and Stabilizes N-Myc in Neuroblastoma. Nat. Med. 2016, 22, 1180–1186. [Google Scholar] [CrossRef]
- Hogarty, M.D.; Norris, M.D.; Davis, K.; Liu, X.; Evageliou, N.F.; Hayes, C.S.; Pawel, B.; Guo, R.; Zhao, H.; Sekyere, E.; et al. ODC1 Is a Critical Determinant of MYCN Oncogenesis and a Therapeutic Target in Neuroblastoma. Cancer Res. 2008, 68, 9735–9745. [Google Scholar] [CrossRef]
- Thomas, T.; Thomas, T.J. Polyamines in Cell Growth and Cell Death: Molecular Mechanisms and Therapeutic Applications. Cell Mol. Life Sci. 2001, 58, 244–258. [Google Scholar] [CrossRef]
- Casero, R.A.; Marton, L.J. Targeting Polyamine Metabolism and Function in Cancer and Other Hyperproliferative Diseases. Nat. Rev. Drug Discov. 2007, 6, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.C.; Kraveka, J.M.; Ferguson, W.; Eslin, D.; Brown, V.I.; Bergendahl, G.; Roberts, W.; Wada, R.K.; Oesterheld, J.; Mitchell, D.; et al. A Subset Analysis of a Phase II Trial Evaluating the Use of DFMO as Maintenance Therapy for High-Risk Neuroblastoma. Int. J. Cancer 2020, 147, 3152–3159. [Google Scholar] [CrossRef] [PubMed]
- Gamble, L.D.; Purgato, S.; Henderson, M.J.; Di Giacomo, S.; Russell, A.J.; Pigini, P.; Murray, J.; Valli, E.; Milazzo, G.; Giorgi, F.M.; et al. A G316A Polymorphism in the Ornithine Decarboxylase Gene Promoter Modulates MYCN-Driven Childhood Neuroblastoma. Cancers 2021, 13, 1807. [Google Scholar] [CrossRef]
- Bouchard, C.; Dittrich, O.; Kiermaier, A.; Dohmann, K.; Menkel, A.; Eilers, M.; Lüscher, B. Regulation of Cyclin D2 Gene Expression by the Myc/Max/Mad Network: Myc-Dependent TRRAP Recruitment and Histone Acetylation at the Cyclin D2 Promoter. Genes Dev. 2001, 15, 2042–2047. [Google Scholar] [CrossRef]
- Woo, C.-W.; Tan, F.; Cassano, H.; Lee, J.; Lee, K.C.; Thiele, C.J. Use of RNA Interference to Elucidate the Effect of MYCN on Cell Cycle in Neuroblastoma. Pediatr. Blood Cancer 2008, 50, 208–212. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Stephens, L.; Hawkins, P. PI3K Signalling: The Path to Discovery and Understanding. Nat. Rev. Mol. Cell Biol. 2012, 13, 195–203. [Google Scholar] [CrossRef]
- Opel, D.; Poremba, C.; Simon, T.; Debatin, K.-M.; Fulda, S. Activation of Akt Predicts Poor Outcome in Neuroblastoma. Cancer Res. 2007, 67, 735–745. [Google Scholar] [CrossRef]
- Loh, A.H.P.; Brennan, R.C.; Lang, W.H.; Hickey, R.J.; Malkas, L.H.; Sandoval, J.A. Dissecting the PI3K Signaling Axis in Pediatric Solid Tumors: Novel Targets for Clinical Integration. Front. Oncol. 2013, 3, 93. [Google Scholar] [CrossRef]
- Bold, R.J.; Kim, H.J.; Ishizuka, J.; Townsend, C.M.; Thompson, J.C. A Human Gastric Cancer Cell Line Possesses a Functional Receptor for Gastrin-Releasing Peptide. Cancer Investig. 1998, 16, 12–17. [Google Scholar] [CrossRef]
- Chen, Y.; Takita, J.; Choi, Y.L.; Kato, M.; Ohira, M.; Sanada, M.; Wang, L.; Soda, M.; Kikuchi, A.; Igarashi, T.; et al. Oncogenic Mutations of ALK Kinase in Neuroblastoma. Nature 2008, 455, 971–974. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Tong, Y.; Tong, J.; Thiele, C.J. Trk Inhibitor Attenuates the BDNF/TrkB-Induced Protection of Neuroblastoma Cells from Etoposide in Vitro and in Vivo. Cancer Biol. Ther. 2015, 16, 477–483. [Google Scholar] [CrossRef]
- Liu, X.; Turbyville, T.; Fritz, A.; Whitesell, L. Inhibition of Insulin-like Growth Factor I Receptor Expression in Neuroblastoma Cells Induces the Regression of Established Tumors in Mice. Cancer Res. 1998, 58, 5432–5438. [Google Scholar] [CrossRef][Green Version]
- Ho, R.; Minturn, J.E.; Hishiki, T.; Zhao, H.; Wang, Q.; Cnaan, A.; Maris, J.; Evans, A.E.; Brodeur, G.M. Proliferation of Human Neuroblastomas Mediated by the Epidermal Growth Factor Receptor. Cancer Res. 2005, 65, 9868–9875. [Google Scholar] [CrossRef] [PubMed]
- Raica, M.; Cimpean, A.M. Platelet-Derived Growth Factor (PDGF)/PDGF Receptors (PDGFR) Axis as Target for Antitumor and Antiangiogenic Therapy. Pharmaceuticals 2010, 3, 572–599. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Rychahou, P.G.; Ishola, T.A.; Mourot, J.M.; Evers, B.M.; Chung, D.H. N-Myc Is a Novel Regulator of PI3K-Mediated VEGF Expression in Neuroblastoma. Oncogene 2008, 27, 3999–4007. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.I.; Ziegler, D.S.; Trahair, T.N.; Marshall, G.M.; Haber, M.; Norris, M.D. Too Many Targets, Not Enough Patients: Rethinking Neuroblastoma Clinical Trials. Nat. Rev. Cancer 2018, 18, 389–400. [Google Scholar] [CrossRef]
- Mossé, Y.P.; Lim, M.S.; Voss, S.D.; Wilner, K.; Ruffner, K.; Laliberte, J.; Rolland, D.; Balis, F.M.; Maris, J.M.; Weigel, B.J.; et al. Safety and Activity of Crizotinib for Paediatric Patients with Refractory Solid Tumours or Anaplastic Large-Cell Lymphoma: A Children’s Oncology Group Phase 1 Consortium Study. Lancet Oncol. 2013, 14, 472–480. [Google Scholar] [CrossRef]
- Domingo-Fernandez, R.; Watters, K.; Piskareva, O.; Stallings, R.L.; Bray, I. The Role of Genetic and Epigenetic Alterations in Neuroblastoma Disease Pathogenesis. Pediatr. Surg. Int. 2013, 29, 101–119. [Google Scholar] [CrossRef]
- Hassell, K.N. Histone Deacetylases and Their Inhibitors in Cancer Epigenetics. Diseases 2019, 7, 57. [Google Scholar] [CrossRef]
- Rocchi, P.; Tonelli, R.; Camerin, C.; Purgato, S.; Fronza, R.; Bianucci, F.; Guerra, F.; Pession, A.; Ferreri, A.M. P21Waf1/Cip1 Is a Common Target Induced by Short-Chain Fatty Acid HDAC Inhibitors (Valproic Acid, Tributyrin and Sodium Butyrate) in Neuroblastoma Cells. Oncol. Rep. 2005, 13, 1139–1144. [Google Scholar] [CrossRef]
- Stockhausen, M.-T.; Sjölund, J.; Manetopoulos, C.; Axelson, H. Effects of the Histone Deacetylase Inhibitor Valproic Acid on Notch Signalling in Human Neuroblastoma Cells. Br. J. Cancer 2005, 92, 751–759. [Google Scholar] [CrossRef]
- Cerna, T.; Hrabeta, J.; Eckschlager, T.; Frei, E.; Schmeiser, H.H.; Arlt, V.M.; Stiborová, M. The Histone Deacetylase Inhibitor Valproic Acid Exerts a Synergistic Cytotoxicity with the DNA-Damaging Drug Ellipticine in Neuroblastoma Cells. Int. J. Mol. Sci. 2018, 19, 164. [Google Scholar] [CrossRef]
- Yang, Q.; Tian, Y.; Liu, S.; Zeine, R.; Chlenski, A.; Salwen, H.R.; Henkin, J.; Cohn, S.L. Thrombospondin-1 Peptide ABT-510 Combined with Valproic Acid Is an Effective Antiangiogenesis Strategy in Neuroblastoma. Cancer Res. 2007, 67, 1716–1724. [Google Scholar] [CrossRef]
- Groh, T.; Hrabeta, J.; Poljakova, J.; Eckschlager, T.; Stiborova, M. Impact of Histone Deacetylase Inhibitor Valproic Acid on the Anticancer Effect of Etoposide on Neuroblastoma Cells. Neuro Endocrinol. Lett. 2012, 33 (Suppl. S3), 16–24. [Google Scholar]
- Groh, T.; Hrabeta, J.; Khalil, M.A.; Doktorova, H.; Eckschlager, T.; Stiborova, M. The Synergistic Effects of DNA-Damaging Drugs Cisplatin and Etoposide with a Histone Deacetylase Inhibitor Valproate in High-Risk Neuroblastoma Cells. Int. J. Oncol. 2015, 47, 343–352. [Google Scholar] [CrossRef]
- De los Santos, M.; Zambrano, A.; Aranda, A. Combined Effects of Retinoic Acid and Histone Deacetylase Inhibitors on Human Neuroblastoma SH-SY5Y Cells. Mol. Cancer Ther. 2007, 6, 1425–1432. [Google Scholar] [CrossRef]
- Mühlethaler-Mottet, A.; Meier, R.; Flahaut, M.; Bourloud, K.B.; Nardou, K.; Joseph, J.-M.; Gross, N. Complex Molecular Mechanisms Cooperate to Mediate Histone Deacetylase Inhibitors Anti-Tumour Activity in Neuroblastoma Cells. Mol. Cancer 2008, 7, 55. [Google Scholar] [CrossRef]
- van den Bijgaart, R.J.E.; Kroesen, M.; Brok, I.C.; Reijnen, D.; Wassink, M.; Boon, L.; Hoogerbrugge, P.M.; Adema, G.J. Anti-GD2 Antibody and Vorinostat Immunocombination Therapy Is Highly Effective in an Aggressive Orthotopic Neuroblastoma Model. Oncoimmunology 2020, 9, 1817653. [Google Scholar] [CrossRef]
- Cheung, B.B.; Tan, O.; Koach, J.; Liu, B.; Shum, M.S.Y.; Carter, D.R.; Sutton, S.; Po’uha, S.T.; Chesler, L.; Haber, M.; et al. Thymosin-Β4 Is a Determinant of Drug Sensitivity for Fenretinide and Vorinostat Combination Therapy in Neuroblastoma. Mol. Oncol. 2015, 9, 1484–1500. [Google Scholar] [CrossRef]
- Huang, J.-M.; Sheard, M.A.; Ji, L.; Sposto, R.; Keshelava, N. Combination of Vorinostat and Flavopiridol Is Selectively Cytotoxic to Multidrug-Resistant Neuroblastoma Cell Lines with Mutant TP53. Mol. Cancer Ther. 2010, 9, 3289–3301. [Google Scholar] [CrossRef]
- Hagiwara, K.; Tokunaga, T.; Iida, H.; Nagai, H. Combined Inhibition of ALK and HDAC Induces Synergistic Cytotoxicity in Neuroblastoma Cell Lines. Anticancer Res. 2019, 39, 3579–3584. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.J.; Richmond, P.A.; Bunker, E.N.; Karman, S.S.; Azofeifa, J.; Garnett, A.T.; Xu, Q.; Wheeler, G.E.; Toomey, C.M.; Zhang, Q.; et al. Genome-Wide Dose-Dependent Inhibition of Histone Deacetylases Studies Reveal Their Roles in Enhancer Remodeling and Suppression of Oncogenic Super-Enhancers. Nucleic Acids Res. 2018, 46, 1756–1776. [Google Scholar] [CrossRef] [PubMed]
- Blavier, L.; Yang, R.-M.; DeClerck, Y.A. The Tumor Microenvironment in Neuroblastoma: New Players, New Mechanisms of Interaction and New Perspectives. Cancers 2020, 12, 2912. [Google Scholar] [CrossRef] [PubMed]
- Layer, J.P.; Kronmüller, M.T.; Quast, T.; van den Boorn-Konijnenberg, D.; Effern, M.; Hinze, D.; Althoff, K.; Schramm, A.; Westermann, F.; Peifer, M.; et al. Amplification of N-Myc Is Associated with a T-Cell-Poor Microenvironment in Metastatic Neuroblastoma Restraining Interferon Pathway Activity and Chemokine Expression. Oncoimmunology 2017, 6, e1320626. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, Y.; Wang, L.; Zhang, H.; Liu, H.; Liu, Y. Cellular Components in Tumor Microenvironment of Neuroblastoma and the Prognostic Value. PeerJ 2019, 7, e8017. [Google Scholar] [CrossRef]
- Joshi, S. Targeting the Tumor Microenvironment in Neuroblastoma: Recent Advances and Future Directions. Cancers 2020, 12, 2057. [Google Scholar] [CrossRef]
- Nazha, B.; Inal, C.; Owonikoko, T.K. Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy. Front. Oncol. 2020, 10, 1000. [Google Scholar] [CrossRef]
- Wu, Z.L.; Schwartz, E.; Seeger, R.; Ladisch, S. Expression of GD2 Ganglioside by Untreated Primary Human Neuroblastomas. Cancer Res. 1986, 46, 440–443. [Google Scholar]
- Schengrund, C.L.; Shochat, S.J. Gangliosides in Neuroblastomas. Neurochem. Pathol. 1988, 8, 189–202. [Google Scholar] [CrossRef]
- Sariola, H.; Terävä, H.; Rapola, J.; Saarinen, U.M. Cell-Surface Ganglioside GD2 in the Immunohistochemical Detection and Differential Diagnosis of Neuroblastoma. Am. J. Clin. Pathol. 1991, 96, 248–252. [Google Scholar] [CrossRef]
- Dhillon, S. Dinutuximab: First Global Approval. Drugs 2015, 75, 923–927. [Google Scholar] [CrossRef]
- Horwacik, I.; Golik, P.; Grudnik, P.; Kolinski, M.; Zdzalik, M.; Rokita, H.; Dubin, G. Structural Basis of GD2 Ganglioside and Mimetic Peptide Recognition by 14G2a Antibody. Mol. Cell Proteom. 2015, 14, 2577–2590. [Google Scholar] [CrossRef]
- Schumacher-Kuckelkorn, R.; Volland, R.; Gradehandt, A.; Hero, B.; Simon, T.; Berthold, F. Lack of Immunocytological GD2 Expression on Neuroblastoma Cells in Bone Marrow at Diagnosis, during Treatment, and at Recurrence. Pediatr. Blood Cancer 2017, 64, 46–56. [Google Scholar] [CrossRef]
- Van den Bijgaart, R.J.E.; Kroesen, M.; Wassink, M.; Brok, I.C.; Kers-Rebel, E.D.; Boon, L.; Heise, T.; van Scherpenzeel, M.; Lefeber, D.J.; Boltje, T.J.; et al. Combined Sialic Acid and Histone Deacetylase (HDAC) Inhibitor Treatment up-Regulates the Neuroblastoma Antigen GD2. J. Biol. Chem. 2019, 294, 4437–4449. [Google Scholar] [CrossRef]
| Gene Name | CHr. | Alteration Type | Known NB Variants | Mutation Effect | MYCN Status ° | References |
|---|---|---|---|---|---|---|
| Single-Gene Alterations | ||||||
| ALK | 2p23 | Point mutation (missense) | Met1166Asn; Ile1171Asn/Thr; Phe1174Leu/Cys/Ile/Val/Ser §; Leu1240Val; Phe1245Ile/Cys §; Arg1275Gln/Leu § | Gain of function | Amp + non-Amp | [37], # |
| Amplification | - | Amp | [38] | |||
| Translocation/ Deletion | - | - | [12,13] | |||
| ATRX | Xq21.1 | Point mutation (nonsense) | Glu285 *; Glu990 *; Leu1645 * | Loss of function | non-Amp | [32], # |
| Point mutation (frameshift deletion) | Phe2113Serfs *9 | |||||
| PHOX2B | 4p13 | Point mutation (missense and frameshift) | Several variants clustered at 200–300 bp and 600–714 bp from the translation start codon | Gain of function | - | [9] |
| TERT | 5p15.33 | Upstream/downstream regions rearrangements | - | Gain of function | non-Amp | [15,30] |
| Segmental Chromosomal Alterations | ||||||
| - | 1p36 | Deletion | - | Loss of function | Mostly amp | [20] |
| - | 17q | Gain | - | Gain of function | Amp | [39] |
| - | 11q | Deletion | - | Loss of function | non-Amp | [27] |
| Compound | Target/Mechanism | FDA Approval | Clinical Trial Status (2021) | References |
|---|---|---|---|---|
| Crizotinib | ALK | NCT03126916 NCT01606878 NCT00939770 NCT03107988 NCT01121588 | Phase III Phase I Phase I/II Phase I Phase I | [108,109,110,111,112] |
| Alectinib | --- | --- | [113,114,115,116] | |
| Lorlatinib | NCT04753658 NCT03107988 | Observational Phase I | [117,118] | |
| Alisertib | AURKA | NCT01601535 NCT02444884 NCT01154816 | Phase I/II Phase I Phase II | [119,120,121,122,123,124,125] |
| JQ1 | BRD2/3/4 | --- | --- | [101,126,127,128,129,130] |
| OTX015 | NCT01713582 NCT02259114 | Phase I Phase I | [101,131,132] | |
| GSK525762 | NCT01587703 | Phase I/II | [133,134] | |
| Palbociclib | CDK4/6 | NCT03526250 NCT03709680 NCT03155620 | Phase II Phase I Phase II | [135,136] |
| Ribociclib (LEE011) | NCT01747876 NCT02780128 NCT03434262 | Phase I Phase I Phase I | [137,138,139,140] | |
| Abemaciclib (LY2835219) | NCT02644460 NCT04238819 | Phase I Phase I | [141] | |
| THZ1 | CDK7 | --- | --- | [23,105,106,127] |
| CYC065 (fadraciclib) | CDK9/2 | NCT02552953 | Phase I | [107] |
| Carboplatin | DNA synthesis | Approved | Approved | [142,143,144,145,146,147] |
| Cisplatin | DNA/RNA synthesis | Approved | Approved | [148,149,150] |
| Cyclophosphamide | DNA replication/RNA synthesis | Approved | Approved | [151,152,153] |
| Doxorubicin | DNA/RNA synthesis | Approved | Approved | [154,155,156,157,158,159,160,161] |
| Etoposide | DNA synthesis/Topo II poison | Approved | Approved | [142,144,162,163,164] |
| GD2 immunotherapy | GD2 ganglioside | NCT01822652 NCT01460901 NCT01576692 NCT01953900 NCT02100930 NCT01953900 NCT04539366 | Phase I Phase I Phase I Phase I Phase I Phase I Phase I | [165,166,167,168,169,170] |
| PU139 | HAT | --- | --- | [61] |
| PU141 | --- | --- | [61] | |
| P22077 | HAUSP | --- | --- | [171] |
| Panobinostat | HDAC | NCT04897880 | Phase II | [23,172,173,174,175] |
| Valproic acid | NCT01204450 | Phase I | [176,177,178,179,180,181] | |
| Vorinostat (SAHA) | NCT01019850 NCT03332667 NCT03561259 NCT01208454 NCT02035137 NCT02559778 NCT01132911 NCT01163383 NCT04308330 NCT00217412 | Phase I Phase I Phase II Phase I Phase II Phase II Phase I Phase II Phase I Phase I | [182,183,184,185,186,187,188] | |
| 10058-F4 | MYC/MAX heterodimer inhibitor | --- | --- | [189] |
| 10074-G5 | --- | --- | [189,190] | |
| IIA6B17 | --- | --- | [191] | |
| MYCi361 | --- | --- | [192] | |
| Omomyc (OMO-103) | NCT04808362 | Phase I/II | [193,194,195] | |
| DFMO | ODC1 | NCT02395666 NCT01586260 NCT04301843 NCT01059071 NCT02679144 NCT02139397 NCT02030964 NCT02030964 | Phase II Phase II Phase II Phase I Phase II Phase I/II Phase I Phase I | [196,197,198,199,200,201,202] |
| WS6 | PA2G4 | --- | --- | [203] |
| AZD8055 | PI3K/AKT/mTOR pathway | NCT01316809 NCT00973076 NCT00731263 NCT01194193 | Phase I Phase I Phase I Phase I | [204,205,206,207] |
| Perifosine | NCT01049841 NCT00776867 | Phase I Phase I | [208,209,210] | |
| Picropodophyllin (PPP) | NCT01721577 NCT01725555 NCT01062620 | Phase I/II Phase I Phase I | [211] | |
| SF1126 | NCT02337309 NCT00907205 | Phase I Phase I | [212] | |
| AMXT 1501 | SLC3A2 | NCT03536728 | Phase I | [196,202] |
| Lapatinib | TK | --- | --- | [106,213] |
| Ponatinib | --- | --- | [106,214,215,216] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciaccio, R.; De Rosa, P.; Aloisi, S.; Viggiano, M.; Cimadom, L.; Zadran, S.K.; Perini, G.; Milazzo, G. Targeting Oncogenic Transcriptional Networks in Neuroblastoma: From N-Myc to Epigenetic Drugs. Int. J. Mol. Sci. 2021, 22, 12883. https://doi.org/10.3390/ijms222312883
Ciaccio R, De Rosa P, Aloisi S, Viggiano M, Cimadom L, Zadran SK, Perini G, Milazzo G. Targeting Oncogenic Transcriptional Networks in Neuroblastoma: From N-Myc to Epigenetic Drugs. International Journal of Molecular Sciences. 2021; 22(23):12883. https://doi.org/10.3390/ijms222312883
Chicago/Turabian StyleCiaccio, Roberto, Piergiuseppe De Rosa, Sara Aloisi, Marta Viggiano, Leonardo Cimadom, Suleman Khan Zadran, Giovanni Perini, and Giorgio Milazzo. 2021. "Targeting Oncogenic Transcriptional Networks in Neuroblastoma: From N-Myc to Epigenetic Drugs" International Journal of Molecular Sciences 22, no. 23: 12883. https://doi.org/10.3390/ijms222312883
APA StyleCiaccio, R., De Rosa, P., Aloisi, S., Viggiano, M., Cimadom, L., Zadran, S. K., Perini, G., & Milazzo, G. (2021). Targeting Oncogenic Transcriptional Networks in Neuroblastoma: From N-Myc to Epigenetic Drugs. International Journal of Molecular Sciences, 22(23), 12883. https://doi.org/10.3390/ijms222312883







