The Receptor AT1 Appears to Be Important for the Maintenance of Bone Mass and AT2 Receptor Function in Periodontal Bone Loss Appears to Be Regulated by AT1 Receptor
Abstract
:1. Introduction
2. Results
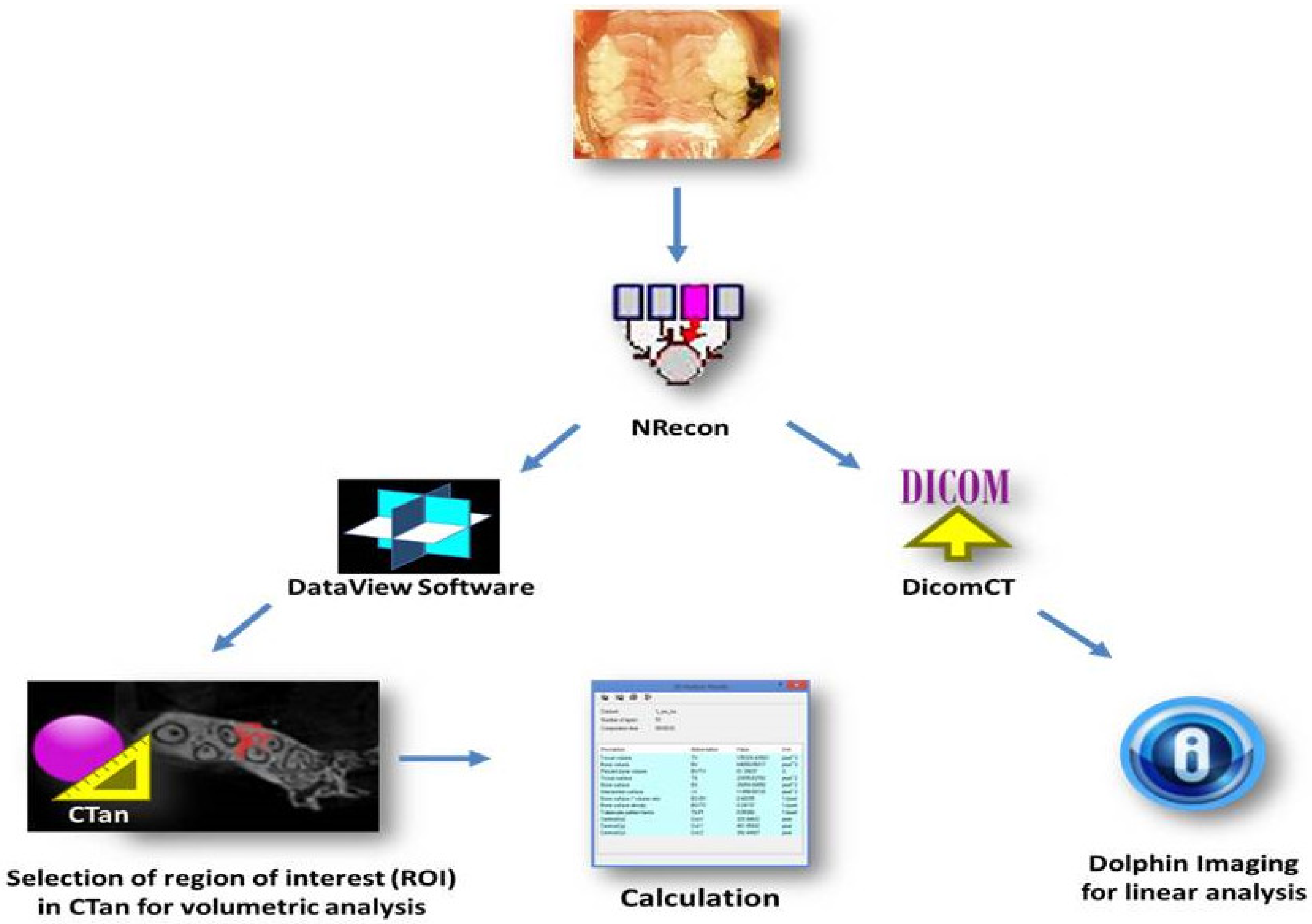
2.1. Analysis of Linear Bone Loss and Bone Volume by Micro-Computed Tomography (Micro-CT)
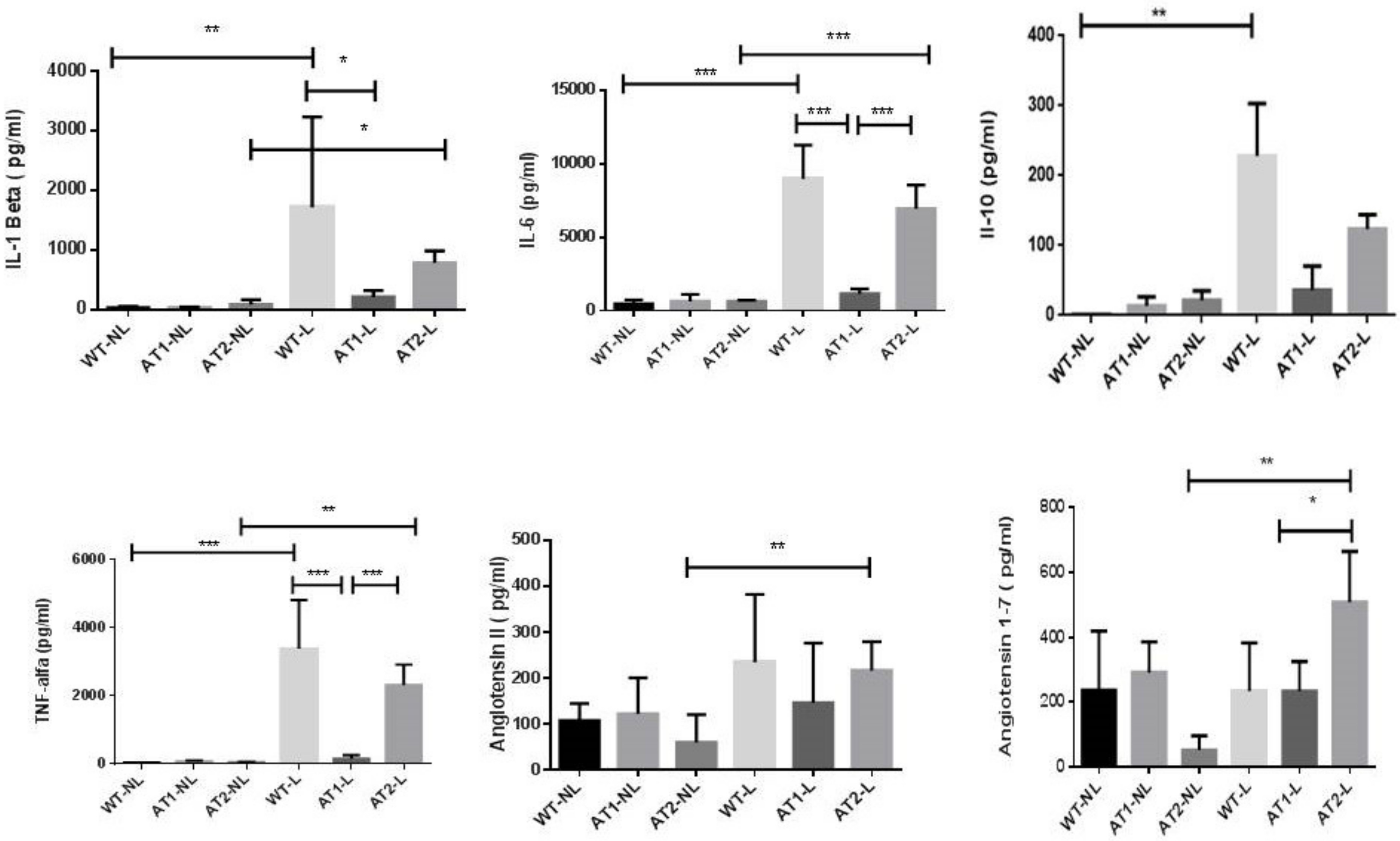
2.2. Analysis of Cytokines and Peptides by ELISA Immunoassay
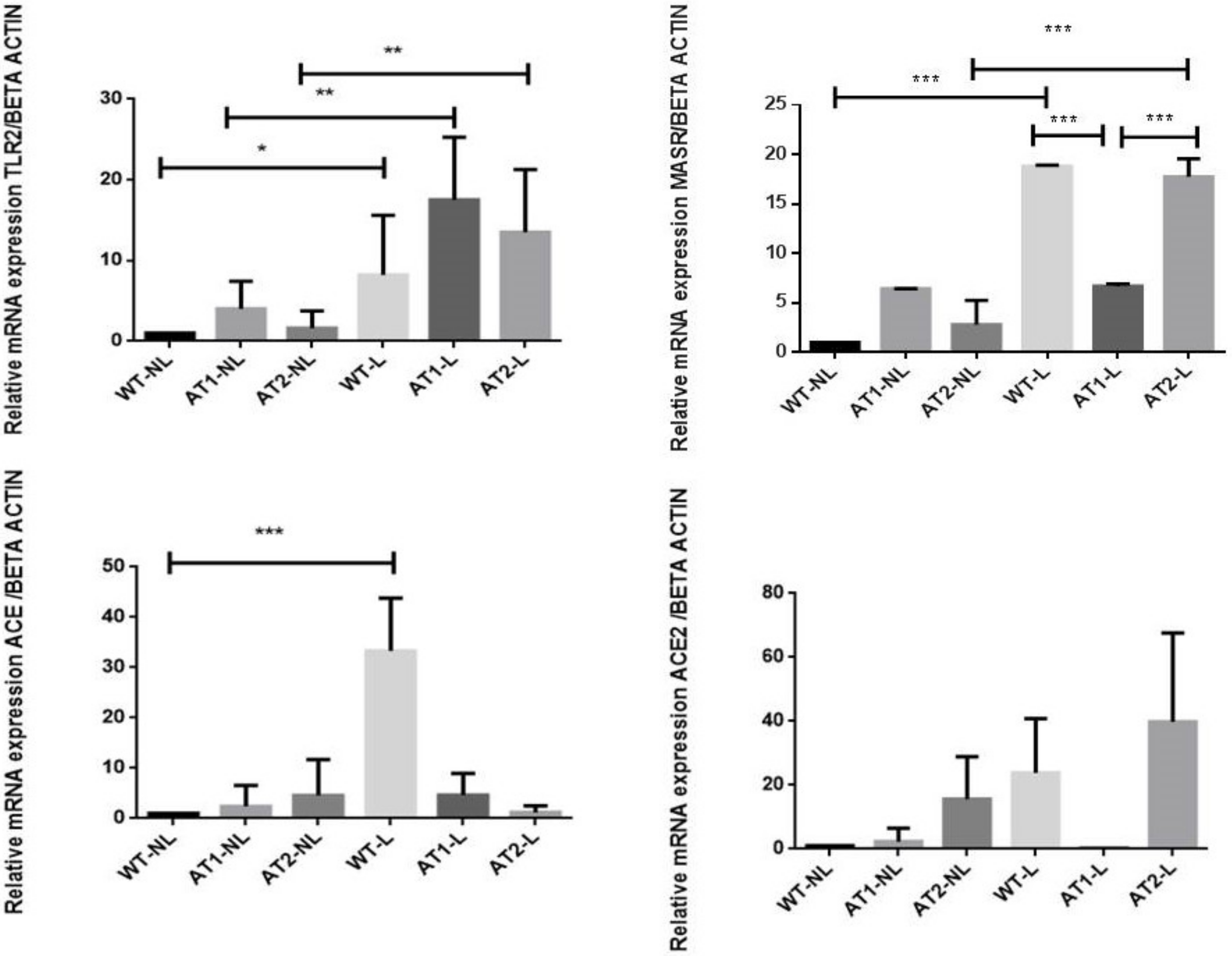
2.3. Gene Expression Analysis by RT-PCR
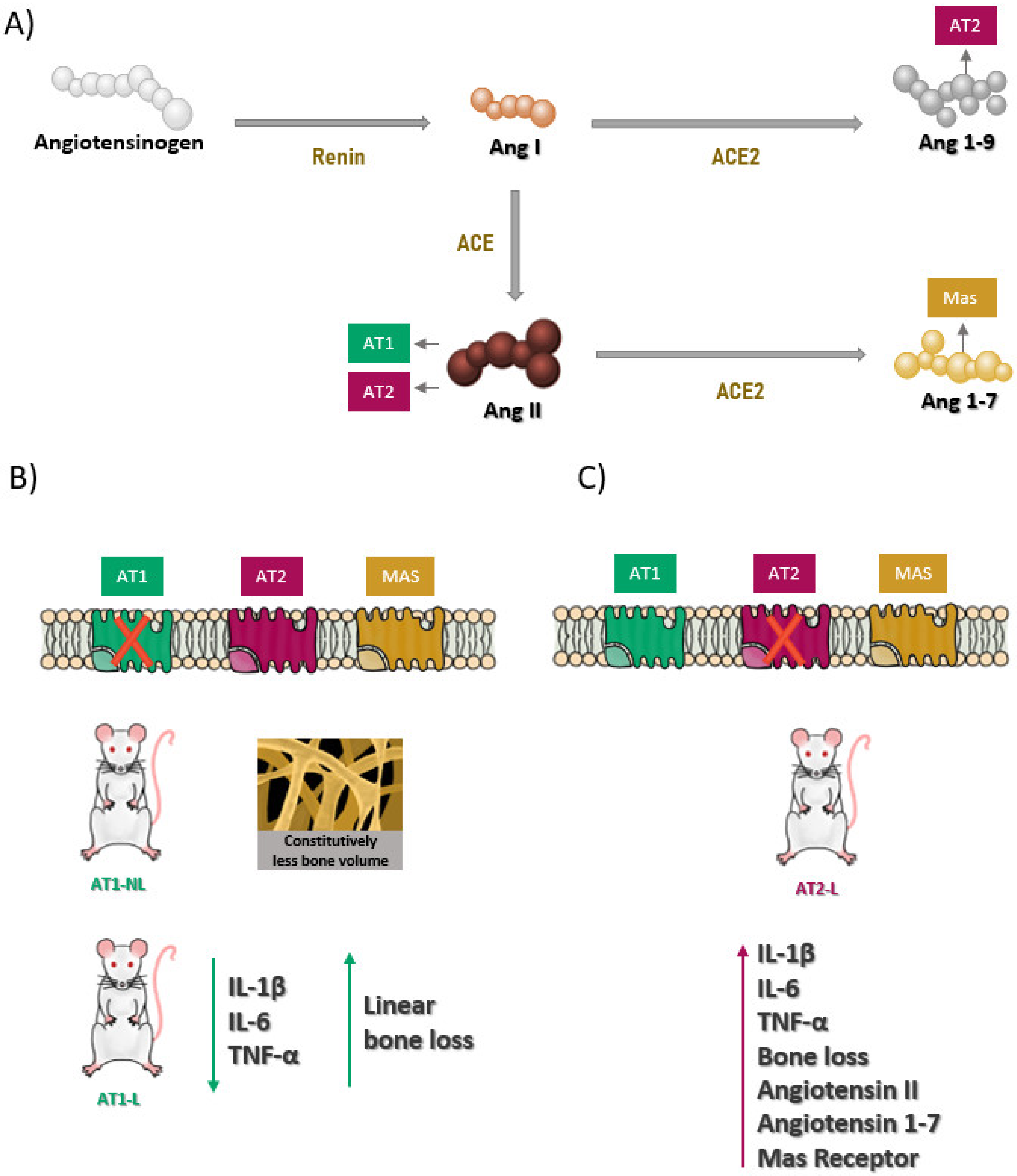
3. Discussion
4. Material and Methods
4.1. Animals
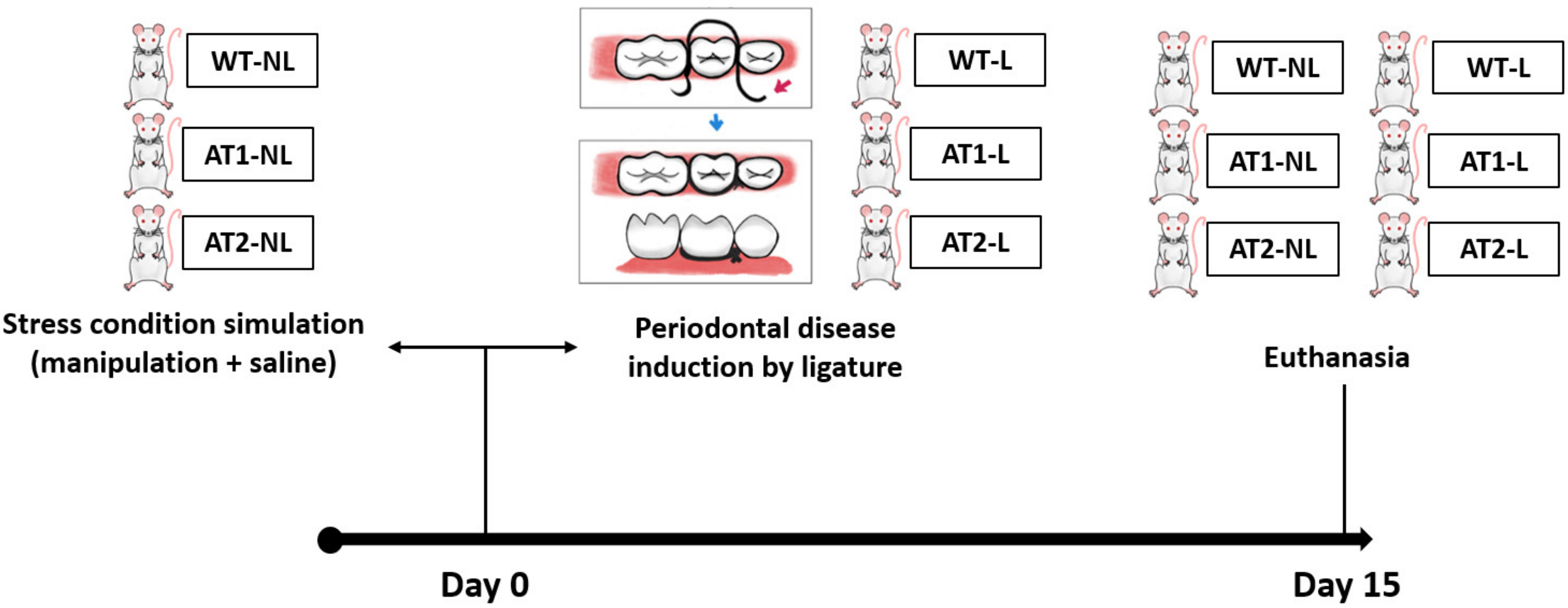
4.2. Study Groups
4.3. Induction of Ligature Periodontitis Experimental Model
4.4. Micro-CT
4.5. Elisa Immunoassay for Detecting IL-1β, IL-6, IL-10, TNF-α and Peptides
4.6. RT-PCR Gene Marker Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kushkevych, I.; Coufalová, M.; Vítězová, M.; Rittmann, S. Sulfate-Reducing Bacteria of the Oral Cavity and Their Relation with Periodontitis-Recent Advances. J. Clin. Med. 2020, 9, 2347. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.H.; Choi, E.Y.; Hyeon, J.Y.; Keum, B.R.; Choi, I.S.; Kim, S.J. Telmisartan, an angiotensin II receptor blocker, attenuates Prevotella intermedia lipopolysaccharide-induced production of nitric oxide and interleukin-1β in murine macrophages. Int. Immunopharmacol. 2019, 75, 105750. [Google Scholar] [CrossRef]
- Herrera, D.; Molina, A.; Buhlin, K.; Klinge, B. Periodontal diseases and association with atherosclerotic disease. Periodontology 2020 2020, 83, 66–89. [Google Scholar] [CrossRef]
- Dionísio, T.J.; Souza, G.P.; Colombini-Ishikiriama, B.L.; Garbieri, T.F.; Parisi, V.A.; Oliveira, G.M.; Cano, I.P.; Rodini, C.O.; Oliveira, S.H.P.; Greene, A.S.; et al. AT1 receptor antagonism promotes bone loss attenuation in experimental periodontitis, block inflammatory mediators, upregulate antioxidant enzymes and bone formation markers. J. Periodontol. 2020, 91, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Brito, V.G.B.; Patrocinio, M.S.; Linjardi, M.C.; Barreto, A.E.A.; Frasnelli, S.C.; Lara, V.S.; Santos, C.F.; Oliveira, S.H.P. Telmisartan Prevents Alveolar Bone Loss by Decreasing the Expression of Osteoclasts Markers in Hypertensive Rats with Periodontal Disease. Front. Pharmacol. 2020, 11, 579926. [Google Scholar] [CrossRef]
- Da Silva Novaes, A.; Ribeiro, R.S.; Pereira, L.G.; Borges, F.T.; Boim, M.A. Intracrine action of angiotensin II in mesangial cells: Subcellular distribution of angiotensin II receptor subtypes AT1 and AT2. Mol. Cell. Biochem. 2018, 448, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.H.P.; Brito, V.G.B.; Frasnelli, S.C.T.; Ribeiro, B.D.S.; Ferreira, M.N.; Queiroz, D.P.; Beltan, C.T.; Lara, V.S.; Santos, C.F. Aliskiren Attenuates the Inflammatory Response and Wound Healing Process in Diabetic Mice with Periodontal Disease. Front. Pharmacol. 2019, 4, 708–723. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.F.; Akashi, A.E.; Dionísio, T.J.; Sipert, C.R.; Didier, D.N.; Greene, A.S.; Oliveira, S.H.; Pereira, H.J.; Becari, C.; Oliveira, E.B.; et al. Characterization of a local renin-angiotensin system in rat gingival tissue. J. Periodontol. 2009, 80, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriele, L.G.; Morandini, A.C.; Dionísio, T.J.; Santos, C.F. Angiotensin II Type 1 Receptor Knockdown Impairs Interleukin-1β-Induced Cytokines in Human Periodontal Fibroblasts. J. Periodontol. 2017, 88, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Lelis, D.F.; Freitas, D.F.; Machado, A.S.; Crespo, T.S.; Santos, S.H.S. Angiotensin-(1-7), Adipokines and Inflammation. Metabolism 2019, 95, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Queiroz-Junior, C.M.; Santos, A.; Galvão, I.; Souto, G.R.; Mesquita, R.A.; Sá, M.A.; Ferreira, A.J. The angiotensin converting enzyme 2/angiotensin-(1-7)/Mas Receptor axis as a key player in alveolar bone remodeling. Bone 2019, 128, 115041. [Google Scholar] [CrossRef]
- Mo, C.; Ke, J.; Zhao, D.; Zhang, B. Role of the renin-angiotensin-aldosterone system in bone metabolism. J. Bone Miner. Metab. 2020, 38, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.F.; Zhang, L.H.; Bai, F.; Wang, N.P.; Garner, R.E.; McKallip, R.J.; Zhao, Z.Q. Attenuation of myocardial fibrosis with curcumin is mediated by modulating expression of angiotensin II AT1/AT2 receptors and ACE2 in rats. Drug Des. Dev. Ther. 2015, 11, 6043–6054. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Jung, Y.; Nam, M.; Sun Kang, M.; Lee, M.K.; Cho, Y.; Choi, E.K.; Hwang, G.S.; Soo Kim, H. Angiotensin II affects inflammation mechanisms via AMPK-related signalling pathways in HL-1 atrial myocytes. Sci. Rep. 2017, 4, 10328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asaba, Y.; Ito, M.; Fumoto, T.; Watanabe, K.; Fukuhara, R.; Takeshita, S.; Nimura, Y.; Ishida, J.; Fukamizu, A.; Ikeda, K. Activation of renin-angiotensin system induces osteoporosis independently of hypertension. J. Bone Miner. Res. 2009, 24, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Namsolleck, P.; Recarti, C.; Foulquier, S.; Steckelings, U.M.; Unger, T. AT(2) receptor and tissue injury: Therapeutic implications. Curr. Hypertens. Rep. 2014, 16, 416. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, E.M.; Reis, C.; Manzanares-Céspedes, M.C. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad. Med. 2018, 130, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, M.P.; Seymour, G.J. Periodontal disease and systemic illness: Will the evidence ever be enough? Periodontology 2020 2013, 62, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Priyamvara, A.; Dey, A.K.; Bandyopadhyay, D.; Katikineni, V.; Zaghlol, R.; Basyal, B.; Barssoum, K.; Amarin, R.; Bhatt, D.L.; Lavie, C.J. Periodontal Inflammation and the Risk of Cardiovascular Disease. Curr. Atheroscler. Rep. 2020, 22, 28. [Google Scholar] [CrossRef]
- Santos, C.F.; Morandini, A.C.; Dionísio, T.J.; Faria, F.A.; Lima, M.C.; Figueiredo, C.M.; Colombini-Ishikiriama, B.L.; Sipert, C.R.; Maciel, R.P.; Akashi, A.P.; et al. Functional Local Renin-Angiotensin System in Human and Rat Periodontal Tissue. PLoS ONE 2015, 10, e0134601. [Google Scholar]
- Usategui-Martín, R.; Lendinez-Tortajada, V.; Pérez-Castrillón, J.L.; Briongos-Figuero, L.; Abadía-Otero, J.; Martín-Vallejo, J.; Lara-Hernandez, F.; Chaves, F.J.; García-Garcia, A.B.; Martín-Escudero, J.C. Polymorphisms in genes involved in inflammation, the NF-kB pathway and the renin-angiotensin-aldosterone system are associated with the risk of osteoporotic fracture. The Hortega Follow-up Study. Bone 2020, 138, 115477. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.; Medeiros, C.; Guerra, G.; Santos, R.; Bader, M.; Pirih, F.Q.; Araújo Júnior, R.F.; Chan, A.B.; Cruz, L.J.; Brito, G.; et al. AT1 and AT2 Receptor Knockout Changed Osteonectin and Bone Density in Mice in Periodontal Inflammation Experimental Model. Int. J. Mol. Sci. 2021, 22, 5217. [Google Scholar] [CrossRef]
- Akagi, T.; Mukai, T.; Mito, T.; Kawahara, K.; Tsuji, S.; Fujita, S.; Uchida, H.A.; Morita, Y. Effect of Angiotensin II on Bone Erosion and Systemic Bone Loss in Mice with Tumor Necrosis Factor-Mediated Arthritis. Int. J. Mol. Sci. 2020, 21, 4145. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Ito, M.; Fumoto, T.; Fukuhara, R.; Ishida, J.; Fukamizu, A.; Ikeda, K. Physiological function of the angiotensin AT1a receptor in bone remodeling. J. Bone Miner. Res. 2011, 26, 2959–2966. [Google Scholar] [CrossRef]
- El-Shoura, E.; Sharkawi, S.; Messiha, B.; Bakr, A.G.; Hemeida, R. Perindopril mitigates LPS-induced cardiopulmonary oxidative and inflammatory damage via inhibition of renin angiotensin system, inflammation and oxidative stress. Immunopharmacol. Immunotoxicol. 2019, 41, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, W.; Ren, F.; Luo, L.; Zhou, J.; Huang, D.; Jiang, M.; Du, H.; Fan, J.; Tang, L. Characteristics of Ang-(1-7)/Mas-Mediated Amelioration of Joint Inflammation and Cardiac Complications in Mice with Collagen-Induced Arthritis. Front. Immunol. 2021, 12, 655614. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Yoshimura, A.; Ukai, T.; Lien, E.; Espevik, T.; Hara, Y. Immunohistochemical localization of Toll-like receptors 2 and 4 in gingival tissue from patients with periodontitis. Oral Microbiol. Immunol. 2003, 18, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Jurdziński, K.T.; Potempa, J.; Grabiec, A.M. Epigenetic regulation of inflammation in periodontitis: Cellular mechanisms and therapeutic potential. Clin. Epigenetics 2020, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Niimi, H.; Ohsugi, Y.; Tsuchiya, Y.; Shimohira, T.; Komatsu, K.; Liu, A.; Shiba, T.; Aoki, A.; Iwata, T.; et al. Application of Ligature-Induced Periodontitis in Mice to Explore the Molecular Mechanism of Periodontal Disease. Int. J. Mol. Sci. 2021, 22, 8900. [Google Scholar] [CrossRef]
- Graves, D.T.; Kang, J.; Andriankaja, O.; Wada, K.; Rossa, C. Animal models to study host-bacteria interactions involved in periodontitis. Front. Oral Biol. 2012, 15, 117–132. [Google Scholar] [PubMed] [Green Version]
- Rojas, C.; García, M.P.; Polanco, A.F.; González-Osuna, L.; Sierra-Cristancho, A.; Melgar-Rodríguez, S.; Cafferata, E.A.; Vernal, R. Humanized Mouse Models for the Study of Periodontitis: An Opportunity to Elucidate Unresolved Aspects of Its Immunopathogenesis and Analyze New Immunotherapeutic Strategies. Front. Immunol. 2021, 12, 663328. [Google Scholar] [CrossRef] [PubMed]

| Primer | Forward | Reverse | Specie |
|---|---|---|---|
| Beta-Actin | AGGCCAACCTGTAAAAGATG | TGTGGTACGAGAGGCATAC | Mouse |
| MASR | AGAAATCCCTTCACGGTCTACA | GTCACCGATAATGTCACGATTGT | Mouse |
| TLR2 | GCAGCCACTCTACCTGAACC | GCGCCACCAAATCATAGATGTTG | Mouse |
| ACE2 | TCCAGACTCCGATCATCAAGC | TGCTCATGGTGTTCAGAATTGT | Mouse |
| TLR-2 | CACCACTGCCCGTAGATGAA | GCCTGCGAATGCCAGCTT | Mouse |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, M.L.d.S.; Martins, A.A.; Medeiros, C.A.C.X.d.; Guerra, G.C.B.; Santos, R.; Bader, M.; Pirih, F.Q.; Araújo Júnior, R.F.d.; Brito, G.A.d.C.; Leitão, R.F.d.C.; et al. The Receptor AT1 Appears to Be Important for the Maintenance of Bone Mass and AT2 Receptor Function in Periodontal Bone Loss Appears to Be Regulated by AT1 Receptor. Int. J. Mol. Sci. 2021, 22, 12849. https://doi.org/10.3390/ijms222312849
Lima MLdS, Martins AA, Medeiros CACXd, Guerra GCB, Santos R, Bader M, Pirih FQ, Araújo Júnior RFd, Brito GAdC, Leitão RFdC, et al. The Receptor AT1 Appears to Be Important for the Maintenance of Bone Mass and AT2 Receptor Function in Periodontal Bone Loss Appears to Be Regulated by AT1 Receptor. International Journal of Molecular Sciences. 2021; 22(23):12849. https://doi.org/10.3390/ijms222312849
Chicago/Turabian StyleLima, Maria Laura de Souza, Agnes Andrade Martins, Caroline Addison Carvalho Xavier de Medeiros, Gerlane Coelho Bernardo Guerra, Robson Santos, Michael Bader, Flavia Q. Pirih, Raimundo Fernandes de Araújo Júnior, Gerly Anne de Castro Brito, Renata Ferreira de Carvalho Leitão, and et al. 2021. "The Receptor AT1 Appears to Be Important for the Maintenance of Bone Mass and AT2 Receptor Function in Periodontal Bone Loss Appears to Be Regulated by AT1 Receptor" International Journal of Molecular Sciences 22, no. 23: 12849. https://doi.org/10.3390/ijms222312849
APA StyleLima, M. L. d. S., Martins, A. A., Medeiros, C. A. C. X. d., Guerra, G. C. B., Santos, R., Bader, M., Pirih, F. Q., Araújo Júnior, R. F. d., Brito, G. A. d. C., Leitão, R. F. d. C., Silva, R. A., Barbosa, S. J. d. A., Melo, R. C. d. O., & Araújo, A. A. d. (2021). The Receptor AT1 Appears to Be Important for the Maintenance of Bone Mass and AT2 Receptor Function in Periodontal Bone Loss Appears to Be Regulated by AT1 Receptor. International Journal of Molecular Sciences, 22(23), 12849. https://doi.org/10.3390/ijms222312849







