Humoral Predictors of Malignancy in IPMN: A Review of the Literature
Abstract
:1. Introduction
2. Circulating Humoral Predictors of Malignancy
2.1. Carbohydrate Antigen 19-9
2.2. Carcinoembryonic Antigen
2.3. Neutrophil to Lymphocyte Ratio
2.4. Platelet to Lymphocyte Ratio
2.5. C-Reactive Protein to Albumin Ratio
2.6. Cyst Fluid Sample
2.7. Carcinoembryonic Antigen
2.8. Cytological Analysis
2.9. Glucose
2.10. Mucin
2.11. Amylase
2.12. Other Intra-Cystic Markers
3. New Perspectives
3.1. Cyst Fluid DNA Sequencing
3.2. MicroRNA (mi-RNA) and Telomeres
3.3. Circulating Microvesicles
3.4. Concept of Liquid Biopsy
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spinelli, K.S.; Fromwiller, T.E.; Daniel, R.A.; Kiely, J.M.; Nakeeb, A.; Komorowski, R.A.; Wilson, S.D.; Pitt, H.A. Cystic Pancreatic Neoplasms: Observe or Operate. Ann. Surg. 2004, 239, 651–659. [Google Scholar] [CrossRef]
- Furukawa, T.; Klöppel, G.; Volkan Adsay, N.; Albores-Saavedra, J.; Fukushima, N.; Horii, A.; Hruban, R.H.; Kato, Y.; Klimstra, D.S.; Longnecker, D.S.; et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: A consensus study. Virchows Arch. 2005, 447, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Mino-Kenudson, M.; Fernández-del Castillo, C.; Baba, Y.; Valsangkar, N.P.; Liss, A.S.; Hsu, M.; Correa-Gallego, C.; Ingkakul, T.; Perez Johnston, R.; Turner, B.G.; et al. Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut 2011, 60, 1712–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, H.; Ziogas, A.; Rhee, J.M.; Lee, J.G.; Lipkin, S.M.; Zell, J.A. A population-based, descriptive analysis of malignant intraductal papillary mucinous neoplasms of the pancreas. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2737–2741. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Matthaei, H.; Maitra, A.; Dal Molin, M.; Wood, L.D.; Eshleman, J.R.; Goggins, M.; Canto, M.I.; Schulick, R.D.; Edil, B.H.; et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci. Transl. Med. 2011, 3, 92ra66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, T.; Kuboki, Y.; Tanji, E.; Yoshida, S.; Hatori, T.; Yamamoto, M.; Shibata, N.; Shimizu, K.; Kamatani, N.; Shiratori, K.; et al. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci. Rep. 2011, 1, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 1, 161. [Google Scholar] [CrossRef]
- Brugge, W.R.; Lewandrowski, K.; Lee-Lewandrowski, E.; Centeno, B.A.; Szydlo, T.; Regan, S.; del Castillo, C.F.; Warshaw, A.L. Diagnosis of Pancreatic Cystic Neoplasms: A Report of the Cooperative Pancreatic Cyst Study. Gastroenterology 2004, 126, 1330–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornton, G.D.; McPhail, M.J.W.; Nayagam, S.; Hewitt, M.J.; Vlavianos, P.; Monahan, K.J. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: A meta-analysis. Pancreatology 2013, 13, 48–57. [Google Scholar] [CrossRef]
- Keane, M.G.; Afghani, E. A Review of the Diagnosis and Management of Premalignant Pancreatic Cystic Lesions. J. Clin. Med. 2021, 10, 1284. [Google Scholar] [CrossRef]
- Koprowski, H.; Steplewski, Z.; Mitchell, K.; Herlyn, M.; Herlyn, D.; Fuhrer, P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979, 5, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Magnani, J.L.; Nilsson, B.; Brockhaus, M.; Zopf, D.; Steplewski, Z.; Koprowski, H.; Ginsburg, V. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J. Biol. Chem. 1982, 257, 14365–14369. [Google Scholar] [CrossRef]
- Steinberg, W. The clinical utility of the CA 19-9 tumor-associated antigen. Am. J. Gastroenterol. 1990, 85, 350–355. [Google Scholar] [PubMed]
- Tempero, M.A.; Uchida, E.; Takasaki, H.; Burnett, D.A.; Steplewski, Z.; Pour, P.M. Relationship of Carbohydrate Antigen 19–9 and Lewis Antigens in Pancreatic Cancer. Cancer Res. 1987, 47, 5501–5503. [Google Scholar]
- Tsen, A.; Barbara, M.; Rosenkranz, L. Dilemma of elevated CA 19-9 in biliary pathology. Pancreatology 2018, 18, 862–867. [Google Scholar] [CrossRef]
- Scarà, S.; Bottoni, P.; Scatena, R. CA 19-9: Biochemical and clinical aspects. Adv. Exp. Med. Biol. 2015, 867, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Ballehaninna, U.K.; Chamberlain, R.S. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J. Gastrointest. Oncol. 2012, 3, 105–119. [Google Scholar] [CrossRef]
- Goonetilleke, K.S.; Siriwardena, A.K. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur. J. Surg. Oncol. 2007, 33, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Kuntz, A.I.; Wadleigh, R.G. Ca 19-9 tumor marker: Is it reliable? A case report in a patient with pancreatic cancer. Clin. Adv. Hematol. Oncol. 2013, 11, 50–52. [Google Scholar]
- Duffy, M.J.; Sturgeon, C.; Lamerz, R.; Haglund, C.; Holubec, V.L.; Klapdor, R.; Nicolini, A.; Topolcan, O.; Heinemann, V. Tumor markers in pancreatic cancer: A European Group on Tumor Markers (EGTM) status report. Ann. Oncol. 2009, 21, 441–447. [Google Scholar] [CrossRef]
- Lee, K.J.; Yi, S.W.; Chung, M.J.; Park, S.W.; Song, S.Y.; Chung, J.B.; Park, J.Y. Serum CA 19-9 and CEA levels as a prognostic factor in pancreatic adenocarcinoma. Yonsei Med. J. 2013, 54, 643–649. [Google Scholar] [CrossRef] [Green Version]
- Fritz, S.; Hackert, T.; Hinz, U.; Hartwig, W.; Büchler, M.W.; Werner, J. Role of serum carbohydrate antigen 19-9 and carcinoembryonic antigen in distinguishing between benign and invasive intraductal papillary mucinous neoplasm of the pancreas. Br. J. Surg. 2011, 98, 104–110. [Google Scholar] [CrossRef]
- Kim, J.R.; Jang, J.Y.; Kang, M.J.; Park, T.; Lee, S.Y.; Jung, W.; Chang, J.; Shin, Y.; Han, Y.; Kim, S.W. Clinical implication of serum carcinoembryonic antigen and carbohydrate antigen 19-9 for the prediction of malignancy in intraductal papillary mucinous neoplasm of pancreas. J. Hepatobiliary Pancreat. Sci. 2015, 22, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, L.; Chen, L.; Wei, J.; Sun, Q.; Xie, Q.; Zhou, X.; Zhou, D.; Huang, P.; Yang, Q.; et al. Serum carcinoembryonic antigen and carbohydrate antigen 19-9 for prediction of malignancy and invasiveness in intraductal papillary mucinous neoplasms of the pancreas: A meta-analysis. Biomed. Rep. 2015, 3, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Fernández-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L.; et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef]
- Ciprani, D.; Morales-Oyarvide, V.; Qadan, M.; Hank, T.; Weniger, M.; Harrison, J.M.; Rodrigues, C.; Horick, N.K.; Mino-Kenudson, M.; Ferrone, C.R.; et al. An elevated CA 19-9 is associated with invasive cancer and worse survival in IPMN. Pancreatology 2020, 20, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Paxton, R.J.; Mooser, G.; Pande, H.; Lee, T.D.; Shively, J.E. Sequence analysis of carcinoembryonic antigen: Identification of glycosylation sites and homology with the immunoglobulin supergene family. Proc. Natl. Acad. Sci. USA 1987, 84, 920–924. [Google Scholar] [CrossRef] [Green Version]
- Hammarström, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef]
- Gold, P.; Freedman, S.O. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J. Exp. Med. 1965, 121, 439–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beauchemin, N.; Arabzadeh, A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013, 32, 643–671. [Google Scholar] [CrossRef] [PubMed]
- Baqar, A.R.; Wilkins, S.; Staples, M.; Lee, C.H.A.; Oliva, K.; McMurrick, P. The role of preoperative CEA in the management of colorectal cancer: A cohort study from two cancer centres. Int. J. Surg. 2019, 64, 10–15. [Google Scholar] [CrossRef]
- Li, Y.; Xing, C.; Wei, M.; Wu, H.; Hu, X.; Li, S.; Sun, G.; Zhang, G.; Wu, B.; Zhang, F.; et al. Combining red blood cell distribution width (RDW-CV) and CEA predict poor prognosis for survival outcomes in colorectal cancer. J. Cancer 2019, 10, 1162–1170. [Google Scholar] [CrossRef] [Green Version]
- Satake, K.; Kanazawa, G.; Kho, I.; Chung, Y.S.; Umeyama, K. Evaluation of Serum Pancreatic Enzymes, Carbohydrate Antigen 19-9, and Carcinoembryonic Antigen in Various Pancreatic Diseases. Am. J. Gastroenterol. 1985, 80, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Wang, J.; Wang, Y.; Tong, M.; Hu, H.; Huang, C.; Li, D. Diagnostic value of CA 19-9 and carcinoembryonic antigen for pancreatic cancer: A meta-analysis. Gastroenterol. Res. Pract. 2018, 2018, 8704751. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Paris, P.L.; Chen, J.; Ngo, V.; Yao, H.; Frazier, M.L.; Killary, A.M.; Liu, C.G.; Liang, H.; Mathy, C.; et al. Next generation sequencing of pancreatic cyst fluid microRNAs from low grade-benign and high grade-invasive lesions. Cancer Lett. 2015, 356, 404–409. [Google Scholar] [CrossRef]
- Moris, D.; Damaskos, C.; Spartalis, E.; Papalampros, A.; Vernadakis, S.; Dimitroulis, D.; Griniatsos, J.; Felekouras, E.; Nikiteas, N. Updates and critical evaluation on novel biomarkers for the malignant progression of intraductal papillary mucinous neoplasms of the pancreas. Anticancer Res. 2017, 37, 2185–2194. [Google Scholar] [CrossRef] [Green Version]
- Paramanathan, A.; Saxena, A.; Morris, D.L. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg. Oncol. 2014, 23, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Mano, Y.; Shirabe, K.; Yamashita, Y.; Harimoto, N.; Tsujita, E.; Takeishi, K.; Aishima, S.; Ikegami, T.; Yoshizumi, T.; Yamanaka, T.; et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: A retrospective analysis. Ann. Surg. 2013, 258, 301–305. [Google Scholar] [CrossRef]
- Arima, K.; Okabe, H.; Hashimoto, D.; Chikamoto, A.; Kuroki, H.; Taki, K.; Kaida, T.; Higashi, T.; Nitta, H.; Komohara, Y.; et al. The Neutrophil-to-Lymphocyte Ratio Predicts Malignant Potential in Intraductal Papillary Mucinous Neoplasms. J. Gastrointest. Surg. 2015, 19, 2171–2177. [Google Scholar] [CrossRef]
- Gemenetzis, G.; Bagante, F.; Griffin, J.F.; Rezaee, N.; Javed, A.A.; Manos, L.L.; Lennon, A.M.; Wood, L.D.; Hruban, R.H.; Zheng, L.; et al. Neutrophil-to-lymphocyte Ratio is a Predictive Marker for Invasive Malignancy in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann. Surg. 2017, 266, 339–345. [Google Scholar] [CrossRef]
- Hata, T.; Mizuma, M.; Motoi, F.; Ishida, M.; Morikawa, T.; Takadate, T.; Nakagawa, K.; Hayashi, H.; Kanno, A.; Masamune, A.; et al. Diagnostic and Prognostic Impact of Neutrophil-to-Lymphocyte Ratio for Intraductal Papillary Mucinous Neoplasms of the Pancreas with High-Grade Dysplasia and Associated Invasive Carcinoma. Pancreas 2019, 48, 99–106. [Google Scholar] [CrossRef]
- Ohno, R.; Kawamoto, R.; Kanamoto, M.; Watanabe, J.; Fujii, M.; Ohtani, H.; Harada, M.; Kumagi, T.; Kawasaki, H. Neutrophil to Lymphocyte Ratio is a Predictive Factor of Malignant Potential for Intraductal Papillary Mucinous Neoplasms of the pancreas. Biomark. Insights 2019, 14, 1177271919851505. [Google Scholar] [CrossRef]
- McIntyre, C.A.; Pulvirenti, A.; Lawrence, S.A.; Seier, K.; Gonen, M.; Balachandran, V.P.; Kingham, T.P.; DʼAngelica, M.I.; Drebin, J.A.; Jarnagin, W.R.; et al. Neutrophil-to-Lymphocyte Ratio as a Predictor of Invasive Carcinoma in Patients with Intraductal Papillary Mucinous Neoplasms of the Pancreas. Pancreas 2019, 48, 832–836. [Google Scholar] [CrossRef]
- Zhou, X.; Du, Y.; Huang, Z.; Xu, J.; Qiu, T.; Wang, J.; Wang, T.; Zhu, W.; Liu, P. Prognostic value of PLR in various cancers: A meta-analysis. PLoS ONE 2014, 9, e101119. [Google Scholar] [CrossRef]
- Goh, B.K.P.; Tan, D.M.; Chan, C.Y.; Lee, S.Y.; Lee, V.T.; Thng, C.H.; Low, A.S.; Tai, D.W.; Cheow, P.C.; Chow, P.K.; et al. Are preoperative blood neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios useful in predicting malignancy in surgically-treated mucin-producing pancreatic cystic neoplasms? J. Surg. Oncol. 2015, 112, 366–371. [Google Scholar] [CrossRef]
- Alagappan, M.; Pollom, E.L.; von Eyben, R.; Kozak, M.M.; Aggarwal, S.; Poultsides, G.A.; Koong, A.C.; Chang, D.T. Albumin and Neutrophil-Lymphocyte Ratio (NLR) Predict Survival in Patients with Pancreatic Adenocarcinoma Treated with SBRT. Am. J. Clin. Oncol. Cancer Clin. Trials 2018, 41, 242–247. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, H.; Li, W.; Chen, J. Prognostic significance of the CRP/Alb and neutrophil to lymphocyte ratios in hepatocellular carcinoma patients undergoing TACE and RFA. J. Clin. Lab. Anal. 2019, 33, e22999. [Google Scholar] [CrossRef] [Green Version]
- Hata, T.; Mizuma, M.; Motoi, F.; Ishida, M.; Morikawa, T.; Nakagawa, K.; Hayashi, H.; Kanno, A.; Masamune, A.; Kamei, T.; et al. An integrated analysis of host- and tumor-derived markers for predicting high-grade dysplasia and associated invasive carcinoma of intraductal papillary mucinous neoplasms of the pancreas. Surg. Today 2020, 50, 1039–1048. [Google Scholar] [CrossRef]
- Asari, S.; Matsumoto, I.; Toyama, H.; Shinzeki, M.; Goto, T.; Ishida, J.; Ajiki, T.; Fukumoto, T.; Ku, Y. Preoperative independent prognostic factors in patients with borderline resectable pancreatic ductal adenocarcinoma following curative resection: The neutrophil-lymphocyte and platelet-lymphocyte ratios. Surg. Today 2016, 46, 583–592. [Google Scholar] [CrossRef]
- Serafini, S.; Friziero, A.; Sperti, C.; Vallese, L.; Grego, A.; Piangerelli, A.; Belluzzi, A.; Moletta, L. The Ratio of C-Reactive Protein to Albumin Is an Independent Predictor of Malignant Intraductal Papillary Mucinous Neoplasms of the Pancreas. J. Clin. Med. 2021, 10, 2058. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Kochman, M.L.; Lewis, J.D.; Ginsberg, G.G. Can EUS alone differentiate between malignant and benign cystic lesions of the pancreas? Am. J. Gastroenterol. 2001, 96, 3295–3300. [Google Scholar] [CrossRef]
- Rockacy, M.; Khalid, A. Update on pancreatic cyst fluid analysis. Ann. Gastroenterol. 2013, 26, 122–127. [Google Scholar]
- Lewandrowski, K.B.; Southern, J.F.; Pins, M.R.; Compton, C.C.; Warshaw, A.L. Cyst fluid analysis in the differential diagnosis of pancreatic cysts: A comparison of pseudocysts, serous cystadenomas, mucinous cystic neoplasms, and mucinous cystadenocarcinoma. Ann. Surg. 1993, 217, 41–47. [Google Scholar] [CrossRef]
- van der Waaij, L.A.; van Dullemen, H.M.; Porte, R.J. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: A pooled analysis. Gastrointest. Endosc. 2005, 62, 383–389. [Google Scholar] [CrossRef]
- Cizginer, S.; Turner, B.; Bilge, A.R.; Karaca, C.; Pitman, M.B.; Brugge, W.R. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas 2011, 40, 1024–1028. [Google Scholar] [CrossRef] [Green Version]
- Ngamruengphong, S.; Bartel, M.J.; Raimondo, M. Cyst carcinoembryonic antigen in differentiating pancreatic cysts: A meta-analysis. Dig. Liver Dis. 2013, 45, 920–926. [Google Scholar] [CrossRef]
- Mallik, M.K.; Qadan, L.R.; Al Naseer, A.; Al Ali, A.; Al Ansari, T.; Naquib, S.A.I.; Das, D.K.; Kapila, K. The applicability of Papanicolaou Society of Cytopathology system on reporting endoscopic ultrasound-guided fine needle aspiration cytology specimens of pancreatic lesions in situations with limited availability of ancillary tests. Experience at a single. Cytopathology 2020, 31, 564–571. [Google Scholar] [CrossRef]
- Pitman, M.B.; Centeno, B.A.; Ali, S.Z.; Genevay, M.; Stelow, E.; Mino-Kenudson, M.; Castillo, C.F.; Schmidt, C.M.; Brugge, W.R.; Layfield, L.J. Standardized terminology and nomenclature for pancreatobiliary cytology: The Papanicolaou Society of Cytopathology Guidelines. Cytojournal 2014, 44, 714. [Google Scholar] [CrossRef]
- Muthusamy, V.R.; Chandrasekhara, V.; Acosta, R.D.; Bruining, D.H.; Chathadi, K.V.; Eloubeidi, M.A.; Faulx, A.L.; Fonkalsrud, L.; Gurudu, S.R.; Khashab, M.A.; et al. The role of endoscopy in the diagnosis and treatment of cystic pancreatic neoplasms. Gastrointest. Endosc. 2016, 84, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Park, W.G.; Wu, M.; Bowen, R.; Zheng, M.; Fitch, W.L.; Pai, R.K.; Wodziak, D.; Visser, B.C.; Poultsides, G.A.; Norton, J.A.; et al. Metabolomic-derived novel cyst fluid biomarkers for pancreatic cysts: Glucose and kynurenine. Gastrointest. Endosc. 2013, 78, 295–302.e2. [Google Scholar] [CrossRef] [Green Version]
- Faias, S.; Pereira, L.; Roque, R.; Chaves, P.; Torres, J.; Cravo, M.; Pereira, A.D. Excellent Accuracy of Glucose Level in Cystic Fluid for Diagnosis of Pancreatic Mucinous Cysts. Dig. Dis. Sci. 2020, 65, 2071–2078. [Google Scholar] [CrossRef]
- Carr, R.A.; Yip-Schneider, M.T.; Simpson, R.E.; Dolejs, S.; Schneider, J.G.; Wu, H.; Ceppa, E.P.; Park, W.; Schmidt, C.M. Pancreatic cyst fluid glucose: Rapid, inexpensive, and accurate diagnosis of mucinous pancreatic cysts. Surgery 2018, 163, 600–605. [Google Scholar] [CrossRef] [Green Version]
- Morris-Stiff, G.; Lentz, G.; Chalikonda, S.; Johnson, M.; Biscotti, C.; Stevens, T.; Matthew Walsh, R. Pancreatic cyst aspiration analysis for cystic neoplasms: Mucin or carcinoembryonic antigen—Which is better? Surgery 2010, 148, 638–645. [Google Scholar] [CrossRef]
- Fu, X.; Tang, N.; Xie, W.Q.; Mao, L.; Qiu, Y.D. MUC1 promotes glycolysis through inhibiting BRCA1 expression in pancreatic cancer. Chin. J. Nat. Med. 2020, 18, 178–185. [Google Scholar] [CrossRef]
- Nissim, S.; Idos, G.E.; Wu, B. Genetic markers of malignant transformation in intraductal papillary mucinous neoplasm of the pancreas: A meta-analysis. Pancreas 2012, 41, 1195–1205. [Google Scholar] [CrossRef] [Green Version]
- Maker, A.V.; Hu, V.; Kadkol, S.S.; Hong, L.; Brugge, W.; Winter, J.; Yeo, C.J.; Hackert, T.; Büchler, M.; Lawlor, R.T.; et al. Cyst Fluid Biosignature to Predict Intraductal Papillary Mucinous Neoplasms of the Pancreas with High Malignant Potential. J. Am. Coll. Surg. 2019, 228, 721–729. [Google Scholar] [CrossRef]
- de Paredes, A.G.G.; Gleeson, F.C.; Rajan, E.; Vazquez-Sequeiros, E. Current clinical and research fluid biomarkers to aid risk stratification of pancreatic cystic lesions. Rev. Esp. Enferm. Dig. 2021, 113, 714–720. [Google Scholar] [CrossRef]
- Stigliano, S.; Zaccari, P.; Severi, C. Pancreatic intra-cystic CA 19-9 dosage in the management of pancreatic cysts: Useful or confounding? Dig. Liver Dis. 2021, 53, 131–133. [Google Scholar] [CrossRef]
- Maker, A.V.; Katabi, N.; Qin, L.X.; Klimstra, D.S.; Schattner, M.; Brennan, M.F.; Jarnagin, W.R.; Allen, P.J. Cyst fluid interleukin-1β (IL1β) levels predict the risk of carcinoma in intraductal papillary mucinous neoplasms of the pancreas. Clin. Cancer Res. 2011, 17, 1502–1508. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, S.; Cen, C.; Peng, S.; Chen, Y.; Li, X.; Diao, N.; Li, Q.; Ma, L.; Han, P. Identification of differentially expressed genes in pancreatic ductal adenocarcinoma and normal pancreatic tissues based on microarray datasets. Mol. Med. Rep. 2019, 20, 1901–1914. [Google Scholar] [CrossRef]
- Mas, L.; Lupinacci, R.M.; Cros, J.; Bachet, J.B.; Coulet, F.; Svrcek, M. Intraductal papillary mucinous carcinoma versus conventional pancreatic ductal adenocarcinoma: A comprehensive review of clinical-pathological features, outcomes, and molecular insights. Int. J. Mol. Sci. 2021, 22, 6756. [Google Scholar] [CrossRef]
- Khalid, A.; Zahid, M.; Finkelstein, S.D.; LeBlanc, J.K.; Kaushik, N.; Ahmad, N.; Brugge, W.R.; Edmundowicz, S.A.; Hawes, R.H.; McGrath, K.M. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: A report of the PANDA study. Gastrointest. Endosc. 2009, 69, 1095–1102. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Tomosugi, T.; Kimura, R.; Nakamura, S.; Miyasaka, Y.; Nakata, K.; Mori, Y.; Morita, M.; Torata, N.; Shindo, K.; et al. Clinical assessment of the GNAS mutation status in patients with intraductal papillary mucinous neoplasm of the pancreas. Surg. Today 2019, 49, 887–893. [Google Scholar] [CrossRef]
- Tamura, K.; Ohtsuka, T.; Date, K.; Fujimoto, T.; Matsunaga, T.; Kimura, H.; Watanabe, Y.; Miyazaki, T.; Ohuchida, K.; Takahata, S.; et al. Distinction of Invasive Carcinoma Derived from Intraductal Papillary Mucinous Neoplasms from Concomitant Ductal Adenocarcinoma of the Pancreas Using Molecular Biomarkers. Pancreas 2016, 45, 826–835. [Google Scholar] [CrossRef]
- McCarty, T.R.; Paleti, S.; Rustagi, T. Molecular analysis of EUS-acquired pancreatic cyst fluid for KRAS and GNAS mutations for diagnosis of intraductal papillary mucinous neoplasia and mucinous cystic lesions: A systematic review and meta-analysis. Gastrointest. Endosc. 2021, 93, 1019–1033. [Google Scholar] [CrossRef]
- Wu, J.; Jiao, Y.; Dal Molin, M.; Maitra, A.; de Wilde, R.F.; Wood, L.D.; Eshleman, J.R.; Goggins, M.G.; Wolfgang, C.L.; Canto, M.I.; et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 21188–21193. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.Y.; Wu, Y.; Jiang, Y.; Wang, P.Y.; Chen, J. RNF43 Mutations in IPMN Cases: A Potential Prognostic Factor. Gastroenterol. Res. Pract. 2020, 2020, 1457452. [Google Scholar] [CrossRef]
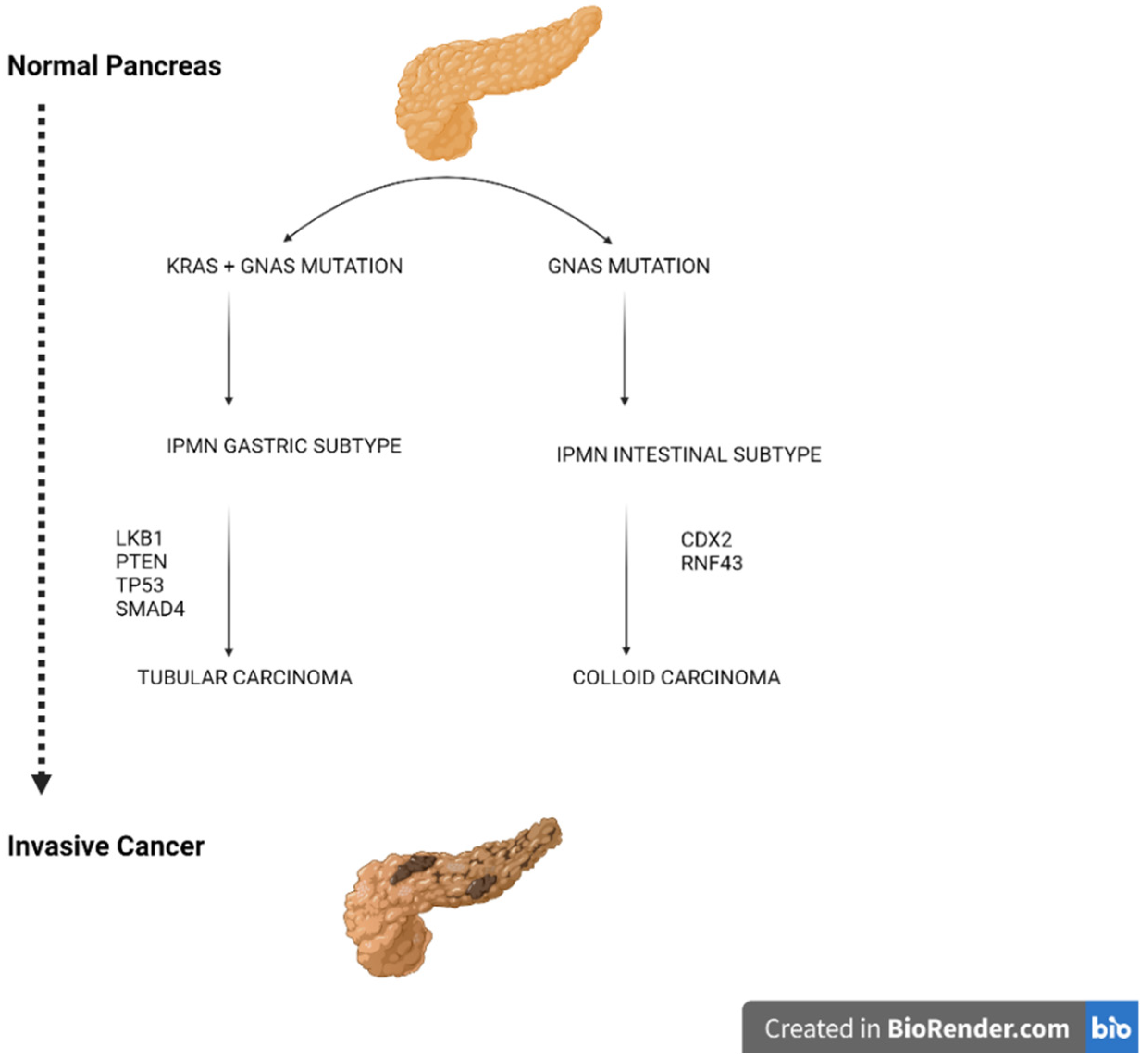
- Omori, Y.; Ono, Y.; Tanino, M.; Karasaki, H.; Yamaguchi, H.; Furukawa, T.; Enomoto, K.; Ueda, J.; Sumi, A.; Katayama, J.; et al. Pathways of Progression from Intraductal Papillary Mucinous Neoplasm to Pancreatic Ductal Adenocarcinoma Based on Molecular Features. Gastroenterology 2019, 156, 647–661. [Google Scholar] [CrossRef] [Green Version]
- Felsenstein, M.; Noë, M.; Masica, D.L.; Hosoda, W.; Chianchiano, P.; Fischer, C.G.; Lionheart, G.; Brosens, L.A.A.; Pea, A.; Yu, J.; et al. IPMNs with co-occurring invasive cancers: Neighbours but not always relatives. Gut 2018, 67, 1652–1662. [Google Scholar] [CrossRef]
- Xia, T.; Chen, X.Y.; Zhang, Y.N. MicroRNAs as biomarkers and perspectives in the therapy of pancreatic cancer. Mol. Cell. Biochem. 2021, 476, 4191–4203. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, K.Y.; Liu, S.M.; Sen, S. Tumor-Associated circulating micrornas as biomarkers of cancer. Molecules 2014, 19, 1912–1938. [Google Scholar] [CrossRef] [Green Version]
- Utomo, W.K.; Looijenga, L.H.; Bruno, M.J.; Hansen, B.E.; Gillis, A.; Biermann, K.; Peppelenbosch, M.P.; Fuhler, G.M.; Braat, H. A MicroRNA Panel in Pancreatic Cyst Fluid for the Risk Stratification of Pancreatic Cysts in a Prospective Cohort. Mol. Ther.-Nucleic Acids 2016, 5, e350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirakami, Y.; Iwashita, T.; Uemura, S.; Imai, H.; Murase, K.; Shimizu, M. Micro-RNA Analysis of Pancreatic Cyst Fluid for Diagnosing Malignant Transformation of Intraductal Papillary Mucinous Neoplasm by Comparing Intraductal Papillary Mucinous Adenoma and Carcinoma. J. Clin. Med. 2021, 10, 2249. [Google Scholar] [CrossRef]
- Shay, J.W. Role of telomeres and telomerase in aging and cancer. Cancer Discov. 2016, 6, 584–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hata, T.; Dal Molin, M.; McGregor-Das, A.; Song, T.J.; Wolfgang, C.; Eshleman, J.R.; Hruban, R.H.; Goggins, M. Simple Detection of Telomere Fusions in Pancreatic Cancer, Intraductal Papillary Mucinous Neoplasm, and Pancreatic Cyst Fluid. J. Mol. Diagn. 2018, 20, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hata, T.; Dal Molin, M.; Suenaga, M.; Yu, J.; Pittman, M.; Weiss, M.; Canto, M.I.; Wolfgang, C.; Lennon, A.M.; Hruban, R.H.; et al. Cyst fluid telomerase activity predicts the histologic grade of cystic neoplasms of the pancreas. Clin. Cancer Res. 2016, 22, 5141–5151. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell 2020, 182, 1044–1061. [Google Scholar] [CrossRef]
- Yang, K.S.; Ciprani, D.; O’Shea, A.; Liss, A.S.; Yang, R.; Fletcher-Mercaldo, S.; Mino-Kenudson, M.; Fernández-Del Castillo, C.; Weissleder, R. Extracellular Vesicle Analysis Allows for Identification of Invasive IPMN. Gastroenterology 2021, 160, 1345–1358. [Google Scholar] [CrossRef]
- Kaur, S.; Kumar, S.; Momi, N.; Sasson, A.R.; Batra, S.K. Mucins in pancreatic cancer and its microenvironment. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 607–620. [Google Scholar] [CrossRef] [Green Version]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Grützmann, R.; Niedergethmann, M.; Pilarsky, C.; Klöppel, G.; Saeger, H.D. Intraductal Papillary Mucinous Tumors of the Pancreas: Biology, Diagnosis, and Treatment. Oncologist 2010, 15, 1294–1309. [Google Scholar] [CrossRef] [Green Version]
- Crowley, E.; di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Mu, Z.; Rademaker, A.W.; Austin, L.K.; Strickland, K.S.; Costa, R.L.B.; Nagy, R.J.; Zagonel, V.; Taxter, T.J.; Behdad, A.; et al. Cell-free DNA and circulating tumor cells: Comprehensive liquid biopsy analysis in advanced breast cancer. Clin. Cancer Res. 2018, 24, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunvald, M.W.; Jacobson, R.A.; Kuzel, T.M.; Pappas, S.G.; Masood, A. Current status of circulating tumor dna liquid biopsy in pancreatic cancer. Int. J. Mol. Sci. 2020, 21, 7651. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, H.; Chen, N.; Hao, J.; Jin, H.; Ma, X. Diagnostic value of various liquid biopsy methods for pancreatic cancer: A systematic review and meta-analysis. Medicine 2020, 99, e18581. [Google Scholar] [CrossRef]
- Berger, A.W.; Schwerdel, D.; Costa, I.G.; Hackert, T.; Strobel, O.; Lam, S.; Barth, T.F.; Schröppel, B.; Meining, A.; Büchler, M.W.; et al. Detection of Hot-Spot Mutations in Circulating Cell-Free DNA from Patients with Intraductal Papillary Mucinous Neoplasms of the Pancreas. Gastroenterology 2016, 151, 267–270. [Google Scholar] [CrossRef]
- Hata, T.; Dal Molin, M.; Hong, S.M.; Tamura, K.; Suenaga, M.; Yu, J.; Sedogawa, H.; Weiss, M.J.; Wolfgang, C.L.; Lennon, A.M.; et al. Predicting the grade of dysplasia of pancreatic cystic neoplasms using cyst fluid DNA methylation markers. Clin. Cancer Res. 2017, 23, 3935–3944. [Google Scholar] [CrossRef] [Green Version]
- Mateos, R.N.; Nakagawa, H.; Hirono, S.; Takano, S.; Fukasawa, M.; Yanagisawa, A.; Yasukawa, S.; Maejima, K.; Oku-Sasaki, A.; Nakano, K.; et al. Genomic analysis of pancreatic juice DNA assesses malignant risk of intraductal papillary mucinous neoplasm of pancreas. Cancer Med. 2019, 8, 4565–4573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhi, A.D.; Wood, L.D. Early detection of pancreatic cancer using DNA-based molecular approaches. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 457–468. [Google Scholar] [CrossRef]
- Storz, P.; Crawford, H.C. Carcinogenesis of Pancreatic Ductal Adenocarcinoma. Gastroenterology 2020, 158, 2072–2081. [Google Scholar] [CrossRef]
- Nasca, V.; Chiaravalli, M.; Piro, G.; Esposito, A.; Salvatore, L.; Tortora, G.; Corbo, V.; Carbone, C. Intraductal pancreatic mucinous neoplasms: A tumor-biology based approach for risk stratification. Int. J. Mol. Sci. 2020, 21, 6386. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.C.; Bardeesy, N.; Mizukami, Y. Diversity of Precursor Lesions for Pancreatic Cancer: The Genetics and Biology of Intraductal Papillary Mucinous Neoplasm. Clin. Transl. Gastroenterol. 2017, 8, e86. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.C.; Kato, Y.; Mizukami, Y.; Widholz, S.; Boukhali, M.; Revenco, I.; Grossman, E.A.; Ji, F.; Sadreyev, R.I.; Liss, A.S.; et al. Mutant GNAS drives pancreatic tumourigenesis by inducing PKA-mediated SIK suppression and reprogramming lipid metabolism. Nat. Cell Biol. 2018, 20, 811–822. [Google Scholar] [CrossRef]
- Taki, K.; Ohmuraya, M.; Tanji, E.; Komatsu, H.; Hashimoto, D.; Semba, K.; Araki, K.; Kawaguchi, Y.; Baba, H.; Furukawa, T. GNASR201H and KrasG12D cooperate to promote murine pancreatic tumorigenesis recapitulating human intraductal papillary mucinous neoplasm. Oncogene 2016, 35, 2407–2412. [Google Scholar] [CrossRef]
- Kopp, J.L.; Dubois, C.L.; Schaeffer, D.F.; Samani, A.; Taghizadeh, F.; Cowan, R.W.; Rhim, A.D.; Stiles, B.L.; Valasek, M.; Sander, M. Loss of Pten and Activation of Kras Synergistically Induce Formation of Intraductal Papillary Mucinous Neoplasia from Pancreatic Ductal Cells in Mice. Gastroenterology 2018, 154, 1509–1523. [Google Scholar] [CrossRef]
- Balduzzi, A.; Marchegiani, G.; Pollini, T.; Biancotto, M.; Caravati, A.; Stigliani, E.; Burelli, A.; Bassi, C.; Salvia, R. Systematic review and meta-analysis of observational studies on BD-IPMNS progression to malignancy. Pancreatology 2021, 21, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Ansari, D.; Amini, J.; Edman, M.; Andersson, R. IPMN of the pancreas–does histological subtyping allow for improved stratification and follow-up? Scand. J. Gastroenterol. 2021, 56, 862–864. [Google Scholar] [CrossRef]
- Rong, Y.; Wang, D.; Xu, C.; Ji, Y.; Jin, D.; Wu, W.; Xu, X.; Kuang, T.; Lou, W. Prognostic value of histological subtype in intraductal papillary mucinous neoplasm of the pancreas. Medicine 2017, 96, e6599. [Google Scholar] [CrossRef] [PubMed]
- Aleotti, F.; Nano, R.; Piemonti, L.; Falconi, M.; Balzano, G. Total pancreatectomy sequelae and quality of life: Results of islet autotransplantation as a possible mitigation strategy. Updates Surg. 2021, 73, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Sledzianowski, J.F.; Duffas, J.P.; Muscari, F.; Suc, B.; Fourtanier, F. Risk factors for mortality and intra-abdominal morbidity after distal pancreatectomy. Surgery 2005, 137, 180–185. [Google Scholar] [CrossRef] [PubMed]
| Worrisome Features | High-Risk Stigmata |
|---|---|
|
|
| Biomarkers | Description |
|---|---|
| Ca 19.9 (>37 U/mL) | 89% sensitivity and 40% specificity in detecting degeneration. |
| CEA (>5 µg/L) | 96.4% sensitivity and 6.1% specificity in detecting degeneration. |
| NLR (>2) | 73.1% sensitivity and 58% specificity in detecting degeneration. |
| PLR | Not well-established cut-off. >200 associated in 83% to degeneration. |
| Cytological analysis | 83–99% sensitivity and 25–88% specificity in detecting degeneration. |
| Cystic fluid mucins | Overexpression of MUC1, MUC2, and MUC4 and a down expression of MUC5A are associated with degeneration. |
| Cystic fluid DNA sequencing | The presence of KRAS, GNAS, and RNF43 is associated with degeneration. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nista, E.C.; Schepis, T.; Candelli, M.; Giuli, L.; Pignataro, G.; Franceschi, F.; Gasbarrini, A.; Ojetti, V. Humoral Predictors of Malignancy in IPMN: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 12839. https://doi.org/10.3390/ijms222312839
Nista EC, Schepis T, Candelli M, Giuli L, Pignataro G, Franceschi F, Gasbarrini A, Ojetti V. Humoral Predictors of Malignancy in IPMN: A Review of the Literature. International Journal of Molecular Sciences. 2021; 22(23):12839. https://doi.org/10.3390/ijms222312839
Chicago/Turabian StyleNista, Enrico C., Tommaso Schepis, Marcello Candelli, Lucia Giuli, Giulia Pignataro, Francesco Franceschi, Antonio Gasbarrini, and Veronica Ojetti. 2021. "Humoral Predictors of Malignancy in IPMN: A Review of the Literature" International Journal of Molecular Sciences 22, no. 23: 12839. https://doi.org/10.3390/ijms222312839
APA StyleNista, E. C., Schepis, T., Candelli, M., Giuli, L., Pignataro, G., Franceschi, F., Gasbarrini, A., & Ojetti, V. (2021). Humoral Predictors of Malignancy in IPMN: A Review of the Literature. International Journal of Molecular Sciences, 22(23), 12839. https://doi.org/10.3390/ijms222312839









