Differential Effects of Low and High Radiation Dose Rates on Mouse Spermatogenesis
Abstract
:1. Introduction
2. Results
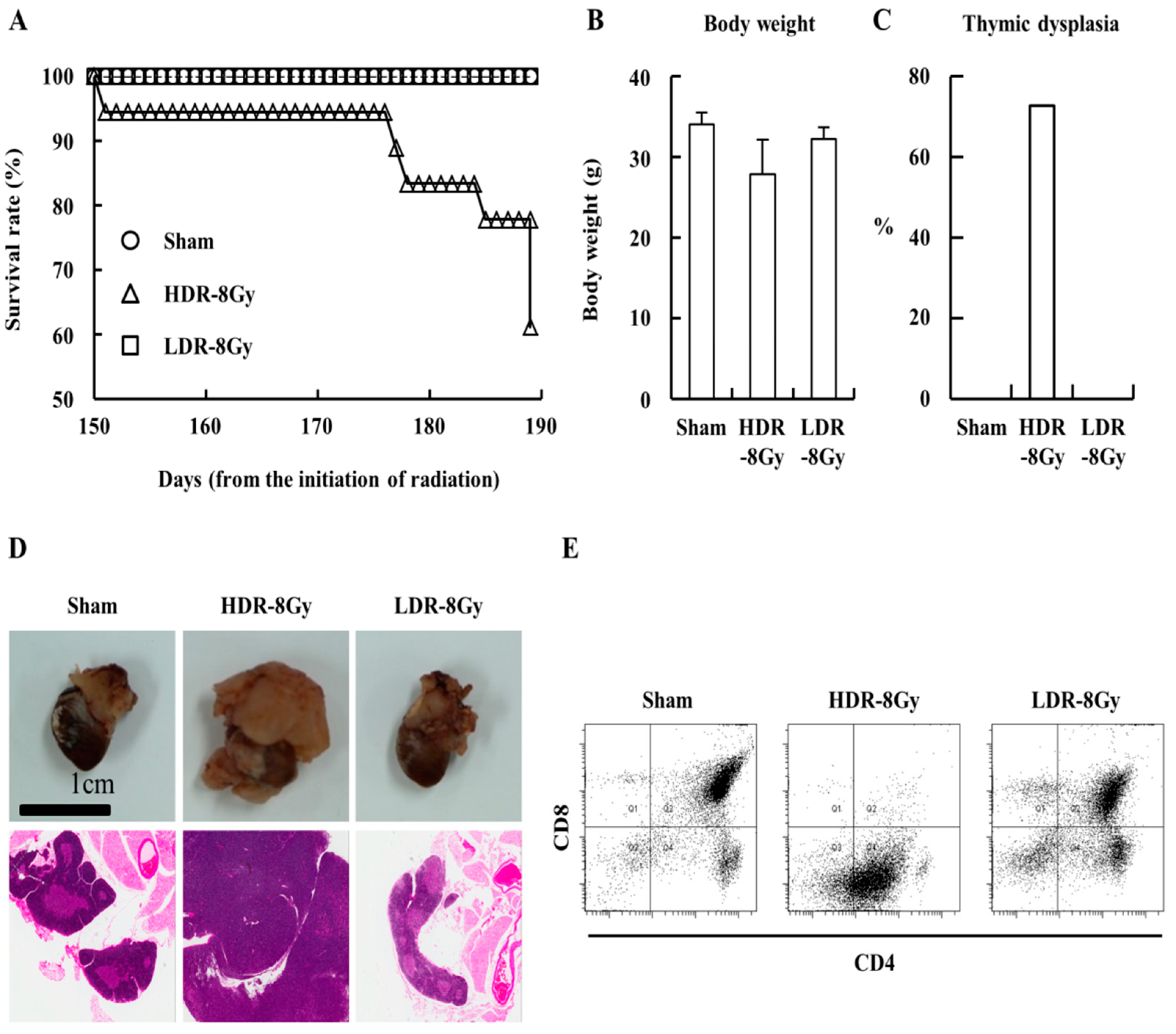
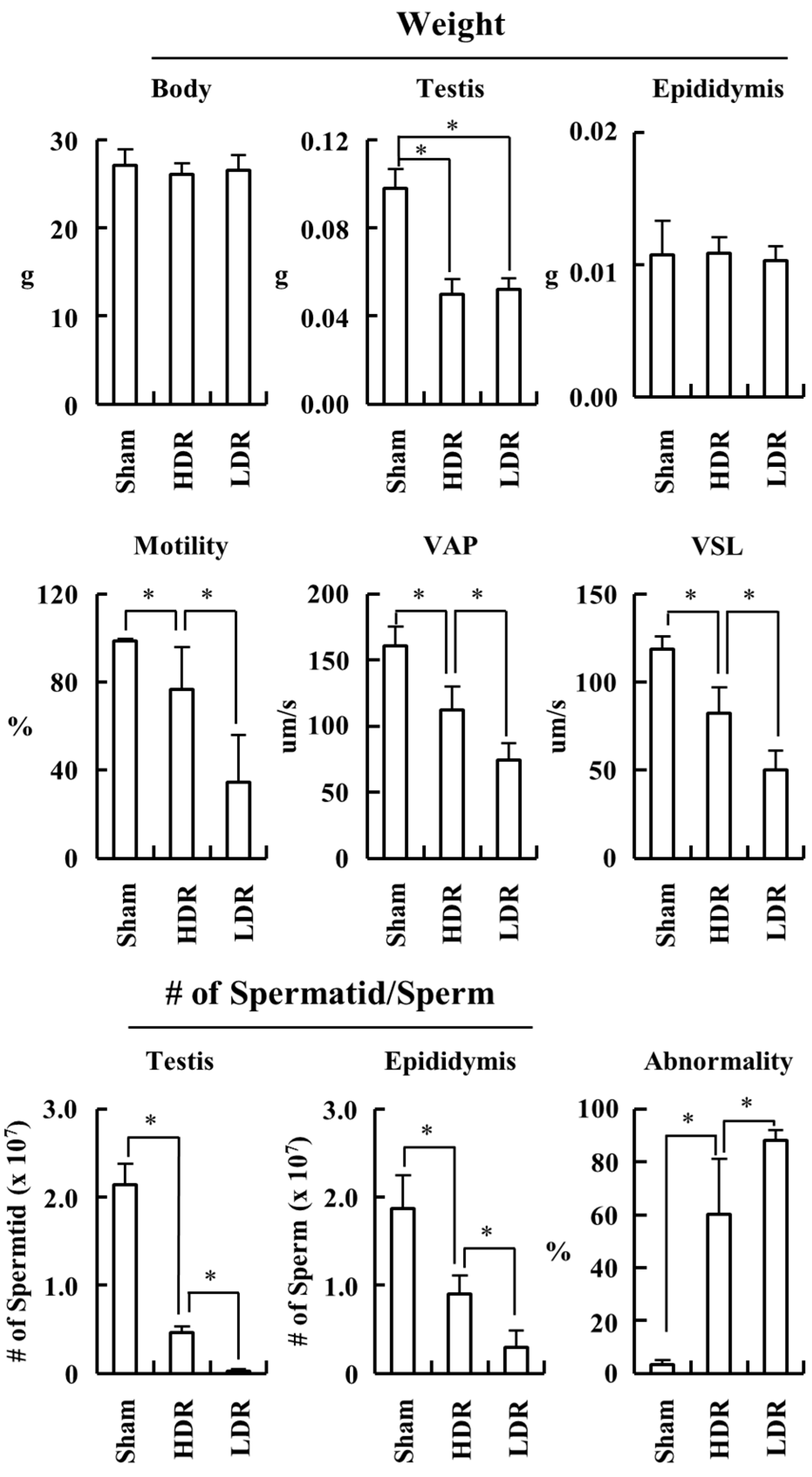
2.1. Low- and High-Dose-Rate Radiation Have Differential Effects on Mouse Organs
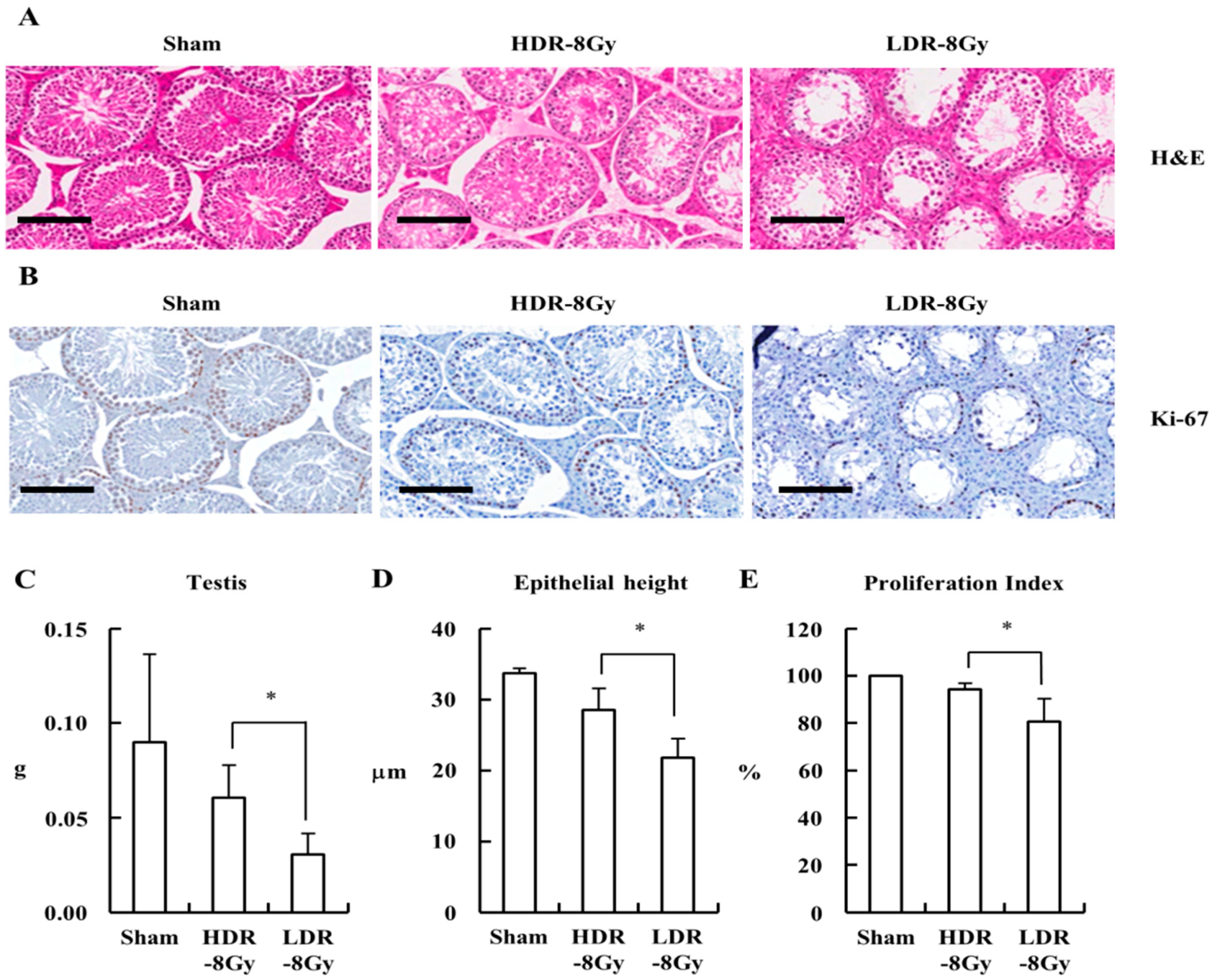
2.2. LDR Radiation Induced More Defects in Sperm Production than HDR Radiation
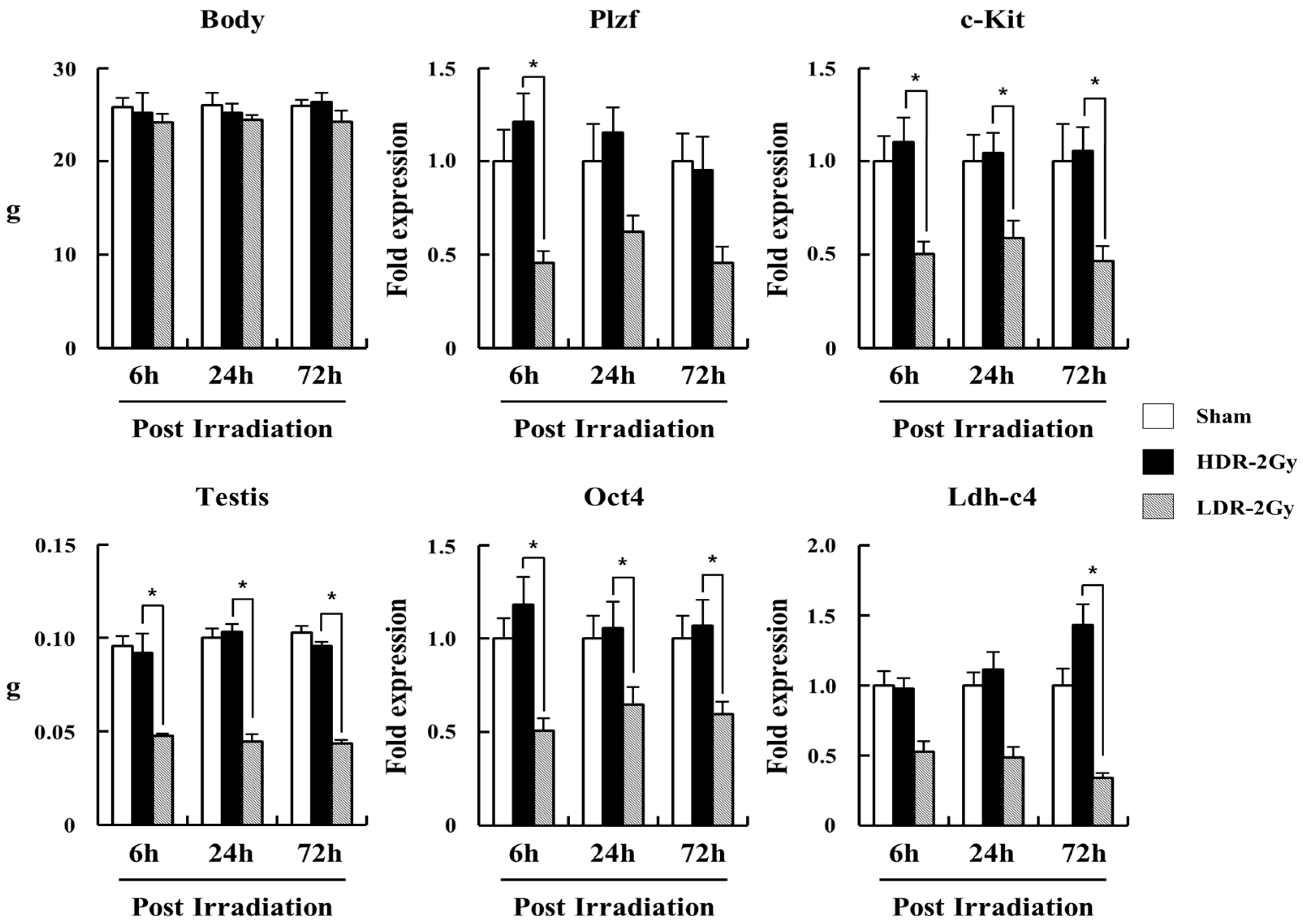
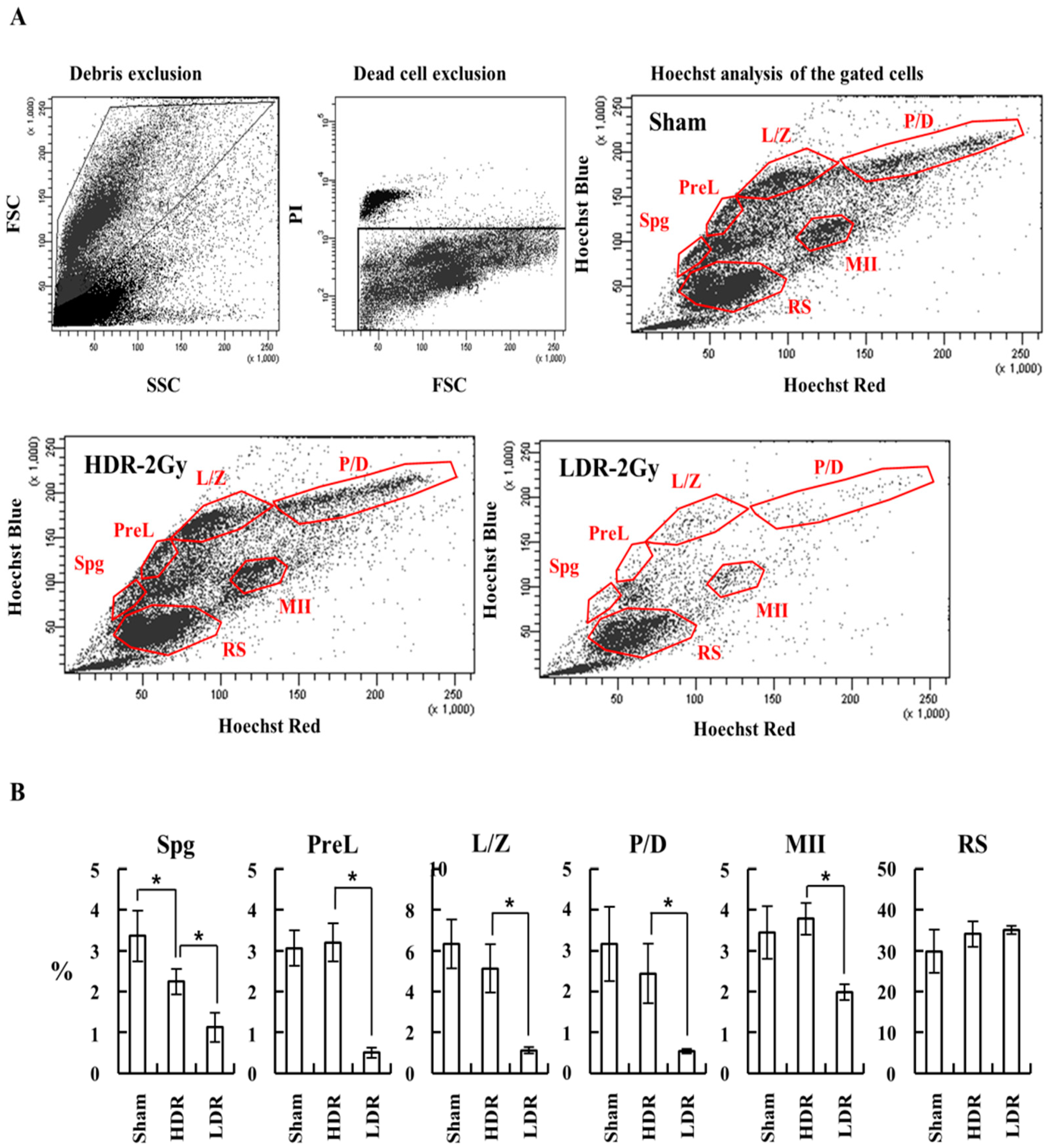
2.3. LDR Radiation Significantly Reduced Spermatogonia Cells Compared to HDR Radiation
3. Discussion
4. Materials and Methods
4.1. Animals
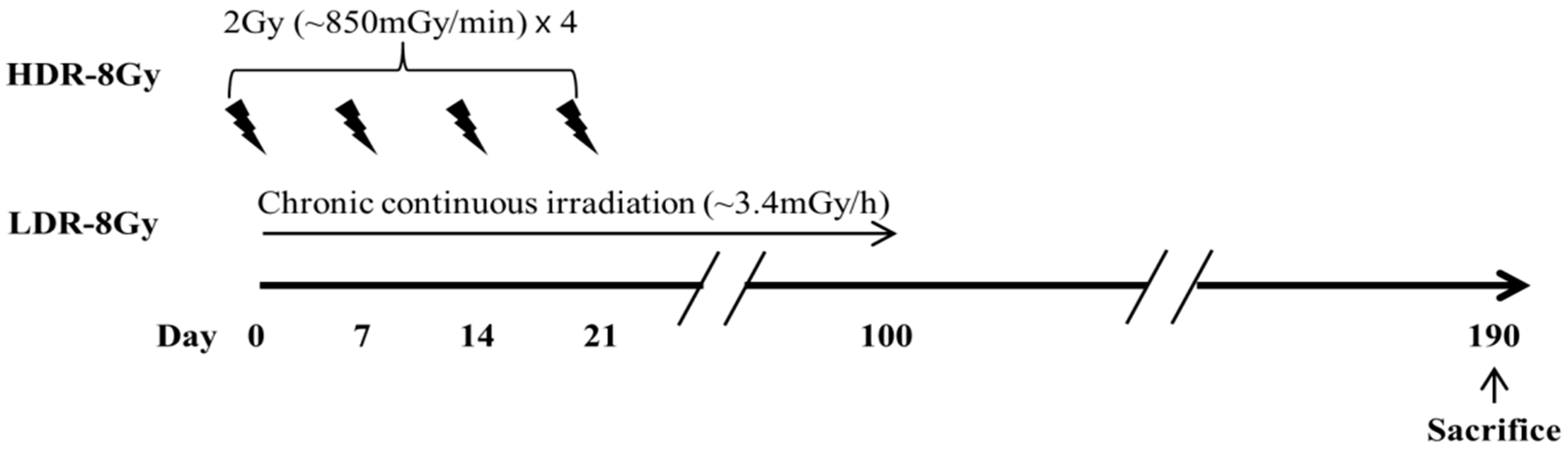
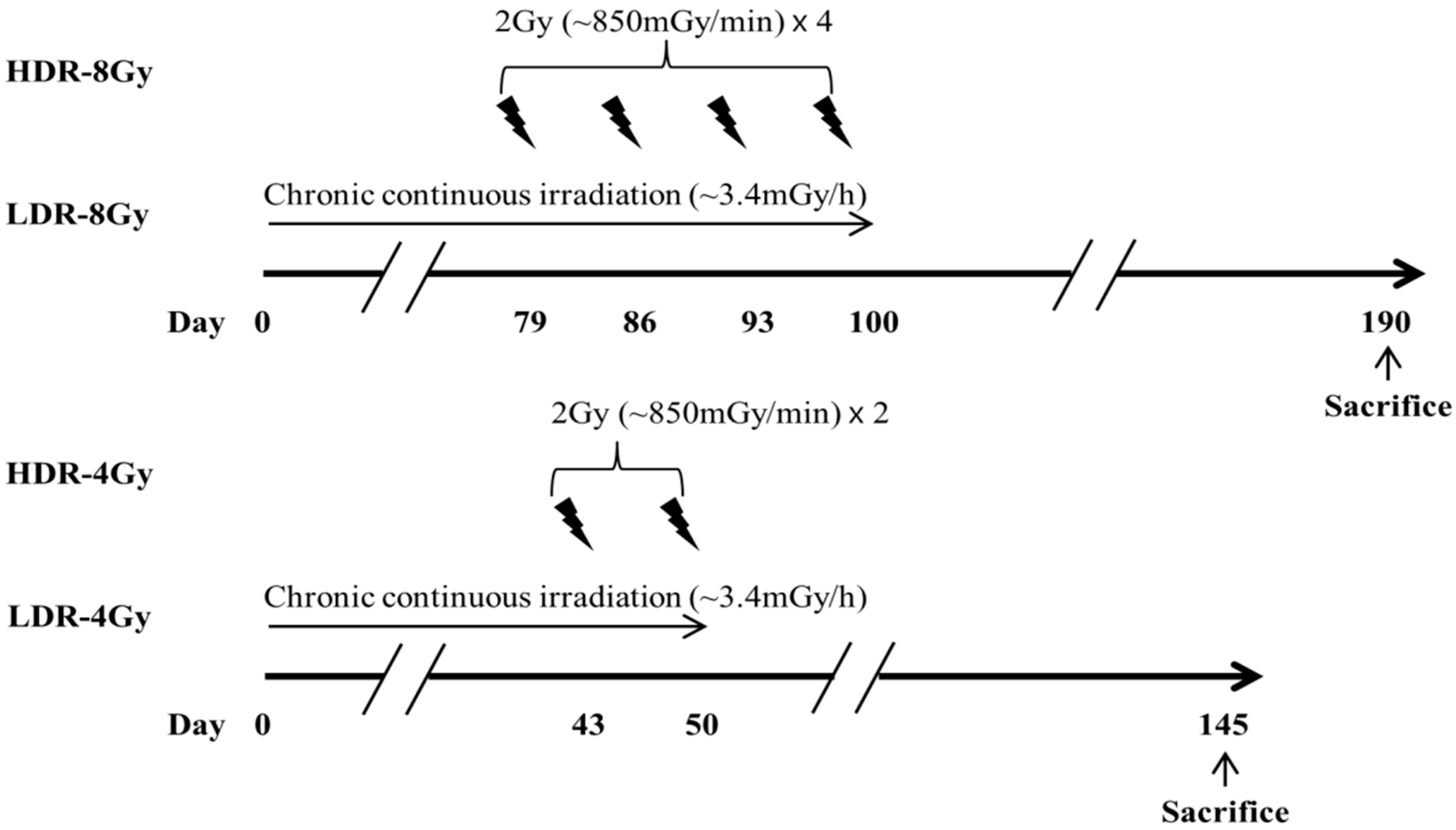
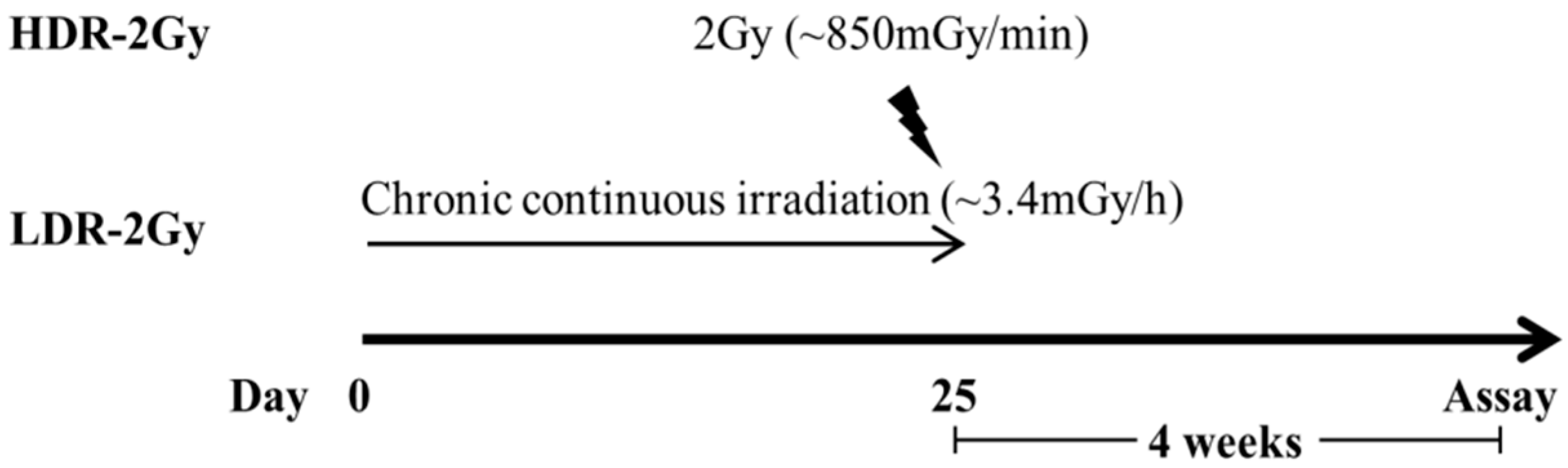
4.2. Irradiation
4.3. Evaluation of Radiation-Induced Toxicity by Histological Examination
4.4. Fluorescence-Activated Cell Sorting (FACS) Analysis of Thymocytes
4.5. Examination of Sperm Motility, Count, and Abnormality
4.6. Purification of Testicular Single Cells
4.7. FACS Analysis of Spermatogenesis Stages
4.8. qRT-PCR Analysis for Spermatogenesis Stage-Specific Markers
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hall, E.J. Weiss lecture. The dose-rate factor in radiation biology. Int. J. Radiat. Biol. 1991, 59, 595–610. [Google Scholar] [CrossRef]
- Rühm, W.; Woloschak, G.; Shore, R.E.; Azizova, T.; Grosche, B.; Niwa, O.; Akiba, S.; Ono, T.; Suzuki, K.; Iwasaki, T.; et al. Dose and dose-rate effects of ionizing radiation: A discussion in the light of radiological protection. Radiat. Environ. Biophys. 2015, 54, 379–401. [Google Scholar] [CrossRef]
- Amundson, S.A.; Chen, D.J. Inverse dose-rate effect for mutation induction by gamma-rays in human lymphoblasts. Int. J. Radiat. Biol. 1996, 69, 555–563. [Google Scholar] [CrossRef]
- Matsuya, Y.; Tsutsumi, K.; Sasaki, K.; Date, H. Evaluation of the cell survival curve under radiation exposure based on the kinetics of lesions in relation to dose-delivery time. J. Radiat. Res. 2015, 56, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, C.R.; Folkard, M.; Joiner, M.C. Effects of exposure to low-dose-rate (60)co gamma rays on human tumor cells in vitro. Radiat. Res. 2002, 158, 311–318. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bradley, S.; Goodhead, D.T.; Hill, M.A. The Influence of Dose Rate on the Induction of Chromosome Aberrations and Gene Mutation after Exposure of Plateau Phase V79-4 Cells with High-LET Alpha Particles. Radiat. Res. 2014, 182, 331–337. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Shinohara, T. Spermatogonial Stem Cell Self-Renewal and Development. Annu. Rev. Cell Dev. Biol. 2013, 29, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.T.; Gassei, K.; Orwig, K.E. Spermatogonial stem cell regulation and spermatogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1663–1678. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, G.T.; Stern, K. Reproductive aspects of cancer treatment: An update. Med. J. Aust. 1999, 170, 495–497. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, D.G.; Russell, L.D. All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 2000, 21, 776–798. [Google Scholar]
- Meistrich, M.L.; Wilson, G.; Mathur, K.; Fuller, L.M.; Rodriguez, M.A.; McLaughlin, P.; Romaguera, J.E.; Cabanillas, F.F.; Ha, C.S.; Lipshultz, L.I.; et al. Rapid recovery of spermatogenesis after mitoxantrone, vincristine, vinblastine, and prednisone chemotherapy for Hodgkin’s disease. J. Clin. Oncol. 1997, 15, 3488–3495. [Google Scholar] [CrossRef]
- Van Der Meer, Y.; Huiskamp, R.; Davids, J.A.; Van Der Tweel, I.; De Rooij, D.G. The sensitivity of quiescent and proliferating mouse spermatogonial stem cells to X irradiation. Radiat. Res. 1992, 130, 289–295. [Google Scholar] [CrossRef]
- Van Der Meer, Y.; Huiskamp, R.; Davids, J.A.; Van Der Tweel, I.; De Rooij, D.G. The sensitivity to X rays of mouse spermatogonia that are committed to differentiate and of differentiating spermatogonia. Radiat. Res. 1992, 130, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Meistrich, M.L.; Trostle, P.K.; Frapart, M.; Erickson, R.P. Biosynthesis and localization of lactate dehydrogenase X in pachytene spermatocytes and spermatids of mouse testes. Dev. Biol. 1977, 60, 428–441. [Google Scholar] [CrossRef]
- Mian, T.A.; Suzuki, N.; Glenn, H.J.; Haynie, T.P.; Meistrich, M.L. Radiation damage to mouse testis cells from [99mTc] pertechnetate. J. Nucl. Med. 1977, 18, 1116–1122. [Google Scholar]
- Van Beek, M.E.A.B.; Meistrich, M.L.; De Rooij, D.G. Probability of self-renewing divisions of spermatogonial stem cells in colonies, formed after fission neutron irradiation. Cell Prolif. 1990, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Boniver, J.; Humblet, C.; Rongy, A.M.; Delvenne, C.; Delvenne, P.; Greimers, R.; Thiry, A.; Courtoy, R.; Defresne, M.P. Cellular aspects of the pathogenesis of radiation--induced thymic lymphomas in C57 BL mice (review). Vivo 1990, 4, 41–43. [Google Scholar]
- Humblet, C.; Greimers, R.; Boniver, J.; Defrense, M.P. Stages in the development of radiation-induced thymic lymphomas in C57 BL/Ka mice: Preleukemic cells become progressively resistant to the tumor preventing effects of a bone marrow graft. Exp. Hematol. 1997, 25, 109–113. [Google Scholar]
- Muto, M.; Sado, T.; Hayata, I.; Nagasawa, F.; Kamisaku, H.; Kubo, E. Reconfirmation of indirect induction of radiogenic lymphomas using thymectomized, irradiated B10 mice grafted with neonatal thymuses from Thy 1 congenic donors. Cancer Res. 1983, 43, 3822–3827. [Google Scholar] [PubMed]
- Barrios, F.; Filipponi, D.; Campolo, F.; Gori, M.; Bramucci, F.; Pellegrini, M.; Ottolenghi, S.; Rossi, P.; Jannini, E.A.; Dolci, S. SOHLH1 and SOHLH2 control Kit expression during postnatal male germ cell development. J. Cell Sci. 2012, 125 Pt 6, 1455–1464. [Google Scholar]
- Filipponi, D.; Hobbs, R.M.; Ottolenghi, S.; Rossi, P.; Jannini, E.A.; Pandolfi, P.P.; Dolci, S. Repression of kit Expression by Plzf in Germ Cells. Mol. Cell. Biol. 2007, 27, 6770–6781. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, E.; Eddy, E.M.; Duan, C.; Odet, F. LDHC: The Ultimate Testis-Specific Gene. J. Androl. 2009, 31, 86–94. [Google Scholar] [CrossRef]
- Fukunaga, H.; Butterworth, K.; Yokoya, A.; Ogawa, T.; Prise, K.M. Low-dose radiation-induced risk in spermatogenesis. Int. J. Radiat. Biol. 2017, 93, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Grewenig, A.; Schuler, N.; Rübe, C.E. Persistent DNA Damage in Spermatogonial Stem Cells After Fractionated Low-Dose Irradiation of Testicular Tissue. Int. J. Radiat. Oncol. 2015, 92, 1123–1131. [Google Scholar] [CrossRef]
- Qi, L.; Li, J.; Le, W.; Zhang, J. Low-dose ionizing irradiation triggers apoptosis of undifferentiated spermatogonia in vivo and in vitro. Transl. Androl. Urol. 2019, 8, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, A.; Skrzypek, M.; Stec, M.; Panasiuk, L. Effect of ionizing radiation on the male reproductive system. Ann. Agric. Environ. Med. 2019, 26, 210–216. [Google Scholar] [CrossRef]
- Vilenchik, M.M.; Knudson, A.G. Inverse radiation dose-rate effects on somatic and germ-line mutations and DNA damage rates. Proc. Natl. Acad. Sci. USA 2000, 97, 5381–5386. [Google Scholar] [CrossRef] [Green Version]
- Gong, E.J.; Shin, I.S.; Son, T.G.; Yang, K.; Heo, K.; Kim, J.S. Low-dose-rate radiation exposure leads to testicular damage with decreases in DNMT1 and HDAC1 in the murine testis. J. Radiat. Res. 2014, 55, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.; Heo, K.; Bae, M.J.; Lee, C.G.; Cho, W.S.; Kim, S.D.; Yang, K.; Shin, I.S.; Lee, M.Y.; Kim, J.S. Injury to the blood-testis barrier after low-dose-rate chronic radiation exposure in mice. Radiat. Prot. Dosim. 2015, 167, 316–320. [Google Scholar] [CrossRef]
- Hou, W.-G.; Zhao, J.; Li, Z.; Li, W.; Li, T.; Xiong, L.-Z.; Zhang, Y.-Q. Effects of Electromagnetic Pulse Irradiation on the Mouse Blood-testicle Barrier. Urology 2012, 80, 225.e1–225.e6. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.C.; Kang, Y.M.; Jin, Y.W.; Kim, H.S. Relative morphological abnormalities of sperm in the caudal epididymis of high- and low-dose-rate gamma-irradiated ICR mice. J. Radiat. Res. 2009, 50, 261–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.; Son, Y.; Jang, H.; Bae, M.J.; Kim, J.; Kang, D.; Kim, J.S. Protective Effect of Administered Rolipram against Radiation-Induced Testicular Injury in Mice. World J. Men’s Health 2015, 33, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvajal, G.; Brukman, N.G.; Muñoz, M.W.; Battistone, M.A.; Guazzone, V.A.; Ikawa, M.; Haruhiko, M.; Lustig, L.; Breton, S.; Cuasnicu, P.S. Impaired male fertility and abnormal epididymal epithelium differentiation in mice lacking CRISP1 and CRISP4. Sci. Rep. 2018, 8, 17531. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.; Marquez, B.; Suarez, S.; Schimenti, J. Sperm Motility Defects and Infertility in Male Mice with a Mutation in Nsun7, a Member of the Sun Domain-Containing Family of Putative RNA Methyltransferases1. Biol. Reprod. 2007, 77, 376–382. [Google Scholar] [CrossRef] [Green Version]
- Rasgele, P.G. Abnormal sperm morphology in mouse germ cells after short-term exposures to acetamiprid, propineb, and their mixture. Arch. Ind. Hyg. Toxicol. 2014, 65, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Gaysinskaya, V.; Soh, I.Y.; van der Heijden, G.W.; Bortvin, A. Optimized flow cytometry isolation of murine spermatocytes. Cytom. Part A 2014, 85, 556–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| % | Small Head | Amorphous Head | Two Head | Excessive Hook | Blunt Hook | Folded Tail | Short Tail | Two Tail | Detached Tail | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sham | 0 | 0 | 0 | 0 | 0 | 0.1 ± 0.2 | 0 | 0 | 3.1 ± 1.9 | |
| 2 Gy | HDR | 0.1 ± 0.2 | 9.3 ± 6.3 | 0 | 0 | 0 | 4.6 ± 2.4 | 0.7 ± 1.0 | 0.3 ± 0.6 | 45.1 ± 12.7 |
| LDR | 0.4 ± 0.4 | 12.8 ± 4.7 | 0 | 0 | 0 | 2.6 ± 1.2 | 1.3 ± 0.7 | 1.2 ± 2.2 | 70.0 ± 3.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, M.J.; Kang, M.K.; Kye, Y.U.; Baek, J.-H.; Sim, Y.-J.; Lee, H.-J.; Kang, Y.-R.; Jo, W.S.; Kim, J.S.; Lee, C.G. Differential Effects of Low and High Radiation Dose Rates on Mouse Spermatogenesis. Int. J. Mol. Sci. 2021, 22, 12834. https://doi.org/10.3390/ijms222312834
Bae MJ, Kang MK, Kye YU, Baek J-H, Sim Y-J, Lee H-J, Kang Y-R, Jo WS, Kim JS, Lee CG. Differential Effects of Low and High Radiation Dose Rates on Mouse Spermatogenesis. International Journal of Molecular Sciences. 2021; 22(23):12834. https://doi.org/10.3390/ijms222312834
Chicago/Turabian StyleBae, Min Ji, Min Kook Kang, Yong Uk Kye, Jeong-Hwa Baek, Ye-Ji Sim, Hae-June Lee, Yeong-Rok Kang, Wol Soon Jo, Joong Sun Kim, and Chang Geun Lee. 2021. "Differential Effects of Low and High Radiation Dose Rates on Mouse Spermatogenesis" International Journal of Molecular Sciences 22, no. 23: 12834. https://doi.org/10.3390/ijms222312834






