The Role of Membrane Transporters in Plant Growth and Development, and Abiotic Stress Tolerance
Abstract
:1. Introduction
2. Role of Membrane Transporters in Plants Growth and Development
2.1. Plant Architecture, Seed Yield and Quality
2.2. Ionic Homeostasis, Detoxification and Transportation of Mineral Elements
2.3. Remobilization of Photosynthates
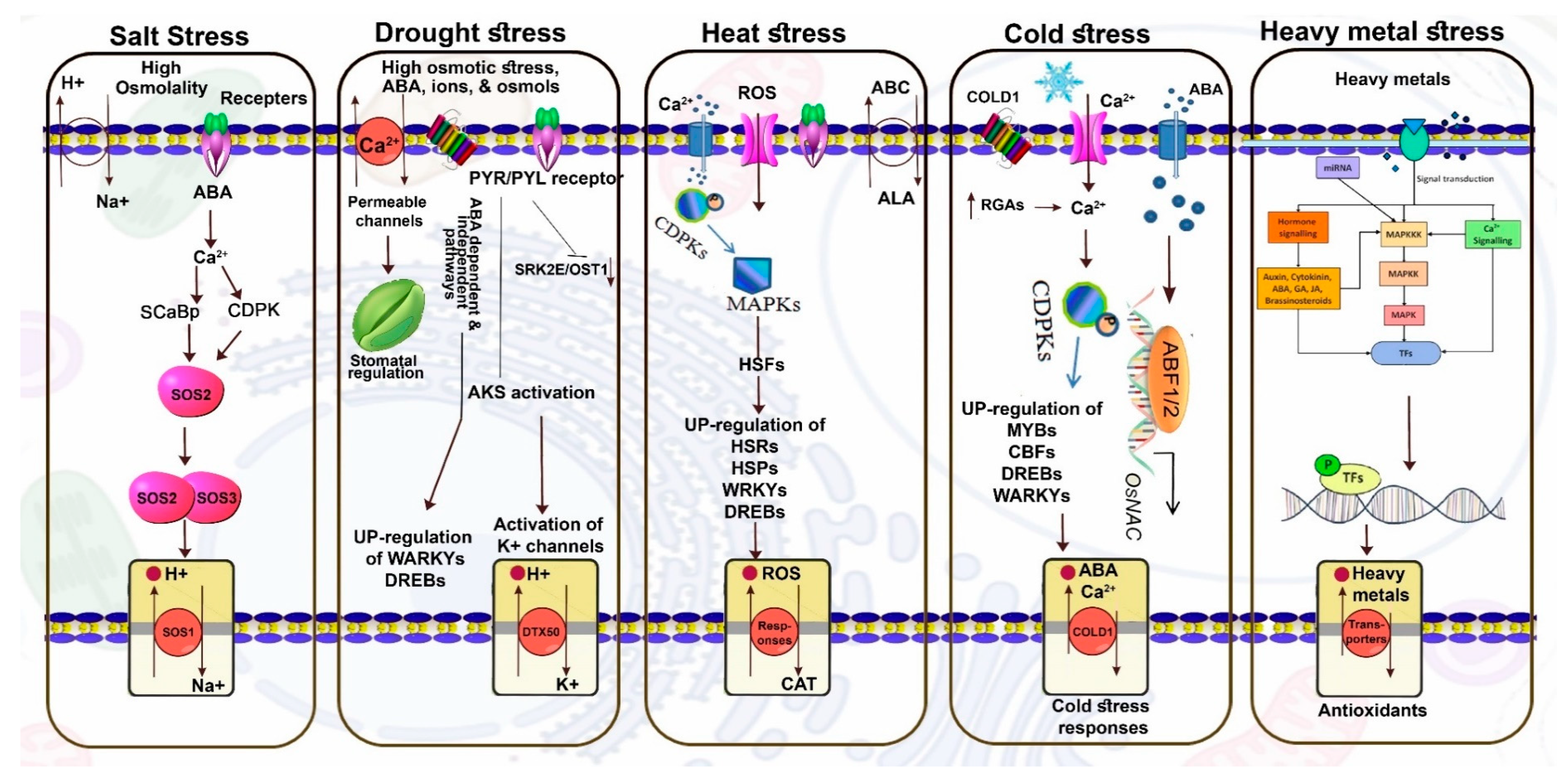
3. Membrane Transporters and Abiotic Stresses
3.1. Salt Stress
3.2. Drought
3.3. Heat Stress
3.4. Cold Stress
3.5. HMs Stress
| Abiotic Stress | Transporter Protein | Plant Species | Tissue/Organ | Biological Role | Ref. |
|---|---|---|---|---|---|
| Salt | AlHKT2;1 | A. lagopoides | Leaf/shoot /root | Na+/K+ co-transporter gene prevents plants from salinity stress | [62] |
| OsCam1–1 | O. sativa | Leaf | It is involved in signaling, hormone-mediated regulation, transcription, lipid, carbohydrate and secondary metabolism, photosynthesis, glycolysis, TCA and glyoxylate cycle under salt stress | [111] | |
| AtFC1 | A. thaliana | Roots, cotyledon, root, shoot, leaf and flower | It enhances K+ accumulation and prevents cell membrane lysis; it also upregulates the expression levels of NHX1 and AVP1 | [56] | |
| ATG8 | A. thaliana | Root/cortex cells | It plays a role in nutrient remobilization following salt induced autophagy | [112] | |
| PRE1/AAP1 | A. thaliana | Root | It enhanced uptake and transportation of proline and prevented proline degradation | [113] | |
| OsAKT1 | O. sativa | Root/elongation zone and shoot | Retain K+ in root to balance Na+/K+ ratio | [59] | |
| ZmHKT1;5 | Z. mays | Leaf | Balances Na+/K+ ratio and improves plant growth | [60] | |
| Drought | CrPIP2;3 | A. thaliana | Germinating seed, seedling and root | It plays pivotal roles in maintaining water and nutrition homeostasis | [67] |
| PIP1;5/PIP2;3 | S. bicolor | Root and leaf | Maintains WUE | [66] | |
| H+-ATPase | C. sinensis | Leaf | Maintenance of K+ homeostasis in mesophyll cells | [114] | |
| OsHAK1 | O. sativa | Root and shoot | Involved in K acquisition, translocation and homeostasis by upregulating OsTPKb and OsAKT1 | [68] | |
| OsNAC5/6/9/10 | O. sativa | Root | Target genes were involved in transmembrane/transporter activity, carbohydrate metabolism, vesicle and plant hormones | [115] | |
| Cold | HSP70-16/VDAC3 | A. thaliana | Seed, endosperm and embryo | Activation of the opening of VDAC3 ion channels, ABA transportation from endosperm to embryo and then inhibits seed germination | [116] |
| CsSWEET16 | A. thaliana | Leaf and flower buds | Sugar transport across vacuoles and cold tolerance | [42] | |
| TsABCG11 | A. thaliana | Root, stem leaf, rosette leaf, flower and silique | Thickening the leaf cuticle layer (wax and cutin) by exporting cuticle lipid molecules to prevent plants from cold stress | [92] | |
| AlTMP2 | A. littoralis | Root and leaf | Improves membrane stability | [117] | |
| AtPIP1;4/AtPIP2;5 | A. thaliana | Root and shoot | Plays a role in cold acclimation and freezing tolerance | [91] | |
| VAB3/NHX2/NHX5 | E. botschantzevii | Shoot | Cold acclimation | [118] | |
| SOS1/VP2/HA3 | E. salsugineum | Shoot | Cold acclimation | [118] | |
| Heat | TaZnFP | A. thaliana | 14-day seedling | Larger primary roots, more lateral branches, increased in leaf size and numbers, promotes early flowering and enhanced fresh biomass | [16] |
| P4-type ATPase | A. thaliana | 14-day seedling, rosette leaf, flower (stamen and pistil) and silique | It is involved in flipping lipids that cope with heat stress | [76] | |
| OsSUS | O. sativa | Flag leaf, stem-sheath and spikelet | It acts as a signalling molecule to mediate source and sink relationships under heat stress | [119] | |
| HMs | TpNRAMP3 | T. polonicum, Polish wheat | Leaf and root | Transport Cd, Co and Mn but does not transport Fe or Zn, which induced HM toxicity | [21] |
| PtABCC1 | P. trichocarpa/A. thaliana | Root | It enhances the accumulation and tolerance to Hg | [101] | |
| PtoABCG36 | A. thaliana | Leaf/stem/root | It acts as an extrusion pump to decrease Cd uptake and enhance tolerance to Cd stress | [102] | |
| PtoABCG36 | O. sativa | Root and shoot | Export Cd from root and enhance Cd tolerance | [103] | |
| OsSMP1 | O. sativa | Leaf | Acts as a positive regulator of Cd and Cu tolerance via ABA-dependent pathway | [100] | |
| LmSAP | N. tabacum | Leaf and root | Enhanced accumulation of Cu, Cd and Mn, decreased H2O2 content, upregulated SOD, POD and CAT activities and stress related metallothioneins, i.e., Met1-5 | [120] | |
| AtCNGC1/10/13/19 | A. thaliana | Primary root and seedling | Plays a role Pb toxicity by reducing its uptake | [121] | |
| AtCNGC11/13/16/20 | A. thaliana | Primary root and seedling | Plays a role Cd toxicity by reducing its uptake | [121] | |
| SaNramp6 | A. thaliana | Root, stem and leaf | Improves Cd accumulation | [122] | |
| OsLCT1/OsHMA2/ OsZIP3 | O. sativa | Root and shoot | Co-expression of HM transporters improved root and shoot lengths under Zn and Cd stress | [123] |
4. Closing Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef] [Green Version]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant. 2021, 171, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Jogawat, A.; LalNITRATE, S.K.; Lakra, N.; Mehta, S.; Shabek, N.; Narayan, O.P. Plant mineral transport systems and the potential for crop improvement. Planta 2021, 253, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Misra, V.; Mall, A. Plant sugar transporters and their role in abiotic stress. In Transporters and Plant Osmotic Stress; Elsevier: Amsterdam, The Netherlands, 2021; pp. 101–112. [Google Scholar]
- Schroeder, J.I.; Delhaize, E.; Frommer, W.B.; Guerinot, M.L.; Harrison, M.J.; Herrera-Estrella, L.; Horie, T.; Kochian, L.V.; Munns, R.; Nishizawa, N.K. Using membrane transporters to improve crops for sustainable food production. Nature 2013, 497, 60–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawat, N.; Singla-Pareek, S.L.; Pareek, A. Membrane dynamics during individual and combined abiotic stresses in plants and tools to study the same. Physiol. Plant. 2021, 171, 653–676. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Cai, Z.; Xia, K.; Wang, Y.; Duan, J.; Zhang, M. Identification and analysis of eight peptide transporter homologs in rice. Plant Sci. 2010, 179, 374–382. [Google Scholar] [CrossRef]
- Fan, X.; Xie, D.; Chen, J.; Lu, H.; Xu, Y.; Ma, C.; Xu, G. Over-expression of OsPTR6 in rice increased plant growth at different nitrogen supplies but decreased nitrogen use efficiency at high ammonium supply. Plant Sci. 2014, 227, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.; Chen, Y.; Chen, F.; Ji, Y.; Zhao, F.-J. OsPTR7 (OsNPF8. 1), a putative peptide transporter in rice, is involved in dimethylarsenate accumulation in rice grain. Plant Cell Physiol. 2017, 58, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liesche, J. Sugar export from Arabidopsis leaves: Actors and regulatory strategies. J. Exp. Bot. 2021, 72, 5275–5284. [Google Scholar] [CrossRef]
- Rottmann, T.M.; Fritz, C.; Lauter, A.; Schneider, S.; Fischer, C.; Danzberger, N.; Dietrich, P.; Sauer, N.; Stadler, R. Protoplast-esculin assay as a new method to assay plant sucrose transporters: Characterization of AtSUC6 and AtSUC7 sucrose uptake activity in Arabidopsis Col-0 ecotype. Front. Plant Sci. 2018, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zhang, Y.; Ruan, B.; Guo, L.; Zeng, D.; Gao, Z.; Zhu, L.; Hu, J.; Ren, D.; Yu, L. OsHAK1 controls the vegetative growth and panicle fertility of rice by its effect on potassium-mediated sugar metabolism. Plant Sci. 2018, 274, 261–270. [Google Scholar] [CrossRef]
- Bazihizina, N.; Colmer, T.D.; Cuin, T.A.; Mancuso, S.; Shabala, S. Friend or foe? Chloride patterning in halophytes. Trends Plant Sci. 2019, 24, 142–151. [Google Scholar] [CrossRef]
- Assaha, D.V.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Mathan, J.; Singh, A.; Ranjan, A. Sucrose transport in response to drought and salt stress involves ABA-mediated induction of OsSWEET13 and OsSWEET15 in rice. Physiol. Plant. 2021, 171, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Khurana, P. Characterization of a novel zinc finger transcription factor (TaZnF) from wheat conferring heat stress tolerance in Arabidopsis. Cell Stress Chaperones 2018, 23, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Zhao, K.X.; Yang, Z.M. Identification of genomic ATP binding cassette (ABC) transporter genes and Cd-responsive ABCs in Brassica napus. Gene 2018, 664, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.A.; Ali, B.; Yang, S.; Tong, C.; Islam, F.; Gill, M.B.; Mwamba, T.M.; Ali, S.; Mao, B.; Liu, S. Reduced Glutathione Mediates Pheno-Ultrastructure, Kinome and Transportome in Chromium-Induced Brassica napus L. Front. Plant Sci. 2017, 8, 2037. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Xu, L.; Wang, Y.; Dong, J.; Zhang, X.; Wang, K.; Ying, J.; Li, C.; Liu, L. Melatonin-induced DNA demethylation of metal transporters and antioxidant genes alleviates lead stress in radish plants. Hortic. Res. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, F.-J. The roles of membrane transporters in arsenic uptake, translocation and detoxification in plants. Crit. Rev. Environ. Sci. Technol. 2020, 1–36. [Google Scholar] [CrossRef]
- Peng, F.; Wang, C.; Cheng, Y.; Kang, H.; Fan, X.; Sha, L.; Zhang, H.; Zeng, J.; Zhou, Y.; Wang, Y. Cloning and characterization of TpNRAMP3, a metal transporter from polish wheat (Triticum polonicum L.). Front. Plant Sci. 2018, 9, 1354. [Google Scholar] [CrossRef]
- Wang, J.; Lu, K.; Nie, H.; Zeng, Q.; Wu, B.; Qian, J.; Fang, Z. Rice nitrate transporter OsNPF7. 2 positively regulates tiller number and grain yield. Rice 2018, 11, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Z.; Bai, G.; Huang, W.; Wang, Z.; Wang, X.; Zhang, M. The rice peptide transporter OsNPF7. 3 is induced by organic nitrogen, and contributes to nitrogen allocation and grain yield. Front. Plant Sci. 2017, 8, 1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, L.; Zhu, Y.; Fan, T.; Peng, C.; Wang, J.; Sun, L.; Chen, C. OsZIP7 functions in xylem loading in roots and inter-vascular transfer in nodes to deliver Zn/Cd to grain in rice. Biochem. Biophys. Res. Commun. 2019, 512, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Senoura, T.; Sakashita, E.; Kobayashi, T.; Takahashi, M.; Aung, M.S.; Masuda, H.; Nakanishi, H.; Nishizawa, N.K. The iron-chelate transporter OsYSL9 plays a role in iron distribution in developing rice grains. Plant Mol. Biol. 2017, 95, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Peris-Peris, C.; Serra-Cardona, A.; Sánchez-Sanuy, F.; Campo, S.; Ariño, J.; San Segundo, B. Two NRAMP6 isoforms function as iron and manganese transporters and contribute to disease resistance in rice. Mol. Plant-Microbe Interact. 2017, 30, 385–398. [Google Scholar] [CrossRef] [Green Version]
- Luan, M.; Tang, R.-j.; Tang, Y.; Tian, W.; Hou, C.; Zhao, F.; Lan, W.; Luan, S. Transport and homeostasis of potassium and phosphate: Limiting factors for sustainable crop production. J. Exp. Bot. 2017, 68, 3091–3105. [Google Scholar] [CrossRef] [Green Version]
- Ai, P.; Sun, S.; Zhao, J.; Fan, X.; Xin, W.; Guo, Q.; Yu, L.; Shen, Q.; Wu, P.; Miller, A.J. Two rice phosphate transporters, OsPht1; 2 and OsPht1; 6, have different functions and kinetic properties in uptake and translocation. Plant J. 2009, 57, 798–809. [Google Scholar] [CrossRef]
- Huang, K.-L.; Wang, H.; Wei, Y.-L.; Jia, H.-X.; Zha, L.; Zheng, Y.; Ren, F.; Li, X.-B. The high-affinity transporter BnPHT1; 4 is involved in phosphorus acquisition and mobilization for facilitating seed germination and early seedling growth of Brassica napus. BMC Plant Biol. 2019, 19, 1–13. [Google Scholar] [CrossRef]
- Cai, H.; Huang, S.; Che, J.; Yamaji, N.; Ma, J.F. The tonoplast-localized transporter OsHMA3 plays an important role in maintaining Zn homeostasis in rice. J. Exp. Bot. 2019, 70, 2717–2725. [Google Scholar] [CrossRef] [Green Version]
- Bashir, K.; Ishimaru, Y.; Nishizawa, N.K. Molecular mechanisms of zinc uptake and translocation in rice. Plant Soil 2012, 361, 189–201. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Ma, J.F. Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J. Exp. Bot. 2014, 65, 6013–6021. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.F.; Xia, J.; Yamaji, N.; Shen, R.F.; Ma, J.F. Effective reduction of cadmium accumulation in rice grain by expressing OsHMA3 under the control of the OsHMA2 promoter. J. Exp. Bot. 2018, 69, 2743–2752. [Google Scholar] [CrossRef]
- Liang, J.; He, Y.; Zhang, Q.; Wang, W.; Zhang, Z. Plasma membrane Ca2+ permeable mechanosensitive channel OsDMT1 is involved in regulation of plant architecture and ion homeostasis in rice. Int. J. Mol. Sci. 2020, 21, 1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajeesh Krishna, T.; Maharajan, T.; Victor Roch, G.; Ignacimuthu, S.; Antony Ceasar, S. Structure, function, regulation and phylogenetic relationship of ZIP family transporters of plants. Front. Plant Sci. 2020, 11, 662. [Google Scholar] [CrossRef]
- Li, H.; Liu, C.; Zhou, L.; Zhao, Z.; Li, Y.; Qu, M.; Huang, K.; Zhang, L.; Lu, Y.; Cao, M. Molecular and functional characterization of the magnesium transporter gene ZmMGT12 in maize. Gene 2018, 665, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Liu, X.-d.; Xie, Q.; He, Z.-h. Two faces of one seed: Hormonal regulation of dormancy and germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Née, G.; Xiang, Y.; Soppe, W.J. The release of dormancy, a wake-up call for seeds to germinate. Curr. Opin. Plant Biol. 2017, 35, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, X.; Zhang, H.; Wang, S.; Ye, X.; Shi, L.; Xu, F.; Ding, G. Molecular identification of the phosphate transporter family 1 (PHT1) genes and their expression profiles in response to phosphorus deprivation and other abiotic stresses in Brassica napus. PLoS ONE 2019, 14, e0220374. [Google Scholar] [CrossRef] [Green Version]
- Leach, K.A.; Tran, T.M.; Slewinski, T.L.; Meeley, R.B.; Braun, D.M. Sucrose transporter2 contributes to maize growth, development, and crop yield. J. Integr. Plant Biol. 2017, 59, 390–408. [Google Scholar] [CrossRef] [Green Version]
- Jeena, G.S.; Kumar, S.; Shukla, R.K. Structure, evolution and diverse physiological roles of SWEET sugar transporters in plants. Plant Mol. Biol. 2019, 100, 351–365. [Google Scholar] [CrossRef]
- Wang, L.; Yao, L.; Hao, X.; Li, N.; Qian, W.; Yue, C.; Ding, C.; Zeng, J.; Yang, Y.; Wang, X. Tea plant SWEET transporters: Expression profiling, sugar transport, and the involvement of CsSWEET16 in modifying cold tolerance in Arabidopsis. Plant Mol. Biol. 2018, 96, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Ma, H.; Feng, S.; Gong, S.; Wang, J. A novel sugar transporter from Dianthus spiculifolius, DsSWEET12, affects sugar metabolism and confers osmotic and oxidative stress tolerance in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Lee, S.-K.; Yoo, Y.; Wei, J.; Kwon, S.-Y.; Lee, S.-W.; Jeon, J.-S.; An, G. Rice transcription factor OsDOF11 modulates sugar transport by promoting expression of sucrose transporter and SWEET genes. Mol. Plant 2018, 11, 833–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.-L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [Green Version]
- Durand, M.; Mainson, D.; Porcheron, B.; Maurousset, L.; Lemoine, R.; Pourtau, N. Carbon source–sink relationship in Arabidopsis thaliana: The role of sucrose transporters. Planta 2018, 247, 587–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, K.; Osakabe, Y.; Yamaguchi-Shinozaki, K. A C-terminal motif contributes to the plasma membrane localization of Arabidopsis STP transporters. PLoS ONE 2017, 12, e0186326. [Google Scholar]
- Zhou, A.; Ma, H.; Feng, S.; Gong, S.; Wang, J. DsSWEET17, a tonoplast-localized sugar transporter from Dianthus spiculifolius, affects sugar metabolism and confers multiple stress tolerance in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 1564. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Zhang, D.; Miao, Q.; Yang, J.; Xuan, Y.; Hu, Y. Essential role of sugar transporter OsSWEET11 during the early stage of rice grain filling. Plant Cell Physiol. 2017, 58, 863–873. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.-G.; Kim, E.J.; Jeon, J.; Jeong, S.W.; Kim, K.-H.; Kim, K.-M.; Park, C.S.; Kang, C.-S.; Park, Y.-I. Expression of wheat Peptide TRansporter 2.1 (TaPTR2. 1) during early seed germination. Plant Biotechnol. Rep. 2020, 14, 627–634. [Google Scholar] [CrossRef]
- Ricachenevsky, F.K.; Punshon, T.; Lee, S.; Oliveira, B.H.N.; Trenz, T.S.; Maraschin, F.d.S.; Hindt, M.N.; Danku, J.; Salt, D.E.; Fett, J.P. Elemental profiling of rice FOX lines leads to characterization of a new Zn plasma membrane transporter, OsZIP7. Front. Plant Sci. 2018, 9, 865. [Google Scholar] [CrossRef] [Green Version]
- Ning, P.; Peng, Y.; Fritschi, F.B. Carbohydrate dynamics in maize leaves and developing ears in response to nitrogen application. Agronomy 2018, 8, 302. [Google Scholar] [CrossRef] [Green Version]
- Erskine, W.; Upadhyaya, H.; Malik, A. Salinity. Plant Genet. Resour. Clim. Chang. 2014, 236–250. [Google Scholar] [CrossRef]
- Ali, A.; Raddatz, N.; Pardo, J.M.; Yun, D.J. HKT sodium and potassium transporters in Arabidopsis thaliana and related halophyte species. Physiol. Plant. 2021, 171, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, G.; Alli, A.; Yu, L. Plant HAK/KUP/KT K+ Transporters: Function and Regulation; Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 133–141. [Google Scholar]
- Zhao, W.T.; Feng, S.J.; Li, H.; Faust, F.; Kleine, T.; Li, L.N.; Yang, Z.M. Salt stress-induced FERROCHELATASE 1 improves resistance to salt stress by limiting sodium accumulation in Arabidopsis thaliana. Sci. Rep. 2017, 7, 1–16. [Google Scholar]
- Jadamba, C.; Kang, K.; Paek, N.-C.; Lee, S.I.; Yoo, S.-C. Overexpression of rice expansin7 (Osexpa7) confers enhanced tolerance to salt stress in rice. Int. J. Mol. Sci. 2020, 21, 454. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Shabala, S.; Zhang, J.; Ma, G.; Chen, D.; Shabala, L.; Zeng, F.; Chen, Z.H.; Zhou, M.; Venkataraman, G. Melatonin improves rice salinity stress tolerance by NADPH oxidase-dependent control of the plasma membrane K+ transporters and K+ homeostasis. Plant Cell Environ. 2020, 43, 2591–2605. [Google Scholar] [CrossRef]
- Liu, J.; Shabala, S.; Shabala, L.; Zhou, M.; Meinke, H.; Venkataraman, G.; Chen, Z.; Zeng, F.; Zhao, Q. Tissue-specific regulation of Na+ and K+ transporters explains genotypic differences in salinity stress tolerance in rice. Front. Plant Sci. 2019, 10, 1361. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Song, G.; Shan, X.; Wei, Z.; Liu, Y.; Jiang, C.; Jiang, Y.; Jin, F.; Li, Y. Association analysis and identification of ZmHKT1; 5 variation with salt-stress tolerance. Front. Plant Sci. 2018, 9, 1485. [Google Scholar] [CrossRef]
- Xu, Q.-F.; Mao, X.-G.; Wang, Y.-X.; Wang, J.-Y.; Xi, Y.-J.; Jing, R.-L. A wheat gene TaSAP17-D encoding an AN1/AN1 zinc finger protein improves salt stress tolerance in transgenic Arabidopsis. J. Integr. Agric. 2018, 17, 507–516. [Google Scholar] [CrossRef] [Green Version]
- Dave, A.; Sanadhya, P.; Joshi, P.S.; Agarwal, P.; Agarwal, P.K. Molecular cloning and characterization of high-affinity potassium transporter (AlHKT2; 1) gene promoter from halophyte Aeluropus lagopoides. Int. J. Biol. Macromol. 2021, 181, 1254–1264. [Google Scholar] [CrossRef]
- Ahmad, P.; Wani, M.R.; Azooz, M.M.; Tran, L.-S.P. Improvement of Crops in the Era of Climatic Changes; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Ojuederie, O.B.; Olanrewaju, O.S.; Babalola, O.O. Plant growth promoting rhizobacterial mitigation of drought stress in crop plants: Implications for sustainable agriculture. Agronomy 2019, 9, 712. [Google Scholar] [CrossRef] [Green Version]
- ElBasyoni, I.; Saadalla, M.; Baenziger, S.; Bockelman, H.; Morsy, S. Cell membrane stability and association mapping for drought and heat tolerance in a worldwide wheat collection. Sustainability 2017, 9, 1606. [Google Scholar] [CrossRef] [Green Version]
- Hasan, S.; Rabei, S.; Nada, R.; Abogadallah, G. Water use efficiency in the drought-stressed sorghum and maize in relation to expression of aquaporin genes. Biol. Plant. 2017, 61, 127–137. [Google Scholar] [CrossRef]
- Zheng, J.; Lin, R.; Pu, L.; Wang, Z.; Mei, Q.; Zhang, M.; Jian, S. Ectopic Expression of CrPIP2; 3, a Plasma Membrane Intrinsic Protein Gene from the Halophyte Canavalia rosea, Enhances Drought and Salt-Alkali Stress Tolerance in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 565. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, C.; Gao, Z.; Zhang, Y.; Jiang, H.; Zhu, L.; Ren, D.; Yu, L.; Xu, G.; Qian, Q. OsHAK1, a high-affinity potassium transporter, positively regulates responses to drought stress in rice. Front. Plant Sci. 2017, 8, 1885. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, J.-U.; Lee, M.; Kim, Y.-Y.; Assmann, S.M.; Martinoia, E.; Lee, Y. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. USA 2010, 107, 2355–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuromori, T.; Sugimoto, E.; Shinozaki, K. Arabidopsis mutants of AtABCG22, an ABC transporter gene, increase water transpiration and drought susceptibility. Plant J. 2011, 67, 885–894. [Google Scholar] [CrossRef]
- Ge, K.; Liu, X.; Li, X.; Hu, B.; Li, L. Isolation of an ABA transporter-like 1 gene from Arachis hypogaea that affects ABA import and reduces ABA sensitivity in Arabidopsis. Front. Plant Sci. 2017, 8, 1150. [Google Scholar] [CrossRef] [Green Version]
- Qin, M.; Li, X.; Tang, S.; Huang, Y.; Li, L.; Hu, B. Expression of AhATL1, an ABA Transport Factor Gene from Peanut, Is Affected by Altered Memory Gene Expression Patterns and Increased Tolerance to Drought Stress in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 3398. [Google Scholar] [CrossRef]
- Raza, A.; Tabassum, J.; Kudapa, H.; Varshney, R.K. Can omics deliver temperature resilient ready-to-grow crops? Crit. Rev. Biotechnol. 2021, 1–24. [Google Scholar] [CrossRef]
- Shinozaki, K.; Uemura, M.; Bailey-Serres, J.; Bray, E.; Weretilnyk, E. Responses to abiotic stress. Biochem. Mol. Biol. Plants 2015, 1051–1100. [Google Scholar]
- Chen, J.-M. A Comprehensive Evolutionary Theory Deduced from Thermodynamics. Preprints 2021. [CrossRef]
- Niu, Y.; Qian, D.; Liu, B.; Ma, J.; Wan, D.; Wang, X.; He, W.; Xiang, Y. ALA6, a P4-type ATPase, is involved in heat stress responses in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 1732. [Google Scholar] [CrossRef] [Green Version]
- Higashi, Y.; Saito, K. Lipidomic studies of membrane glycerolipids in plant leaves under heat stress. Prog. Lipid Res. 2019, 75, 100990. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Mesihovic, A.; Chaturvedi, P.; Ghatak, A.; Weckwerth, W.; Böhmer, M.; Schleiff, E. Structural and functional heat stress responses of chloroplasts of Arabidopsis thaliana. Genes 2020, 11, 650. [Google Scholar] [CrossRef]
- Wu, C.; Tang, S.; Li, G.; Wang, S.; Fahad, S.; Ding, Y. Roles of phytohormone changes in the grain yield of rice plants exposed to heat: A review. PeerJ 2019, 7, e7792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallis, J.G.; Browse, J. Mutants of Arabidopsis reveal many roles for membrane lipids. Prog. Lipid Res. 2002, 41, 254–278. [Google Scholar] [CrossRef]
- Penfield, S. Temperature perception and signal transduction in plants. New Phytol. 2008, 179, 615–628. [Google Scholar] [CrossRef]
- Ruelland, E.; Zachowski, A. How plants sense temperature. Environ. Exp. Bot. 2010, 69, 225–232. [Google Scholar] [CrossRef]
- Furt, F.; Simon-Plas, F.; Mongrand, S. Lipids of the plant plasma membrane. In The Plant Plasma Membrane; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–30. [Google Scholar]
- Aguilar, P.S.; De Mendoza, D. Control of fatty acid desaturation: A mechanism conserved from bacteria to humans. Mol. Microbiol. 2006, 62, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Xiang, Y. An overview of biomembrane functions in plant responses to high-temperature stress. Front. Plant Sci. 2018, 9, 915. [Google Scholar] [CrossRef] [Green Version]
- Ward, J.M.; Mäser, P.; Schroeder, J.I. Plant ion channels: Gene families, physiology, and functional genomics analyses. Annu. Rev. Physiol. 2009, 71, 59–82. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Han, X.; Wu, J.; Zheng, S.; Shang, Z.; Sun, D.; Zhou, R.; Li, B. A heat-activated calcium-permeable channel–Arabidopsis cyclic nucleotide-gated ion channel 6–is involved in heat shock responses. Plant J. 2012, 70, 1056–1069. [Google Scholar] [CrossRef]
- von Koskull-Döring, P.; Scharf, K.-D.; Nover, L. The diversity of plant heat stress transcription factors. Trends Plant Sci. 2007, 12, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Saidi, Y.; Finka, A.; Muriset, M.; Bromberg, Z.; Weiss, Y.G.; Maathuis, F.J.; Goloubinoff, P. The heat shock response in moss plants is regulated by specific calcium-permeable channels in the plasma membrane. Plant Cell 2009, 21, 2829–2843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishwakarma, K.; Mishra, M.; Patil, G.; Mulkey, S.; Ramawat, N.; Pratap Singh, V.; Deshmukh, R.; Kumar Tripathi, D.; Nguyen, H.T.; Sharma, S. Avenues of the membrane transport system in adaptation of plants to abiotic stresses. Crit. Rev. Biotechnol. 2019, 39, 861–883. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Kawamura, Y.; Maeshima, M.; Rahman, A.; Uemura, M. Plasma membrane aquaporin members pips act in concert to regulate cold acclimation and freezing tolerance responses in Arabidopsis thaliana. Plant Cell Physiol. 2020, 61, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Song, B.; Tang, S.; He, J.; Zhou, Y.; Feng, J.; Shi, S.; Xu, X. Overexpression of the ABC transporter gene TsABCG11 increases cuticle lipids and abiotic stress tolerance in Arabidopsis. Plant Biotechnol. Rep. 2018, 12, 303–313. [Google Scholar] [CrossRef]
- Pan, Y.; Liang, H.; Gao, L.; Dai, G.; Chen, W.; Yang, X.; Qing, D.; Gao, J.; Wu, H.; Huang, J. Transcriptomic profiling of germinating seeds under cold stress and characterization of the cold-tolerant gene LTG5 in rice. BMC Plant Biol. 2020, 20, 1–17. [Google Scholar] [CrossRef]
- Peng, M.; Shahzad, R.; Gul, A.; Subthain, H.; Shen, S.; Lei, L.; Zheng, Z.; Zhou, J.; Lu, D.; Wang, S. Differentially evolved glucosyltransferases determine natural variation of rice flavone accumulation and UV-tolerance. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Le Roy, J.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Front. Plant Sci. 2016, 7, 735. [Google Scholar] [CrossRef] [Green Version]
- Dong, N.-Q.; Sun, Y.; Guo, T.; Shi, C.-L.; Zhang, Y.-M.; Kan, Y.; Xiang, Y.-H.; Zhang, H.; Yang, Y.-B.; Li, Y.-C. UDP-glucosyltransferase regulates grain size and abiotic stress tolerance associated with metabolic flux redirection in rice. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.M.; Ishikawa, S.; Takahashi, F.; Suzuki, K.; Kamo, M.; Umezawa, T.; Shinozaki, K.; Kawamura, Y.; Uemura, M. Large-Scale Phosphoproteomic Study of Arabidopsis Membrane Proteins Reveals Early Signaling Events in Response to Cold. Int. J. Mol. Sci. 2020, 21, 8631. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Takahashi, D.; Kawamura, Y.; Uemura, M. Plasma membrane proteome analyses of Arabidopsis thaliana suspension-cultured cells during cold or ABA treatment: Relationship with freezing tolerance and growth phase. J. Proteom. 2020, 211, 103528. [Google Scholar] [CrossRef] [PubMed]
- Goering, R.; Larsen, S.; Tan, J.; Whelan, J.; Makarevitch, I. QTL mapping of seedling tolerance to exposure to low temperature in the maize IBM RIL population. PLoS ONE 2021, 16, e0254437. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, S.; Feng, J.; Wang, W.; Wang, Y.; Yu, Q.; Liao, Y.; Mo, Y.; Xu, Z.; Li, L. Overexpression of a stress response membrane protein gene OsSMP1 enhances rice tolerance to salt, cold and heavy metal stress. Environ. Exp. Bot. 2021, 182, 104327. [Google Scholar] [CrossRef]
- Sun, L.; Ma, Y.; Wang, H.; Huang, W.; Wang, X.; Han, L.; Sun, W.; Han, E.; Wang, B. Overexpression of PtABCC1 contributes to mercury tolerance and accumulation in Arabidopsis and poplar. Biochem. Biophys. Res. Commun. 2018, 497, 997–1002. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Peng, Z.; Li, J.; Huang, W.; Liu, Y.; Wang, X.; Xie, S.; Sun, L.; Han, E. Ectopic expression of poplar ABC transporter PtoABCG36 confers Cd tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 3293. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.; Lu, Y.; Zhang, X.; Yang, G.; Chao, D.; Wang, Z.; Shi, M.; Chen, J.; Chao, D.-Y.; Li, R. The ABC transporter ABCG36 is required for cadmium tolerance in rice. J. Exp. Bot. 2019, 70, 5909–5918. [Google Scholar] [CrossRef] [PubMed]
- Qiao, K.; Gong, L.; Tian, Y.; Wang, H.; Chai, T. The metal-binding domain of wheat heavy metal ATPase 2 (TaHMA2) is involved in zinc/cadmium tolerance and translocation in Arabidopsis. Plant Cell Rep. 2018, 37, 1343–1352. [Google Scholar] [CrossRef]
- Zhiguo, E.; Tingting, L.; Chen, C.; Lei, W. Genome-wide survey and expression analysis of P1B-ATPases in rice, maize and sorghum. Rice Sci. 2018, 25, 208–217. [Google Scholar] [CrossRef]
- Gill, R.; Hu, X.; Ali, B.; Yang, C.; Shou, J.; Wu, Y.; Zhou, W. Genotypic variation of the responses to chromium toxicity in four oilseed rape cultivars. Biol. Plant. 2014, 58, 539–550. [Google Scholar] [CrossRef]
- Gill, R.A.; Ali, B.; Cui, P.; Shen, E.; Farooq, M.A.; Islam, F.; Ali, S.; Mao, B.; Zhou, W. Comparative transcriptome profiling of two Brassica napus cultivars under chromium toxicity and its alleviation by reduced glutathione. BMC Genom. 2016, 17, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, R.A.; Zang, L.; Ali, B.; Farooq, M.A.; Cui, P.; Yang, S.; Ali, S.; Zhou, W. Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 2015, 120, 154–164. [Google Scholar] [CrossRef]
- Gill, R.A.; Zhang, N.; Ali, B.; Farooq, M.A.; Xu, J.; Gill, M.B.; Mao, B.; Zhou, W. Role of exogenous salicylic acid in regulating physio-morphic and molecular changes under chromium toxicity in black-and yellow-seeded Brassica napus L. Environ. Sci. Pollut. Res. 2016, 23, 20483–20496. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.A.; Ali, B.; Islam, F.; Farooq, M.A.; Gill, M.B.; Mwamba, T.M.; Zhou, W. Physiological and molecular analyses of black and yellow seeded Brassica napus regulated by 5-aminolivulinic acid under chromium stress. Plant Physiol. Biochem. 2015, 94, 130–143. [Google Scholar] [CrossRef]
- Yuenyong, W.; Chinpongpanich, A.; Comai, L.; Chadchawan, S.; Buaboocha, T. Downstream components of the calmodulin signaling pathway in the rice salt stress response revealed by transcriptome profiling and target identification. BMC Plant Biol. 2018, 18, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Zhang, P.; Zhu, R.; Fu, J.; Su, J.; Zheng, J.; Wang, Z.; Wang, D.; Gong, Q. Autophagy is rapidly induced by salt stress and is required for salt tolerance in Arabidopsis. Front. Plant Sci. 2017, 8, 1459. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Chen, Y.; Zhang, M.; Chen, J.; Liu, J.; Han, H.; Hua, X. Arabidopsis AMINO ACID PERMEASE1 contributes to salt stress-induced proline uptake from exogenous sources. Front. Plant Sci. 2017, 8, 2182. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wu, H.; Chen, L.; Liu, L.; Wan, X. Maintenance of mesophyll potassium and regulation of plasma membrane H+-ATPase are associated with physiological responses of tea plants to drought and subsequent rehydration. Crop J. 2018, 6, 611–620. [Google Scholar] [CrossRef]
- Chung, P.J.; Jung, H.; Do Choi, Y.; Kim, J.-K. Genome-wide analyses of direct target genes of four rice NAC-domain transcription factors involved in drought tolerance. BMC Genom. 2018, 19, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Mao, Q.; Hong, J.; Shi, L.; Ran, X.; Liaquat, F.; Uzair, M.; Liang, W.; Fernie, A.R.; Shi, J. HSP70-16 and VDAC3 jointly inhibit seed germination under cold stress in Arabidopsis. Plant Cell Environ. 2021. [CrossRef]
- Ben-Romdhane, W.; Ben-Saad, R.; Meynard, D.; Zouari, N.; Mahjoub, A.; Fki, L.; Guiderdoni, E.; Al-Doss, A.; Hassairi, A. Overexpression of AlTMP2 gene from the halophyte grass Aeluropus littoralis in transgenic tobacco enhances tolerance to different abiotic stresses by improving membrane stability and deregulating some stress-related genes. Protoplasma 2018, 255, 1161–1177. [Google Scholar] [CrossRef]
- Shamustakimova, A.; Leonova, T.; Taranov, V.; de Boer, A.; Babakov, A. Cold stress increases salt tolerance of the extremophytes Eutrema salsugineum (Thellungiella salsuginea) and Eutrema (Thellungiella) botschantzevii. J. Plant Physiol. 2017, 208, 128–138. [Google Scholar] [CrossRef]
- Zhang, C.-X.; Feng, B.-H.; Chen, T.-T.; Fu, W.-M.; Li, H.-B.; Li, G.-Y.; Jin, Q.-Y.; Tao, L.-X.; Fu, G.-F. Heat stress-reduced kernel weight in rice at anthesis is associated with impaired source-sink relationship and sugars allocation. Environ. Exp. Bot. 2018, 155, 718–733. [Google Scholar] [CrossRef]
- Saad, R.B.; Hsouna, A.B.; Saibi, W.; Hamed, K.B.; Brini, F.; Ghneim-Herrera, T. A stress-associated protein, LmSAP, from the halophyte Lobularia maritima provides tolerance to heavy metals in tobacco through increased ROS scavenging and metal detoxification processes. J. Plant Physiol. 2018, 231, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Belloeil, C.; Ianna, M.L.; Shin, R. Arabidopsis CNGC family members contribute to heavy metal ion uptake in plants. Int. J. Mol. Sci. 2019, 20, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Han, X.; Fang, J.; Lu, Z.; Qiu, W.; Liu, M.; Sang, J.; Jiang, J.; Zhuo, R. Sedum alfredii SaNramp6 metal transporter contributes to cadmium accumulation in transgenic Arabidopsis thaliana. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, S.; Liang, S.; Qiao, K.; Wang, F.; Zhang, Y.; Chai, T. Co-expression of multiple heavy metal transporters changes the translocation, accumulation, and potential oxidative stress of Cd and Zn in rice (Oryza sativa). J. Hazard. Mater. 2019, 380, 120853. [Google Scholar] [CrossRef] [PubMed]
- Jarzyniak, K.M.; Jasiński, M. Membrane transporters and drought resistance–a complex issue. Front. Plant Sci. 2014, 5, 687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.-Y.; Park, J.; Eisenach, C.; Maeshima, M.; Lee, Y.; Martinoia, E. ABC transporters and heavy metals. In Plant ABC Transporters; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–17. [Google Scholar]
- Guo, X.; Liu, D.; Chong, K. Cold signaling in plants: Insights into mechanisms and regulation. J. Integr. Plant Biol. 2018, 60, 745–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalmi, S.K.; Bhagat, P.K.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A.K. Traversing the links between heavy metal stress and plant signaling. Front. Plant Sci. 2018, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Conde, A.; Chaves, M.M.; Gerós, H. Membrane transport, sensing and signaling in plant adaptation to environmental stress. Plant Cell Physiol. 2011, 52, 1583–1602. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Kurjak, D.; Petek, A.; Khan, M.M.A. Drought: Sensing, signalling, effects and tolerance in higher plants. Physiol. Plant. 2021, 172, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.-J.; Luan, M.; Wang, C.; Lhamo, D.; Yang, Y.; Zhao, F.-G.; Lan, W.-Z.; Fu, A.-G.; Luan, S. Plant membrane transport research in the post-genomic era. Plant Commun. 2020, 1, 100013. [Google Scholar] [CrossRef]
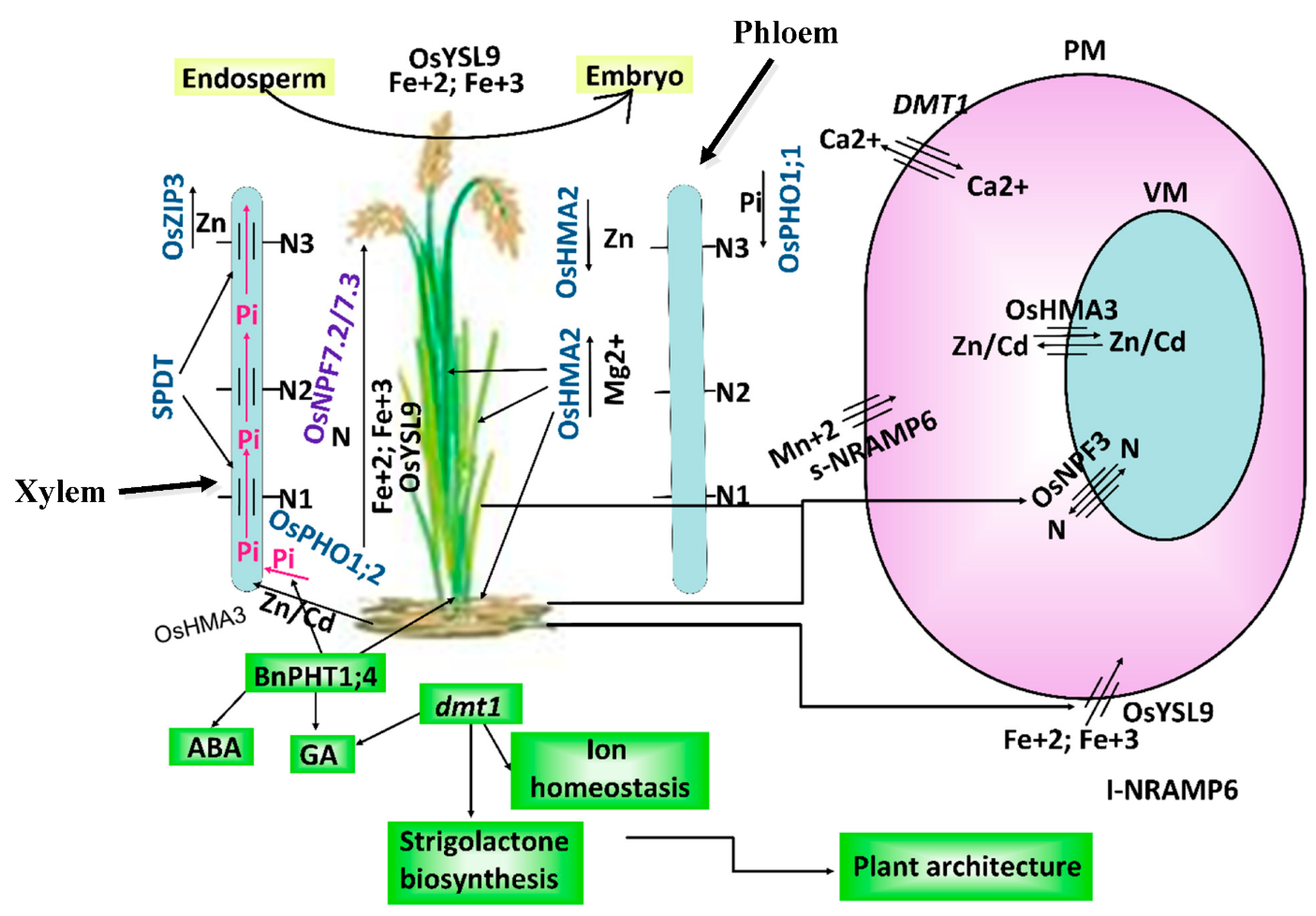

| Transporter Protein | Plant Species | Localization in Tissue/Organ/Cell | Biological Role | Ref. |
|---|---|---|---|---|
| OsNPF7.3 | O. sativa | Lateral roots and stems | Contribute to NUE and grain yield | [23] |
| OsNPF7.2 | O. sativa | Enhance tiller number and grain yield | [22] | |
| OsZIP7 | O. sativa | Parenchyma cells of vascular bundles in roots and nodes | Xylem loading in roots and transfer of Zn/Cd to grain | [24] |
| OsYSL9 | O. sativa | Roots and non-juvenile leaves | Distribute iron to developing grains | [25] |
| OsHMA3 | O. sativa | Tonoplast/roots | Zn detoxification in roots and storage in vacuoles | [30] |
| OsDMT1 | O. sativa | Regulate plant architecture and ion homeostasis | [34] | |
| NRAMP6 | O. sativa | PM | Transport Fe and Mg and disease resistance | [26] |
| ZmMGT12 | Z. mays | Root, stem and leaves | Maintain Mg homeostasis in chloroplast | [36] |
| ZmSUT2 | Z. mays | Tonoplast | It acts as sucrose/H+ symporter on the vacuolar membrane and remobilizes stored sucrose for subsequent growing tissues | [40] |
| TaPTR2.1 | T. aestivum | Tonoplast | Regulates water status during seed germination at early stage | [50] |
| BnaPHT1 | B. napus | PM | Pi acquisition and homeostasis and responds to various nutrient stresses including N, K, S and Fe | [39] |
| BnaPHT1;4 | B. napus | Cotyledons of early developing seedlings | Pi homeostasis, seed germination and seedling growth through modification in biosynthesis of ABA and GA | [29] |
| OsZIP7 | A. thaliana | PM | Increases Zn concentration by 25% in the shoot of transgenic plants | [51] |
| DsSWEET12 | A. thaliana | PM | Increased sugar supply and enhanced seedling growth (larger roots and fresh biomass) | [43] |
| DsSWEET17 | A. thaliana | Tonoplast | It enhanced root length and fresh weight | [48] |
| OsDOF11 | O. sativa | Photosynthetic cells | It upregulated OsSUT1 and OsSWEET11 and 14 genes expression and transported sucrose through apoplastic loading and enhanced resistance against Xanthomonas | [44] |
| OsSWEET11 | O. sativa | Ovular vascular trace, nucellar epidermis and cross cells | It remobilizes the sugar from maternal tissues towards maternal–filial interface during early caryopsis developmental stage | [49] |
| ZmSWEETa/b/c | Z. mays | Leaf | Influence on the sugar dynamics from leaves towards developing ears | [52] |
| AtSUC6 | A. thaliana | PM | Sugar accumulation in pollen tube and synergid cells | [11] |
| AtSTP1/13 | A. thaliana | PM | Involved in sugar transport across cell membranes | [47] |
| OsHAK1 | O. sativa | PM | It is involved in controlling vegetative growth, panicle fertility and K+ mediated sugar homeostasis | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gill, R.A.; Ahmar, S.; Ali, B.; Saleem, M.H.; Khan, M.U.; Zhou, W.; Liu, S. The Role of Membrane Transporters in Plant Growth and Development, and Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 12792. https://doi.org/10.3390/ijms222312792
Gill RA, Ahmar S, Ali B, Saleem MH, Khan MU, Zhou W, Liu S. The Role of Membrane Transporters in Plant Growth and Development, and Abiotic Stress Tolerance. International Journal of Molecular Sciences. 2021; 22(23):12792. https://doi.org/10.3390/ijms222312792
Chicago/Turabian StyleGill, Rafaqat Ali, Sunny Ahmar, Basharat Ali, Muhammad Hamzah Saleem, Muhammad Umar Khan, Weijun Zhou, and Shengyi Liu. 2021. "The Role of Membrane Transporters in Plant Growth and Development, and Abiotic Stress Tolerance" International Journal of Molecular Sciences 22, no. 23: 12792. https://doi.org/10.3390/ijms222312792
APA StyleGill, R. A., Ahmar, S., Ali, B., Saleem, M. H., Khan, M. U., Zhou, W., & Liu, S. (2021). The Role of Membrane Transporters in Plant Growth and Development, and Abiotic Stress Tolerance. International Journal of Molecular Sciences, 22(23), 12792. https://doi.org/10.3390/ijms222312792










