Mesenchymal Stem Cell-Derived Extracellular Vesicle: A Promising Alternative Therapy for Osteoporosis
Abstract
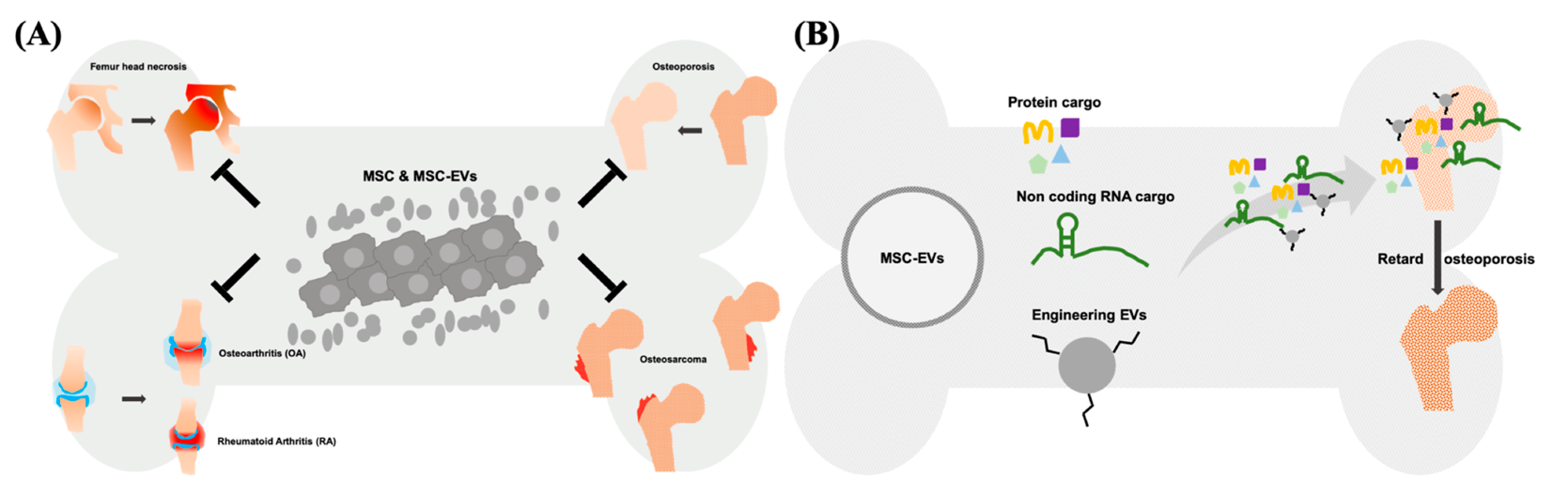
1. Introduction
2. MSC-EV with Carried Molecules for Diseases Treatment
3. Applications of Modified EVs for Therapy
4. Therapeutic Potential of Exogenous MSC-EVs for Osteoporosis
4.1. Rebalancing the Bone Homeostasis by Regulation of Bone Formation and Resorption
4.2. miRNAs and lncRNAs Carried by MSC-EVs Serve as Osteoporosis Medications
4.3. MSC-EVs’ Protein Act as Therapeutic Cargo in the Treatment of Osteoporosis
5. Modification of MSC-EVs in Osteoporosis Therapy
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonyadi, M.; Waldman, S.D.; Liu, D.; Aubin, J.E.; Grynpas, M.D.; Stanford, W.L. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc. Natl. Acad. Sci. USA 2003, 100, 5840–5845. [Google Scholar] [CrossRef]
- Kapinas, K.; Delany, A.M. MicroRNA biogenesis and regulation of bone remodeling. Arthritis Res. Ther. 2011, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tian, Y.; Wang, Q.; Fu, M.; Xue, C.; Wang, J. Comparative Study of DHA with Different Molecular Forms for Ameliorating Osteoporosis by Promoting Chondrocyte-to-Osteoblast Transdifferentiation in the Growth Plate of Ovariectomized Mice. J. Agric. Food Chem. 2021, 69, 10562–10571. [Google Scholar] [CrossRef] [PubMed]
- Epsley, S.; Tadros, S.; Farid, A.; Kargilis, D.; Mehta, S.; Rajapakse, C.S. The Effect of Inflammation on Bone. Front. Physiol. 2020, 11, 511799. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, L.; Abtahi, J. Bisphosphonate associated osteonecrosis of the jaw: An update on pathophysiology, risk factors, and treatment. Int. J. Dent. 2014, 2014, 471035. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Crandall, C.J. Bisphosphonates for Postmenopausal Osteoporosis. JAMA 2019, 49, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, N.; Pedrazzoni, M.; Negri, G.; Passeri, G.; Impicciatore, M.; Girasole, G. Bisphosphonates stimulate formation of osteoblast precursors and mineralized nodules in murine and human bone marrow cultures in vitro and promote early osteoblastogenesis in young and aged mice in vivo. Bone 1998, 22, 455–461. [Google Scholar] [CrossRef]
- Papapetrou, P.D. Bisphosphonate-associated adverse events. Hormones (Athens) 2009, 8, 96–110. [Google Scholar] [CrossRef]
- Eguia, A.; Bagan-Debon, L.; Cardona, F. Review and update on drugs related to the development of osteonecrosis of the jaw. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e71–e83. [Google Scholar] [CrossRef]
- Lindner, U.; Kramer, J.; Rohwedel, J.; Schlenke, P. Mesenchymal Stem or Stromal Cells: Toward a Better Understanding of Their Biology? Transfus. Med. Hemotherapy 2010, 37, 75–83. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Zeng, R.; Wang, L.W.; Hu, Z.B.; Guo, W.T.; Wei, J.S.; Lin, H.; Sun, X.; Chen, L.X.; Yang, L.J. Differentiation of human bone marrow mesenchymal stem cells into neuron-like cells in vitro. Spine (Phila Pa 1976) 2011, 36, 997–1005. [Google Scholar] [CrossRef]
- James, A.W. Review of Signaling Pathways Governing MSC Osteogenic and Adipogenic Differentiation. Scientifica (Cairo) 2013, 2013, 684736. [Google Scholar] [CrossRef] [PubMed]
- Antebi, B.; Pelled, G.; Gazit, D. Stem cell therapy for osteoporosis. Curr. Osteoporos. Rep. 2014, 12, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kangari, P.; Talaei-Khozani, T.; Razeghian-Jahromi, I.; Razmkhah, M. Mesenchymal stem cells: Amazing remedies for bone and cartilage defects. Stem Cell Res. Ther. 2020, 11, 492. [Google Scholar] [CrossRef]
- Behnke, J.; Kremer, S.; Shahzad, T.; Chao, C.M.; Bottcher-Friebertshauser, E.; Morty, R.E.; Bellusci, S.; Ehrhardt, H. MSC Based Therapies-New Perspectives for the Injured Lung. J. Clin. Med. 2020, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- de Klerk, E.; Hebrok, M. Stem Cell-Based Clinical Trials for Diabetes Mellitus. Front. Endocrinol. (Lausanne) 2021, 12, 631463. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Z.; Gu, P. Stem/progenitor cell-based transplantation for retinal degeneration: A review of clinical trials. Cell Death Discov. 2020, 11, 793. [Google Scholar] [CrossRef] [PubMed]
- Golchin, A. Cell-Based Therapy for Severe COVID-19 Patients: Clinical Trials and Cost-Utility. Stem Cell Rev. Rep. 2021, 17, 56–62. [Google Scholar] [CrossRef]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transpl. 2016, 25, 829–848. [Google Scholar] [CrossRef]
- Hoogduijn, M.J.; Lombardo, E. Mesenchymal Stromal Cells Anno 2019: Dawn of the Therapeutic Era? Concise Review. Stem Cells Transl. Med. 2019, 8, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Abofila, M.T.; Azab, A.E. Stem Cells: Insights into Niche, Classification, Identification, Characterization, Mechanisms of Regeneration by Using Stem Cells, and Applications in Joint Disease Remedy. J. Biotech. Bioprocess. 2021, 2, 2766-2314. [Google Scholar]
- Arjmand, B.; Sarvari, M.; Alavi-Moghadam, S.; Payab, M.; Goodarzi, P.; Gilany, K.; Mehrdad, N.; Larijani, B. Prospect of Stem Cell Therapy and Regenerative Medicine in Osteoporosis. Front. Endocrinol. (Lausanne) 2020, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Linero, I.; Chaparro, O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS ONE 2014, 9, e107001. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, J.A. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res. Int. 2014, 2014, 965849. [Google Scholar] [CrossRef]
- Tofino-Vian, M.; Guillen, M.I.; Perez Del Caz, M.D.; Silvestre, A.; Alcaraz, M.J. Microvesicles from Human Adipose Tissue-Derived Mesenchymal Stem Cells as a New Protective Strategy in Osteoarthritic Chondrocytes. Cell. Physiol. Biochem. 2018, 47, 11–25. [Google Scholar] [CrossRef]
- Collino, F.; Pomatto, M.; Bruno, S.; Lindoso, R.S.; Tapparo, M.; Sicheng, W.; Quesenberry, P.; Camussi, G. Exosome and Microvesicle-Enriched Fractions Isolated from Mesenchymal Stem Cells by Gradient Separation Showed Different Molecular Signatures and Functions on Renal Tubular Epithelial Cells. Stem Cell Rev. Rep. 2017, 13, 226–243. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Gupta, D.; Liang, X.; Pavlova, S.; Wiklander, O.P.B.; Corso, G.; Zhao, Y.; Saher, O.; Bost, J.; Zickler, A.M.; Piffko, A.; et al. Quantification of extracellular vesicles in vitro and in vivo using sensitive bioluminescence imaging. J. Extracell. Vesicles 2020, 9, 1800222. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Chaparro Padilla, A.; Weber Aracena, L.; Realini Fuentes, O.; Albers Busquetts, D.; Hernandez Rios, M.; Ramirez Lobos, V.; Pascual La Rocca, A.; Nart Molina, J.; Beltran Varas, V.; Acuna-Gallardo, S.; et al. Molecular signatures of extracellular vesicles in oral fluids of periodontitis patients. Oral Dis. 2020, 26, 1318–1325. [Google Scholar] [CrossRef]
- Lasser, C.; Eldh, M.; Lotvall, J. Isolation and characterization of RNA-containing exosomes. J. Vis. Exp. 2012, 59, e3037. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2019, 10, 359. [Google Scholar] [CrossRef]
- Chen, Y.A.; Lu, C.H.; Ke, C.C.; Chiu, S.J.; Jeng, F.S.; Chang, C.W.; Yang, B.H.; Liu, R.S. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Alzheimer’s Disease Pathology and Improve Cognitive Deficits. Biomedicines 2021, 9, 594. [Google Scholar] [CrossRef]
- Wiklander, O.P.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mager, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef]
- Kordelas, L.; Schwich, E.; Dittrich, R.; Horn, P.A.; Beelen, D.W.; Borger, V.; Giebel, B.; Rebmann, V. Individual Immune-Modulatory Capabilities of MSC-Derived Extracellular Vesicle (EV) Preparations and Recipient-Dependent Responsiveness. Int. J. Mol. Sci. 2019, 20, 1642. [Google Scholar] [CrossRef]
- Basalova, N.; Sagaradze, G.; Arbatskiy, M.; Evtushenko, E.; Kulebyakin, K.; Grigorieva, O.; Akopyan, Z.; Kalinina, N.; Efimenko, A. Secretome of Mesenchymal Stromal Cells Prevents Myofibroblasts Differentiation by Transferring Fibrosis-Associated microRNAs within Extracellular Vesicles. Cells 2020, 9, 1272. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Buller, B.; Katakowski, M.; Zhang, Y.; Wang, X.; Shang, X.; Zhang, Z.G.; Chopp, M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 2012, 30, 1556–1564. [Google Scholar] [CrossRef]
- Wang, X.; Gu, H.; Qin, D.; Yang, L.; Huang, W.; Essandoh, K.; Wang, Y.; Caldwell, C.C.; Peng, T.; Zingarelli, B.; et al. Exosomal miR-223 Contributes to Mesenchymal Stem Cell-Elicited Cardioprotection in Polymicrobial Sepsis. Sci. Rep. 2015, 5, 13721. [Google Scholar] [CrossRef] [PubMed]
- Dexheimer, P.J.; Cochella, L. MicroRNAs: From Mechanism to Organism. Front. Cell Dev. Biol. 2020, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Kocijan, R.; Weigl, M.; Skalicky, S.; Geiger, E.; Ferguson, J.; Leinfellner, G.; Heimel, P.; Pietschmann, P.; Grillari, J.; Redl, H.; et al. MicroRNA levels in bone and blood change during bisphosphonate and teriparatide therapy in an animal model of postmenopausal osteoporosis. Bone 2020, 131, 115104. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, R.C.; Wu, Y. The role of microRNAs in self-renewal and differentiation of mesenchymal stem cells. Exp. Hematol. 2011, 39, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Valinezhad Orang, A.; Safaralizadeh, R.; Kazemzadeh-Bavili, M. Mechanisms of miRNA-Mediated Gene Regulation from Common Downregulation to mRNA-Specific Upregulation. Int. J. Genomics 2014, 2014, 970607. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.W.; Wang, Y.; Chen, L.L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019, 21, 542–551. [Google Scholar] [CrossRef]
- Karakas, D.; Ozpolat, B. The Role of LncRNAs in Translation. Noncoding RNA 2021, 7, 16. [Google Scholar] [CrossRef]
- Loayza-Puch, F.; Agami, R. Lncing protein translation to metastasis. EMBO J. 2013, 32, 2657–2658. [Google Scholar] [CrossRef][Green Version]
- Sun, Q.; Hao, Q.; Prasanth, K.V. Nuclear Long Noncoding RNAs: Key Regulators of Gene Expression. Trends Genet. 2018, 34, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Khorkova, O.; Hsiao, J.; Wahlestedt, C. Basic biology and therapeutic implications of lncRNA. Adv. Drug Deliv. Rev. 2015, 87, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Luan, S.; Chen, J.; Zhou, Y.; Wang, T.; Li, Z.; Fu, Y.; Zhai, A.; Bi, C. The MSC-Derived Exosomal lncRNA H19 Promotes Wound Healing in Diabetic Foot Ulcers by Upregulating PTEN via MicroRNA-152-3p. Mol. Ther. Nucleic Acids 2020, 19, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Lai, R.C.; Zhang, B.; Lim, S.K. MSC exosome works through a protein-based mechanism of action. Biochem. Soc. Trans. 2018, 46, 843–853. [Google Scholar] [CrossRef]
- Crain, S.K.; Robinson, S.R.; Thane, K.E.; Davis, A.M.; Meola, D.M.; Barton, B.A.; Yang, V.K.; Hoffman, A.M. Extracellular Vesicles from Wharton’s Jelly Mesenchymal Stem Cells Suppress CD4 Expressing T Cells Through Transforming Growth Factor Beta and Adenosine Signaling in a Canine Model. Stem Cells Dev. 2019, 28, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-King, H.; Garcia, N.A.; Ontoria-Oviedo, I.; Ciria, M.; Montero, J.A.; Sepulveda, P. Hypoxia Inducible Factor-1alpha Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells 2017, 35, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhao, S.; Wang, J.; Luo, L.; Li, E.; Zhu, Z.; Liu, Y.; Kang, R.; Zhao, Z. Bone marrow mesenchymal stem cells reduce ureteral stricture formation in a rat model via the paracrine effect of extracellular vesicles. J. Cell. Mol. Med. 2018, 22, 4449–4459. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, B.; Yu, Q. Genetic Engineering-Facilitated Coassembly of Synthetic Bacterial Cells and Magnetic Nanoparticles for Efficient Heavy Metal Removal. ACS Appl. Mater. Interfaces 2020, 12, 22948–22957. [Google Scholar] [CrossRef] [PubMed]
- Carnino, J.M.; Ni, K.; Jin, Y. Post-translational Modification Regulates Formation and Cargo-Loading of Extracellular Vesicles. Front. Immunol. 2020, 11, 948. [Google Scholar] [CrossRef]
- Chen, C.C.; Chang, D.Y.; Li, J.J.; Chan, H.W.; Chen, J.T.; Chang, C.H.; Liu, R.S.; Chang, C.A.; Chen, C.L.; Wang, H.E. Investigation of biodistribution and tissue penetration of PEGylated gold nanostars and their application for photothermal cancer treatment in tumor-bearing mice. J. Mater. Chem. B 2020, 8, 65–77. [Google Scholar] [CrossRef]
- Brezhneva, N.; Dezhkunov, N.V.; Ulasevich, S.A.; Skorb, E.V. Characterization of transient cavitation activity during sonochemical modification of magnesium particles. Ultrason. Sonochemistry 2021, 70, 105315. [Google Scholar] [CrossRef] [PubMed]
- Hwang, O.K.; Noh, Y.W.; Hong, J.T.; Lee, J.W. Hypoxia Pretreatment Promotes Chondrocyte Differentiation of Human Adipose-Derived Stem Cells via Vascular Endothelial Growth Factor. Tissue Eng. Regen. Med. 2020, 17, 335–350. [Google Scholar] [CrossRef]
- Olmo, A.; Yuste, Y.; Serrano, J.A.; Maldonado-Jacobi, A.; Perez, P.; Huertas, G.; Pereira, S.; Yufera, A.; de la Portilla, F. Electrical Modeling of the Growth and Differentiation of Skeletal Myoblasts Cell Cultures for Tissue Engineering. Sensors 2020, 20, 3152. [Google Scholar] [CrossRef]
- Resnik, D.B.; Vorhaus, D.B. Genetic modification and genetic determinism. Philos. Ethics Humanit. Med. 2006, 1, E9. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- King, H.W.; Michael, M.Z.; Gleadle, J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 2012, 12, 421. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, L.; Kumar, P.; Wang, A. Engineering Extracellular Vesicles as Nanotherapeutics for Regenerative Medicine. Biomolecules 2019, 10, 48. [Google Scholar] [CrossRef]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.A.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef]
- Kwon, S.; Shin, S.; Do, M.; Oh, B.H.; Song, Y.; Bui, V.D.; Lee, E.S.; Jo, D.G.; Cho, Y.W.; Kim, D.H.; et al. Engineering approaches for effective therapeutic applications based on extracellular vesicles. J. Control. Release 2021, 330, 15–30. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Fliervoet, L.A.L.; van der Meel, R.; Fens, M.; Heijnen, H.F.G.; van Bergen En Henegouwen, P.M.P.; Vader, P.; Schiffelers, R.M. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Control. Release 2016, 224, 77–85. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Stremersch, S.; Braeckmans, K.; de Smedt, S.C.; Hendrix, A.; Wood, M.J.A.; Schiffelers, R.M.; Raemdonck, K.; Vader, P. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Release 2013, 172, 229–238. [Google Scholar] [CrossRef]
- Pomatto, M.A.C.; Bussolati, B.; D’Antico, S.; Ghiotto, S.; Tetta, C.; Brizzi, M.F.; Camussi, G. Improved Loading of Plasma-Derived Extracellular Vesicles to Encapsulate Antitumor miRNAs. Mol. Ther. Methods Clin. Dev. 2019, 13, 133–144. [Google Scholar] [CrossRef]
- Jeyaram, A.; Lamichhane, T.N.; Wang, S.; Zou, L.; Dahal, E.; Kronstadt, S.M.; Levy, D.; Parajuli, B.; Knudsen, D.R.; Chao, W.; et al. Enhanced Loading of Functional miRNA Cargo via pH Gradient Modification of Extracellular Vesicles. Mol. Ther. 2020, 28, 975–985. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Jeyaram, A.; Patel, D.B.; Parajuli, B.; Livingston, N.K.; Arumugasaamy, N.; Schardt, J.S.; Jay, S.M. Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell. Mol. Bioeng. 2016, 9, 315–324. [Google Scholar] [CrossRef]
- Syn, N.L.; Wang, L.; Chow, E.K.; Lim, C.T.; Goh, B.C. Exosomes in Cancer Nanomedicine and Immunotherapy: Prospects and Challenges. Trends Biotechnol. 2017, 35, 665–676. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Ocansey, D.K.W.; Xu, W.; Qian, H. Extracellular vesicles: A bright star of nanomedicine. Biomaterials 2021, 269, 120467. [Google Scholar] [CrossRef]
- Wang, K.X.; Xu, L.L.; Rui, Y.F.; Huang, S.; Lin, S.E.; Xiong, J.H.; Li, Y.H.; Lee, W.Y.; Li, G. The effects of secretion factors from umbilical cord derived mesenchymal stem cells on osteogenic differentiation of mesenchymal stem cells. PLoS ONE 2015, 10, e0120593. [Google Scholar] [CrossRef]
- Yang, J.; Yu, K.; Liu, D.; Yang, J.; Tan, L.; Zhang, D. Irisin enhances osteogenic differentiation of mouse MC3T3-E1 cells via upregulating osteogenic genes. Exp. Ther. Med. 2021, 21, 580. [Google Scholar] [CrossRef]
- Liu, Y.; Shan, H.; Zong, Y.; Lin, Y.; Xia, W.; Wang, N.; Zhou, L.; Gao, Y.; Ma, X.; Jiang, C.; et al. IKKe in osteoclast inhibits the progression of methylprednisolone-induced osteonecrosis. Int. J. Biol. Sci. 2021, 17, 1353–1360. [Google Scholar] [CrossRef]
- Hayrapetyan, A.; Jansen, J.A.; van den Beucken, J.J. Signaling pathways involved in osteogenesis and their application for bone regenerative medicine. Tissue Eng. Part B Rev. 2015, 21, 75–87. [Google Scholar] [CrossRef]
- Baker, N.; Sohn, J.; Tuan, R.S. Promotion of human mesenchymal stem cell osteogenesis by PI3-kinase/Akt signaling, and the influence of caveolin-1/cholesterol homeostasis. Stem Cell Res. Ther. 2015, 6, 238. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, H.; Lin, J.; Xiao, T.; Xu, R.; Fu, Y.; Zhang, Y.; Du, Y.; Cheng, J.; Jiang, H. Insulin impedes osteogenesis of BMSCs by inhibiting autophagy and promoting premature senescence via the TGF-beta1 pathway. Aging (Albany NY) 2020, 12, 2084–2100. [Google Scholar] [CrossRef]
- Rossini, M.; Gatti, D.; Adami, S. Involvement of WNT/beta-catenin signaling in the treatment of osteoporosis. Calcif. Tissue Int. 2013, 93, 121–132. [Google Scholar] [CrossRef]
- Holliday, L.S.; Patel, S.S.; Rody, W.J., Jr. RANKL and RANK in extracellular vesicles: Surprising new players in bone remodeling. Extracell. Vesicles Circ. Nucleic Acids 2021, 2, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Li, P.; Yuan, K.; Zhao, F.; Zhu, X.; Zhang, P.; Huang, Z. Extracellular vesicles derived from human dental pulp stem cells promote osteogenesis of adipose-derived stem cells via the MAPK pathway. J. Tissue Eng. 2020, 11, 2041731420975569. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, Y.; Qin, H.; Tong, S.; Sun, Q.; Wang, T.; Zhang, H.; Cui, M.; Guo, S. Osteogenicainduced exosomes stimulate osteogenesis of human adipose-derived stem cells. Cell Tissue Bank. 2021, 22, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Fukuda, T.; Zhang, Q.; Sanui, T.; Shinjo, T.; Kou, X.; Chen, C.; Liu, D.; Watanabe, Y.; Hayashi, C.; et al. Exosomes from TNF-alpha-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021, 122, 306–324. [Google Scholar] [CrossRef]
- Zhao, A.G.; Shah, K.; Cromer, B.; Sumer, H. Mesenchymal Stem Cell-Derived Extracellular Vesicles and Their Therapeutic Potential. Stem Cells Int. 2020, 2020, 8825771. [Google Scholar] [CrossRef]
- Li, L.; Zhou, X.; Zhang, J.T.; Liu, A.F.; Zhang, C.; Han, J.C.; Zhang, X.Q.; Wu, S.; Zhang, X.Y.; Lv, F.Q. Exosomal miR-186 derived from BMSCs promote osteogenesis through hippo signaling pathway in postmenopausal osteoporosis. J. Orthop. Surg. Res. 2021, 16, 23. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Wang, Y.; Zhao, H.; Han, X.; Zhao, T.; Qu, P. Extracellular Vesicle-Encapsulated miR-29b-3p Released From Bone Marrow-Derived Mesenchymal Stem Cells Underpins Osteogenic Differentiation. Front. Cell Dev. Biol. 2020, 8, 581545. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, X. The role and mechanism of exosomes from umbilical cord mesenchymal stem cells in inducing osteogenesis and preventing osteoporosis. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, R.; Chen, C.Y.; Rao, S.S.; Xia, K.; Huang, J.; Yin, H.; Wang, Z.X.; Cao, J.; Liu, Z.Z.; et al. Extracellular vesicles from human umbilical cord blood ameliorate bone loss in senile osteoporotic mice. Metabolism 2019, 95, 93–101. [Google Scholar] [CrossRef]
- Hu, R.; Liu, W.; Li, H.; Yang, L.; Chen, C.; Xia, Z.Y.; Guo, L.J.; Xie, H.; Zhou, H.D.; Wu, X.P.; et al. A Runx2/miR-3960/miR-2861 regulatory feedback loop during mouse osteoblast differentiation. J. Biol. Chem. 2011, 286, 12328–12339. [Google Scholar] [CrossRef]
- Lu, G.D.; Cheng, P.; Liu, T.; Wang, Z. BMSC-Derived Exosomal miR-29a Promotes Angiogenesis and Osteogenesis. Front. Cell Dev. Biol. 2020, 8, 608521. [Google Scholar] [CrossRef]
- Behera, J.; Kelly, K.E.; Voor, M.J.; Metreveli, N.; Tyagi, S.C.; Tyagi, N. Hydrogen Sulfide Promotes Bone Homeostasis by Balancing Inflammatory Cytokine Signaling in CBS-Deficient Mice through an Epigenetic Mechanism. Sci. Rep. 2018, 8, 15226. [Google Scholar] [CrossRef] [PubMed]
- Behera, J.; Kumar, A.; Voor, M.J.; Tyagi, N. Exosomal lncRNA-H19 promotes osteogenesis and angiogenesis through mediating Angpt1/Tie2-NO signaling in CBS-heterozygous mice. Theranostics 2021, 11, 7715–7734. [Google Scholar] [CrossRef]
- Chevillet, J.R.; Kang, Q.; Ruf, I.K.; Briggs, H.A.; Vojtech, L.N.; Hughes, S.M.; Cheng, H.H.; Arroyo, J.D.; Meredith, E.K.; Gallichotte, E.N.; et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. USA 2014, 111, 14888–14893. [Google Scholar] [CrossRef] [PubMed]
- Albanese, M.; Chen, Y.-F.A.; Hüls, C.; Gärtner, K.; Tagawa, T.; Mejias-Perez, E.; Keppler, O.T.; Göbel, C.; Zeidler, R.; Shein, M. Micro RNAs are minor constituents of extracellular vesicles and are hardly delivered to target cells. BioRxiv 2020. [Google Scholar] [CrossRef]
- Kim, H.S.; Choi, D.Y.; Yun, S.J.; Choi, S.M.; Kang, J.W.; Jung, J.W.; Hwang, D.; Kim, K.P.; Kim, D.W. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J. Proteome Res. 2012, 11, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Tan, S.S.; Teh, B.J.; Sze, S.K.; Arslan, F.; de Kleijn, D.P.; Choo, A.; Lim, S.K. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int. J. Proteom. 2012, 2012, 971907. [Google Scholar] [CrossRef]
- McBride, J.D.; Rodriguez-Menocal, L.; Guzman, W.; Khan, A.; Myer, C.; Liu, X.; Bhattacharya, S.K.; Badiavas, E.V. Proteomic analysis of bone marrow-derived mesenchymal stem cell extracellular vesicles from healthy donors: Implications for proliferation, angiogenesis, Wnt signaling, and the basement membrane. Stem Cell Res. Ther. 2021, 12, 328. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Ni, C.Y.; Chen, C.Y.; Rao, S.S.; Yin, H.; Huang, J.; Tan, Y.J.; Wang, Z.X.; Cao, J.; et al. Human umbilical cord mesenchymal stromal cells-derived extracellular vesicles exert potent bone protective effects by CLEC11A-mediated regulation of bone metabolism. Theranostics 2020, 10, 2293–2308. [Google Scholar] [CrossRef]
- Shen, B.; Vardy, K.; Hughes, P.; Tasdogan, A.; Zhao, Z.; Yue, R.; Crane, G.M.; Morrison, S.J. Integrin alpha11 is an Osteolectin receptor and is required for the maintenance of adult skeletal bone mass. Elife 2019, 8, e42274. [Google Scholar] [CrossRef]
- Huang, B.; Su, Y.; Shen, E.; Song, M.; Liu, D.; Qi, H. Extracellular vesicles from GPNMB-modified bone marrow mesenchymal stem cells attenuate bone loss in an ovariectomized rat model. Life Sci. 2021, 272, 119208. [Google Scholar] [CrossRef] [PubMed]
- Roubelakis, M.G.; Pappa, K.I.; Bitsika, V.; Zagoura, D.; Vlahou, A.; Papadaki, H.A.; Antsaklis, A.; Anagnou, N.P. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: Comparison to bone marrow mesenchymal stem cells. Stem Cells Dev. 2007, 16, 931–952. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, G.; Li, P.; Zhou, X.; Zhang, Y. Urine-derived stem cells: A novel and versatile progenitor source for cell-based therapy and regenerative medicine. Genes Dis. 2014, 1, 8–17. [Google Scholar] [CrossRef]
- Chen, C.Y.; Rao, S.S.; Tan, Y.J.; Luo, M.J.; Hu, X.K.; Yin, H.; Huang, J.; Hu, Y.; Luo, Z.W.; Liu, Z.Z.; et al. Extracellular vesicles from human urine-derived stem cells prevent osteoporosis by transferring CTHRC1 and OPG. Bone Res. 2019, 7, 18. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z. Bone marrow mesenchymal stem cell-derived exosomes enhance osteoclastogenesis during alveolar bone deterioration in rats. RSC Adv. 2017, 7, 21153–21163. [Google Scholar] [CrossRef]
- Shojaei, S.; Hashemi, S.M.; Ghanbarian, H.; Salehi, M.; Mohammadi-Yeganeh, S. Effect of mesenchymal stem cells-derived exosomes on tumor microenvironment: Tumor progression versus tumor suppression. J. Cell. Physiol. 2019, 234, 3394–3409. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Lai, L.C. The potential roles of stem cell-derived extracellular vesicles as a therapeutic tool. Ann. Transl. Med. 2019, 7, 693. [Google Scholar] [CrossRef] [PubMed]
- Turturici, G.; Tinnirello, R.; Sconzo, G.; Geraci, F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: Advantages and disadvantages. Am. J. Physiol. Cell Physiol. 2014, 306, C621–C633. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.; Weng, J.; Guo, L.; Chen, X.; Du, X. Novel insights into MSC-EVs therapy for immune diseases. Biomark Res. 2019, 7, 6. [Google Scholar] [CrossRef]
- Trindade, R.P.; Renault, N.; El Harane, N.; Menasché, P.; Williams, G.R. Extracellular vesicles can be processed by electrospinning without loss of structure or function. Mater. Lett. 2021, 282, 128671. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, Q.; Chen, D.; Yu, H.Y.; Luo, A.X.; Suo, L.P.; Cai, Y.; Cai, D.Y.; Luo, J.; Huang, J.F.; et al. Extracellular vesicles derived from mesenchymal stem cells: A platform that can be engineered. Histol. Histopathol. 2021, 36, 615–632. [Google Scholar] [CrossRef]
- Gowen, A.; Shahjin, F.; Chand, S.; Odegaard, K.E.; Yelamanchili, S.V. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Front. Cell Dev. Biol. 2020, 8, 149. [Google Scholar] [CrossRef]
- Zhang, C.; Shang, Y.; Chen, X.; Midgley, A.C.; Wang, Z.; Zhu, D.; Wu, J.; Chen, P.; Wu, L.; Wang, X.; et al. Supramolecular Nanofibers Containing Arginine-Glycine-Aspartate (RGD) Peptides Boost Therapeutic Efficacy of Extracellular Vesicles in Kidney Repair. ACS Nano 2020, 14, 12133–12147. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Brammer, K.S.; Li, Y.S.; Teng, D.; Engler, A.J.; Chien, S.; Jin, S. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. USA 2009, 106, 2130–2135. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Y.; Xiao, L.; Lu, H.; Ma, Y.; Liu, Q.; Wang, X. Surface engineering of titania nanotubes incorporated with double-layered extracellular vesicles to modulate inflammation and osteogenesis. Regen. Biomater. 2021, 8, rbab010. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, J.; Cai, L.; Liu, T.; Wang, X.; Zhang, Y.; Zhou, Z.; Li, T.; Liu, M.; Lai, R.; et al. Bone-Targeted Extracellular Vesicles from Mesenchymal Stem Cells for Osteoporosis Therapy. Int. J. Nanomed. 2020, 15, 7967–7977. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Zhang, X. Aptamers as Versatile Ligands for Biomedical and Pharmaceutical Applications. Int. J. Nanomed. 2020, 15, 1059–1071. [Google Scholar] [CrossRef]
- Bouvier-Muller, A.; Duconge, F. Application of aptamers for in vivo molecular imaging and theranostics. Adv. Drug Deliv. Rev. 2018, 134, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.W.; Li, F.X.; Liu, Y.W.; Rao, S.S.; Yin, H.; Huang, J.; Chen, C.Y.; Hu, Y.; Zhang, Y.; Tan, Y.J.; et al. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale 2019, 11, 20884–20892. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Beretti, F.; Zavatti, M.; Bertucci, E.; Ribeiro Luz, S.; Palumbo, C.; Maraldi, T. Amniotic Fluid Stem Cell-Derived Extracellular Vesicles Counteract Steroid-Induced Osteoporosis In Vitro. Int. J. Mol. Sci. 2020, 22, 38. [Google Scholar] [CrossRef]
- Zhao, P.; Xiao, L.; Peng, J.; Qian, Y.Q.; Huang, C.C. Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3962–3970. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huang, J.; Chen, F.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Li, X.; Li, X.; Yang, L. MSC-derived small extracellular vesicles overexpressing miR-20a promoted the osteointegration of porous titanium alloy by enhancing osteogenesis via targeting BAMBI. Stem Cell Res. Ther. 2021, 12, 348. [Google Scholar] [CrossRef] [PubMed]
| No. | NCT04501354 | NCT04499105 | NCT04414592 | NCT04759105 | NCT05066334 | NCT04297813 | NCT03692221 | NCT04735185 | No. | NCT04849429 | NCT04998058 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase | Mesenchymal stem cells (MSCs) | Phase 2 | Phase 2 | Not Applicable | Phase 2 | Phase 2 | Phase 3 | Early Phase 1 | Not Applicable | Phase | Extracellular vesicles (EVs) | Phase 1 | Phase 1 |
| Intervention/treatment | Mesenchymal Stem Cell | Mesenchymal Stem Cell + NaCl 0,9% 2 mL | Mesenchymal stem cells | Mesenchymal stem cells | Mesenchymal stem cells | Combination Product: Advanced medicinal Therapy (MSC combined with biomaterial) Procedure: Autologous bone graft | Mesenchymal stem cells | Other: Autologous stem cells Drug: CorticosteroidDrug: Local anesthetic | Intervention/treatment | Biological: Platelet rich plasma (PRP) with exosomes | Procedure: Maxillary sinus floor elevation grafting with synthetic bone substitute. | ||
| Intervention model | Single Group Assignment | Single Group Assignment | Single Group Assignment | Parallel Assignment | Parallel Assignment | Parallel Assignment | Parallel Assignment | Parallel Assignment | Intervention Model | Parallel Assignment | Parallel Assignment | ||
| Cell sources | Umbilical cord | Umbilical cord | Human umbilical cord | Bone marrow | Bone marrow | - | Bone marrow | Bone marrow | EV sources/term | Platelet-rich Plasma/Exosomes | Adipose tissue-derived mesenchymal stem cells/Conditioned medium | ||
| Condition or disease | Osteoporosis | Degenerative Disc DiseaseLow Back Pain Disc Degeneration | Lumbar Disc Degeneration Lumbar Disc Herniation | Intervertebral Disc Degeneration Chronic Low-back Pain | Intervertebral Disc Degeneration Chronic Low-back Pain | Alveolar Bone Atrophy | Disc Degeneration | Chronic Low Back Pain Degenerative Disc Disease | Condition or disease | Chronic Low Back Pain Degenerative Disc Disease | Bone Loss, Osteoclastic Bone Loss, Alveolar Alveolar Bone Loss Alveolar Bone Atrophy Grafting Bone | ||
| Last update posted | 7 August 2020 | 6 August 2020 | 4 June 2020 | 18 February 2021 | 4 October 2021 | 12 March 2020 | 4 April 2019 | 10 May 2021 | Last Update Posted | 19 April 2021 | 10 August 2021 | ||
| Sponsor | Indonesia University | Indonesia University | Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine | Campus Bio-Medico University of Rome | Campus Bio-Medico University of Rome | University of Bergen | University Hospitals Cleveland Medical Center | Johns Hopkins University | Sponsor | Dr. Himanshu Bansal Foundation | Pontifical Catholic University of Rio Grande do Sul |
| Animal Model | Terminology | Source of EVs | Administration Route | EVs Cargos | Duration of Treatment | Time Point of Sacrifice | Target | Refs. |
|---|---|---|---|---|---|---|---|---|
| In vitro (steroid-related osteoporosis) | EVs | AFSCs | In vitro model | Not revealed | 4 days (Dose not revealed) | Not applicable | Related proteins (SIRT1, FoxO, Nrf2, p21, Galectin-3, AP-1 complex) | [120] |
| OVX mice | Aptamer-functionalized exosomes | BMSCs | IV injection | miR-26a | 100 μg EVs, once per week for 2 months | endpoint of treatment | Not revealed | [119] |
| Healthy mice | Exosomes | BMSCs | IV injection | miR-29a | 100 μg EVs, twice per week for 2 months | endpoint of treatment | VASH1 | [91] |
| CBS+/− heterozygous mice | Exosomes | BMSCs | IV injection | lnc-H19 | 100 μg EVs, 3 times per week for 2 months | endpoint of treatment | miR-106a/Angpt1 | [93] |
| OVX rat | Exosomes | BMSCs | IV injection | miR-186 | 1013/mL EVs, once per week for 1 months | endpoint of treatment | Hippo signaling pathway | [86] |
| In vitro | Exosomes | BMSCs | In vitro model | Not revealed | Not revealed | Not revealed | MAPK signaling pathway | [121] |
| OVX rat | GPNMB-modified BMSC-EV | BMSCs | IV injection | GPNMB | 100 μg EVs, once per week for 2 months | endpoint of treatment | Wnt/β-catenin signaling pathway | [101] |
| Aged male mice (16 months old) | EVs | hUCB | IV injection | miR-3960 | 100 μg EVs, once per week for 8 weeks | 1, 2 and 8 weeks after the first treatment | HOXA2 | [89] |
| OVX mice | EVs | hucMSCs | IV injection | CLEC11A | 100 μg EVs, once per week for 2 months | endpoint of treatment | Integrin α11 | [99] |
| In vitro | EVs isolated from OVX mice with agomiR-miR-29b-3p injection | BMSCs | In vitro model | miR-29b-3p | Not revealed | Not applicable | KDM5A/NF-kB pathway | [87] |
| OVX mice | Exosomes | WJ-MSCs | IP injection | miR-328-3p, miR-2110 | 0.5 mg/kg EVs, every 3 days for 6 weeks | endpoint of treatment | CHRD, TNF | [88] |
| OVX rat | sEV | BMSCs | Not revealed | miR-20a/BAMBI | 3 weeks (Dose not revealed) | endpoint of treatment | BAMBI | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.-H.; Chen, Y.-A.; Ke, C.-C.; Liu, R.-S. Mesenchymal Stem Cell-Derived Extracellular Vesicle: A Promising Alternative Therapy for Osteoporosis. Int. J. Mol. Sci. 2021, 22, 12750. https://doi.org/10.3390/ijms222312750
Lu C-H, Chen Y-A, Ke C-C, Liu R-S. Mesenchymal Stem Cell-Derived Extracellular Vesicle: A Promising Alternative Therapy for Osteoporosis. International Journal of Molecular Sciences. 2021; 22(23):12750. https://doi.org/10.3390/ijms222312750
Chicago/Turabian StyleLu, Cheng-Hsiu, Yi-An Chen, Chien-Chih Ke, and Ren-Shyan Liu. 2021. "Mesenchymal Stem Cell-Derived Extracellular Vesicle: A Promising Alternative Therapy for Osteoporosis" International Journal of Molecular Sciences 22, no. 23: 12750. https://doi.org/10.3390/ijms222312750
APA StyleLu, C.-H., Chen, Y.-A., Ke, C.-C., & Liu, R.-S. (2021). Mesenchymal Stem Cell-Derived Extracellular Vesicle: A Promising Alternative Therapy for Osteoporosis. International Journal of Molecular Sciences, 22(23), 12750. https://doi.org/10.3390/ijms222312750








