BRAF Mutation Is Associated with Hyperplastic Polyp-Associated Gastric Cancer
Abstract
:1. Introduction
2. Results
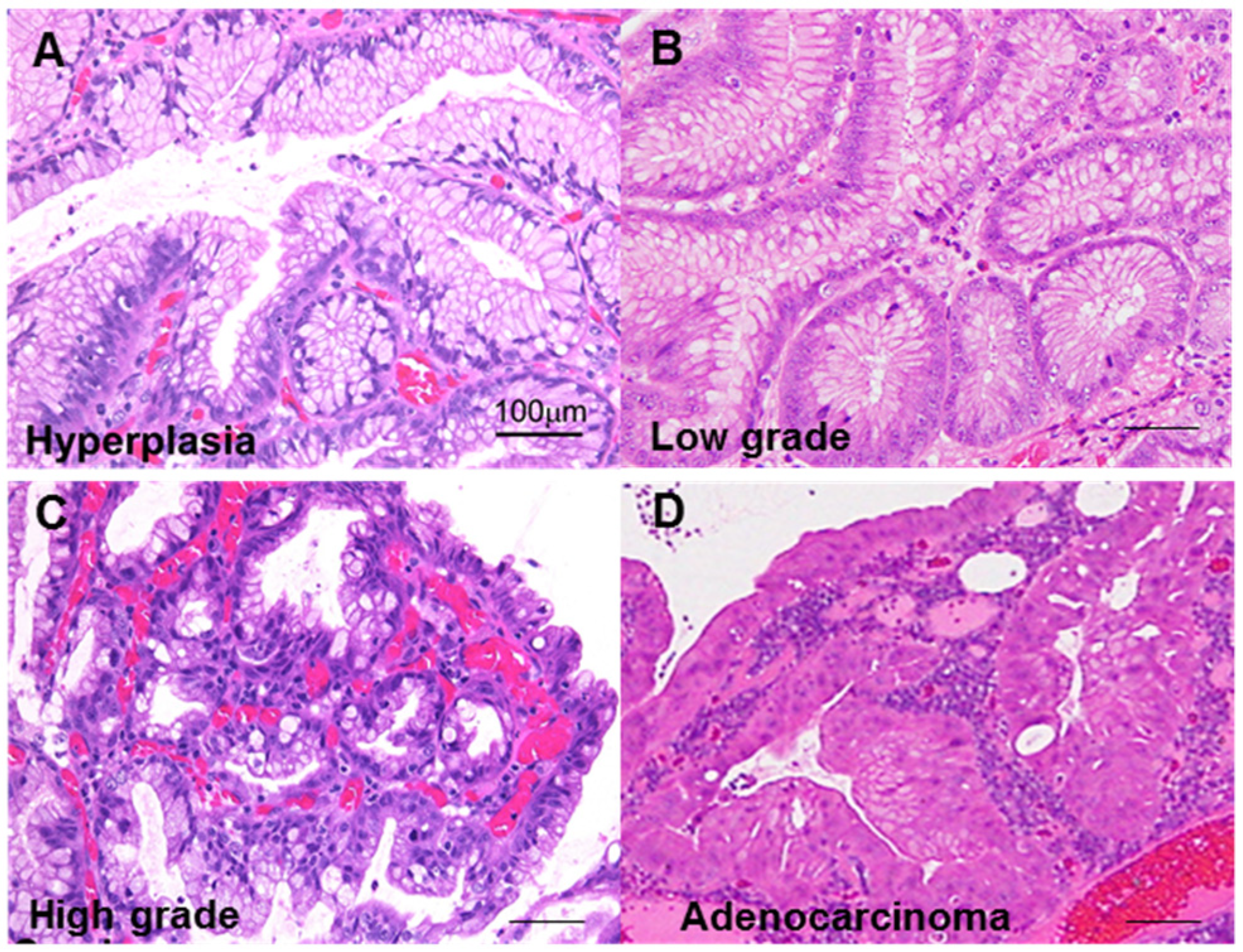
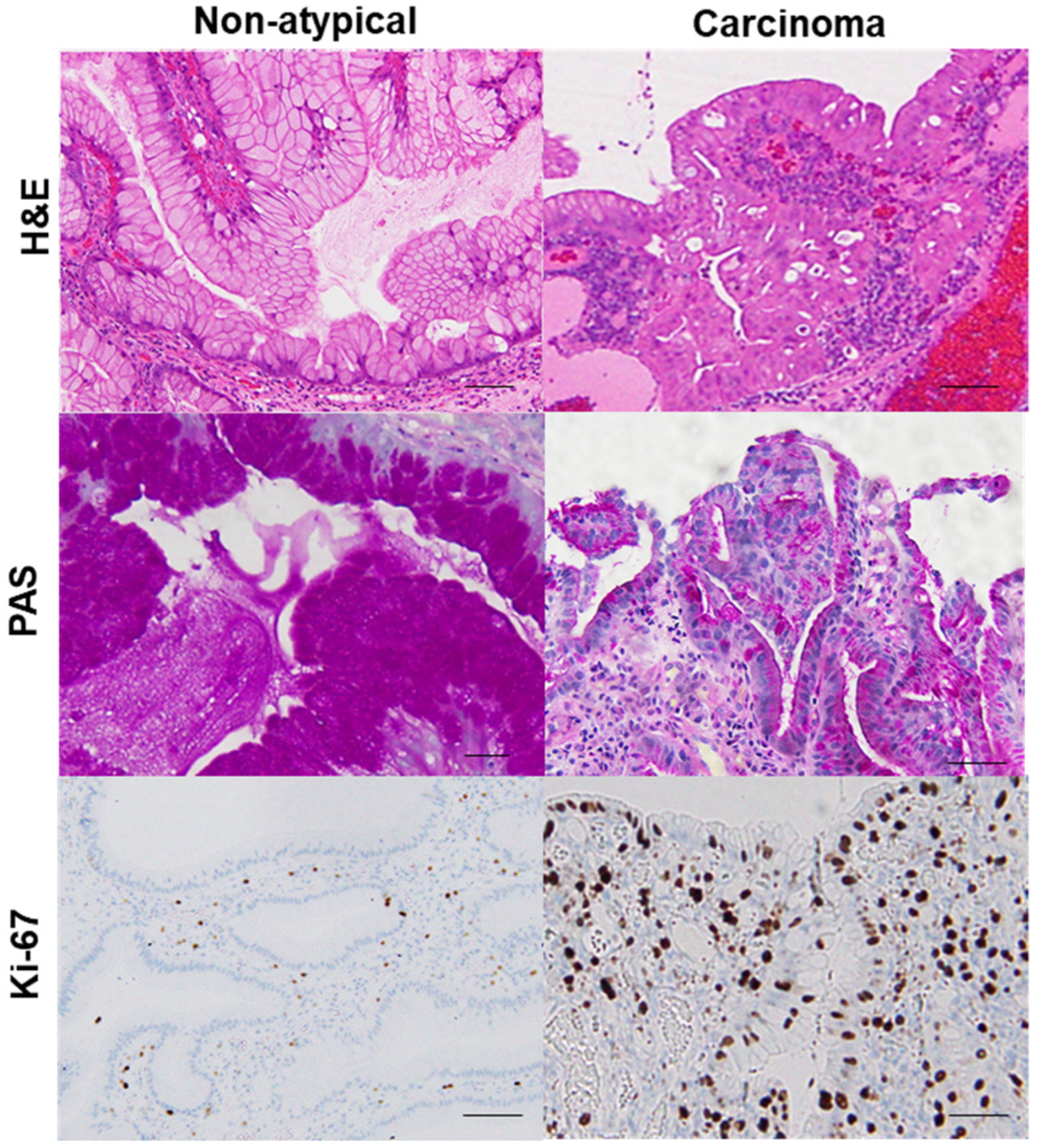
2.1. GHP Atypia and the Associated Clinical and Morphological Characteristics
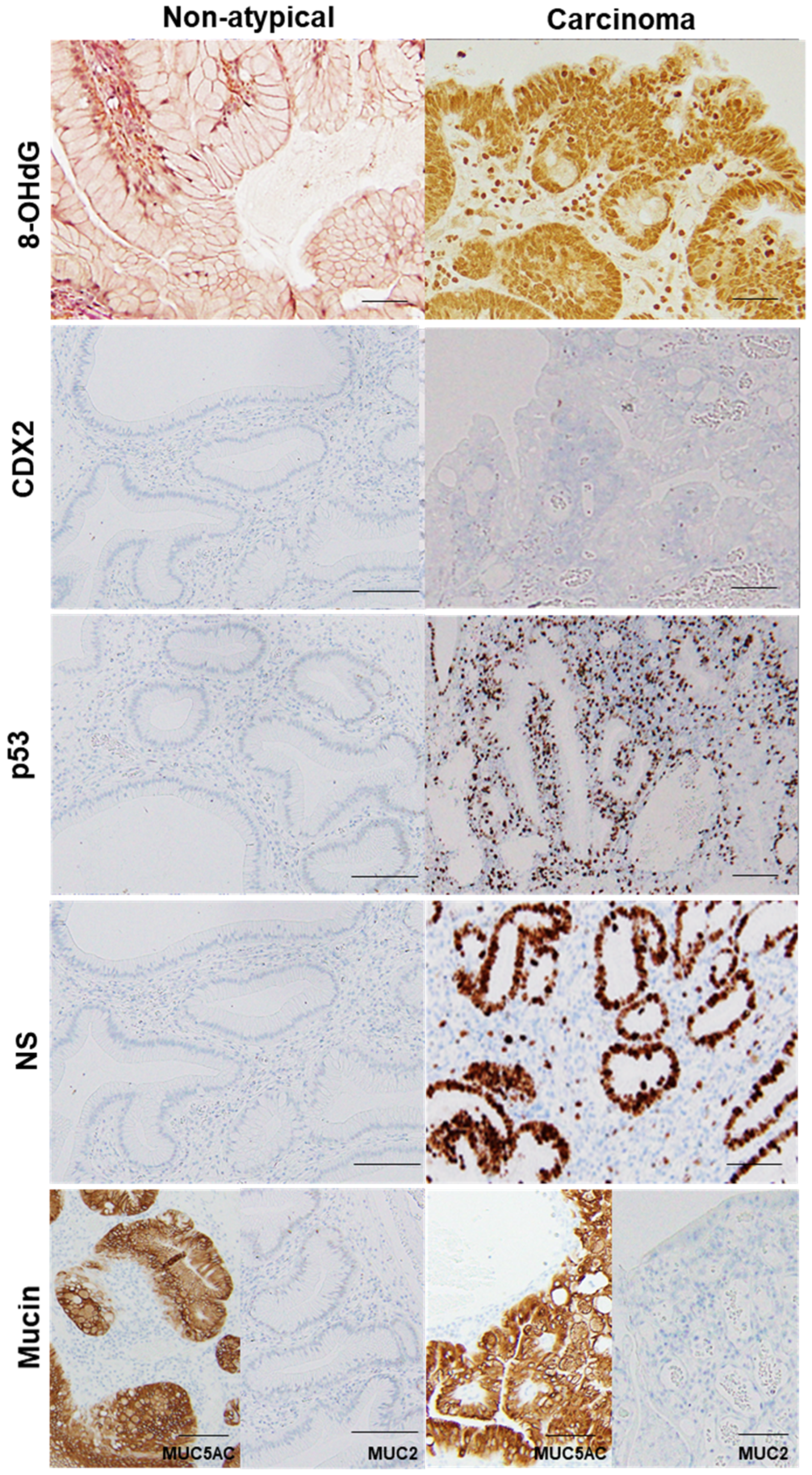
2.2. GHP Atypia and Oxidative Stress
2.3. GHP Atypia and Expression of Cancer-Associated Proteins
2.4. Features of HPAGC
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Histological Analyses
4.3. Immunohistochemistry
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Mutation Analysis
4.6. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GHP | gastric hyperplastic polyps |
| CDX | caudal type homeobox transcription factor |
| HPAGC | hyperplastic polyp-associated gastric cancer |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 |
| AKT | v-akt murine thymoma viral oncogene homolog |
| TCGA | the cancer genome atlas |
| PAS | periodic acid–Schiff |
| HNE | hydroxy nonenal |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| NS | nucleostemin |
| PIK3CA | phosphatidylinositol 3-kinase catalytic subunit alpha |
References
- Statistics Bureau Ministry of Internal Affairs and Communications Japan. Statistical Handbook of Japan 2017; Statistics Bureau Ministry of Internal Affairs and Communications Japan: Tokyo, Japan, 2017.
- Murata, M. Inflammation and cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef] [Green Version]
- Watari, J.; Chen, N.; Amenta, P.S.; Fukui, H.; Oshima, T.; Tomita, T.; Miwa, H.; Lim, K.J.; Das, K.M. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J. Gastroenterol. 2014, 20, 5461–5473. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Kuniyasu, H.; Luo, Y.; Kitayoshi, M.; Tanabe, E.; Kato, D.; Shinya, S.; Fujii, K.; Ohmori, H.; Yamashita, Y. Increased phosphorylation of AKT in high-risk gastric mucosa. Anticancer Res. 2013, 33, 3295–3300. [Google Scholar]
- Kuniyasu, H.; Kitadai, Y.; Mieno, H.; Yasui, W. Helicobactor pylori infection is closely associated with telomere reduction in gastric mucosa. Oncology 2003, 65, 275–282. [Google Scholar] [CrossRef]
- Kuniyasu, H.; Yasui, W.; Yokozaki, H.; Tahara, E. Helicobacter pylori infection and carcinogenesis of the stomach. Langenbeck Arch 2000, 385, 69–74. [Google Scholar] [CrossRef]
- Tahara, E. Genetic pathways of two types of gastric cancer. IARC Sci. Pub. 2004, 157, 327–349. [Google Scholar]
- Yu, X.; Wang, Z.; Wang, L.; Meng, X.; Zhou, C.; Xin, Y.; Sun, W.; Dong, Q. Gastric hyperplastic polyps inversely associated with current Helicobacter pylori infection. Exp. Ther. Med. 2020, 19, 3143–3149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, R.; Chetty, R. Gastric hyperplastic polyps: A review. Dig. Dis. Sci. 2009, 54, 1839–1846. [Google Scholar] [CrossRef]
- Takeuchi, C.; Yamamichi, N.; Shimamoto, T.; Takahashi, Y.; Mitsushima, T.; Koike, K. Gastric polyps diagnosed by double-contrast upper gastrointestinal barium X-ray radiography mostly arise from the Helicobacter pylori-negative stomach with low risk of gastric cancer in Japan. Gastric Cancer 2017, 20, 314–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushima, R.; Hattori, T. Histogenesis and characteristics of gastric-type adenocarcinomas in the stomach. J. Cancer Res. Clin. Oncol. 1993, 120, 103–111. [Google Scholar] [CrossRef]
- Daibo, M.; Itabashi, M.; Hirota, T. Malignant transformation of gastric hyperplastic polyps. Am. J. Gastroenterol. 1987, 82, 1016–1025. [Google Scholar] [PubMed]
- Orlowska, J.; Jarosz, D.; Pachlewski, J.; Butruk, E. Malignant transformation of benign epithelial gastric polyps. Am. J. Gastroenterol. 1995, 90, 2152–2159. [Google Scholar]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011, 14, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Bornschein, J.; Malfertheiner, P. Helicobacter pylori and gastric cancer. Dig. Dis. 2014, 32, 249–264. [Google Scholar] [CrossRef]
- Dirschmid, K.; Platz-Baudin, C.; Stolte, M. Why is the hyperplastic polyp a marker for the precancerous condition of the gastric mucosa? Virchows Arch. 2006, 448, 80–84. [Google Scholar] [CrossRef]
- Nam, S.Y.; Lee, S.W.; Jeon, S.W.; Kwon, Y.H.; Lee, H.S. Helicobacter pylori Eradication Regressed Gastric Hyperplastic Polyp: A Randomized Controlled Trial. Dig. Dis. Sci. 2020, 65, 3652–3659. [Google Scholar] [CrossRef] [PubMed]
- Horvath, B.; Pai, R.K. Prevalence of Helicobacter pylori in Gastric Hyperplastic Polyps. Int. J. Surg. Pathol. 2016, 24, 704–708. [Google Scholar] [CrossRef]
- Gonzalez-Obeso, E.; Fujita, H.; Deshpande, V.; Ogawa, F.; Lisovsky, M.; Genevay, M.; Grzyb, K.; Brugge, W.; Lennerz, J.K.; Shimizu, M.; et al. Gastric hyperplastic polyps: A heterogeneous clinicopathologic group including a distinct subset best categorized as mucosal prolapse polyp. Am. J. Surg. Pathol. 2011, 35, 670–677. [Google Scholar] [CrossRef]
- Camilo, V.; Barros, R.; Sousa, S.; Magalhães, A.M.; Lopes, T.; Mário Santos, A.; Pereira, T.; Figueiredo, C.; David, L.; Almeida, R. Helicobacter pylori and the BMP pathway regulate CDX2 and SOX2 expression in gastric cells. Carcinogenesis 2012, 33, 1985–1992. [Google Scholar] [CrossRef]
- Akhavan-Niaki, H.; Samadani, A.A. Molecular insight in gastric cancer induction: An overview of cancer stemness genes. Cell Biochem. Biophys. 2014, 68, 463–473. [Google Scholar] [CrossRef]
- Hatakeyama, M. Helicobacter pylori and gastric carcinogenesis. J. Gastroenterol. 2009, 44, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.P.; Meltzer, S.J. A review of the genomics of gastric cancer. Clin. Gastroenterol. Hepatol. 2006, 4, 416–425. [Google Scholar] [CrossRef]
- Hatakeyama, M. Helicobacter pylori CagA and gastric cancer: A paradigm for hit-and-run carcinogenesis. Cell Host Microbe 2014, 15, 306–316. [Google Scholar] [CrossRef] [Green Version]
- Butcher, L.D.; den Hartog, G.; Ernst, P.B.; Crowe, S.E. Oxidative Stress Resulting From Helicobacter pylori Infection Contributes to Gastric Carcinogenesis. Cell Mol. Gastroenterol. Hepatol. 2017, 3, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Okada, F. Beyond foreign-body-induced carcinogenesis: Impact of reactive oxygen species derived from inflammatory cells in tumorigenic conversion and tumor progression. Int. J. Cancer 2007, 121, 2364–2372. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.P.; Cobb, C.A.; Chapman, R.W.; Kettlewell, M.; Hoang, P.; Haot, B.J.; Jewell, D.P. Cap polyposis—an unusual cause of diarrhoea. Gut 1993, 34, 562–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, G.T.; Bussey, H.J.R.; Morson, B.C. Inflammatory ’cap’ polyps of the large intestine. Br. J. Surg. 1985, 72, S133. [Google Scholar]
- Chetty, R.; Bhathal, P.S.; Slavin, J.L. Prolapse-induced inflammatory polyps of the colorectum and anal transitional zone. Histopathology 1993, 23, 63–67. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.H.; Láng, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Huo, S.; Sui, Y.; Du, Z.; Zhao, H.; Liu, Y.; Li, W.; Wan, X.; Liu, T.; Zhang, G. Mutation Status and Immunohistochemical Correlation of KRAS, NRAS, and BRAF in 260 Chinese Colorectal and Gastric Cancers. Front. Oncol. 2018, 8, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, N.; Yamada, Y.; Taniguchi, H.; Fukahori, M.; Sasaki, Y.; Shoji, H.; Honma, Y.; Iwasa, S.; Takashima, A.; Kato, K.; et al. Clinicopathological features and prognostic roles of KRAS, BRAF, PIK3CA and NRAS mutations in advanced gastric cancer. BMC Res. Notes 2014, 7, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, C.; Pinto, M.; Duval, A.; Brennetot, C.; Domingo, E.; Espín, E.; Armengol, M.; Yamamoto, H.; Hamelin, R.; Seruca, R.; et al. BRAF mutations characterize colon but not gastric cancer with mismatch repair deficiency. Oncogene 2003, 22, 9192–9196. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, N.; Feng, Y.; Stolfi, C.; Kurosu, Y.; Green, M.; Lin, J.; Green, M.E.; Sentani, K.; Yasui, W.; McMahon, M.; et al. BRAF(V600E) cooperates with CDX2 inactivation to promote serrated colorectal tumorigenesis. eLife 2017, 6, e20331. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Ono, Y.; Mizukami, Y.; Itoh, H.; Nakajima, N.; Arai, H.; Tanaka, S.; Nobusawa, S.; Yokoo, H.; Onozato, Y. Comparative genome-wide analysis of gastric adenocarcinomas with hyperplastic polyp components. Virchows Arch. 2019, 475, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Imai, Y.; Ichihara, T.; Miyagawa, K.; Kanemitsu, K.; Ajiki, T.; Kawasaki, K.; Kamigaki, T.; Ikuta, H.; Ohbayashi, C.; et al. Adenocarcinoma arising in gastric inverted hyperplastic polyp: A case report and review of the literature. Pathol. Res. Pract. 2007, 203, 53–56. [Google Scholar] [CrossRef]
- Murakami, K.; Mitomi, H.; Yamashita, K.; Tanabe, S.; Saigenji, K.; Okayasu, I. p53, but not c-Ki-ras, mutation and down-regulation of p21WAF1/CIP1 and cyclin D1 are associated with malignant transformation in gastric hyperplastic polyps. Am. J. Clin. Pathol. 2001, 115, 224–234. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.H.; Chen, Z.T.; Wang, W.H.; Fan, X.J.; Huang, Y.; Wu, X.B.; Huang, J.L.; Wang, J.X.; Lin, H.J.; Tan, X.L.; et al. KRAS G12V Mutation is an Adverse Prognostic Factor of Chinese Gastric Cancer Patients. J. Cancer 2019, 10, 821–828. [Google Scholar] [CrossRef]
- Pilié, P.G.; Tang, C.; Mills, G.B.; Yap, T.A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 81–104. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Van Cutsem, E.; Rougier, P.; Ciardiello, F.; Heeger, S.; Schlichting, M.; Celik, I.; Köhne, C.H. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: Pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur. J. Cancer 2012, 48, 1466–1475. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. New Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuniyasu, H.; Luo, Y.; Fujii, K.; Sasahira, T.; Moriwaka, Y.; Tatsumoto, N.; Sasaki, T.; Yamashita, Y.; Ohmori, H. CD10 enhances metastasis of colorectal cancer by abrogating the anti-tumoural effect of methionine-enkephalin in the liver. Gut 2010, 59, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Bando, H.; Yoshino, T.; Shinozaki, E.; Nishina, T.; Yamazaki, K.; Yamaguchi, K.; Yuki, S.; Kajiura, S.; Fujii, S.; Yamanaka, T.; et al. Simultaneous identification of 36 mutations in KRAS codons 61 and 146, BRAF, NRAS, and PIK3CA in a single reaction by multiplex assay kit. BMC Cancer 2013, 13, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Parameter | Polyp Atypia (1) | p-Value | |||
|---|---|---|---|---|---|
| None | Low Grade | High Grade | Cancer | ||
| Number | 72 | 20 | 7 | 5 | |
| Age (yrs) | 55 ± 13 | 60 ± 11 | 63 ± 10 | 70 ± 5 | 0.0191 |
| Sex (male: female) | 22:22 | 12:8 | 4:3 | 3:2 | NS |
| Size (mm) | 4.7 ± 1.8 | 6.9 ± 1.7 | 12.3 ± 5.9 | 14.6 ± 5.4 | <0.0001 |
| H. pylori infection | |||||
| Incidence (%) | 9 | 10 | 0 | 20 | NS |
| Grade (2) | 0.18 ± 0.65 | 0.10 ± 0.31 | 0 | 0.20 ± 0.45 | NS |
| Intestinal metaplasia (2) | |||||
| Incidence (%) | 32 | 55 | 29 | 40 | NS |
| Grade (2) | 0.5 ± 0.88 | 0.55 ± 0.51 | 0.29 ± 0.49 | 0.40 ± 0.55 | NS |
| PAS staining (3) | 2.0 ± 0.1 | 1.4 ± 0.5 | 0.8 ± 0.3 | 0.6 ± 0.2 | <0.0001 |
| Ki-67 index (%) (4) | 26 ± 13 | 52 ± 23 | 74 ± 18 | 86 ± 10 | <0.0001 |
| Parameter | Polyp Atypia (1) | p-Value | |||
|---|---|---|---|---|---|
| None | Low Grade | High Grade | Cancer | ||
| Number | 44 | 20 | 7 | 5 | |
| Granulation tissue (%) (2) | 15 ± 12 | 48 ± 23 | 79 ± 7 | 87 ± 7 | <0.0001 |
| Tumor 8-OHdG index (3) | 23 ± 12 | 52 ± 18 | 86 ± 8 | 94 ± 5 | <0.0001 |
| 4-HNE (ng/g) (4) | 0.5 ± 0.1 | 0.8 ± 0.2 | 1.8 ± 0.3 | 2.4 ± 0.3 | <0.0001 |
| CDX2 incidence (%) (3) | 25 | 50 | 29 | 40 | NS |
| p53 incidence (%) (3) | 0 | 0 | 43 | 100 | 0.0075 |
| NS index (%) (3) | 18 ± 8 | 53 ± 17 | 80 ± 2 | 84 ± 6 | <0.0001 |
| Case | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Polyp size (mm) | 11 | 8 | 20 | 20 | 14 |
| Cancer lesion (mm) | 2 | 4 | 3 | 3 | 2 |
| Histology (1) | tub1 | Pap + tub1 | tub1 | tub1 | Pap + tub1 |
| Invasion | In situ | In situ | Invasive | In situ | In situ |
| Mucin type (2) | Mixed | Gastric | Gastric | Mixed | Mixed |
| Ki-67 index (%) | 94 | 92 | 85 | 70 | 90 |
| 8-OHdG index (%) (3) | 100 | 100 | 92 | 88 | 90 |
| CDX2 index (%) (3) | 24 | 0 | 0 | 5 | 80 |
| p53 index (%) (3) | 78 | 72 | 19 | 17 | 89 |
| NS index (%) (3) | 78 | 87 | 85 | 82 | 88 |
| KRAS G12D/G13D | -/- | -/- | -/- | -/- | -/- |
| BRAF V600E | - | + | + | - | - |
| Target (1) | Manufacturer (2) | Clone | Code | Working Concentration (μg/mL) |
|---|---|---|---|---|
| Ki-67 | DAKO-Agilent | MIB-1 | M7240 | 0.5 |
| 8-OHdG | JaIKA | N45.1 | MOG-100P | 5 |
| CDX2 | Abcam | CDX2-88 | ab157524 | 0.2 |
| p53 | Abcam | PAb240 | ab26 | 1 |
| NS | Abcam | - | ab70346 | 0.5 |
| MUC5AC | DAKO-Agilent | CLH2 | M731601 | 0.5 |
| MUC2 | DAKO-Agilent | Ccp58 | M7313 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujiwara-Tani, R.; Okamoto, A.; Katsuragawa, H.; Ohmori, H.; Fujii, K.; Mori, S.; Kishi, S.; Sasaki, T.; Nakashima, C.; Kawahara, I.; et al. BRAF Mutation Is Associated with Hyperplastic Polyp-Associated Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 12724. https://doi.org/10.3390/ijms222312724
Fujiwara-Tani R, Okamoto A, Katsuragawa H, Ohmori H, Fujii K, Mori S, Kishi S, Sasaki T, Nakashima C, Kawahara I, et al. BRAF Mutation Is Associated with Hyperplastic Polyp-Associated Gastric Cancer. International Journal of Molecular Sciences. 2021; 22(23):12724. https://doi.org/10.3390/ijms222312724
Chicago/Turabian StyleFujiwara-Tani, Rina, Ayaka Okamoto, Hiroyuki Katsuragawa, Hitoshi Ohmori, Kiyomu Fujii, Shiori Mori, Shingo Kishi, Takamitsu Sasaki, Chie Nakashima, Isao Kawahara, and et al. 2021. "BRAF Mutation Is Associated with Hyperplastic Polyp-Associated Gastric Cancer" International Journal of Molecular Sciences 22, no. 23: 12724. https://doi.org/10.3390/ijms222312724
APA StyleFujiwara-Tani, R., Okamoto, A., Katsuragawa, H., Ohmori, H., Fujii, K., Mori, S., Kishi, S., Sasaki, T., Nakashima, C., Kawahara, I., Hojo, Y., Nishiguchi, Y., Mori, T., Mizumoto, T., Nagai, K., Luo, Y., & Kuniyasu, H. (2021). BRAF Mutation Is Associated with Hyperplastic Polyp-Associated Gastric Cancer. International Journal of Molecular Sciences, 22(23), 12724. https://doi.org/10.3390/ijms222312724






