Abstract
This review focuses on the effects of hydrogen sulfide (H2S) on the unique bioenergetic molecular machines in mitochondria and bacteria—the protein complexes of electron transport chains and associated enzymes. H2S, along with nitric oxide and carbon monoxide, belongs to the class of endogenous gaseous signaling molecules. This compound plays critical roles in physiology and pathophysiology. Enzymes implicated in H2S metabolism and physiological actions are promising targets for novel pharmaceutical agents. The biological effects of H2S are biphasic, changing from cytoprotection to cytotoxicity through increasing the compound concentration. In mammals, H2S enhances the activity of FoF1-ATP (adenosine triphosphate) synthase and lactate dehydrogenase via their S-sulfhydration, thereby stimulating mitochondrial electron transport. H2S serves as an electron donor for the mitochondrial respiratory chain via sulfide quinone oxidoreductase and cytochrome c oxidase at low H2S levels. The latter enzyme is inhibited by high H2S concentrations, resulting in the reversible inhibition of electron transport and ATP production in mitochondria. In the branched respiratory chain of Escherichia coli, H2S inhibits the bo3 terminal oxidase but does not affect the alternative bd-type oxidases. Thus, in E. coli and presumably other bacteria, cytochrome bd permits respiration and cell growth in H2S-rich environments. A complete picture of the impact of H2S on bioenergetics is lacking, but this field is fast-moving, and active ongoing research on this topic will likely shed light on additional, yet unknown biological effects.
1. Introduction
For a long time, hydrogen sulfide (H2S) had been considered merely as a highly toxic and occasionally lethal gas. However, it was discovered that in mammals H2S has physiological relevance and is endogenously generated [,]. Currently, H2S is considered to be a member of the class of gasotransmitters or, in other words, endogenous gaseous signaling molecules, along with nitric oxide and carbon monoxide [,,,,]. Although still debated, cyanide has also recently been proposed to be part of this class [,]. H2S contributes to the regulation of important physiological processes in the cardiovascular, gastrointestinal, nervous, and respiratory systems. It shows various physiological effects in mammalian cells, but in a biphasic, concentration-dependent manner. At low, nanomolar concentrations, H2S exhibits cytoprotective effects. At higher levels the compound induces cytotoxicity. For instance, H2S exerts vasorelaxant effects through the opening of KATP (adenosine triphosphate) channels in vascular smooth muscle cells []. It also functions as a neuromodulator in the brain [] and serves as a stimulator of angiogenesis []. At concentrations of 200 µM and higher, H2S induces apoptosis of aorta smooth muscle cells through the activation of mitogen-activated protein kinases and caspase-3 []. In mammalian systems, H2S is proposed to signal via four different mechanisms: (i) serving as an antioxidant that detoxifies reactive oxygen species (ROS) and/or reactive nitrogen species (RNS); (ii) blocking and/or reducing metal active sites in metalloproteins, e.g., heme Fe sites in heme proteins; (iii) performing protein S-persulfidation, also known as S-sulfhydration (post-translational modification of a cysteine residue by adding a thiol group); (iv) performing the chemical reduction of disulfide bonds in proteins (see [,] and references therein) (Figure 1).
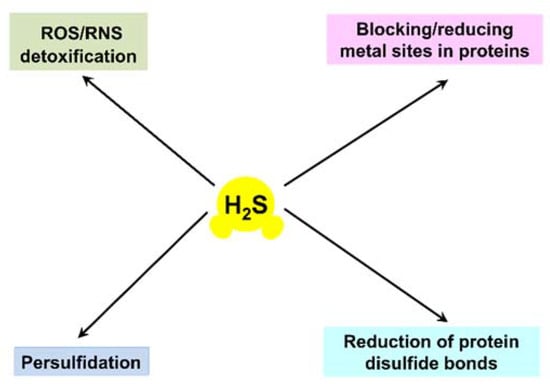
Figure 1.
Proposed H2S signaling mechanisms in mammalian systems.
The physiological roles of H2S are not limited to mammals but appear to be inherent in all kingdoms of life. In higher plants, H2S signaling functions seem to occur mainly through a persulfidation-based mechanism []. It regulates important physiological functions, including fruit ripening; stomatal movement; senescence in flowers, leaves, and fruits; photosynthesis via promotion of photosynthetic enzyme expression and chloroplast biogenesis; and the promotion of root organogenesis, seed germination, nodulation, and N2 fixation. H2S can also activate antioxidant systems in plant cells, thereby contributing to defense against adverse environment situations, such as drought-induced oxidative stress, salinity stress, temperature stress (high and low temperatures), toxic heavy metal stress, as well as different biotic stresses (see [] and references therein).
Although most bacteria can generate and sense H2S, the exact physiological roles of this compound in the prokaryotes are not yet wholly understood. In particular, whether endogenous H2S is a true signaling effector molecule that induces a response in the same microbial cell, or whether the H2S-mediated effect is more a response to environmental and/or host-derived H2S, remains to be established []. Meanwhile, Shatalin et al. reported that inhibition of H2S production through inactivation of H2S-generating enzymes in Bacillus anthracis Sterne, Pseudomonas aeruginosa PA14, Staphylococcus aureus (MSSA RN4220 and MRSA MW2), and Escherichia coli MG1655 makes these pathogenic species very sensitive to different classes of antibiotics []. The addition of exogenous H2S reverses this effect. The authors concluded that endogenously generated H2S increases bacterial resistance to oxidative stress imposed by antibiotics. The antibiotic-induced ROS cause DNA damage via the Fenton reaction. It is suggested that H2S prevents oxidative DNA damage in bacteria via the following cytoprotective mechanisms: (i) direct reduction of H2O2 into H2O; (ii) depletion of Fe2+, a catalyst of the Fenton reaction; (iii) transient depletion of free cysteine, a reducing agent that fuels the Fenton reaction; and (iv) stimulation of the activities of superoxide dismutase (SOD) and catalase []. The latter two enzymes are the well-known ROS scavenging systems []. It should be noted, however, that Shatalin et al. [] did not suggest a mechanism for the depletion of Fe2+ and free cysteine by H2S.
H2S is a colorless flammable gas, soluble in water (100 mM at 25 °C []), and with high cell membrane permeability []. It is a weak acid; therefore in aqueous solutions, it equilibrates with hydrosulfide (HS−) and sulfide (S2−) according to Equation (1):
According to the reported pKa1 values that varied from 6.97 to 7.06 at 25 °C (see [] and references therein), at physiological pH 7.4, 69–73% of the total hydrogen sulfide pool in the solution is in the form of HS−, and 27–31% exists as H2S. Taking into account the reported pKa2 values (from 12.2 to 19), the concentration of S2− in the solution at pH 7.4 is negligible. The mitochondrial matrix in eukaryotic cells is usually alkaline, with a pH value of about 8 [,]. Accordingly, in the matrix, the concentration of HS− is higher, 90–91%, and the remainder is in the form of H2S. On the contrary, intralysosomal pH in living cells is highly acidic, 4.7–4.8 []. Therefore, inside lysosomes, the undissociated form of hydrogen sulfide, H2S, dominates (>99%). H2S acts as a reducing agent. Respectively, the standard redox potential (vs. the standard hydrogen electrode, pH 7) E0′(S0/H2S) is −230 mV (E0′(S0/HS−) = −270 mV) []. Herein, unless otherwise stated, we use the term “H2S” to designate the total hydrogen sulfide pool (H2S + HS− + S2−).
This review focuses on the effects of H2S on the respiratory chains of mammalian mitochondria and bacteria, mammalian FoF1-ATP synthase, and mammalian lactate dehydrogenase (LDH) in light of recent findings.
2. Endogenous Production of H2S
In mammalian tissues, H2S can be endogenously produced via both non-enzymatic and enzymatic pathways.
2.1. Non-Enzymatic Production of H2S
Non-enzymatic formation of H2S usually takes place in the reactions of thiols or thiol derivatives with other molecules [,,]. Inorganic polysulfides, persulfides, and thiosulfate can be reduced with reduced glutathione (GSH) to yield H2S (Figure 2). This demands the presence of reducing equivalents, such as NADPH (reduced nicotinamide adenine dinucleotide phosphate), because glutathione disulfide (GSSG), also produced in the reaction, needs to be converted back to GSH by NADPH-dependent glutathione reductase. Inorganic sulfide salts, such as Na2S or NaHS, can undergo hydrolysis. Yang et al. also reported that cysteine is the preferred substrate for the non-enzymatic pathway, and that the reaction required coordinated catalysis by Fe3+ and pyridoxal phosphate (PLP) [] (Figure 2).
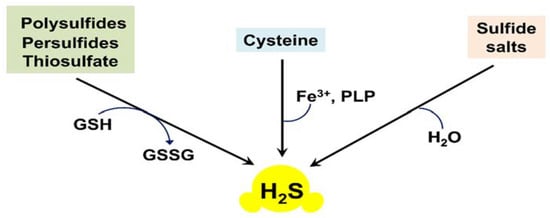
Figure 2.
Non-enzymatic endogenous production of H2S in mammalian tissues.
2.2. Enzymatic Production of H2S
Enzymatic production of H2S in mammalian systems is carried out primarily by cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE), and 3-mercaptopyruvate-sulfurtransferase (3MST) [,,,,]. The main enzymatic reactions resulting in the biosynthesis of H2S are shown in Figure 3. CBS can catalyze the condensation of homocysteine and L-cysteine to produce L-cystathionine and H2S (Figure 3, reaction 1). In the presence of L-cysteine, CBS can also produce H2S, with the formation of L-serine as a byproduct (Figure 3, reaction 2). CSE can decompose homocysteine to yield H2S, α-ketobutyrate, and ammonia (Figure 3, reaction 3). Furthermore, CSE can catalyze the conversion of L-cysteine into H2S, pyruvate, and ammonia (Figure 3, reaction 4). Both CBS and CSE can generate H2S through β-replacement of cysteine by a second cysteine, with the formation of lanthionine as a byproduct (Figure 3, reaction 5). Additionally, CSE can catalyze the γ-replacement reaction between two homocysteine molecules, with the production of H2S and homolanthionine as a by-product (Figure 3, reaction 6). 3MST generates H2S from 3-mercaptopyruvate coupled with either of the two enzymes, mitochondrial cysteine aminotransferase (CAT) or peroxisomal D-amino acid oxidase (DAO). 3MST transfers a sulfur atom from 3-mercaptopyruvate onto itself, resulting in the formation of the enzyme-bound persulfide (3MST-SS) and pyruvate (Figure 3, reaction 7). H2S is then released from the persulfide in the presence of a reductant, e.g., thioredoxin (Trx). CAT and DAO in turn produce 3-mercaptopyruvate from L-cysteine and D-cysteine, respectively [].
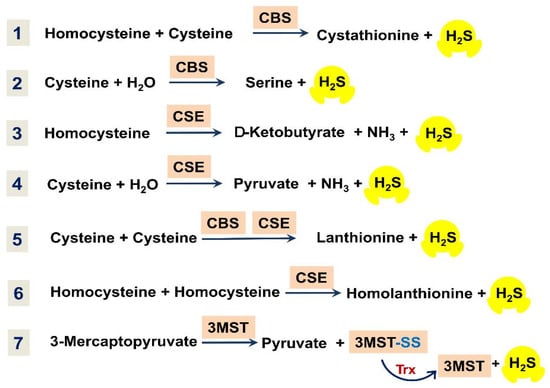
Figure 3.
Overview of main reactions for enzymatic production of H2S in mammalian tissues.
The majority of bacterial species whose genomes were completely sequenced have the orthologs of mammalian genes encoding CBS, CSE, or 3MST []. Since H2S provides defense against modern antibiotics in bacteria, suppression of H2S-producing enzymes in pathogens by new drugs would be a promising antimicrobial treatment strategy [,]. The use of H2S biogenesis as a target for versatile antibiotic potentiators may have therapeutic potential for the fight against difficult-to-treat infections based on bacterial antibiotic tolerance.
3. S-Sulfhydration of ATP Synthase
In eukaryotes, one of the main targets of H2S signaling is the mitochondria. These cell organelles are known to be the power plants of the eukaryotic cell. Energy transduction events in mitochondria occur in the O2-dependent respiratory electron transport chain. The mammalian respiratory chain is unbranched [,]. It consists of four membrane-bound multi-subunit complexes: I, (reduced nicotinamide adenine dinucleotide) NADH: ubiquinone reductase or type I NADH dehydrogenase; II, succinate dehydrogenase; III, ubiquinol: cytochrome c reductase or cytochrome bc1 complex; and IV, cytochrome c oxidase or cytochrome aa3. The chain transfers electrons from NADH and succinate to O2. The electron transfer reactions catalyzed by complexes I, III, and IV are coupled to the generation of the proton motive force. The latter is used by ATP synthase (FoF1-ATP synthase or complex V) to produce ATP.
Modis et al. observed S-sulfhydration of subunit α of the synthase (ATP5A1) in HepG2 and HEK293 cell lysates in response to exposure to H2S, using a biotin switch assay []. Sulfhydration of subunit α of the synthase increases with increasing the H2S concentration, 50–300 μM. H2S at low concentrations (10–100 nM) stimulates the specific activity of ATP synthase, while at higher concentrations (1–10 μM) a tendency for inhibition of the activity is detected (Figure 4). Such a bell-shaped concentration–response curve is quite typical for the effects of H2S. Sulfhydration occurs at two highly conserved cysteine residues in subunit α of the synthase, Cys244, or Cys294. Mutation of either of the two cysteines (C244S or C294S) leads to a slight reduction in the catalytic activity of ATP synthase. The double mutant (C244S/C294S) exhibits more than 50% inhibition of the enzyme activity. In vivo, subunit α of the synthase is basally sulfhydrated. The basal sulfhydration is mostly due to CSE-derived endogenous H2S generation because it is suppressed in liver homogenates harvested from CSE−/− mice. Burn injuries that upregulate CSE and increase H2S generation result in an increase in S-sulfhydration of subunit α of the synthase. Thus, S-sulfhydration of subunit α of the synthase could be a physiological mechanism to maintain ATP synthase in a physiologically activated state, thereby supporting mitochondrial bioenergetics [].
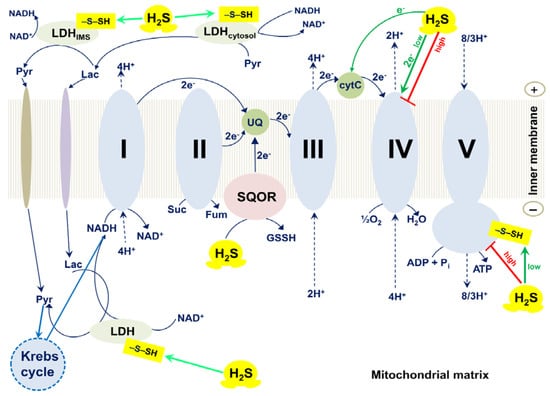
Figure 4.
Effects of H2S on mammalian mitochondrial electron transport chain, ATP synthase, and lactate dehydrogenase. The mammalian respiratory chain includes four different membrane-bound complexes: I, II, III, and IV. H2S at low concentrations stimulates the activity of FoF1-ATP synthase (also known as complex V) via S-sulfhydration of Cys244 or Cys294 of its α subunit []. H2S increases the activity of lactate dehydrogenase (LDH) via S-sulfhydration of its Cys163 that, in turn, stimulates mitochondrial electron transport []. H2S can also donate electrons to the respiratory chain via sulfide quinone oxidoreductase (SQOR) by directly reducing ubiquinone (UQ) []. At high (toxic) concentrations H2S inhibits complex IV (cytochrome c oxidase) and FoF1-ATP synthase that leads to reversible inhibition of mitochondrial electron transport and ATP production [,,,]. At low concentrations H2S serves as an electron donor for complex IV, either directly [,,] or indirectly, via reduction of its native substrate cytochrome c []. The outer mitochondrial membrane is not shown for simplicity.
4. S-Sulfhydration of Lactate Dehydrogenase (LDH)
LDH catalyzes the reversible conversion of lactate to pyruvate with the reduction of NAD+ to NADH, and vice versa. The enzyme is a tetramer that is usually composed of the two most common types of subunits, LDHA and LDHB []. LDHA and LDHB can assemble into five different isoenzymes: LDH1, LDH2, LDH3, LDH4, and LDH5. Isoenzymes that are rich in LDHA catabolize pyruvate to lactate with the concomitant production of NAD+ from NADH. Conversely, isoenzymes rich in LDHB facilitate lactate-to-pyruvate conversion with the concomitant formation of NADH from NAD+.
Untereiner et al. reported that in the colon cancer cell line HCT116, LDHA catalyzes the conversion of pyruvate to lactate, and H2S donation increases the total cellular lactate levels []. Notably, H2S enhances the catalytic activity of LDHA, which is primary cytosolic, doing so via S-sulfhydration of its Cys163 (Figure 4). Experiments with whole HCT116 cell extracts showed that H2S also stimulates the LDHB activity, although to a smaller extent. Importantly, H2S stimulates oxidative phosphorylation in HCT116 cells in an LDHA-dependent manner. Thus, in colon cancer cells, H2S-induced stimulation of the catalytic activity of LDHA leads to the stimulation of mitochondrial electron transport. The authors hypothesize that the increase in the LDHA activity causes an increase in cytosolic lactate. This enhances the flux of lactate into the mitochondria through the intracellular lactate shuttle. Lactate enters the mitochondrial intermembrane space. Lactate and pyruvate also enter the mitochondrial matrix. Lactate is converted to pyruvate via the mitochondrial LDH that is primarily made up of LDHB and also activated by H2S. Pyruvate in the matrix is oxidized via the Krebs cycle to generate NADH that stimulates the activity of the mitochondrial respiratory chain. Additionally, the oxidation of lactate to pyruvate by the mitochondrial LDH is coupled to the reduction of NAD+ to NADH. This NADH, in turn, is utilized by the mitochondrial respiratory chain to further support electron transport.
5. H2S Donates Electrons to the Mitochondrial Respiratory Chain via Sulfide Quinone Oxidoreductase (SQOR)
Powell and Somero first reported that the oxidation of H2S can occur in mitochondria, and that the process is coupled to oxidative phosphorylation []. The mitochondrial oxidation of H2S was observed in the gill and foot tissue of Solemya reidi, a gutless clam living in sulfide-rich habitats. Later, Goubern et al., using permeabilized human colon adenocarcinoma HT29 cells, showed that H2S at low micromolar concentrations can serve as an electron donor for the mammalian respiratory chain []. This is accompanied by mitochondrial energization. The latter is extremely sensitive to the amount of H2S delivered instantaneously to mitochondria. H2S donates electrons at the respiratory chain at the level of UQ.
The enzyme that catalyzes the electron transfer from H2S to UQ is SQOR []. SQOR belongs to group four of the flavin disulfide reductase (FDR) superfamily. The enzyme contains a tightly bound FAD (flavin adenine dinucleotide) and two spatially proximal redox-active cysteines. A peculiar feature of human SQOR is the fact that the two cysteines are linked via a bridging sulfur atom which forms the redox-active cysteine trisulfide configuration. The trisulfide appears to be the active form of SQOR, being present at the start and end of the catalytic cycle [].
When H2S is oxidized in the mitochondrial matrix by the action of SQOR under physiological conditions, GSH serves as the primary sulfur acceptor (Figure 4). In the reaction that is coupled to the reduction of UQ to UQH2, glutathione persulfide (GSSH) is produced. GSSH is then oxidized by persulfide dioxygenase (ETHE1) to yield sulfite (SO32−) and regenerate GSH. GSSH can also be a substrate for rhodanese or thiosulfate sulfurtransferase (TST). TST catalyzes the reaction of GSSH with SO32− to generate GSH and thiosulfate (S2O32−). Alternatively, SO32− can be oxidized to sulfate (SO42−) by sulfite oxidase (SUOX) located in the intermembrane space, with the concomitant reduction of cytochrome c. Thiosulfate and sulfate can be excreted with urine (see [] and references therein). Thus, electrons from the sulfide oxidation pathway can enter the mitochondrial respiratory chain at the level of the cytochrome bc1 complex (from SQOR) and cytochrome c oxidase (from SUOX).
6. H2S at Toxic Concentrations Inhibits Mitochondrial Cytochrome c Oxidase
H2S exposure, at high concentrations and high rates, is extremely toxic to mammals, including humans []. The acute toxicity of H2S is generally attributed to the suppression of the mitochondrial cytochrome c oxidase (complex IV). Complex IV couples the transfer of electrons from cytochrome c to O2 with the generation of the transmembrane proton gradient using the mechanism of proton pumping [,,,,,,,,,,,,,,,,,,,,,,]. The enzyme carries four redox-active metal sites: CuA, heme a, heme a3, and CuB. Electrons from cytochrome c primarily accepted by CuA are transferred to heme a and then to the catalytic binuclear center composed of heme a3 and CuB in which the reduction of oxygen to water occurs [,,,]. There are two forms of cytochrome c oxidase: slow (resting oxidase as obtained from the preparation) and fast (the completely reduced enzyme exposed to a “pulse” of O2) []. The fast form is also called pulsed or unrelaxed. The slow form differs from the fast form in lower reactivity for inhibitors [] and slower intramolecular electron transfer [].
H2S at high concentrations was reported to inhibit the O2 consumption by the beef heart cytochrome c oxidase in the isolated form and in submitochondrial particles (Figure 4) [,,]. The inhibition by H2S appears to be non-competitive with respect to both substrates, cytochrome c and O2 []. The inhibition efficiency increases with decreasing pH: the Ki values measured at pH 8.05, 7.48, and 6.28, are 2.6, 0.55, and 0.07 μM, respectively []. The initial rate of inactivation of the fast form of the isolated cytochrome c oxidase is proportional to total H2S concentration, with an initial rate constant, kon, of 2.2 × 104 M−1 s−1 at pH 7.47 []. The inhibition of complex IV leads to blockage of the mitochondrial respiratory chain and, as a consequence, to the dissipation of the membrane potential, the cessation of oxidative phosphorylation, and enhanced ROS production []. Consistently, examination of the cytotoxic effects of inhalational H2S exposure showed that sublethal (>50 ppm) concentrations of the inhaled gas caused inhibition of cytochrome c oxidase activity in the lung and heart, which can be observed ex vivo and is associated with pathological alterations [,,,,,]. That complex IV is suppressed by high (usually 10–100 μM) concentrations of H2S in vitro in various cell lines is well documented [,,,,,,]. The H2S-induced inhibition is reversible, e.g., 10–30 min after the exposure of tissue homogenates to H2S, the activity of complex IV gets back to its initial level, simultaneously with the decomposition of the inhibitor [].
H2S, presumably in the form of HS−, binds to the ferric heme a3 and CuB in either state (either cupric or cuprous) []. The final enzyme product of H2S inhibition is a mixed-valence state of the oxidase in which CuA and heme a are reduced and heme a3 is in the ferric H2S-bound form [,]. CuB in the final inhibited enzyme remains reduced even after reoxidation of the H2S-bound oxidase, as evidenced by an electron paramagnetic resonance spectroscopy study []. The fact that CuB remains cuprous in the presence of ferricyanide suggests that H2S binding to CuB1+ increases its redox potential and makes re-oxidation difficult. In other words, CuB1+ in this state may also be H2S-bound []. Nicholls et al. [] suggested a model for the H2S-mediated inhibition of the mitochondrial cytochrome c oxidase (Figure 5). According to this model, during the steady-state turnover of complex IV, the first molecule of HS− transiently binds to cupric or cuprous CuB. Then, it is transferred to the ferric heme a3 that blocks the catalytic reaction of the heme with O2. In the final protein-inhibitor adduct, CuB is likely reduced and presumably bound to the second molecule of HS−. Heme a and CuA are most likely reduced in the adduct.
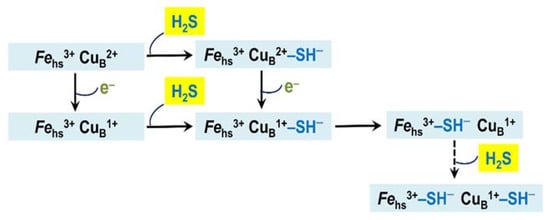
Figure 5.
Proposed mechanism of inhibition of mitochondrial cytochrome c oxidase and Escherichia coli cytochrome bo3 by H2S. Shown is the catalytic binuclear center in different redox and ligation states. The center consists of the copper ion CuB and the high-spin heme (Fehs). The latter is heme a3 in cytochrome c oxidase and heme o3 in cytochrome bo3. The metal redox groups which are not part of the center are not shown.
7. H2S at Low Concentrations Serves as Electron Donor for Mitochondrial Cytochrome c Oxidase
H2S at low concentrations can act as a substrate for mitochondrial complex IV (Figure 4) [,]. The fast form of the oxidized cytochrome c oxidase reacts aerobically with low H2S levels in the absence of reduced substrates. However, the initial product of this reaction is not the inhibited enzyme but a catalytic intermediate, compound ‘P’ (Figure 6) []. Compound ‘P’ then decays into another catalytic intermediate, compound ‘F’. It is proposed that in this reaction two H2S molecules donate two electrons to the fully oxidized binuclear center, a33+CuB2+, converting it to the fully reduced state, a32+CuB+. The concomitant H2S oxidation product is possibly hydrogen persulfide (HSSH), although a form of free sulfur (S0 or S8) cannot be excluded []. Then the fully reduced binuclear center reacts with O2, producing compound ‘P’. It remains unclear whether cytochrome c oxidase contributes significantly to a dissimilatory mechanism for the endogenously generated H2S, or if SQOR is the major contributor in vivo. Notably, H2S can also reduce complex IV indirectly, via reduction of its native substrate cytochrome c (Figure 4). The reaction between H2S and cytochrome c presumably leads to the initial formation of the HS•/S•− radical. HS•/S•− could then be trapped by proteins producing protein persulfides and superoxide, or be trapped by O2 yielding HSO2• [].
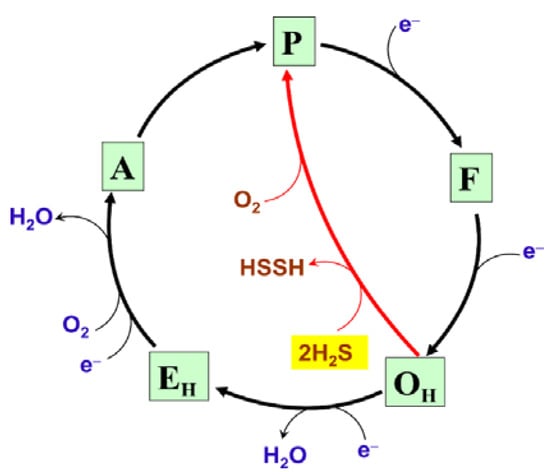
Figure 6.
Proposed molecular mechanism of the reaction of the fast form of mitochondrial cytochrome c oxidase with low H2S levels. OH, EH, A, P, and F are unrelaxed oxidized, unrelaxed one-electron-reduced, compound ‘A’, compound ‘P’, and compound ‘F’ catalytic intermediates, respectively. Chemical and pumped protons involved in the catalytic cycle are not shown for clarity.
8. Effect of H2S on the Operation of the Branched Respiratory Chain of E. coli and Bacterial Growth
Under steady-state conditions, the mammalian tissue concentration of H2S is usually in the low nanomolar range, e.g., it is around 15 nM in mouse brain and liver tissues []. The mammalian gut, in this sense, is unique among other body compartments. Millimolar concentrations (1.0–2.4 mM) of H2S are commonly present in the gut []. The reason is that in the gastrointestinal tract, unlike other compartments, H2S is generated by both the mammalian CBS, CSE, and 3MST, and by the microbial communities inhabiting the gut, of which sulfate-reducing bacteria are the key H2S-producing species [,]. At such extremely high H2S levels, bacterial respiratory chains which terminate in cytochrome c oxidase or other heme-copper oxidases should be inhibited. The question then arises as to how under these conditions bacteria inhabiting the mammalian gut can maintain aerobic respiration. It turned out that E. coli, and possibly other bacteria inhabiting H2S-rich environments, has a unique bd-type terminal oxidase that is not inhibited by H2S, even at toxic concentrations [,,,].
E. coli is an essential member of the intestinal microbiome of humans and warm-blooded animals. The large intestine of humans normally harbors several E. coli strains at a given point in time []. In contrast to the electron transport chain of mammals that is unbranched, E. coli, like many other prokaryotes, possesses the branched aerobic respiratory chain (Figure 7) [,,,,,,]. The E. coli chain comprises of type I and type II NADH dehydrogenases which transfer electrons from NADH to ubiquinol-8 (UQ8) or menaquinol-8 (MQ8); succinate dehydrogenase transferring electrons from succinate to UQ8; and three terminal oxidases, cytochromes bo3, bd-I, and bd-II which transfer electrons from UQ8 or MQ8 to O2 producing H2O [,,,,,,,,,]. The proton motive force generated by type I NADH dehydrogenase and the terminal oxidases is used by FoF1-ATP synthase to make ATP [,,]. Being a true proton pump, cytochrome bo3 produces the proton motive force with higher efficiency as compared to the evolutionarily unrelated bd-type oxidases [,,,,,]. The three-dimensional structures of cytochrome bo3 and cytochrome bd-I were determined [,,,]. The bo3 oxidase is a member of type A-1 heme-copper oxidase superfamily [,,,,,,,,,,,]. Cytochrome bo3 contains the UQ8 binding site, heme b, and the catalytic binuclear center formed by heme o3 and CuB [,]. Cytochrome bd-I and cytochrome bd-II are members of the L subfamily of the cytochrome bd oxygen reductase family [,]. According to recent work by Murali et al. [], the latter family should be expanded into a huge superfamily. The bd oxidase has the UQ8/MQ8 binding site, and three heme prosthetic groups, b558, b595, and d but lacks any copper site [,,,,,,,]. Hemes b595 and d may form a di-heme active site for oxygen chemistry, as evidenced by a number of studies [,,,,,,,,,,,,,]. These hemes are in van der Waals contacts [,], enabling fast electron transfers between them [,,]. Thus, the functional di-heme active site in the bd oxidase implies that heme b595 is able to rapidly donate an electron and a proton to heme d to perform a concerted four-electron reduction of oxygen to water. This role of heme b595 in cytochrome bd is similar to that of CuB in the catalytic binuclear center of cytochrome bo3. Cytochromes bo3 and bd have low and high O2 affinity, respectively [,,,,,]. As a consequence, the bo3 enzyme dominates in E. coli under conditions of high O2 concentration, whereas the bd oxidase is expressed at low aeration [,,,,]. Cytochrome bd performs vital physiological functions in E. coli and other prokaryotes [,,,,,,]. In particular, the enzyme endows the microbes with resistance to nitric oxide [,,,,,,,,,], peroxynitrite [,], hydrogen peroxide [,,,,,,], cyanide [,,], and ammonia []. The bd-type oxidases are present in the respiratory chains of bacteria and archaea but not in humans and animals. For this reason, they could serve as suitable protein targets for next-generation antibiotics [,,,,,,,,].
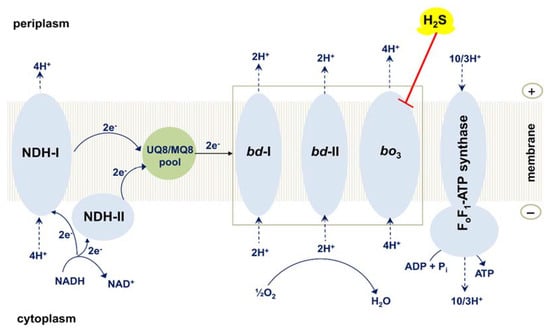
Figure 7.
Effect of H2S on the operation of the branched respiratory chain of E. coli. Shown is the schematic view of the branched aerobic respiratory chain of E. coli. H2S inhibits cytochrome bo3 but does not affect cytochrome bd-I and cytochrome bd-II. Succinate dehydrogenase and other substrate dehydrogenases are not shown for clarity.
Forte et al. studied the effect of H2S on the oxygen consumption by the purified terminal oxidases bo3, bd-I, and bd-II from E. coli []. It turned out that the activity of cytochrome bo3 is quickly inhibited with an apparent half-maximal inhibitory concentration IC50 of 1.1 μM H2S (pH 7.4). This value is similar to the Ki of 0.55 μM H2S obtained by Nicholls and Kim for the beef heart cytochrome c oxidase at pH 7.48 []. The inhibition of the bo3 oxidase appeared to be fully reversible. The rapid and total recovery of the enzymatic activity occurs following the removal of H2S from the solution by the H2S scavenger, the Entamoeba histolytica O-acetylserine sulfhydrylase (EhOASS), in the presence of excess O-acetyl-L-serine (OAS). In contrast, neither the bd-I oxidase nor the bd-II oxidase is inhibited under identical conditions, even at a high concentration of H2S (58 μM) []. Similar results were obtained when examining the effect of H2S on the oxygen consumption by cell suspensions of the E. coli mutant strains which possess bo3, bd-I, and bd-II as the only terminal oxidase. The oxygen uptake by cells having bo3 as the sole oxidase is rapidly inhibited by 50 µM H2S. As in the case of the purified enzyme, the inhibition is promptly and completely restored after H2S depletion by the EhOASS/OAS system. Conversely, H2S at the same concentration does not affect the oxygen uptake by mutant cells having either bd-I or bd-II as the only oxidase []. Similar data on the E. coli membrane vesicles were reported by Korshunov et al. [].
Forte et al. also tested if bd-I and/or bd-II oxidases, besides enabling aerobic respiration, promote the growth of E. coli cells in the presence of H2S []. A quantity of 200 μM H2S appeared to severely inhibit the growth of mutant cells with only cytochrome bo3. On the contrary, virtually no effect on cell growth was detected following the addition of H2S at the same concentration to the strains which express either bd-I or bd-II as the sole oxidase. Thus, in contrast with cytochrome bo3, which is potently and reversibly inhibited by H2S, both cytochrome bd-I and cytochrome bd-II are H2S-insensitive, and therefore, able to sustain respiration and cell growth in the presence of high levels of H2S. We assume that the mechanism of the inhibition of the E. coli cytochrome bo3 by H2S is very similar to that suggested for the H2S-mediated inhibition of another heme-copper terminal oxidase, mitochondrial complex IV (Figure 5).
The H2S resistance of cytochrome bd is probably a key trait not only found in E. coli. Saini et al. [] reported that H2S promotes the respiration and growth of Mycobacterium tuberculosis, Mycobacterium smegmatis, and Mycobacterium bovis BCG. The authors concluded that the suppression of the cytochrome bcc-aa3 supercomplex by H2S leads to the switching of the electron flow from MQ to cytochrome bd. The latter, in turn, stimulates respiration and ATP production, leading to the increased growth of the mycobacteria []. Furthermore, Kunota et al. [] showed that multidrug-resistant and drug-susceptible clinical M. tuberculosis strains (but not non-pathogenic M. smegmatis) generate H2S endogenously, maintaining bioenergetic homeostasis by stimulating respiration primarily via the bd-type terminal oxidase. These findings are in agreement with the fact that the bd oxidases of the E. coli respiratory chain are H2S-insensitive [,].
9. Concluding Remarks
Accumulated evidence has shown that H2S is an effector molecule that controls energy metabolism. Depending on the concentration, H2S can either stimulate or inhibit the mammalian mitochondrial respiratory chain and FoF1-ATP synthase. An involvement of LDHA in the stimulatory effects of H2S on mitochondrial respiration has also been suggested. High H2S inhibits the bacterial respiratory chain terminating in cytochrome bo3 but not cytochrome bd. The effects of H2S on a bacterial ATP synthase are still unknown. Although the role of H2S in microorganisms has been investigated less, there is currently an explosion of interest in the link between H2S and bacterial energy metabolism as a result of the recognition that this gaseous signaling molecule is critically important for the growth and colonization potential of pathogens, such as M. tuberculosis. A clearer picture of the effects of H2S on energy metabolism would represent a significant advance for the development of novel therapeutics.
Author Contributions
V.B.B. and E.F. performed the literature review and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Russian Foundation for Basic Research (http://www.rfbr.ru/rffi/eng—research project № 19-04-00094) and Sapienza grants n. RP120172B8B36A98.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We are grateful to the anonymous reviewers for their comments and suggestions on this review.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Bakalarz, D.; Korbut, E.; Yuan, Z.; Yu, B.; Wojcik, D.; Danielak, A.; Magierowska, K.; Kwiecien, S.; Brzozowski, T.; Marcinkowska, M.; et al. Novel hydrogen sulfide (H2S)-releasing BW-HS-101 and its non-H2S releasing derivative in modulation of microscopic and molecular parameters of gastric mucosal barrier. Int. J. Mol. Sci. 2021, 22, 5211. [Google Scholar] [CrossRef] [PubMed]
- Nowaczyk, A.; Kowalska, M.; Nowaczyk, J.; Grzesk, G. Carbon monoxide and nitric oxide as examples of the youngest class of transmitters. Int. J. Mol. Sci. 2021, 22, 6029. [Google Scholar] [CrossRef]
- Kimura, H. Production and physiological effects of hydrogen sulfide. Antioxid. Redox Signal. 2014, 20, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Southam, H.M.; Poole, R.K. Do nitric oxide, carbon monoxide and hydrogen sulfide really qualify as ’gasotransmitters’ in bacteria? Biochem. Soc. Trans. 2018, 46, 1107–1118. [Google Scholar] [CrossRef]
- Randi, E.B.; Zuhra, K.; Pecze, L.; Panagaki, T.; Szabo, C. Physiological concentrations of cyanide stimulate mitochondrial Complex IV and enhance cellular bioenergetics. Proc. Natl. Acad. Sci. USA 2021, 118, e2026245118. [Google Scholar] [CrossRef]
- Giamogante, F.; Cali, T.; Malatesta, F. Physiological cyanide concentrations do not stimulate mitochondrial cytochrome c oxidase activity. Proc. Natl. Acad. Sci. USA 2021, 118, e2112373118. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. Embo J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Papapetropoulos, A.; Pyriochou, A.; Altaany, Z.; Yang, G.; Marazioti, A.; Zhou, Z.; Jeschke, M.G.; Branski, L.K.; Herndon, D.N.; Wang, R.; et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21972–21977. [Google Scholar] [CrossRef]
- Yang, G.; Sun, X.; Wang, R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 2004, 18, 1782–1784. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.; Bhattacharya, R.; Mukherjee, P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019, 33, 13098–13125. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ding, L.; Xie, Z.Z.; Yang, Y.; Whiteman, M.; Moore, P.K.; Bian, J.S. A review of hydrogen sulfide synthesis, metabolism, and measurement: Is modulation of hydrogen sulfide a novel therapeutic for cancer? Antioxid. Redox Signal. 2019, 31, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Palma, J.M. H2S signaling in plants and applications in agriculture. J. Adv. Res. 2020, 24, 131–137. [Google Scholar] [CrossRef]
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. H2S: A universal defense against antibiotics in bacteria. Science 2011, 334, 986–990. [Google Scholar] [CrossRef]
- Borisov, V.B.; Siletsky, S.A.; Nastasi, M.R.; Forte, E. ROS defense systems and terminal oxidases in bacteria. Antioxidants 2021, 10, 839. [Google Scholar] [CrossRef]
- Calhoun, D.B.; Englander, S.W.; Wright, W.W.; Vanderkooi, J.M. Quenching of room temperature protein phosphorescence by added small molecules. Biochemistry 1988, 27, 8466–8474. [Google Scholar] [CrossRef]
- Riahi, S.; Rowley, C.N. Why can hydrogen sulfide permeate cell membranes? J. Am. Chem. Soc. 2014, 136, 15111–15113. [Google Scholar] [CrossRef]
- Li, Q.; Lancaster, J.R., Jr. Chemical foundations of hydrogen sulfide biology. Nitric Oxide 2013, 35, 21–34. [Google Scholar] [CrossRef]
- Llopis, J.; McCaffery, J.M.; Miyawaki, A.; Farquhar, M.G.; Tsien, R.Y. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl. Acad. Sci. USA 1998, 95, 6803–6808. [Google Scholar] [CrossRef]
- Abad, M.F.; Di Benedetto, G.; Magalhaes, P.J.; Filippin, L.; Pozzan, T. Mitochondrial pH monitored by a new engineered green fluorescent protein mutant. J. Biol. Chem. 2004, 279, 11521–11529. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, S.; Poole, B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl. Acad. Sci. USA 1978, 75, 3327–3331. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef]
- Yang, J.; Minkler, P.; Grove, D.; Wang, R.; Willard, B.; Dweik, R.; Hine, C. Non-enzymatic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B6. Commun. Biol. 2019, 2, 194. [Google Scholar] [CrossRef]
- Kabil, O.; Vitvitsky, V.; Xie, P.; Banerjee, R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid. Redox Signal. 2011, 15, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Giuffre, A.; Vicente, J.B. Hydrogen sulfide biochemistry and interplay with other gaseous mediators in mammalian physiology. Oxid. Med. Cell. Longev. 2018, 2018, 6290931. [Google Scholar] [CrossRef] [PubMed]
- Shatalin, K.; Nuthanakanti, A.; Kaushik, A.; Shishov, D.; Peselis, A.; Shamovsky, I.; Pani, B.; Lechpammer, M.; Vasilyev, N.; Shatalina, E.; et al. Inhibitors of bacterial H2S biogenesis targeting antibiotic resistance and tolerance. Science 2021, 372, 1169–1175. [Google Scholar] [CrossRef]
- Caruana, N.J.; Stroud, D.A. The road to the structure of the mitochondrial respiratory chain supercomplex. Biochem. Soc. Trans. 2020, 48, 621–629. [Google Scholar] [CrossRef]
- Cogliati, S.; Cabrera-Alarcon, J.L.; Enriquez, J.A. Regulation and functional role of the electron transport chain supercomplexes. Biochem. Soc. Trans. 2021, BST20210460. [Google Scholar] [CrossRef]
- Modis, K.; Ju, Y.; Ahmad, A.; Untereiner, A.A.; Altaany, Z.; Wu, L.; Szabo, C.; Wang, R. S-Sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics. Pharmacol. Res. 2016, 113, 116–124. [Google Scholar] [CrossRef]
- Untereiner, A.A.; Olah, G.; Modis, K.; Hellmich, M.R.; Szabo, C. H2S-induced S-sulfhydration of lactate dehydrogenase a (LDHA) stimulates cellular bioenergetics in HCT116 colon cancer cells. Biochem. Pharmacol. 2017, 136, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Goubern, M.; Andriamihaja, M.; Nubel, T.; Blachier, F.; Bouillaud, F. Sulfide, the first inorganic substrate for human cells. FASEB J. 2007, 21, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, P. The effect of sulphide on cytochrome aa3. Isosteric and allosteric shifts of the reduced α-peak. Biochim. Biophys. Acta 1975, 396, 24–35. [Google Scholar] [CrossRef]
- Petersen, L.C. The effect of inhibitors on the oxygen kinetics of cytochrome c oxidase. Biochim. Biophys. Acta 1977, 460, 299–307. [Google Scholar] [CrossRef]
- Szabo, C.; Ransy, C.; Modis, K.; Andriamihaja, M.; Murghes, B.; Coletta, C.; Olah, G.; Yanagi, K.; Bouillaud, F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br. J. Pharmacol. 2014, 171, 2099–2122. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, P.; Kim, J.K. Oxidation of sulphide by cytochrome aa3. Biochim. Biophys. Acta 1981, 637, 312–320. [Google Scholar] [CrossRef]
- Nicholls, P.; Kim, J.K. Sulphide as an inhibitor and electron donor for the cytochrome c oxidase system. Can. J. Biochem. 1982, 60, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, P.; Marshall, D.C.; Cooper, C.E.; Wilson, M.T. Sulfide inhibition of and metabolism by cytochrome c oxidase. Biochem. Soc. Trans. 2013, 41, 1312–1316. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Miljkovic, J.L.; Bostelaar, T.; Adhikari, B.; Yadav, P.K.; Steiger, A.K.; Torregrossa, R.; Pluth, M.D.; Whiteman, M.; Banerjee, R.; et al. Cytochrome c reduction by H2S potentiates sulfide signaling. ACS Chem. Biol. 2018, 13, 2300–2307. [Google Scholar] [CrossRef]
- Doherty, J.R.; Cleveland, J.L. Targeting lactate metabolism for cancer therapeutics. J. Clin. Invest. 2013, 123, 3685–3692. [Google Scholar] [CrossRef]
- Powell, M.A.; Somero, G.N. Hydrogen sulfide oxidation Is coupled to oxidative phosphorylation in mitochondria of Solemya reidi. Science 1986, 233, 563–566. [Google Scholar] [CrossRef]
- Landry, A.P.; Ballou, D.P.; Banerjee, R. Hydrogen sulfide oxidation by sulfide quinone oxidoreductase. Chembiochem 2021, 22, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Landry, A.P.; Moon, S.; Kim, H.; Yadav, P.K.; Guha, A.; Cho, U.S.; Banerjee, R. A catalytic trisulfide in human sulfide quinone oxidoreductase catalyzes Coenzyme A persulfide synthesis and inhibits butyrate oxidation. Cell Chem. Biol. 2019, 26, 1515–1525.e1514. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, F.; Antonini, G.; Sarti, P.; Brunori, M. Structure and function of a molecular machine: Cytochrome c oxidase. Biophys. Chem. 1995, 54, 1–33. [Google Scholar] [CrossRef]
- Melo, A.M.; Teixeira, M. Supramolecular organization of bacterial aerobic respiratory chains: From cells and back. Biochim. Biophys. Acta 2016, 1857, 190–197. [Google Scholar] [CrossRef]
- Siletsky, S.A.; Borisov, V.B.; Mamedov, M.D. Photosystem II and terminal respiratory oxidases: Molecular machines operating in opposite directions. Front. Biosci. 2017, 22, 1379–1426. [Google Scholar] [CrossRef]
- Pereira, M.M.; Sousa, F.L.; Verissimo, A.F.; Teixeira, M. Looking for the minimum common denominator in haem-copper oxygen reductases: Towards a unified catalytic mechanism. Biochim. Biophys. Acta 2008, 1777, 929–934. [Google Scholar] [CrossRef]
- Pereira, M.M.; Santana, M.; Teixeira, M. A novel scenario for the evolution of haem-copper oxygen reductases. Biochim. Biophys. Acta 2001, 1505, 185–208. [Google Scholar] [CrossRef]
- Pereira, M.M.; Gomes, C.M.; Teixeira, M. Plasticity of proton pathways in haem-copper oxygen reductases. FEBS Lett. 2002, 522, 14–18. [Google Scholar] [CrossRef][Green Version]
- Pereira, M.M.; Teixeira, M. Proton pathways, ligand binding and dynamics of the catalytic site in haem-copper oxygen reductases: A comparison between the three families. Biochim. Biophys. Acta 2004, 1655, 340–346. [Google Scholar] [CrossRef]
- Papa, S.; Capitanio, N.; Capitanio, G.; Palese, L.L. Protonmotive cooperativity in cytochrome c oxidase. Biochim. Biophys. Acta 2004, 1658, 95–105. [Google Scholar] [CrossRef]
- Borisov, V.B. Defects in mitochondrial respiratory complexes III and IV, and human pathologies. Mol. Aspects Med. 2002, 23, 385–412. [Google Scholar] [CrossRef]
- Borisov, V.B. Mutations in respiratory chain complexes and human diseases. Ital. J. Biochem. 2004, 53, 34–40. [Google Scholar]
- Borisov, V.B.; Siletsky, S.A. Features of organization and mechanism of catalysis of two families of terminal oxidases: Heme-copper and bd-type. Biochemistry 2019, 84, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Hederstedt, L. Molecular biology of Bacillus subtilis cytochromes anno 2020. Biochemistry 2021, 86, 8–21. [Google Scholar] [CrossRef]
- Sousa, F.L.; Alves, R.J.; Ribeiro, M.A.; Pereira-Leal, J.B.; Teixeira, M.; Pereira, M.M. The superfamily of heme-copper oxygen reductases: Types and evolutionary considerations. Biochim. Biophys. Acta 2012, 1817, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Wikstrom, M.; Krab, K.; Sharma, V. Oxygen activation and energy conservation by cytochrome c oxidase. Chem. Rev. 2018, 118, 2469–2490. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, N.; Palese, L.L.; Capitanio, G.; Martino, P.L.; Richter, O.M.; Ludwig, B.; Papa, S. Allosteric interactions and proton conducting pathways in proton pumping aa3 oxidases: Heme a as a key coupling element. Biochim. Biophys. Acta 2012, 1817, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Maneg, O.; Malatesta, F.; Ludwig, B.; Drosou, V. Interaction of cytochrome c with cytochrome oxidase: Two different docking scenarios. Biochim. Biophys. Acta 2004, 1655, 274–281. [Google Scholar] [CrossRef]
- Gavrikova, E.V.; Grivennikova, V.G.; Borisov, V.B.; Cecchini, G.; Vinogradov, A.D. Assembly of a chimeric respiratory chain from bovine heart submitochondrial particles and cytochrome bd terminal oxidase of Escherichia coli. FEBS Lett. 2009, 583, 1287–1291. [Google Scholar] [CrossRef]
- von Ballmoos, C.; Adelroth, P.; Gennis, R.B.; Brzezinski, P. Proton transfer in ba3 cytochrome c oxidase from Thermus thermophilus. Biochim. Biophys. Acta 2012, 1817, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Rich, P.R. Mitochondrial cytochrome c oxidase: Catalysis, coupling and controversies. Biochem. Soc. Trans. 2017, 45, 813–829. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Shimada, A. Reaction mechanism of cytochrome c oxidase. Chem. Rev. 2015, 115, 1936–1989. [Google Scholar] [CrossRef]
- Forte, E.; Giuffre, A.; Huang, L.S.; Berry, E.A.; Borisov, V.B. Nitric oxide does not inhibit but is metabolized by the cytochrome bcc-aa3 supercomplex. Int. J. Mol. Sci. 2020, 21, 8521. [Google Scholar] [CrossRef] [PubMed]
- Siletsky, S.A.; Borisov, V.B. Proton pumping and non-pumping terminal respiratory oxidases: Active sites intermediates of these molecular machines and their derivatives. Int. J. Mol. Sci. 2021, 22, 10852. [Google Scholar] [CrossRef]
- Tsukihara, T.; Aoyama, H.; Yamashita, E.; Tomizaki, T.; Yamaguchi, H.; Shinzawa-Itoh, K.; Nakashima, R.; Yaono, R.; Yoshikawa, S. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science 1996, 272, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Yano, N.; Muramoto, K.; Shimada, A.; Takemura, S.; Baba, J.; Fujisawa, H.; Mochizuki, M.; Shinzawa-Itoh, K.; Yamashita, E.; Tsukihara, T.; et al. The Mg2+-containing water cluster of mammalian cytochrome c oxidase collects four pumping proton equivalents in each catalytic cycle. J. Biol. Chem. 2016, 291, 23882–23894. [Google Scholar] [CrossRef]
- Shimada, A.; Etoh, Y.; Kitoh-Fujisawa, R.; Sasaki, A.; Shinzawa-Itoh, K.; Hiromoto, T.; Yamashita, E.; Muramoto, K.; Tsukihara, T.; Yoshikawa, S. X-ray structures of catalytic intermediates of cytochrome c oxidase provide insights into its O2 activation and unidirectional proton-pump mechanisms. J. Biol. Chem. 2020, 295, 5818–5833. [Google Scholar] [CrossRef]
- Shimada, A.; Hara, F.; Shinzawa-Itoh, K.; Kanehisa, N.; Yamashita, E.; Muramoto, K.; Tsukihara, T.; Yoshikawa, S. Critical roles of the CuB site in efficient proton pumping as revealed by crystal structures of mammalian cytochrome c oxidase catalytic intermediates. J. Biol. Chem. 2021, 297, 100967. [Google Scholar] [CrossRef]
- Antonini, E.; Brunori, M.; Colosimo, A.; Greenwood, C.; Wilson, M.T. Oxygen “pulsed” cytochrome c oxidase: Functional properties and catalytic relevance. Proc. Natl. Acad. Sci. USA 1977, 74, 3128–3132. [Google Scholar] [CrossRef]
- Moody, A.J. “As prepared” forms of fully oxidised haem/Cu terminal oxidases. Biochim. Biophys. Acta 1996, 1276, 6–20. [Google Scholar] [CrossRef][Green Version]
- Moody, A.J.; Cooper, C.E.; Rich, P.R. Characterisation of ‘fast’ and ‘slow’ forms of bovine heart cytochrome-c oxidase. Biochim. Biophys. Acta 1991, 1059, 189–207. [Google Scholar] [CrossRef]
- Cooper, C.E.; Brown, G.C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008, 40, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Schuler, M.M.; Prior, M.G.; Yong, S.; Coppock, R.W.; Florence, L.Z.; Lillie, L.E. Effects of hydrogen sulfide exposure on lung mitochondrial respiratory chain enzymes in rats. Toxicol. Appl. Pharmacol. 1990, 103, 482–490. [Google Scholar] [CrossRef]
- Khan, A.A.; Yong, S.; Prior, M.G.; Lillie, L.E. Cytotoxic effects of hydrogen sulfide on pulmonary alveolar macrophages in rats. J. Toxicol. Environ. Health 1991, 33, 57–64. [Google Scholar] [CrossRef]
- Struve, M.F.; Brisbois, J.N.; James, R.A.; Marshall, M.W.; Dorman, D.C. Neurotoxicological effects associated with short-term exposure of Sprague-Dawley rats to hydrogen sulfide. Neurotoxicology 2001, 22, 375–385. [Google Scholar] [CrossRef]
- Dorman, D.C.; Moulin, F.J.; McManus, B.E.; Mahle, K.C.; James, R.A.; Struve, M.F. Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: Correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicol. Sci. 2002, 65, 18–25. [Google Scholar] [CrossRef]
- Dorman, D.C.; Struve, M.F.; Gross, E.A.; Brenneman, K.A. Respiratory tract toxicity of inhaled hydrogen sulfide in Fischer-344 rats, Sprague-Dawley rats, and B6C3F1 mice following subchronic (90-day) exposure. Toxicol. Appl. Pharmacol. 2004, 198, 29–39. [Google Scholar] [CrossRef]
- Wu, N.; Du, X.; Wang, D.; Hao, F. Myocardial and lung injuries induced by hydrogen sulfide and the effectiveness of oxygen therapy in rats. Clin. Toxicol. 2011, 49, 161–166. [Google Scholar] [CrossRef]
- Thompson, R.W.; Valentine, H.L.; Valentine, W.M. Cytotoxic mechanisms of hydrosulfide anion and cyanide anion in primary rat hepatocyte cultures. Toxicology 2003, 188, 149–159. [Google Scholar] [CrossRef]
- Leschelle, X.; Goubern, M.; Andriamihaja, M.; Blottiere, H.M.; Couplan, E.; Gonzalez-Barroso, M.D.; Petit, C.; Pagniez, A.; Chaumontet, C.; Mignotte, B.; et al. Adaptative metabolic response of human colonic epithelial cells to the adverse effects of the luminal compound sulfide. Biochim. Biophys. Acta 2005, 1725, 201–212. [Google Scholar] [CrossRef]
- Truong, D.H.; Eghbal, M.A.; Hindmarsh, W.; Roth, S.H.; O’Brien, P.J. Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab. Rev. 2006, 38, 733–744. [Google Scholar] [CrossRef]
- Sun, W.H.; Liu, F.; Chen, Y.; Zhu, Y.C. Hydrogen sulfide decreases the levels of ROS by inhibiting mitochondrial complex IV and increasing SOD activities in cardiomyocytes under ischemia/reperfusion. Biochem. Biophys. Res. Commun. 2012, 421, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Groeger, M.; Matallo, J.; McCook, O.; Wagner, F.; Wachter, U.; Bastian, O.; Gierer, S.; Reich, V.; Stahl, B.; Huber-Lang, M.; et al. Temperature and cell-type dependency of sulfide effects on mitochondrial respiration. Shock 2012, 38, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Buckler, K.J. Effects of exogenous hydrogen sulphide on calcium signalling, background (TASK) K channel activity and mitochondrial function in chemoreceptor cells. Pflugers Arch. 2012, 463, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, I.; Fagiolari, G.; Prelle, A.; Viscomi, C.; Zeviani, M.; Tiranti, V. Chronic exposure to sulfide causes accelerated degradation of cytochrome c oxidase in ethylmalonic encephalopathy. Antioxid. Redox Signal. 2011, 15, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, P.; Petersen, L.C.; Miller, M.; Hansen, F.B. Ligand-induced spectral changes in cytochrome c oxidase and their possible significance. Biochim. Biophys. Acta 1976, 449, 188–196. [Google Scholar] [CrossRef]
- Hill, B.C.; Woon, T.C.; Nicholls, P.; Peterson, J.; Greenwood, C.; Thomson, A.J. Interactions of sulphide and other ligands with cytochrome c oxidase. An electron-paramagnetic-resonance study. Biochem. J. 1984, 224, 591–600. [Google Scholar] [CrossRef]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1479–R1485. [Google Scholar] [CrossRef]
- Dordevic, D.; Jancikova, S.; Vitezova, M.; Kushkevych, I. Hydrogen sulfide toxicity in the gut environment: Meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J. Adv. Res. 2020, 27, 55–69. [Google Scholar] [CrossRef]
- Carbonero, F.; Benefiel, A.C.; Alizadeh-Ghamsari, A.H.; Gaskins, H.R. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 2012, 3, 448. [Google Scholar] [CrossRef]
- Forte, E.; Borisov, V.B.; Falabella, M.; Colaco, H.G.; Tinajero-Trejo, M.; Poole, R.K.; Vicente, J.B.; Sarti, P.; Giuffre, A. The terminal oxidase cytochrome bd promotes sulfide-resistant bacterial respiration and growth. Sci. Rep. 2016, 6, 23788. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, S.; Imlay, K.R.; Imlay, J.A. The cytochrome bd oxidase of Escherichia coli prevents respiratory inhibition by endogenous and exogenous hydrogen sulfide. Mol. Microbiol. 2016, 101, 62–77. [Google Scholar] [CrossRef]
- Forte, E.; Giuffre, A. How bacteria breathe in hydrogen sulphide-rich environments. Biochemist 2016, 38, 8–11. [Google Scholar] [CrossRef]
- Borisov, V.B.; Forte, E. Terminal oxidase cytochrome bd protects bacteria against hydrogen sulfide toxicity. Biochemistry 2021, 86, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Karami, N.; Nowrouzian, F.; Adlerberth, I.; Wold, A.E. Tetracycline resistance in Escherichia coli and persistence in the infantile colonic microbiota. Antimicrob. Agents Chemother. 2006, 50, 156–161. [Google Scholar] [CrossRef]
- Poole, R.K.; Cook, G.M. Redundancy of aerobic respiratory chains in bacteria? Routes, reasons and regulation. Adv. Microb. Physiol. 2000, 43, 165–224. [Google Scholar] [CrossRef]
- Ingledew, W.J.; Poole, R.K. The respiratory chains of Escherichia coli. Microbiol. Rev. 1984, 48, 222–271. [Google Scholar] [CrossRef]
- Azarkina, N.; Borisov, V.; Konstantinov, A.A. Spontaneous spectral changes of the reduced cytochrome bd. FEBS Lett. 1997, 416, 171–174. [Google Scholar] [CrossRef]
- Erhardt, H.; Dempwolff, F.; Pfreundschuh, M.; Riehle, M.; Schafer, C.; Pohl, T.; Graumann, P.; Friedrich, T. Organization of the Escherichia coli aerobic enzyme complexes of oxidative phosphorylation in dynamic domains within the cytoplasmic membrane. Microbiologyopen 2014, 3, 316–326. [Google Scholar] [CrossRef]
- Borisov, V.B.; Verkhovsky, M.I. Oxygen as Acceptor. EcoSal Plus 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Azarkina, N.; Siletsky, S.; Borisov, V.; von Wachenfeldt, C.; Hederstedt, L.; Konstantinov, A.A. A cytochrome bb′-type quinol oxidase in Bacillus subtilis strain 168. J. Biol. Chem. 1999, 274, 32810–32817. [Google Scholar] [CrossRef] [PubMed]
- Melin, F.; Sabuncu, S.; Choi, S.K.; Leprince, A.; Gennis, R.B.; Hellwig, P. Role of the tightly bound quinone for the oxygen reaction of cytochrome bo3 oxidase from Escherichia coli. FEBS Lett. 2018, 592, 3380–3387. [Google Scholar] [CrossRef] [PubMed]
- Szundi, I.; Kittredge, C.; Choi, S.K.; McDonald, W.; Ray, J.; Gennis, R.B.; Einarsdottir, O. Kinetics and intermediates of the reaction of fully reduced Escherichia coli bo3 ubiquinol oxidase with O2. Biochemistry 2014, 53, 5393–5404. [Google Scholar] [CrossRef]
- Mogi, T.; Tsubaki, M.; Hori, H.; Miyoshi, H.; Nakamura, H.; Anraku, Y. Two terminal quinol oxidase families in Escherichia coli: Variations on molecular machinery for dioxygen reduction. J. Biochem. Mol. Biol. Biophys. 1998, 2, 79–110. [Google Scholar]
- Svensson Ek, M.; Brzezinski, P. Oxidation of ubiquinol by cytochrome bo3 from Escherichia coli: Kinetics of electron and proton transfer. Biochemistry 1997, 36, 5425–5431. [Google Scholar] [CrossRef]
- Yang, K.; Borisov, V.B.; Konstantinov, A.A.; Gennis, R.B. The fully oxidized form of the cytochrome bd quinol oxidase from E. coli does not participate in the catalytic cycle: Direct evidence from rapid kinetics studies. FEBS Lett. 2008, 582, 3705–3709. [Google Scholar] [CrossRef]
- Junemann, S. Cytochrome bd terminal oxidase. Biochim. Biophys. Acta 1997, 1321, 107–127. [Google Scholar] [CrossRef]
- Borisov, V.B.; Forte, E.; Sarti, P.; Giuffre, A. Catalytic intermediates of cytochrome bd terminal oxidase at steady-state: Ferryl and oxy-ferrous species dominate. Biochim. Biophys. Acta 2011, 1807, 503–509. [Google Scholar] [CrossRef]
- Li, M.; Jorgensen, S.K.; McMillan, D.G.; Krzeminski, L.; Daskalakis, N.N.; Partanen, R.H.; Tutkus, M.; Tuma, R.; Stamou, D.; Hatzakis, N.S.; et al. Single enzyme experiments reveal a long-lifetime proton leak state in a heme-copper oxidase. J. Am. Chem. Soc. 2015, 137, 16055–16063. [Google Scholar] [CrossRef]
- Asseri, A.H.; Godoy-Hernandez, A.; Goojani, H.G.; Lill, H.; Sakamoto, J.; McMillan, D.G.G.; Bald, D. Cardiolipin enhances the enzymatic activity of cytochrome bd and cytochrome bo3 solubilized in dodecyl-maltoside. Sci. Rep. 2021, 11, 8006. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, A.; Safarian, S.; Thesseling, A.; Wohlwend, D.; Friedrich, T.; Michel, H.; Kusumoto, T.; Sakamoto, J.; Melin, F.; Hellwig, P. Electrocatalytic evidence of the diversity of the oxygen reaction in the bacterial bd oxidase from different organisms. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148436. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi-Matsui, M.; Sekiya, M.; Futai, M. ATP synthase from Escherichia coli: Mechanism of rotational catalysis, and inhibition with the epsilon subunit and phytopolyphenols. Biochim. Biophys. Acta 2016, 1857, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Deckers-Hebestreit, G.; Greie, J.; Stalz, W.; Altendorf, K. The ATP synthase of Escherichia coli: Structure and function of F0 subunits. Biochim. Biophys. Acta 2000, 1458, 364–373. [Google Scholar] [CrossRef]
- Sobti, M.; Walshe, J.L.; Wu, D.; Ishmukhametov, R.; Zeng, Y.C.; Robinson, C.V.; Berry, R.M.; Stewart, A.G. Cryo-EM structures provide insight into how E. coli F1Fo ATP synthase accommodates symmetry mismatch. Nat. Commun. 2020, 11, 2615. [Google Scholar] [CrossRef]
- Puustinen, A.; Finel, M.; Haltia, T.; Gennis, R.B.; Wikstrom, M. Properties of the two terminal oxidases of Escherichia coli. Biochemistry 1991, 30, 3936–3942. [Google Scholar] [CrossRef]
- Jasaitis, A.; Borisov, V.B.; Belevich, N.P.; Morgan, J.E.; Konstantinov, A.A.; Verkhovsky, M.I. Electrogenic reactions of cytochrome bd. Biochemistry 2000, 39, 13800–13809. [Google Scholar] [CrossRef]
- Belevich, I.; Borisov, V.B.; Zhang, J.; Yang, K.; Konstantinov, A.A.; Gennis, R.B.; Verkhovsky, M.I. Time-resolved electrometric and optical studies on cytochrome bd suggest a mechanism of electron-proton coupling in the di-heme active site. Proc. Natl. Acad. Sci. USA 2005, 102, 3657–3662. [Google Scholar] [CrossRef]
- Belevich, I.; Borisov, V.B.; Verkhovsky, M.I. Discovery of the true peroxy intermediate in the catalytic cycle of terminal oxidases by real-time measurement. J. Biol. Chem. 2007, 282, 28514–28519. [Google Scholar] [CrossRef]
- Borisov, V.B.; Belevich, I.; Bloch, D.A.; Mogi, T.; Verkhovsky, M.I. Glutamate 107 in subunit I of cytochrome bd from Escherichia coli is part of a transmembrane intraprotein pathway conducting protons from the cytoplasm to the heme b595/heme d active site. Biochemistry 2008, 47, 7907–7914. [Google Scholar] [CrossRef]
- Borisov, V.B.; Murali, R.; Verkhovskaya, M.L.; Bloch, D.A.; Han, H.; Gennis, R.B.; Verkhovsky, M.I. Aerobic respiratory chain of Escherichia coli is not allowed to work in fully uncoupled mode. Proc. Natl. Acad. Sci. USA 2011, 108, 17320–17324. [Google Scholar] [CrossRef]
- Abramson, J.; Riistama, S.; Larsson, G.; Jasaitis, A.; Svensson-Ek, M.; Laakkonen, L.; Puustinen, A.; Iwata, S.; Wikstrom, M. The structure of the ubiquinol oxidase from Escherichia coli and its ubiquinone binding site. Nat. Struct. Biol. 2000, 7, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, L.; Vallese, F.; Ding, Z.; Choi, S.K.; Hong, S.; Luo, Y.; Liu, B.; Chan, C.K.; Tajkhorshid, E.; et al. Cryo-EM structures of Escherichia coli cytochrome bo3 reveal bound phospholipids and ubiquinone-8 in a dynamic substrate binding site. Proc. Natl. Acad. Sci. USA 2021, 118, e2106750118. [Google Scholar] [CrossRef] [PubMed]
- Safarian, S.; Hahn, A.; Mills, D.J.; Radloff, M.; Eisinger, M.L.; Nikolaev, A.; Meier-Credo, J.; Melin, F.; Miyoshi, H.; Gennis, R.B.; et al. Active site rearrangement and structural divergence in prokaryotic respiratory oxidases. Science 2019, 366, 100–104. [Google Scholar] [CrossRef]
- Thesseling, A.; Rasmussen, T.; Burschel, S.; Wohlwend, D.; Kagi, J.; Muller, R.; Bottcher, B.; Friedrich, T. Homologous bd oxidases share the same architecture but differ in mechanism. Nat. Commun. 2019, 10, 5138. [Google Scholar] [CrossRef]
- Choi, S.K.; Schurig-Briccio, L.; Ding, Z.; Hong, S.; Sun, C.; Gennis, R.B. Location of the substrate binding site of the cytochrome bo3 ubiquinol oxidase from Escherichia coli. J. Am. Chem. Soc. 2017, 139, 8346–8354. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.B.; Gennis, R.B.; Hemp, J.; Verkhovsky, M.I. The cytochrome bd respiratory oxygen reductases. Biochim. Biophys. Acta 2011, 1807, 1398–1413. [Google Scholar] [CrossRef]
- Arutyunyan, A.M.; Sakamoto, J.; Inadome, M.; Kabashima, Y.; Borisov, V.B. Optical and magneto-optical activity of cytochrome bd from Geobacillus thermodenitrificans. Biochim. Biophys. Acta 2012, 1817, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Gennis, R.B.; Hemp, J. Evolution of the cytochrome bd oxygen reductase superfamily and the function of CydAA’ in Archaea. ISME J. 2021. [Google Scholar] [CrossRef]
- Borisov, V.B. Cytochrome bd: Structure and properties. Biochemistry 1996, 61, 565–574. [Google Scholar]
- Forte, E.; Borisov, V.B.; Vicente, J.B.; Giuffre, A. Cytochrome bd and gaseous ligands in bacterial physiology. Adv. Microb. Physiol. 2017, 71, 171–234. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.B. Effect of membrane environment on ligand-binding properties of the terminal oxidase cytochrome bd-I from Escherichia coli. Biochemistry 2020, 85, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.B.; Siletsky, S.A.; Paiardini, A.; Hoogewijs, D.; Forte, E.; Giuffre, A.; Poole, R.K. Bacterial oxidases of the cytochrome bd family: Redox enzymes of unique structure, function and utility as drug targets. Antioxid. Redox Signal. 2021, 34, 1280–1318. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.J.; Alben, J.O.; Gennis, R.B. Spectroscopic evidence for a heme-heme binuclear center in the cytochrome bd ubiquinol oxidase from Escherichia coli. Proc. Natl. Acad. Sci. USA 1993, 90, 5863–5867. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, M.; Hori, H.; Mogi, T.; Anraku, Y. Cyanide-binding site of bd-type ubiquinol oxidase from Escherichia coli. J. Biol. Chem. 1995, 270, 28565–28569. [Google Scholar] [CrossRef]
- Borisov, V.; Arutyunyan, A.M.; Osborne, J.P.; Gennis, R.B.; Konstantinov, A.A. Magnetic circular dichroism used to examine the interaction of Escherichia coli cytochrome bd with ligands. Biochemistry 1999, 38, 740–750. [Google Scholar] [CrossRef]
- Vos, M.H.; Borisov, V.B.; Liebl, U.; Martin, J.L.; Konstantinov, A.A. Femtosecond resolution of ligand-heme interactions in the high-affinity quinol oxidase bd: A di-heme active site? Proc. Natl. Acad. Sci. USA 2000, 97, 1554–1559. [Google Scholar] [CrossRef]
- Borisov, V.B.; Sedelnikova, S.E.; Poole, R.K.; Konstantinov, A.A. Interaction of cytochrome bd with carbon monoxide at low and room temperatures: Evidence that only a small fraction of heme b595 reacts with CO. J. Biol. Chem. 2001, 276, 22095–22099. [Google Scholar] [CrossRef]
- Borisov, V.B.; Liebl, U.; Rappaport, F.; Martin, J.L.; Zhang, J.; Gennis, R.B.; Konstantinov, A.A.; Vos, M.H. Interactions between heme d and heme b595 in quinol oxidase bd from Escherichia coli: A photoselection study using femtosecond spectroscopy. Biochemistry 2002, 41, 1654–1662. [Google Scholar] [CrossRef]
- Arutyunyan, A.M.; Borisov, V.B.; Novoderezhkin, V.I.; Ghaim, J.; Zhang, J.; Gennis, R.B.; Konstantinov, A.A. Strong excitonic interactions in the oxygen-reducing site of bd-type oxidase: The Fe-to-Fe distance between hemes d and b595 is 10 A. Biochemistry 2008, 47, 1752–1759. [Google Scholar] [CrossRef]
- Borisov, V.B. Interaction of bd-type quinol oxidase from Escherichia coli and carbon monoxide: Heme d binds CO with high affinity. Biochemistry 2008, 73, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Bloch, D.A.; Borisov, V.B.; Mogi, T.; Verkhovsky, M.I. Heme/heme redox interaction and resolution of individual optical absorption spectra of the hemes in cytochrome bd from Escherichia coli. Biochim. Biophys. Acta 2009, 1787, 1246–1253. [Google Scholar] [CrossRef]
- Rappaport, F.; Zhang, J.; Vos, M.H.; Gennis, R.B.; Borisov, V.B. Heme-heme and heme-ligand interactions in the di-heme oxygen-reducing site of cytochrome bd from Escherichia coli revealed by nanosecond absorption spectroscopy. Biochim. Biophys. Acta 2010, 1797, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.B.; Verkhovsky, M.I. Accommodation of CO in the di-heme active site of cytochrome bd terminal oxidase from Escherichia coli. J. Inorg. Biochem. 2013, 118, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Siletsky, S.A.; Zaspa, A.A.; Poole, R.K.; Borisov, V.B. Microsecond time-resolved absorption spectroscopy used to study CO compounds of cytochrome bd from Escherichia coli. PLoS ONE 2014, 9, e95617. [Google Scholar] [CrossRef]
- Siletsky, S.A.; Rappaport, F.; Poole, R.K.; Borisov, V.B. Evidence for fast electron transfer between the high-spin haems in cytochrome bd-I from Escherichia coli. PLoS ONE 2016, 11, e0155186. [Google Scholar] [CrossRef]
- Siletsky, S.A.; Dyuba, A.V.; Elkina, D.A.; Monakhova, M.V.; Borisov, V.B. Spectral-kinetic analysis of recombination reaction of heme centers of bd-type quinol oxidase from Escherichia coli with carbon monoxide. Biochemistry 2017, 82, 1354–1366. [Google Scholar] [CrossRef]
- Svensson, M.; Nilsson, T. Flow-flash study of the reaction between cytochrome bo and oxygen. Biochemistry 1993, 32, 5442–5447. [Google Scholar] [CrossRef]
- Borisov, V.B.; Smirnova, I.A.; Krasnosel’skaya, I.A.; Konstantinov, A.A. Oxygenated cytochrome bd from Escherichia coli can be converted into the oxidized form by lipophilic electron acceptors. Biochemistry 1994, 59, 437–443. [Google Scholar]
- D’Mello, R.; Hill, S.; Poole, R.K. The oxygen affinity of cytochrome bo′ in Escherichia coli determined by the deoxygenation of oxyleghemoglobin and oxymyoglobin: Km values for oxygen are in the submicromolar range. J. Bacteriol. 1995, 177, 867–870. [Google Scholar] [CrossRef][Green Version]
- D’mello, R.; Hill, S.; Poole, R.K. The cytochrome bd quinol oxidase in Escherichia coli has an extremely high oxygen affinity and two-oxygen-binding haems: Implicaitons for regulation of activity in vivo by oxygen inihibition. Microbiology 1996, 142, 755–763. [Google Scholar] [CrossRef]
- Belevich, I.; Borisov, V.B.; Konstantinov, A.A.; Verkhovsky, M.I. Oxygenated complex of cytochrome bd from Escherichia coli: Stability and photolability. FEBS Lett. 2005, 579, 4567–4570. [Google Scholar] [CrossRef] [PubMed]
- Belevich, I.; Borisov, V.B.; Bloch, D.A.; Konstantinov, A.A.; Verkhovsky, M.I. Cytochrome bd from Azotobacter vinelandii: Evidence for high-affinity oxygen binding. Biochemistry 2007, 46, 11177–11184. [Google Scholar] [CrossRef]
- Cotter, P.A.; Chepuri, V.; Gennis, R.B.; Gunsalus, R.P. Cytochrome o (cyoABCDE) and d (cydAB) oxidase gene expression in Escherichia coli is regulated by oxygen, pH, and the fnr gene product. J. Bacteriol. 1990, 172, 6333–6338. [Google Scholar] [CrossRef]
- Alexeeva, S.; Hellingwerf, K.; Teixeira de Mattos, M.J. Quantitative assessment of oxygen availability: Perceived aerobiosis and its effect on flux distribution in the respiratory chain of Escherichia coli. J. Bacteriol. 2002, 184, 1402–1406. [Google Scholar] [CrossRef]
- Rolfe, M.D.; Ter Beek, A.; Graham, A.I.; Trotter, E.W.; Asif, H.M.; Sanguinetti, G.; de Mattos, J.T.; Poole, R.K.; Green, J. Transcript profiling and inference of Escherichia coli K-12 ArcA activity across the range of physiologically relevant oxygen concentrations. J. Biol. Chem. 2011, 286, 10147–10154. [Google Scholar] [CrossRef]
- Ederer, M.; Steinsiek, S.; Stagge, S.; Rolfe, M.D.; Ter Beek, A.; Knies, D.; Teixeira de Mattos, M.J.; Sauter, T.; Green, J.; Poole, R.K.; et al. A mathematical model of metabolism and regulation provides a systems-level view of how Escherichia coli responds to oxygen. Front. Microbiol. 2014, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Bettenbrock, K.; Bai, H.; Ederer, M.; Green, J.; Hellingwerf, K.J.; Holcombe, M.; Kunz, S.; Rolfe, M.D.; Sanguinetti, G.; Sawodny, O.; et al. Towards a systems level understanding of the oxygen response of Escherichia coli. Adv. Microb. Physiol. 2014, 64, 65–114. [Google Scholar] [CrossRef] [PubMed]
- Forte, E.; Borisov, V.B.; Konstantinov, A.A.; Brunori, M.; Giuffre, A.; Sarti, P. Cytochrome bd, a key oxidase in bacterial survival and tolerance to nitrosative stress. Ital. J. Biochem. 2007, 56, 265–269. [Google Scholar]
- Borisov, V.B.; Forte, E.; Siletsky, S.A.; Arese, M.; Davletshin, A.I.; Sarti, P.; Giuffre, A. Cytochrome bd protects bacteria against oxidative and nitrosative stress: A potential target for next-generation antimicrobial agents. Biochemistry 2015, 80, 565–575. [Google Scholar] [CrossRef]
- Giuffre, A.; Borisov, V.B.; Mastronicola, D.; Sarti, P.; Forte, E. Cytochrome bd oxidase and nitric oxide: From reaction mechanisms to bacterial physiology. FEBS Lett. 2012, 586, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Giuffre, A.; Borisov, V.B.; Arese, M.; Sarti, P.; Forte, E. Cytochrome bd oxidase and bacterial tolerance to oxidative and nitrosative stress. Biochim. Biophys. Acta 2014, 1837, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Forte, E.; Borisov, V.B.; Siletsky, S.A.; Petrosino, M.; Giuffre, A. In the respiratory chain of Escherichia coli cytochromes bd-I and bd-II are more sensitive to carbon monoxide inhibition than cytochrome bo3. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 148088. [Google Scholar] [CrossRef]
- Borisov, V.B.; Forte, E.; Konstantinov, A.A.; Poole, R.K.; Sarti, P.; Giuffre, A. Interaction of the bacterial terminal oxidase cytochrome bd with nitric oxide. FEBS Lett. 2004, 576, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.B.; Forte, E.; Sarti, P.; Brunori, M.; Konstantinov, A.A.; Giuffre, A. Nitric oxide reacts with the ferryl-oxo catalytic intermediate of the CuB-lacking cytochrome bd terminal oxidase. FEBS Lett. 2006, 580, 4823–4826. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.B.; Forte, E.; Sarti, P.; Brunori, M.; Konstantinov, A.A.; Giuffre, A. Redox control of fast ligand dissociation from Escherichia coli cytochrome bd. Biochem. Biophys. Res. Commun. 2007, 355, 97–102. [Google Scholar] [CrossRef]
- Mason, M.G.; Shepherd, M.; Nicholls, P.; Dobbin, P.S.; Dodsworth, K.S.; Poole, R.K.; Cooper, C.E. Cytochrome bd confers nitric oxide resistance to Escherichia coli. Nat. Chem. Biol. 2009, 5, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.B.; Forte, E.; Giuffre, A.; Konstantinov, A.; Sarti, P. Reaction of nitric oxide with the oxidized di-heme and heme-copper oxygen-reducing centers of terminal oxidases: Different reaction pathways and end-products. J. Inorg. Biochem. 2009, 103, 1185–1187. [Google Scholar] [CrossRef]
- Shepherd, M.; Achard, M.E.; Idris, A.; Totsika, M.; Phan, M.D.; Peters, K.M.; Sarkar, S.; Ribeiro, C.A.; Holyoake, L.V.; Ladakis, D.; et al. The cytochrome bd-I respiratory oxidase augments survival of multidrug-resistant Escherichia coli during infection. Sci. Rep. 2016, 6, 35285. [Google Scholar] [CrossRef]
- Holyoake, L.V.; Hunt, S.; Sanguinetti, G.; Cook, G.M.; Howard, M.J.; Rowe, M.L.; Poole, R.K.; Shepherd, M. CydDC-mediated reductant export in Escherichia coli controls the transcriptional wiring of energy metabolism and combats nitrosative stress. Biochem. J. 2016, 473, 693–701. [Google Scholar] [CrossRef]
- Jones-Carson, J.; Husain, M.; Liu, L.; Orlicky, D.J.; Vazquez-Torres, A. Cytochrome bd-dependent bioenergetics and antinitrosative defenses in Salmonella pathogenesis. mBio 2016, 7, e02052-16. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Yin, J.; Jin, M.; Gao, H. Distinct nitrite and nitric oxide physiologies in Escherichia coli and Shewanella oneidensis. Appl. Environ. Microbiol. 2018, 84, e00559-18. [Google Scholar] [CrossRef] [PubMed]
- Beebout, C.J.; Eberly, A.R.; Werby, S.H.; Reasoner, S.A.; Brannon, J.R.; De, S.; Fitzgerald, M.J.; Huggins, M.M.; Clayton, D.B.; Cegelski, L.; et al. Respiratory heterogeneity shapes biofilm formation and host colonization in uropathogenic Escherichia coli. mBio 2019, 10, e02400-18. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.B.; Forte, E.; Siletsky, S.A.; Sarti, P.; Giuffre, A. Cytochrome bd from Escherichia coli catalyzes peroxynitrite decomposition. Biochim. Biophys. Acta 2015, 1847, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.; Gennis, R.; Konstantinov, A.A. Peroxide complex of cytochrome bd: Kinetics of generation and stability. Biochem. Mol. Biol. Int. 1995, 37, 975–982. [Google Scholar]
- Borisov, V.B.; Gennis, R.B.; Konstantinov, A.A. Interaction of cytochrome bd from Escherichia coli with hydrogen peroxide. Biochemistry 1995, 60, 231–239. [Google Scholar]
- Borisov, V.B.; Davletshin, A.I.; Konstantinov, A.A. Peroxidase activity of cytochrome bd from Escherichia coli. Biochemistry 2010, 75, 428–436. [Google Scholar] [CrossRef]
- Borisov, V.B.; Forte, E.; Davletshin, A.; Mastronicola, D.; Sarti, P.; Giuffre, A. Cytochrome bd oxidase from Escherichia coli displays high catalase activity: An additional defense against oxidative stress. FEBS Lett. 2013, 587, 2214–2218. [Google Scholar] [CrossRef]
- Forte, E.; Borisov, V.B.; Davletshin, A.; Mastronicola, D.; Sarti, P.; Giuffre, A. Cytochrome bd oxidase and hydrogen peroxide resistance in Mycobacterium tuberculosis. mBio 2013, 4, e01006-13. [Google Scholar] [CrossRef]
- Al-Attar, S.; Yu, Y.; Pinkse, M.; Hoeser, J.; Friedrich, T.; Bald, D.; de Vries, S. Cytochrome bd displays significant quinol peroxidase activity. Sci. Rep. 2016, 6, 27631. [Google Scholar] [CrossRef]
- Kita, K.; Konishi, K.; Anraku, Y. Terminal oxidases of Escherichia coli aerobic respiratory chain. II. Purification and properties of cytochrome b558-d complex from cells grown with limited oxygen and evidence of branched electron-carrying systems. J. Biol. Chem. 1984, 259, 3375–3381. [Google Scholar] [CrossRef]
- Sakamoto, J.; Koga, E.; Mizuta, T.; Sato, C.; Noguchi, S.; Sone, N. Gene structure and quinol oxidase activity of a cytochrome bd-type oxidase from Bacillus stearothermophilus. Biochim. Biophys. Acta 1999, 1411, 147–158. [Google Scholar] [CrossRef]
- Forte, E.; Siletsky, S.A.; Borisov, V.B. In Escherichia coli ammonia inhibits cytochrome bo3 but activates cytochrome bd-I. Antioxidants 2021, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, L.; Bald, D. Cytochrome bd in Mycobacterium tuberculosis: A respiratory chain protein involved in the defense against antibacterials. Prog. Biophys. Mol. Biol. 2020, 152, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Sviriaeva, E.; Pethe, K. Targeting the cytochrome oxidases for drug development in mycobacteria. Prog. Biophys. Mol. Biol. 2020, 152, 45–54. [Google Scholar] [CrossRef]
- Cook, G.M.; Hards, K.; Dunn, E.; Heikal, A.; Nakatani, Y.; Greening, C.; Crick, D.C.; Fontes, F.L.; Pethe, K.; Hasenoehrl, E.; et al. Oxidative phosphorylation as a target space for tuberculosis: Success, caution, and future directions. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Bald, D.; Villellas, C.; Lu, P.; Koul, A. Targeting energy metabolism in Mycobacterium tuberculosis, a new paradigm in antimycobacterial drug discovery. mBio 2017, 8, e00272-17. [Google Scholar] [CrossRef]
- Hards, K.; Cook, G.M. Targeting bacterial energetics to produce new antimicrobials. Drug Resist. Updat. 2018, 36, 1–12. [Google Scholar] [CrossRef]
- Winstedt, L.; Frankenberg, L.; Hederstedt, L.; von Wachenfeldt, C. Enterococcus faecalis V583 contains a cytochrome bd-type respiratory oxidase. J. Bacteriol. 2000, 182, 3863–3866. [Google Scholar] [CrossRef]
- Lee, B.S.; Hards, K.; Engelhart, C.A.; Hasenoehrl, E.J.; Kalia, N.P.; Mackenzie, J.S.; Sviriaeva, E.; Chong, S.M.S.; Manimekalai, M.S.S.; Koh, V.H.; et al. Dual inhibition of the terminal oxidases eradicates antibiotic-tolerant Mycobacterium tuberculosis. EMBO Mol. Med. 2021, 13, e13207. [Google Scholar] [CrossRef]
- Saini, V.; Chinta, K.C.; Reddy, V.P.; Glasgow, J.N.; Stein, A.; Lamprecht, D.A.; Rahman, M.A.; Mackenzie, J.S.; Truebody, B.E.; Adamson, J.H.; et al. Hydrogen sulfide stimulates Mycobacterium tuberculosis respiration, growth and pathogenesis. Nat. Commun. 2020, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Kunota, T.T.R.; Rahman, M.A.; Truebody, B.E.; Mackenzie, J.S.; Saini, V.; Lamprecht, D.A.; Adamson, J.H.; Sevalkar, R.R.; Lancaster, J.R., Jr.; Berney, M.; et al. Mycobacterium tuberculosis H2S functions as a sink to modulate central metabolism, bioenergetics, and drug susceptibility. Antioxidants 2021, 10, 1285. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).