Fibroblast Activation Protein Targeted Photodynamic Therapy Selectively Kills Activated Skin Fibroblasts from Systemic Sclerosis Patients and Prevents Tissue Contraction
Abstract
:1. Introduction
2. Results
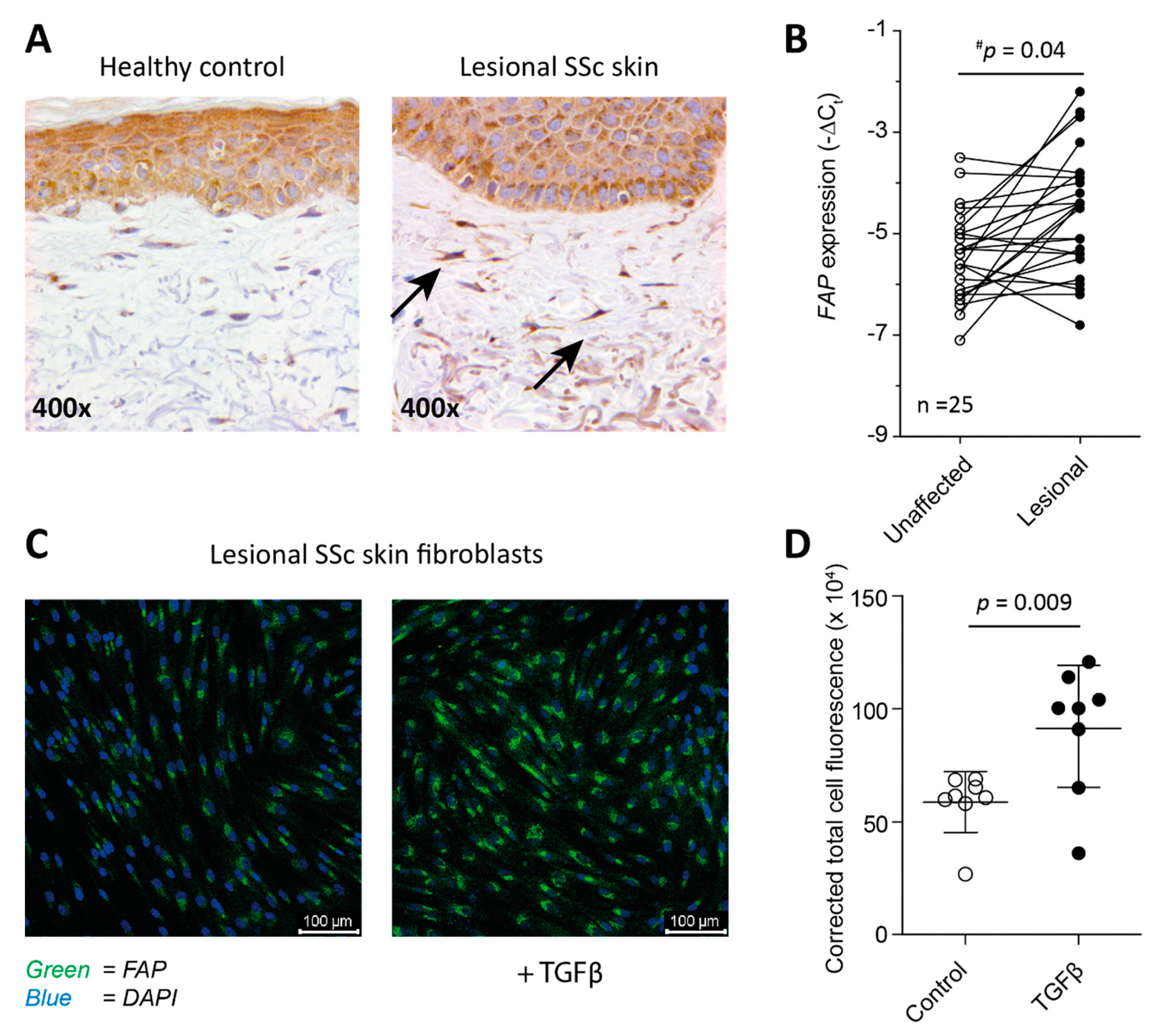
2.1. Fibroblasts from SSc Patient-Derived Skin Express FAP
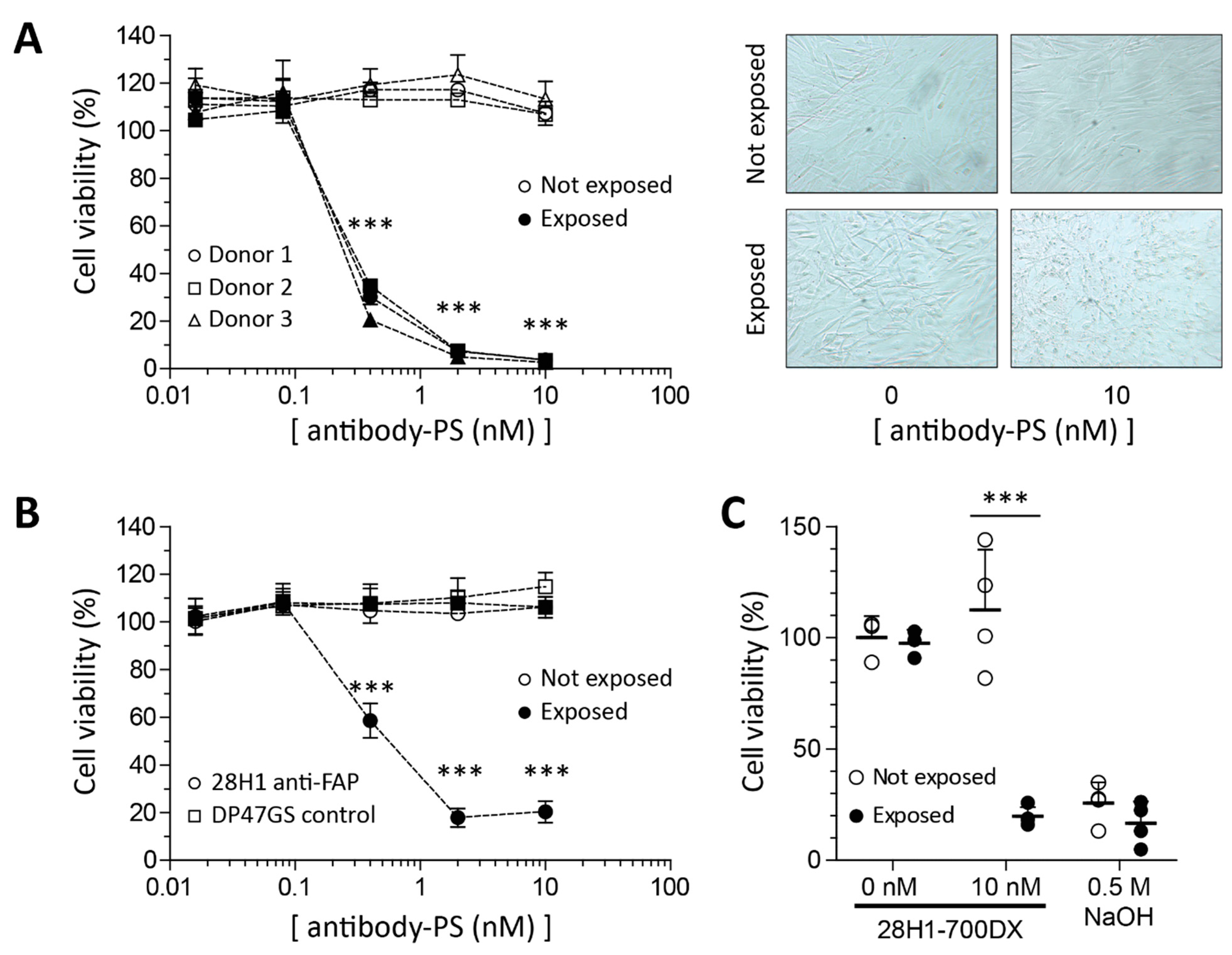
2.2. Reduced Viability in SSc Skin Fibroblasts in Response to FAP-tPDT
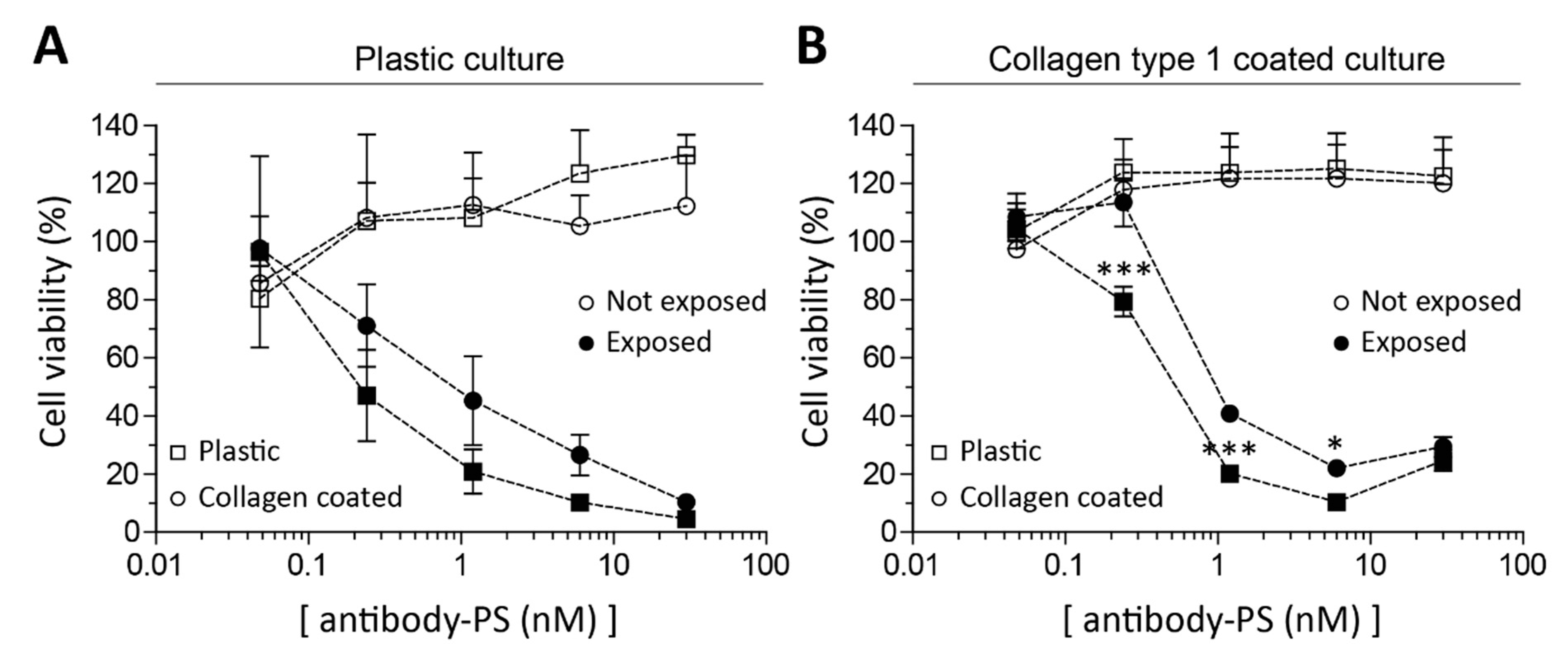
2.3. Fibroblasts’ Culture Substrate Affects FAP-tPDT Efficiency
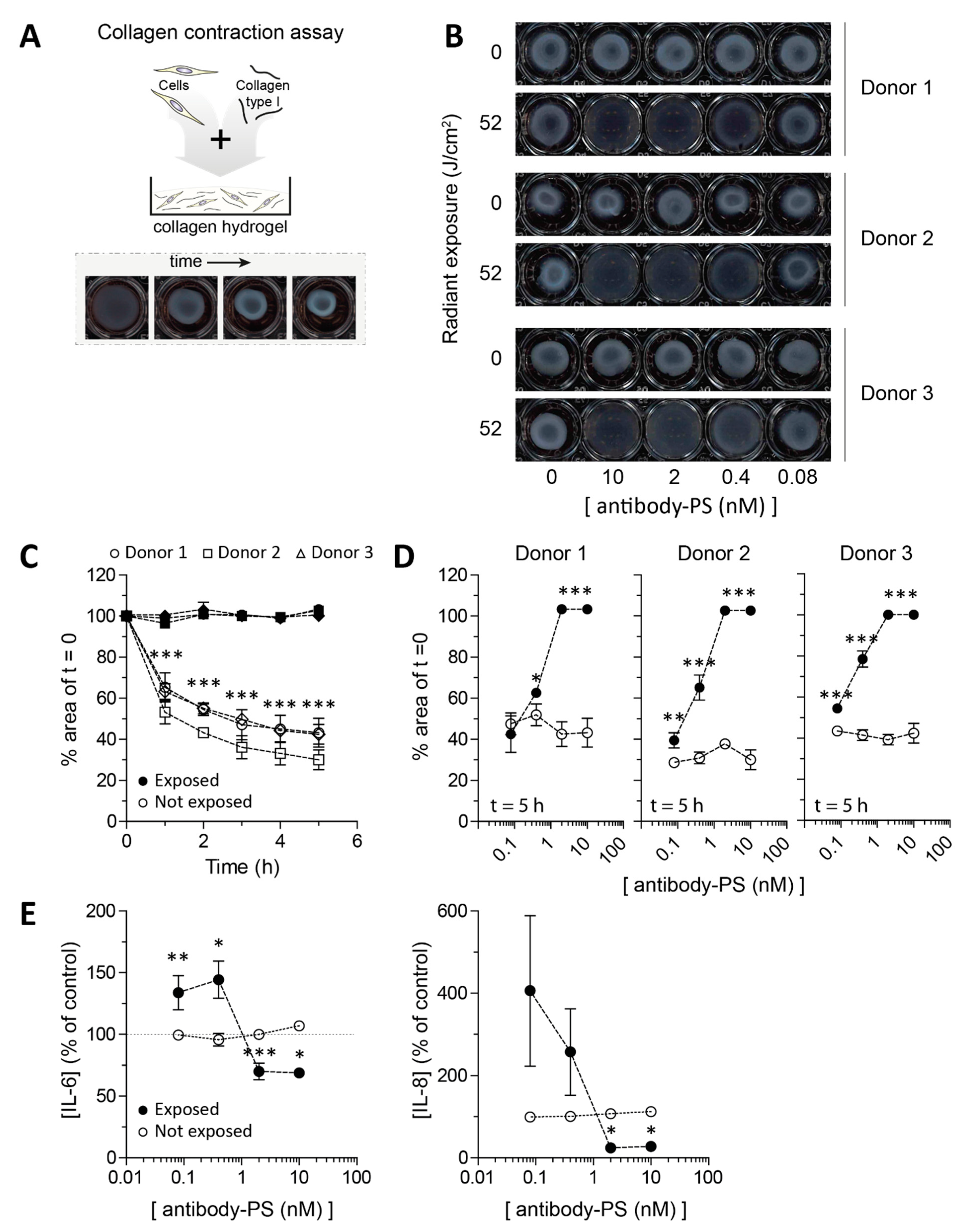
2.4. Impaired Contraction of SSc Skin Fibroblasts after FAP-tPDT
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Collection and Processing of Patient-Derived Skin Biopsies
5.2. Cell Culture
5.3. Antibody Conjugation
5.4. In Vitro 2D FAP-tPDT
5.5. FAP-tPDT on Different Substrates
5.6. In Vitro 3D FAP-tPDT on Collagen Plugs
5.7. Immunohistochemistry
5.8. Immunofluorescence
5.9. RNA Isolation and Quantitative Real-Time PCR
5.10. Cytokine Measurements
5.11. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kowal-Bielecka, O.; Fransen, J.; Avouac, J.; Becker, M.; Kulak, A.; Allanore, Y.; Distler, O.; Clements, P.J.; Cutolo, M.; Czirjak, L.; et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 1327–1339. [Google Scholar] [CrossRef] [Green Version]
- Van Bijnen, S.; De Vries-Bouwstra, J.; Ende, C.H.V.D.; Boonstra, M.; Kroft, L.; Geurts, B.; Snoeren, M.; Schouffoer, A.; Spierings, J.; Van Laar, J.M.; et al. Predictive factors for treatment-related mortality and major adverse events after autologous haematopoietic stem cell transplantation for systemic sclerosis: Results of a long-term follow-up multicentre study. Ann. Rheum. Dis. 2020, 79, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Van Caam, A.; Vonk, M.; Hoogen, F.V.D.; Van Lent, P.; Van Der Kraan, P. Unraveling SSc Pathophysiology; The Myofibroblast. Front. Immunol. 2018, 9, 2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, B.; Lagares, D. Evasion of apoptosis by myofibroblasts: A hallmark of fibrotic diseases. Nat. Rev. Rheumatol. 2020, 16, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Niedermeyer, J.; Kriz, M.; Hilberg, F.; Garin-Chesa, P.; Bamberger, U.; Lenter, M.C.; Park, J.; Viertel, B.; Puschner, H.; Mauz, M.; et al. Targeted Disruption of Mouse Fibroblast Activation Protein. Mol. Cell. Biol. 2000, 20, 1089–1094. [Google Scholar] [CrossRef] [Green Version]
- Croft, A.; Campos, J.; Jansen, K.; Turner, J.; Marshall, J.; Attar, M.; Savary, L.; Wehmeyer, C.; Naylor, A.; Kemble, S.; et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nat. Cell Biol. 2019, 570, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Liu, Y.; Kaneda-Nakashima, K.; Shirakami, Y.; Lindner, T.; Ooe, K.; Toyoshima, A.; Nagata, K.; Shimosegawa, E.; Haberkorn, U.; et al. Theranostics Targeting Fibroblast Activation Protein in the Tumor Stroma: 64Cu- and 225Ac-Labeled FAPI-04 in Pancreatic Cancer Xenograft Mouse Models. J. Nucl. Med. 2020, 61, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.; Deonarine, A.; Jones, J.O.; Denton, A.; Feig, C.; Lyons, S.; Espéli, M.; Kraman, M.; McKenna, B.; Wells, R.J.; et al. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J. Exp. Med. 2013, 210, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Park, C.W.; Son, H.K.; Ju, H.K.; Paik, D.; Koh, G.Y.; Kim, J.; Kim, H.; Jeon, C.J. Fibroblast activation protein α identifies mesenchymal stromal cells from human bone marrow. Br. J. Haematol. 2008, 142, 827–830. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.A.; Schubert, R.D.; Peter, R.U.; Kraut, N.; Park, J.E.; Rettig, W.J.; Garin-Chesa, P. Fibroblast Activation Protein: Differential Expression and Serine Protease Activity in Reactive Stromal Fibroblasts of Melanocytic Skin Tumors. J. Investig. Dermatol. 2003, 120, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Maehara, T.; Kaneko, N.; Perugino, C.A.; Mattoo, H.; Kers, J.; Allard-Chamard, H.; Mahajan, V.S.; Liu, H.; Murphy, S.J.; Ghebremichael, M.; et al. Cytotoxic CD4+ T lymphocytes may induce endothelial cell apoptosis in systemic sclerosis. J. Clin. Investig. 2020, 130, 2451–2464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verjee, L.S.; Verhoekx, J.S.N.; Chan, J.K.K.; Krausgruber, T.; Nicolaidou, V.; Izadi, D.; Davidson, D.; Feldmann, M.; Midwood, K.S.; Nanchahal, J. Unraveling the signaling pathways promoting fibrosis in Dupuytren’s disease reveals TNF as a therapeutic target. Proc. Natl. Acad. Sci. USA 2013, 110, E928–E937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobie, R.; West, C.C.; Henderson, B.E.; Wilson-Kanamori, J.R.; Markose, D.; Kitto, L.J.; Portman, J.R.; Beltran, M.; Sohrabi, S.; Akram, A.R.; et al. Deciphering Mesenchymal Drivers of Human Dupuytren’s Disease at Single-Cell Level. J. Investig. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, M.; Kurniawan, N.A. Mechanical and Physical Regulation of Fibroblast–Myofibroblast Transition: From Cellular Mechanoresponse to Tissue Pathology. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, J.L.; Chaudhry, S.; Sarrazy, V.; Koehler, A.; Hinz, B. The mechanical memory of lung myofibroblasts. Integr. Biol. 2012, 4, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, S.; Bansal, R.; Prakash, J. Drug targeting to myofibroblasts: Implications for fibrosis and cancer. Adv. Drug Deliv. Rev. 2017, 121, 101–116. [Google Scholar] [CrossRef]
- Soare, A.; Györfi, H.A.; Matei, A.E.; Dees, C.; Rauber, S.; Wohlfahrt, T.; Chen, C.-W.; Ludolph, I.; Horch, R.E.; Baeuerle, T.; et al. Dipeptidylpeptidase 4 as a Marker of Activated Fibroblasts and a Potential Target for the Treatment of Fibrosis in Systemic Sclerosis. Arthritis Rheumatol. 2020, 72, 137–149. [Google Scholar] [CrossRef]
- Park, J.-S.; Oh, Y.; Park, Y.J.; Park, O.; Yang, H.; Slania, S.; Hummers, L.K.; Shah, A.A.; An, H.-T.; Jang, J.; et al. Targeting of dermal myofibroblasts through death receptor 5 arrests fibrosis in mouse models of scleroderma. Nat. Commun. 2019, 10, 1128. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.G. Tissue mechanics and fibrosis. Biochim. et Biophys. Acta (BBA) Mol. Basis Dis. 2013, 1832, 884–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Yang, N.; Fiore, V.F.; Barker, T.H.; Sun, Y.; Morris, S.W.; Ding, Q.; Thannickal, V.J.; Zhou, Y. Matrix Stiffness–Induced Myofibroblast Differentiation Is Mediated by Intrinsic Mechanotransduction. Am. J. Respir. Cell Mol. Biol. 2012, 47, 340–348. [Google Scholar] [CrossRef] [Green Version]
- Van Doeveren, T.E.; Bouwmans, R.; Wassenaar, N.P.M.; Schreuder, W.H.; Van Alphen, M.J.; Van Der Heijden, F.; Tan, I.B.; Karakullukçu, M.B.; Van Veen, R.L. On the Development of a Light Dosimetry Planning Tool for Photodynamic Therapy in Arbitrary Shaped Cavities: Initial Results. Photochem. Photobiol. 2020, 96, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C.; Schett, G.; Gay, S.; Distler, O.; Distler, J.H. Hypoxia. Hypoxia in the pathogenesis of systemic sclerosis. Arthritis Res. Ther. 2009, 11, 220. [Google Scholar] [CrossRef] [Green Version]
- Cohen, D.K.; Lee, P.K. Photodynamic Therapy for Non-Melanoma Skin Cancers. Cancers 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, S.A.; Wolfsen, H.C. The Use of Photodynamic Therapy for Diseases of the Esophagus. J. Environ. Pathol. Toxicol. Oncol. 2008, 27, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Dorst, D.N.; Rijpkema, M.; Boss, M.; Walgreen, B.; Helsen, M.M.A.; Bos, D.L.; Brom, M.; Klein, C.; Laverman, P.; van der Kraan, P.M.; et al. Targeted photodynamic therapy selectively kills activated fibroblasts in experimental arthritis. Rheumatology 2020, 59, 3952–3960. [Google Scholar] [CrossRef]
- De Boer, E.; Warram, J.M.; Hartmans, E.; Bremer, P.J.; Bijl, B.; Crane, L.M.; Nagengast, W.B.; Rosenthal, E.L.; van Dam, G.M. A Standardized Light-Emitting Diode Device for Photoimmunotherapy. J. Nucl. Med. 2014, 55, 1893–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gene | Forward Primer 5′-->3′ | Reverse Primer 5′-->3′ |
|---|---|---|
| GAPDH * | ATCTTCTTTTGCGTCGCCAG | TTCCCCATGGTGTCTGAGC |
| HPRT * | CCTGGCGTCGTGATTAGTGA | TCTCGAGCAAGACGTTCAGT |
| TBP * | GCTTCGGAGAGTTCTGGGATTG | GCAGCAAACCGCTTGGGATTA |
| RPS27A * | TGGCTGTCCTGAAATATTATAAGGT | CCCCAGCACCACATTCATCA |
| FAP | GCTTTGAAAAATATCCAGCTGCC | ACCACCATACACTTGAATTAGCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorst, D.N.; van Caam, A.P.M.; Vitters, E.L.; Walgreen, B.; Helsen, M.M.A.; Klein, C.; Gudi, S.; Wubs, T.; Kumari, J.; Vonk, M.C.; et al. Fibroblast Activation Protein Targeted Photodynamic Therapy Selectively Kills Activated Skin Fibroblasts from Systemic Sclerosis Patients and Prevents Tissue Contraction. Int. J. Mol. Sci. 2021, 22, 12681. https://doi.org/10.3390/ijms222312681
Dorst DN, van Caam APM, Vitters EL, Walgreen B, Helsen MMA, Klein C, Gudi S, Wubs T, Kumari J, Vonk MC, et al. Fibroblast Activation Protein Targeted Photodynamic Therapy Selectively Kills Activated Skin Fibroblasts from Systemic Sclerosis Patients and Prevents Tissue Contraction. International Journal of Molecular Sciences. 2021; 22(23):12681. https://doi.org/10.3390/ijms222312681
Chicago/Turabian StyleDorst, Daphne N., Arjan P. M. van Caam, Elly L. Vitters, Birgitte Walgreen, Monique M. A. Helsen, Christian Klein, Shreya Gudi, Tirza Wubs, Jyoti Kumari, Madelon C. Vonk, and et al. 2021. "Fibroblast Activation Protein Targeted Photodynamic Therapy Selectively Kills Activated Skin Fibroblasts from Systemic Sclerosis Patients and Prevents Tissue Contraction" International Journal of Molecular Sciences 22, no. 23: 12681. https://doi.org/10.3390/ijms222312681
APA StyleDorst, D. N., van Caam, A. P. M., Vitters, E. L., Walgreen, B., Helsen, M. M. A., Klein, C., Gudi, S., Wubs, T., Kumari, J., Vonk, M. C., van der Kraan, P. M., & Koenders, M. I. (2021). Fibroblast Activation Protein Targeted Photodynamic Therapy Selectively Kills Activated Skin Fibroblasts from Systemic Sclerosis Patients and Prevents Tissue Contraction. International Journal of Molecular Sciences, 22(23), 12681. https://doi.org/10.3390/ijms222312681








