Multifaceted Analysis of IL-23A- and/or EBI3-Including Cytokines Produced by Psoriatic Keratinocytes
Abstract
:1. Introduction
2. Results
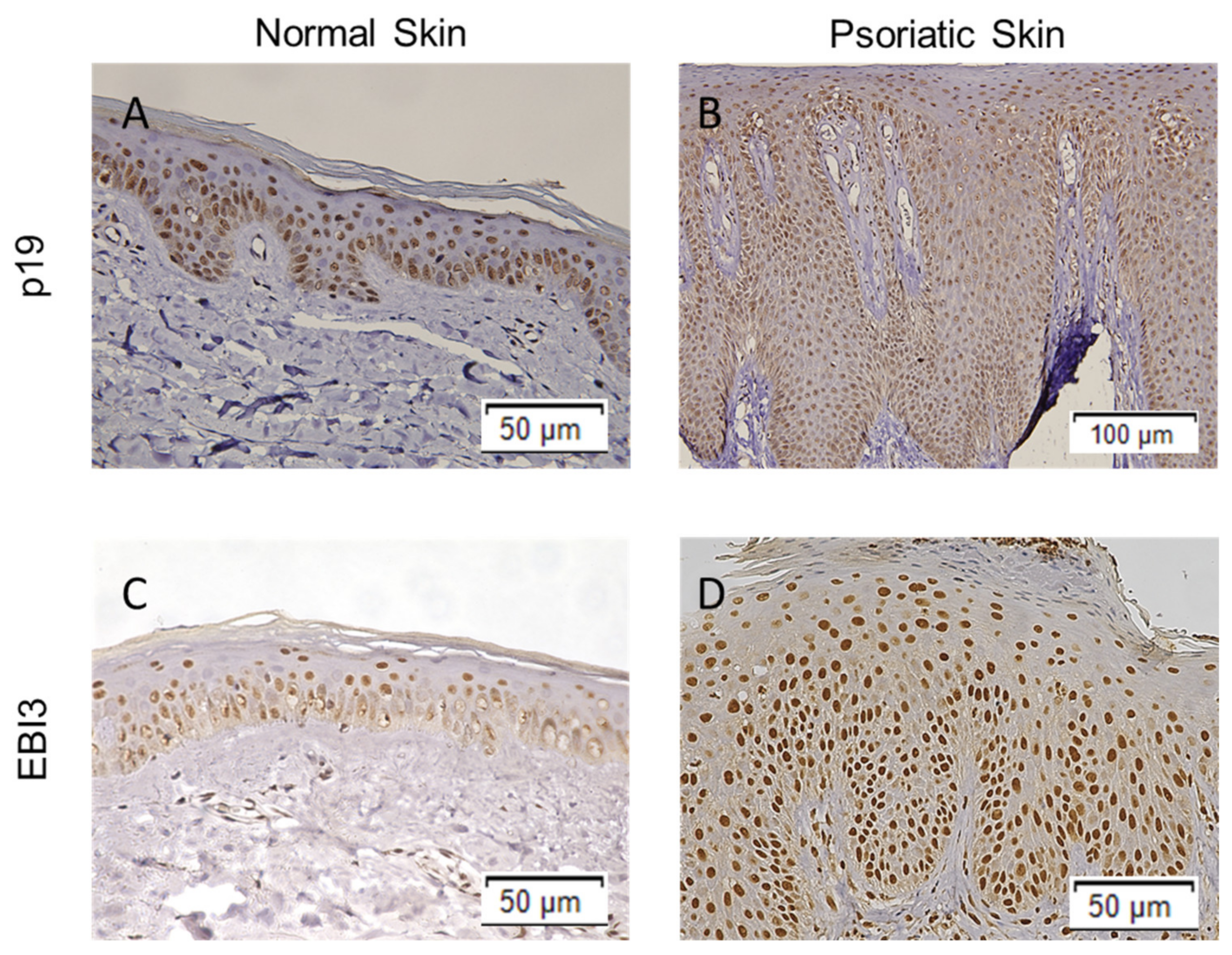
2.1. The Expression of IL-23Ap19 and EBI3 Is Upregulated in Psoriatic Keratinocytes
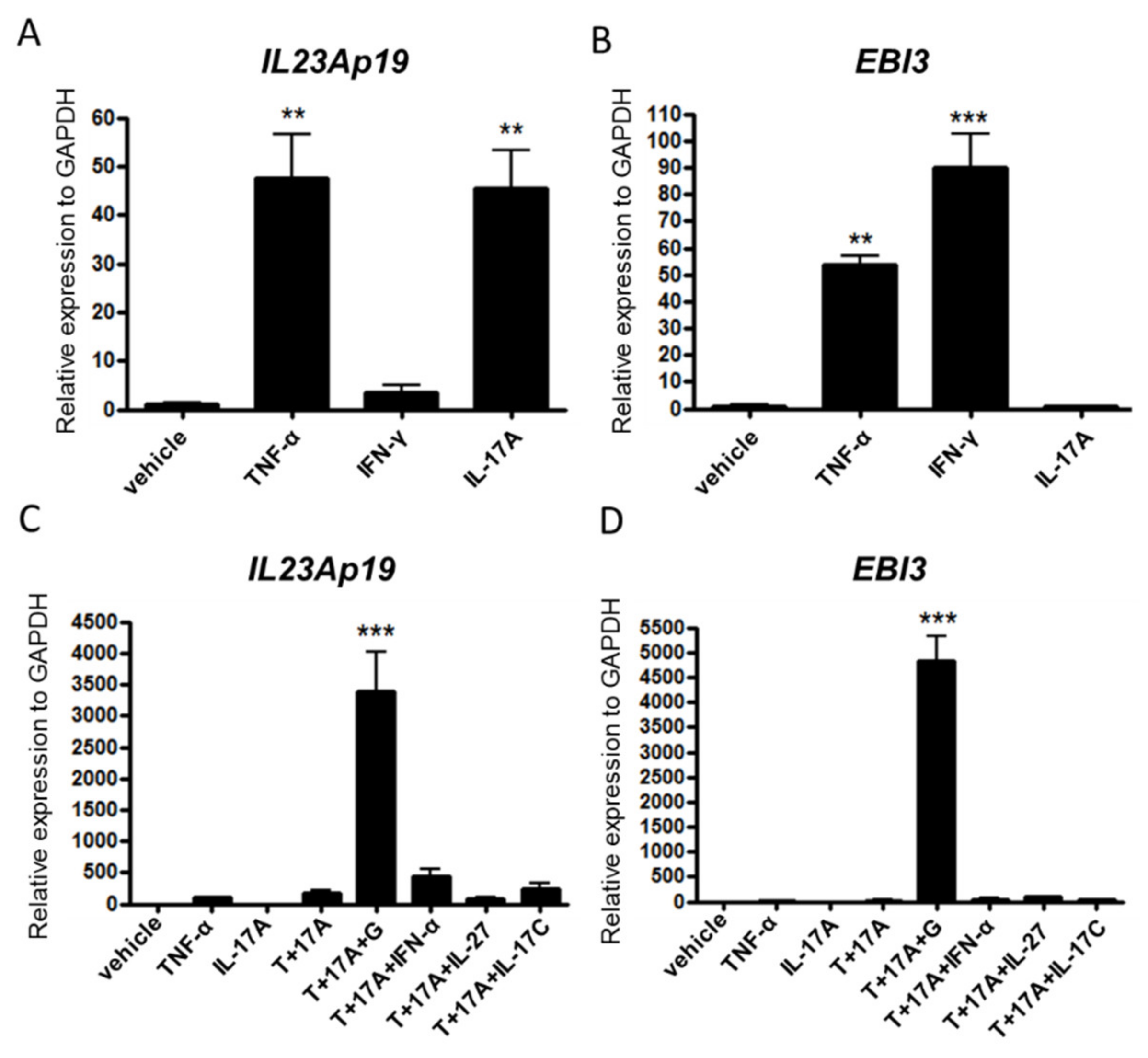
2.2. TNF-α, IL-17A, and IFN-γ Synergistically Induce IL-23Ap19 and EBI3 Expression in NHEKs
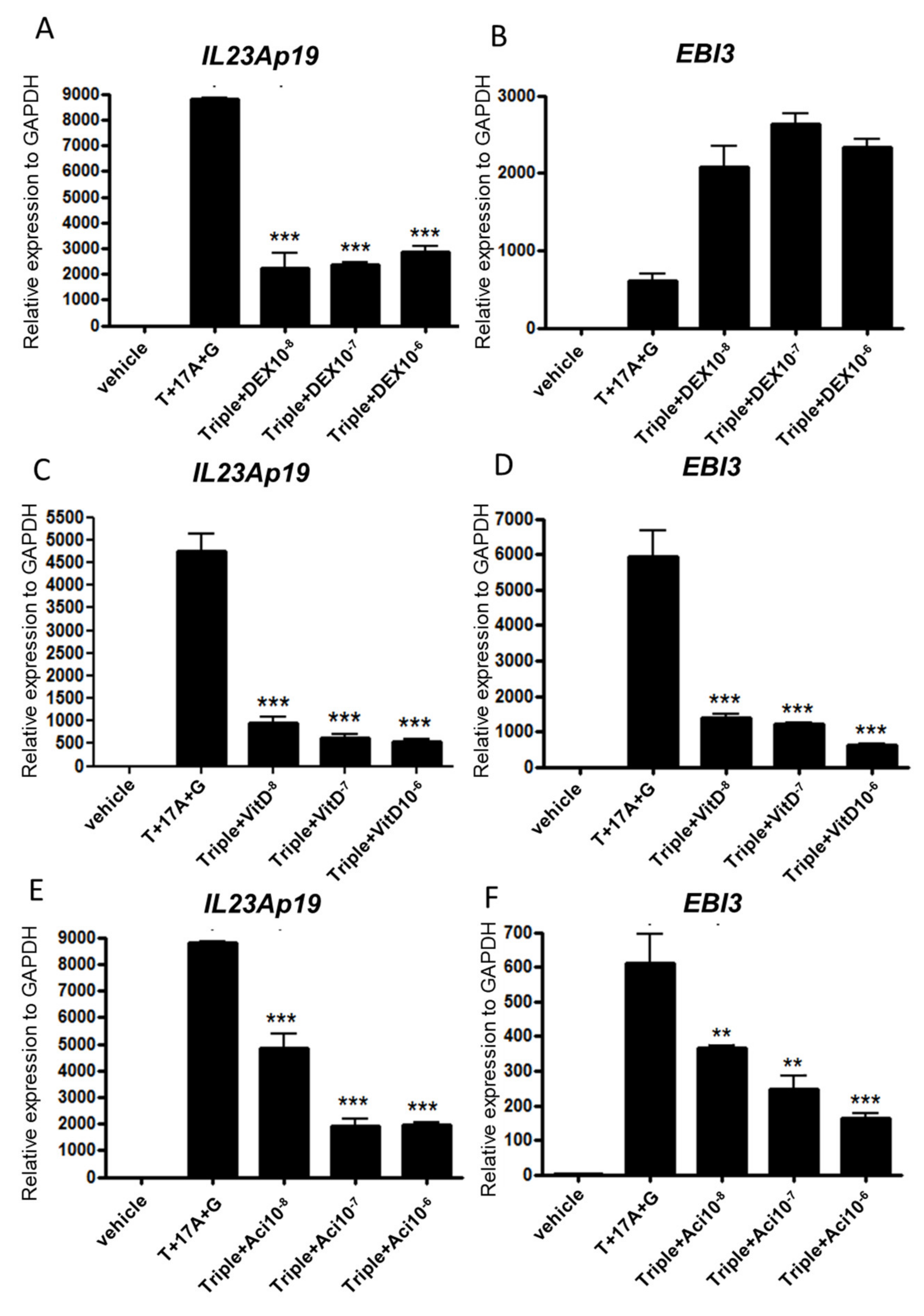
2.3. Topical Treatment by Dexamethasone, Vitamin D3, and Acitretin Suppress p19 and EBI3 Expression in Epidermal Keratinocytes
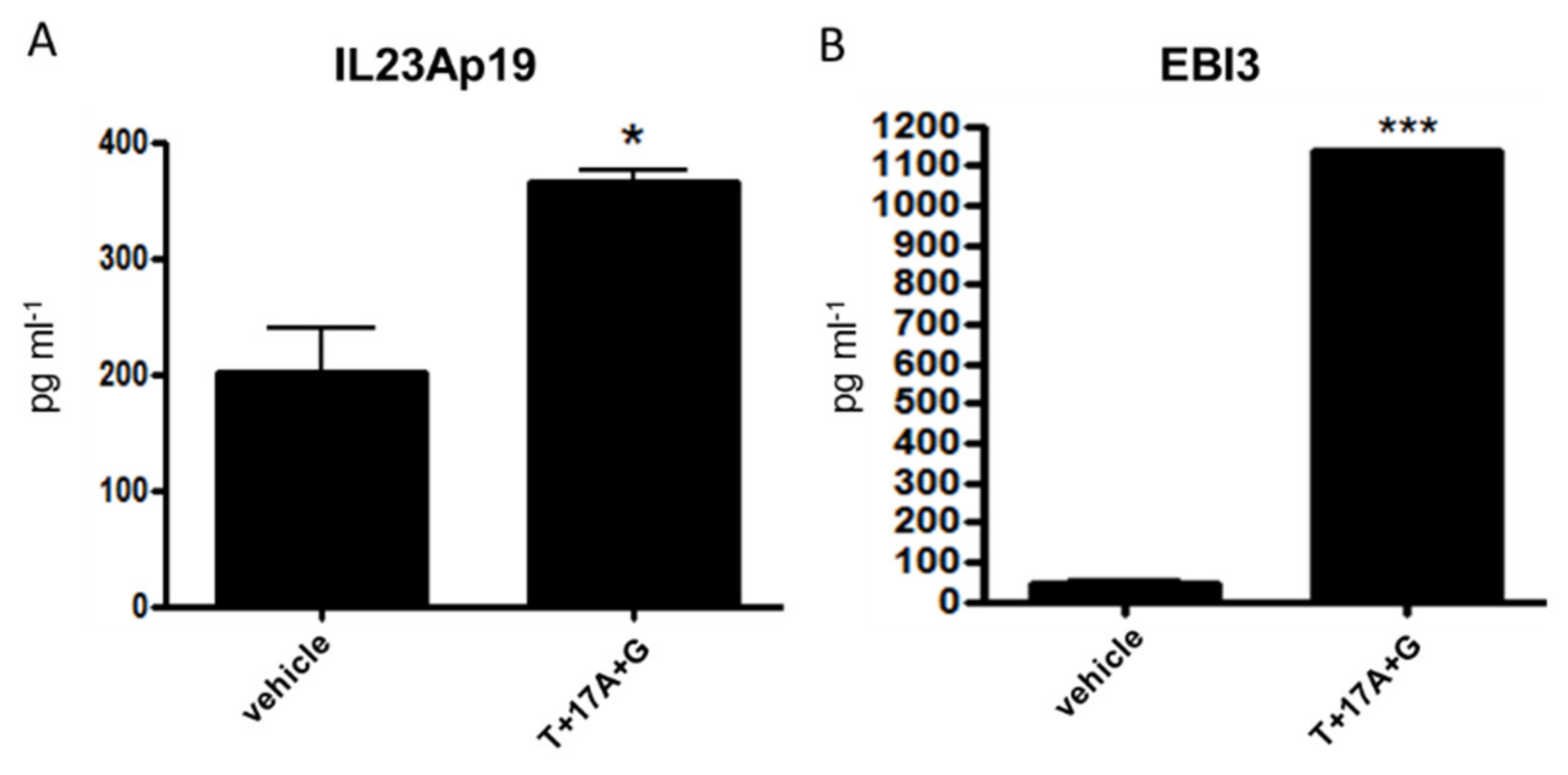
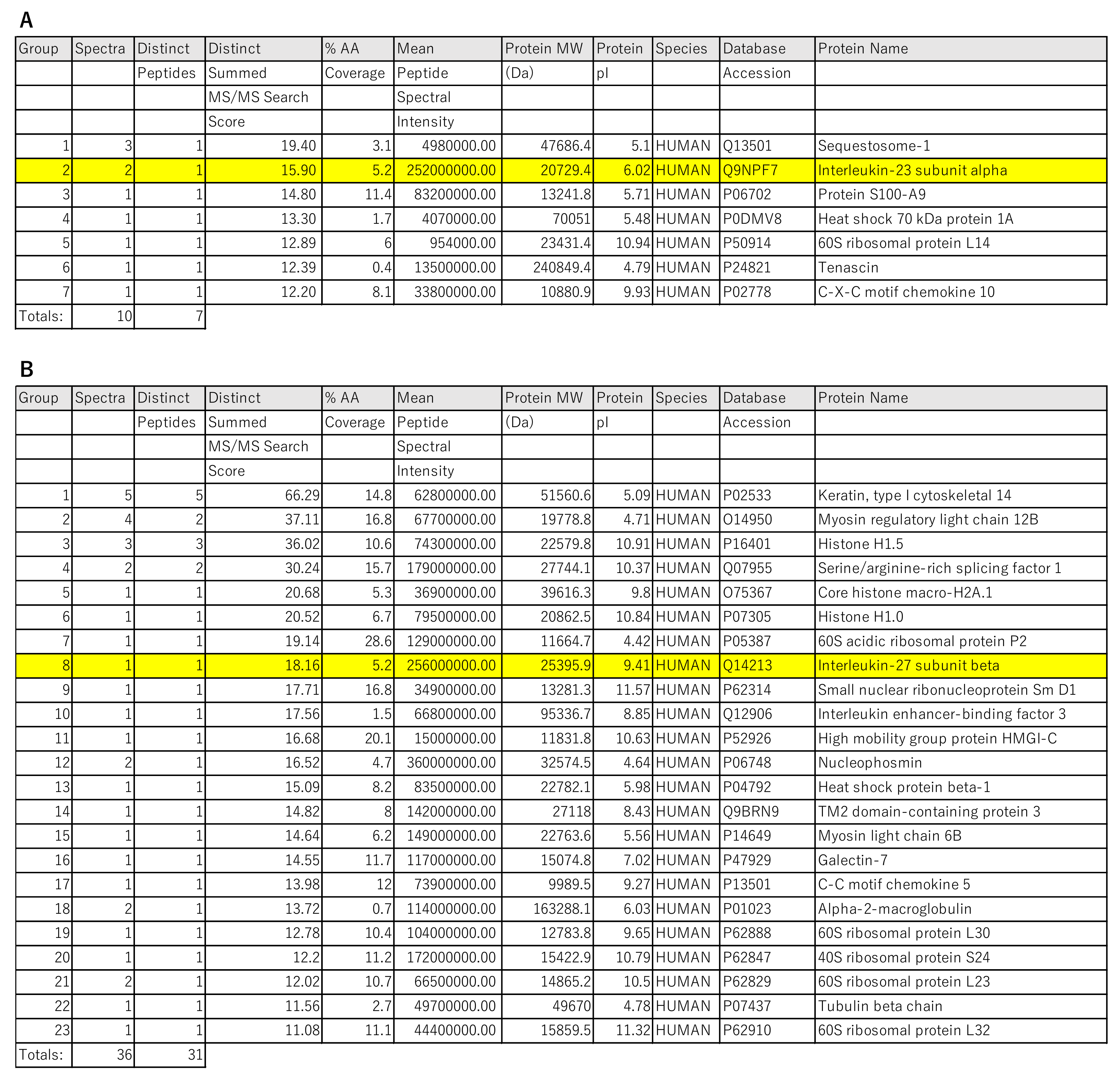
2.4. The Analysis of IL-23Ap19 and EBI3 Protein Expressions in Epidermal Keratinocytes
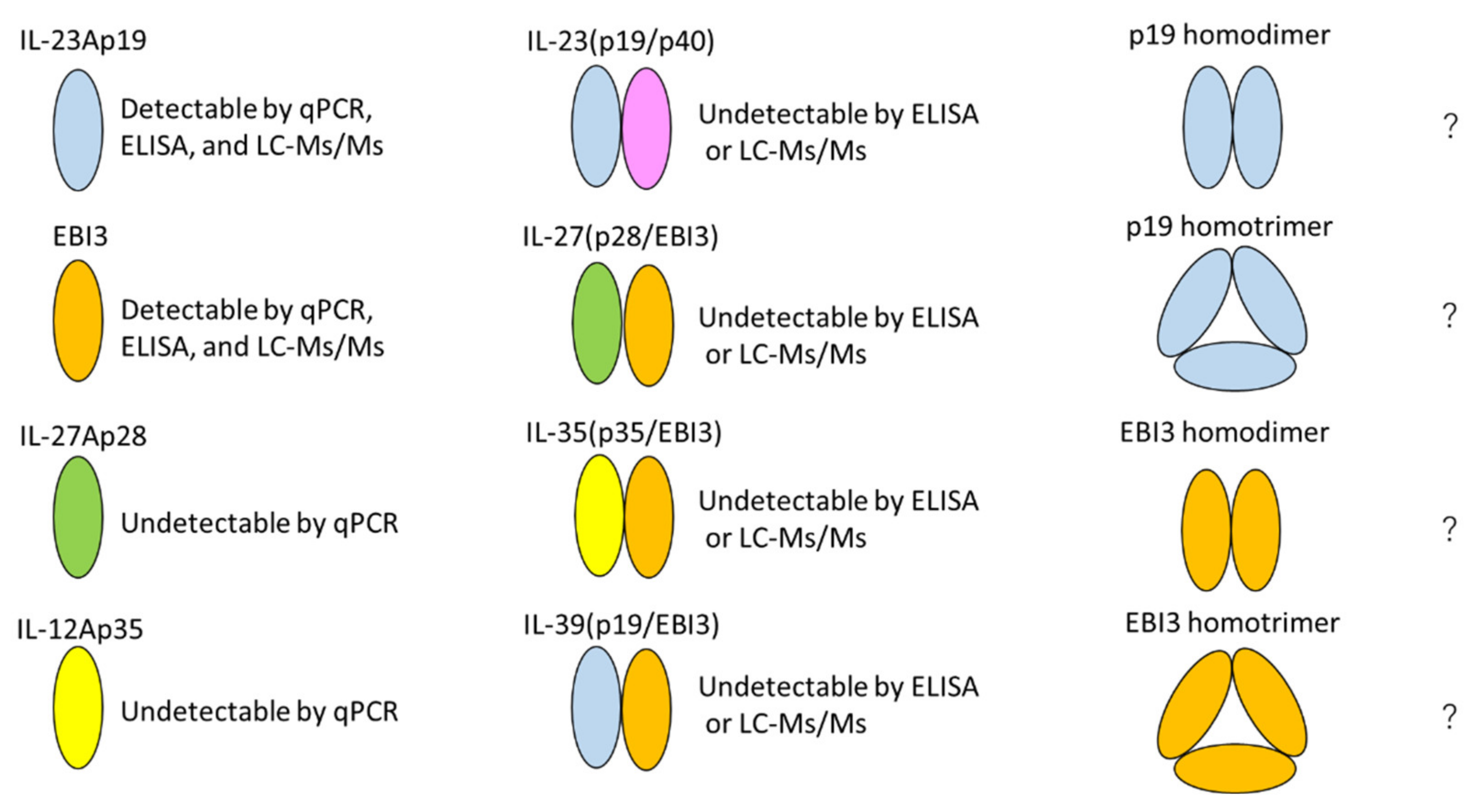
3. Discussion
4. Subjects and Methods
4.1. Immunohistochemistry
4.2. Cell Culture and Stimuli
4.3. Quantitative Real-Time PCR
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Liquid Chromatography-Electrospray Tandem Mass Spectrometry (LC-Ms/Ms)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EBI3 | Epstein–Barr virus-induced 3 |
| ELISA | Enzyme-linked immunosorbent assay |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| IFN | Interferon |
| IL | Interleukin |
| LC-Ms/Ms | Liquid chromatography-electrospray tandem mass spectrometry |
| NHEKs | Normal human epidermal keratinocytes |
| Poly (I:C) | Polycytidylic acid |
| RT-PCR | Real-time polymerase chain reaction |
| STAT | Signal transducer and activator of transcription |
| TNF | Tumor necrosis factor |
References
- Hawkes, J.E.; Chan, T.C.; Krueger, J.G. Psoriasis pathogenesis and the development of novel targeted immune therapies. J. Allergy Clin. Immunol. 2017, 140, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tollefson, M.M.; Crowson, C.S.; McEvoy, M.T.; Maradit Kremers, H. Incidence of psoriasis in children: A population-based study. J. Am. Acad. Dermatol. 2010, 62, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.L.; Petrie, M.; O’Rourke, K.S.; Feldman, S.R. Rheumatologists’ recommendations on what to do in the dermatology office to evaluate and manage psoriasis patients’ joint symptoms. J. Dermatol. Treat. 2009, 20, 350–353. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Neimann, A.L.; Shin, D.B.; Wang, X.; Margolis, D.J.; Troxel, A.B. Risk of myocardial infarction in patients with psoriasis. JAMA 2006, 296, 1735–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gisondi, P.; Tessari, G.; Conti, A.; Piaserico, S.; Schianchi, S.; Peserico, A.; Giannetti, A.; Girolomoni, G. Prevalence of metabolic syndrome in patients with psoriasis: A hospital-based case-control study. Br. J. Dermatol. 2007, 157, 68–73. [Google Scholar] [CrossRef]
- Neimann, A.L.; Shin, D.B.; Wang, X.; Margolis, D.J.; Troxel, A.B.; Gelfand, J.M. Prevalence of cardiovascular risk factors in patients with psoriasis. J. Am. Acad. Dermatol. 2006, 55, 829–835. [Google Scholar] [CrossRef]
- Brauchli, Y.B.; Jick, S.S.; Miret, M.; Meier, C.R. Psoriasis and risk of incident cancer: An inception cohort study with a nested case-control analysis. J. Investig. Dermatol. 2009, 129, 2604–2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, A.A.; Choi, H.K.; Setty, A.R.; Curhan, G.C. Psoriasis and the risk of diabetes and hypertension: A prospective study of US female nurses. Arch. Dermatol. 2009, 145, 379–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piskin, G.; Tursen, U.; Sylva-Steenland, R.M.R.; Bos, J.D.; Teunissen, M.B.M. Clinical improvement in chronic plaque-type psoriasis lesions after narrow-band UVB therapy is accompanied by a decrease in the expression of IFN-gamma inducers—IL-12, IL-18 and IL-23. Exp. Dermatol. 2004, 13, 764–772. [Google Scholar] [CrossRef]
- Piskin, G.; Sylva-Steenland, R.M.; Bos, J.D.; Teunissen, M.B. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: Enhanced expression in psoriatic skin. J. Immunol. 2006, 176, 1908–1915. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Lowes, M.A.; Fuentes-Duculan, J.; Zaba, L.C.; Cardinale, I.; Nograles, K.E.; Khatcherian, A.; Novitskaya, I.; Carucci, J.A.; Bergman, R.; et al. Low Expression of the IL-23/Th17 Pathway in Atopic Dermatitis Compared to Psoriasis. J. Immunol. 2008, 181, 7420–7427. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Trepicchio, W.L.; Oestreicher, J.L.; Pittman, D.; Wang, F.; Chamian, F.; Dhodapkar, M.; Krueger, J.G. Increased Expression of Interleukin 23 p19 and p40 in Lesional Skin of Patients with Psoriasis Vulgaris. J. Exp. Med. 2004, 199, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yao, Q.; Mariscal, A.G.; Wu, X.; Hulse, J.; Pedersen, E.; Helin, K.; Waisman, A.; Vinkel, C.; Thomsen, S.F.; et al. Epigenetic control of IL-23 expression in keratinocytes is important for chronic skin inflammation. Nat. Commun. 2018, 9, 1420. [Google Scholar] [CrossRef] [PubMed]
- Oppmann, B.; Lesley, R.; Blom, B.; Timans, J.C.; Xu, Y.; Hunte, B.; Vega, F.; Yu, N.; Wang, J.; Singh, K.; et al. Novel p19 Protein Engages IL-12p40 to Form a Cytokine, IL-23, with Biological Activities Similar as Well as Distinct from IL-12. Immunity 2000, 13, 715–725. [Google Scholar] [CrossRef] [Green Version]
- Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; Mattson, J.; Basham, B.; Sedgwick, J.D.; McClanahan, T.; Kastelein, R.A.; Cua, D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mus, A.M.C.; Cornelissen, F.; Asmawidjaja, P.S.; Van Hamburg, J.P.; Boon, L.; Hendriks, R.W.; Lubberts, E. Interleukin-23 promotes Th17 differentiation by inhibiting T-bet and FoxP3 and is required for elevation of interleukin-22, but not interleukin-21, in autoimmune experimental arthritis. Arthritis Rheum. 2010, 62, 1043–1050. [Google Scholar] [CrossRef]
- Warren, R.B.; Blauvelt, A.; Poulin, Y.; Beeck, S.; Kelly, M.; Wu, T.; Geng, Z.; Paul, C. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): Results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br. J. Dermatol. 2021, 184, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Warren, R.B.; Iversen, L.; Puig, L.; Pau-Charles, I.; Igarashi, A.; Ohtsuki, M.; Falqués, M.; Harmut, M.; Rozzo, S.; et al. Long-term efficacy and safety of tildrakizumab for moderate-to-severe psoriasis: Pooled analyses of two randomized phase III clinical trials (re SURFACE 1 and re SURFACE 2) through 14. Br. J. Dermatol. 2020, 182, 605–617. [Google Scholar] [CrossRef]
- Blauvelt, A.; Papp, K.A.; Griffiths, C.E.M.; Randazzo, B.; Wasfi, Y.; Shen, Y.-K.; Li, S.; Kimball, A.B. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator–. J. Am. Acad. Dermatol. 2017, 76, 405–417. [Google Scholar] [CrossRef] [Green Version]
- Langley, R.G.; Tsai, T.F.; Flavin, S.; Song, M.; Randazzo, B.; Wasfi, Y.; Jiang, J.; Li, S.; Puig, L. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: Results of the randomized, double-blind, phase III NAVIGATE trial. Br. J. Dermatol. 2018, 178, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Kulig, P.; Musiol, S.; Freiberger, S.N.; Schreiner, B.; Gyülveszi, G.; Russo, G.; Pantelyushin, S.; Kishihara, K.; Alessandrini, F.; Kündig, T.; et al. IL-12 protects from psoriasiform skin inflammation. Nat. Commun. 2016, 7, 13466. [Google Scholar] [CrossRef]
- Ramnath, D.; Tunny, K.; Hohenhaus, D.M.; Pitts, C.M.; Bergot, A.S.; Hogarth, P.M.; Hamilton, J.A.; Kapetanovic, R.; Sturm, R.A.; Scholz, G.M.; et al. TLR3 drives IRF6-dependent IL-23p19 expression and p19/EBI3 heterodimer formation in keratinocytes. Immunol. Cell Biol. 2015, 93, 771–779. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wei, Y.; Xiao, H.; Liu, X.; Zhang, Y.; Han, G.; Chen, G.; Hou, C.; Ma, N.; Shen, B.; et al. A novel IL-23p19/Ebi3 (IL-39) cytokine mediates inflammation in Lupus-like mice. Eur. J. Immunol. 2016, 46, 1343–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Liu, X.; Zhang, Y.; Wang, Z.; Zhu, G.; Han, G.; Chen, G.; Hou, C.; Wang, T.; Ma, N.; et al. Interleukin (IL)-39 [IL-23p19/Epstein-Barr virus-induced 3 (Ebi3)] induces differentiation/expansion of neutrophils in lupus-prone mice. Clin. Exp. Immunol. 2016, 186, 144–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhang, Y.; Wang, Z.; Liu, X.; Zhu, G.; Han, G.; Chen, G.; Hou, C.; Wang, T.; Shen, B.; et al. Anti-IL-39 (IL-23p19/Ebi3) polyclonal antibodies ameliorate autoimmune symptoms in lupus-like mice. Mol. Med. Rep. 2017, 17, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Bridgewood, C.; Alase, A.; Watad, A.; Wittmann, M.; Cuthbert, R.; McGonagle, D. The IL-23p19/EBI3 heterodimeric cytokine termed IL-39 remains a theoretical cytokine in man. Inflamm. Res. 2019, 68, 423–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ecoeur, F.; Weiss, J.; Schleeger, S.; Guntermann, C. Lack of evidence for expression and function of IL-39 in human immune cells. PLoS ONE 2020, 15, e0242329. [Google Scholar] [CrossRef]
- Detry, S.; Skladanowska, K.; Vuylsteke, M.; Savvides, S.N.; Bloch, Y. Revisiting the combinatorial potential of cytokine subunits in the IL-12 family. Biochem. Pharm. 2019, 165, 240–248. [Google Scholar] [CrossRef]
- Scholz, G.M.; Heath, J.E.; Walsh, K.A.; Reynolds, E.C. MEK-ERK signaling diametrically controls the stimulation of IL-23p19 and EBI3 expression in epithelial cells by IL-36γ. Immunol. Cell Biol. 2018, 96, 646–655. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Guttman-Yassky, E.; Suárez-Fariñas, M.; Nograles, K.E.; Tian, S.; Cardinale, I.; Chimenti, S.; Krueger, J.G. Integrative Responses to IL-17 and TNF-α in Human Keratinocytes Account for Key Inflammatory Pathogenic Circuits in Psoriasis. J. Investig. Dermatol. 2011, 131, 677–687. [Google Scholar] [CrossRef]
- Johansen, C.; Bertelsen, T.; Ljungberg, C.; Mose, M.; Iversen, L. Characterization of TNF-α– and IL-17A–Mediated Synergistic Induction of DEFB4 Gene Expression in Human Keratinocytes through IκBζ. J. Investig. Dermatol. 2016, 136, 1608–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morizane, S.; Nomura, H.; Tachibana, K.; Nakagawa, Y.; Iwatsuki, K. The synergistic activities of the combination of tumour necrosis factor-alpha, interleukin-17A and interferon-gamma in epidermal keratinocytes. Br. J. Dermatol. 2018, 179, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Ling, P.; Gately, M.K.; Gubler, U.; Stern, A.S.; Lin, P.; Hollfelder, K.; Su, C.; Pan, Y.C.; Hakimi, J. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J. Immunol. 1995, 154, 116–127. [Google Scholar]
- Shimozato, O.; Sato, A.; Kawamura, K.; Chiyo, M.; Ma, G.; Li, Q.; Tagawa, M. The secreted form of p28 subunit of interleukin (IL)-27 inhibits biological functions of IL-27 and suppresses anti-allogeneic immune responses. Immunology 2009, 128, e816–e825. [Google Scholar] [CrossRef]
- Stumhofer, J.S.; Tait, E.D.; Iii, W.J.Q.; Hosken, N.; Spudy, B.; Goenka, R.; Fielding, C.A.; O’Hara, A.C.; Chen, Y.; Jones, M.L.; et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat. Immunol. 2010, 11, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Maaser, C.; Egan, L.J.; Birkenbach, M.P.; Eckmann, L.; Kagnoff, M.F. Expression of Epstein-Barr virus-induced gene 3 and other interleukin-12-related molecules by human intestinal epithelium. Immunology 2004, 112, 437–445. [Google Scholar] [CrossRef]
- Devergne, O.; Hummel, M.; Koeppen, H.; Le Beau, M.M.; Nathanson, E.C.; Kieff, E.; Birkenbach, M. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J. Virol. 1996, 70, 1143–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, H.; Wang, Z.; Jinnin, M.; Nakayama, W.; Inoue, K.; Honda, N.; Nakashima, T.; Kajihara, I.; Makino, K.; Makino, T.; et al. EBI3 Downregulation Contributes to Type I Collagen Overexpression in Scleroderma Skin. J. Immunol. 2015, 195, 3565–3573. [Google Scholar] [CrossRef] [Green Version]
- Gerosa, F.; Baldani-Guerra, B.; Lyakh, L.A.; Batoni, G.; Esin, S.; Winkler-Pickett, R.T.; Consolaro, M.R.; De Marchi, M.; Giachino, D.; Robbiano, A.; et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J. Exp. Med. 2008, 205, 1447–1461. [Google Scholar] [CrossRef]
- Martínez-Barricarte, R.; Markle, J.G.; Ma, C.S.; Deenick, E.K.; Ramírez-Alejo, N.; Mele, F.; Latorre, D.; Mahdaviani, S.A.; Aytekin, C.; Mansouri, D.; et al. Human IFN-γ immunity to mycobacteria is governed by both IL-12 and IL-23. Sci. Immunol. 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Ehst, B.; Wang, Z.; Leitenberger, J.; McClanahan, D.; De La Torre, R.; Sawka, E.; Ortega-Loayza, A.G.; Strunck, J.; Greiling, T.; Simpson, E.; et al. Synergistic induction of IL-23 by TNFα, IL-17A, and EGF in keratinocytes. Cytokine 2021, 138, 155357. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Li, G.G.; Liu, Q.; Niu, X.; Li, R.; Ma, H. Short-Term Efficacy and Safety of IL-17, IL-12/23, and IL-23 Inhibitors Brodalumab, Secukinumab, Ixekizumab, Ustekinumab, Guselkumab, Tildrakizumab, and Risankizumab for the Treatment of Moderate to Severe Plaque Psoriasis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J. Immunol. Res. 2019, 2019, 2546161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tachibana, K.; Tang, N.; Urakami, H.; Kajita, A.; Kobashi, M.; Nomura, H.; Sasakura, M.; Sugihara, S.; Jiang, F.; Tomonobu, N.; et al. Multifaceted Analysis of IL-23A- and/or EBI3-Including Cytokines Produced by Psoriatic Keratinocytes. Int. J. Mol. Sci. 2021, 22, 12659. https://doi.org/10.3390/ijms222312659
Tachibana K, Tang N, Urakami H, Kajita A, Kobashi M, Nomura H, Sasakura M, Sugihara S, Jiang F, Tomonobu N, et al. Multifaceted Analysis of IL-23A- and/or EBI3-Including Cytokines Produced by Psoriatic Keratinocytes. International Journal of Molecular Sciences. 2021; 22(23):12659. https://doi.org/10.3390/ijms222312659
Chicago/Turabian StyleTachibana, Kota, Nina Tang, Hitoshi Urakami, Ai Kajita, Mina Kobashi, Hayato Nomura, Minori Sasakura, Satoru Sugihara, Fan Jiang, Nahoko Tomonobu, and et al. 2021. "Multifaceted Analysis of IL-23A- and/or EBI3-Including Cytokines Produced by Psoriatic Keratinocytes" International Journal of Molecular Sciences 22, no. 23: 12659. https://doi.org/10.3390/ijms222312659
APA StyleTachibana, K., Tang, N., Urakami, H., Kajita, A., Kobashi, M., Nomura, H., Sasakura, M., Sugihara, S., Jiang, F., Tomonobu, N., Sakaguchi, M., Ouchida, M., & Morizane, S. (2021). Multifaceted Analysis of IL-23A- and/or EBI3-Including Cytokines Produced by Psoriatic Keratinocytes. International Journal of Molecular Sciences, 22(23), 12659. https://doi.org/10.3390/ijms222312659






