X-ray Diffraction Analysis to Explore Molecular Traces of Eccentric Contraction on Rat Skeletal Muscle Parallelly Evaluated with Signal Protein Phosphorylation Levels
Abstract
1. Introduction
2. Results
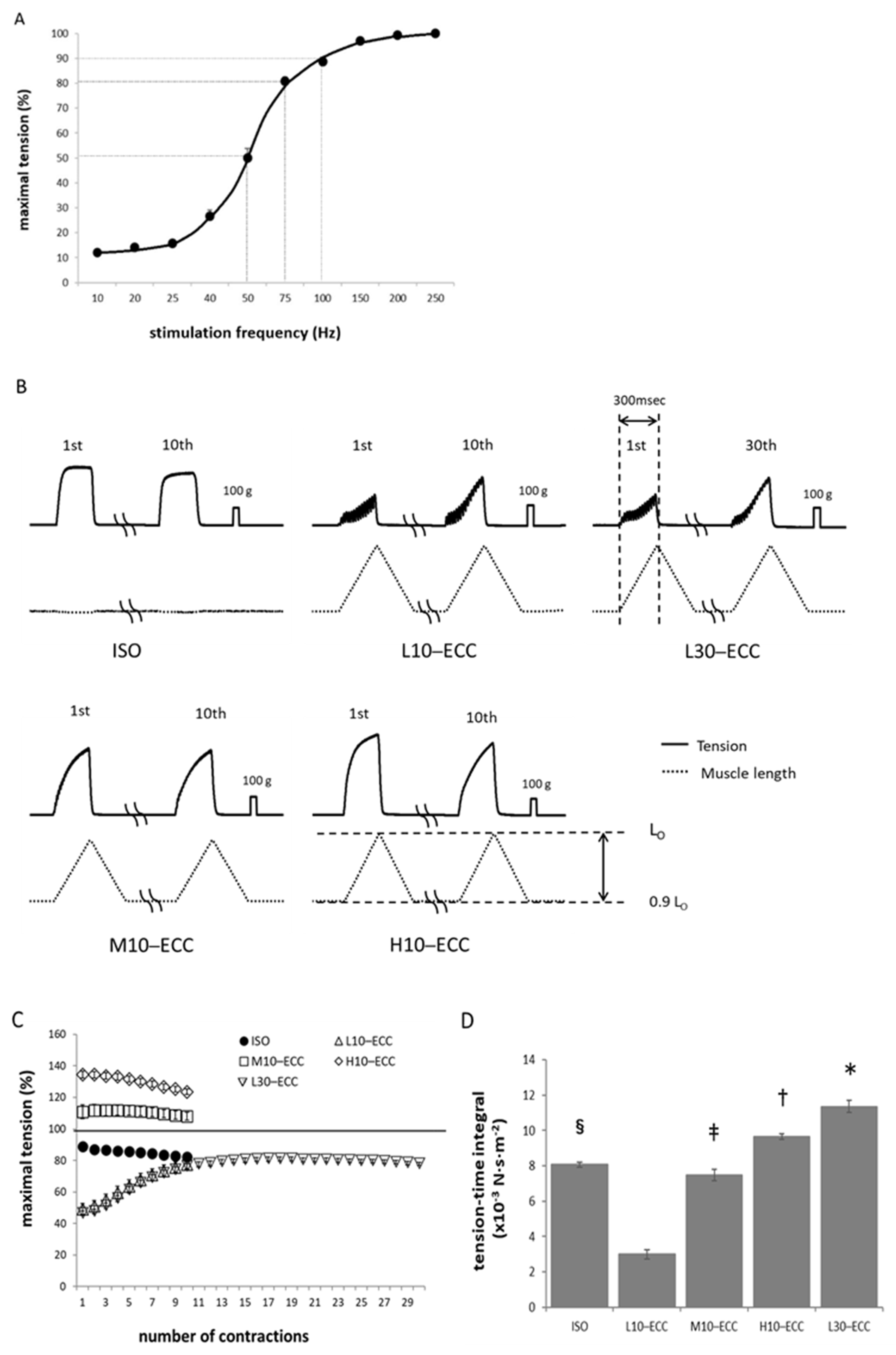
2.1. Contractile Performance during ECC Sessions
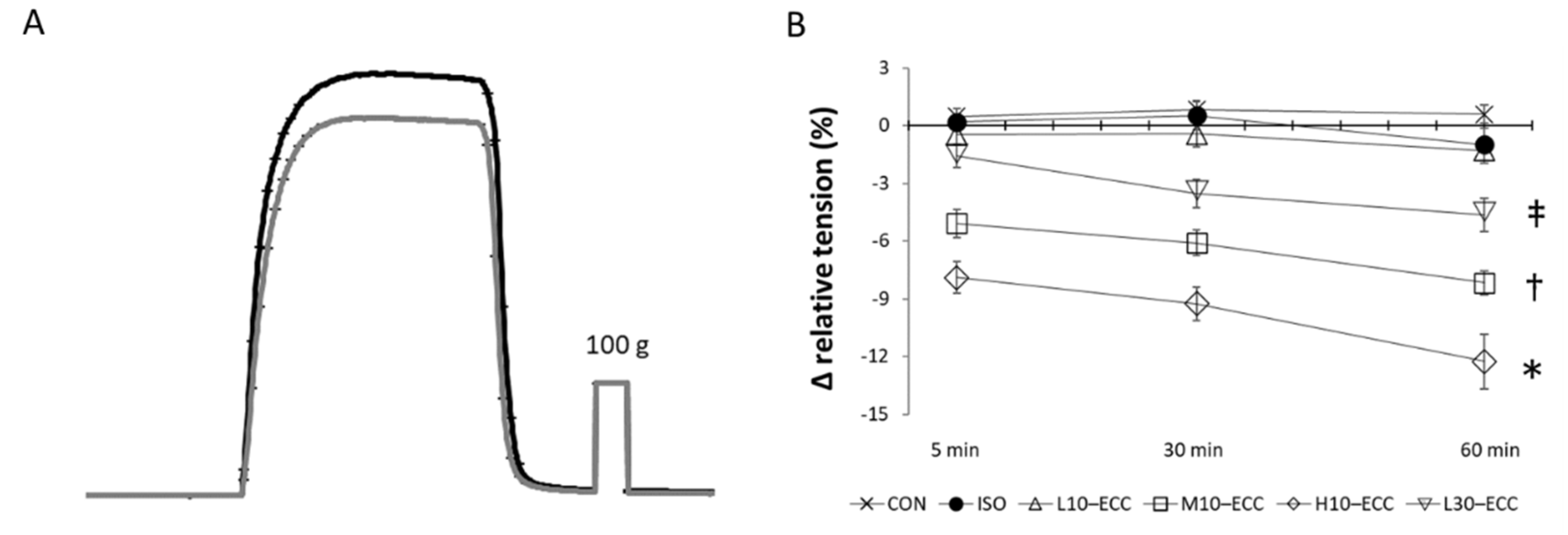
2.2. Contractility after the Session Series
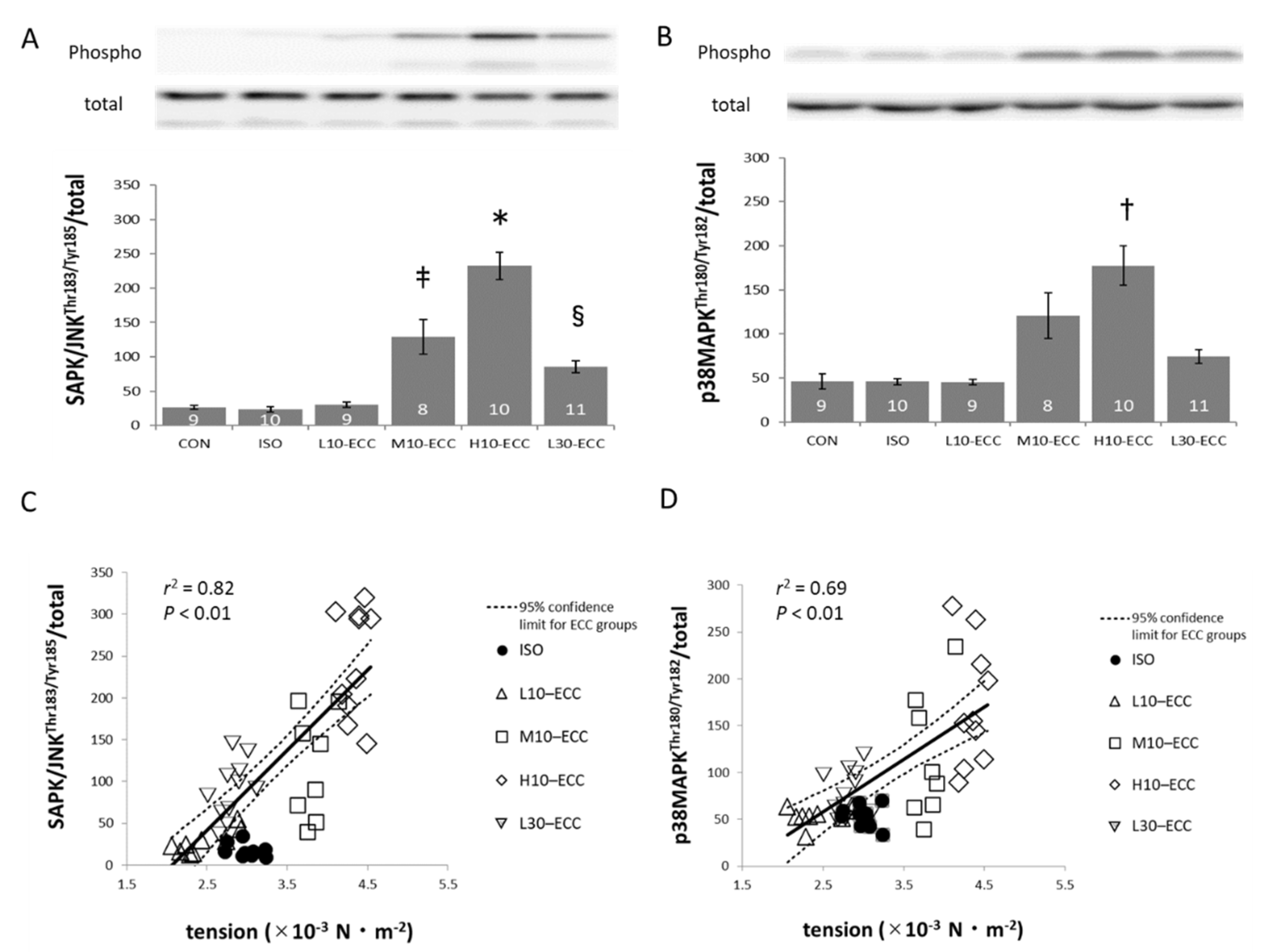
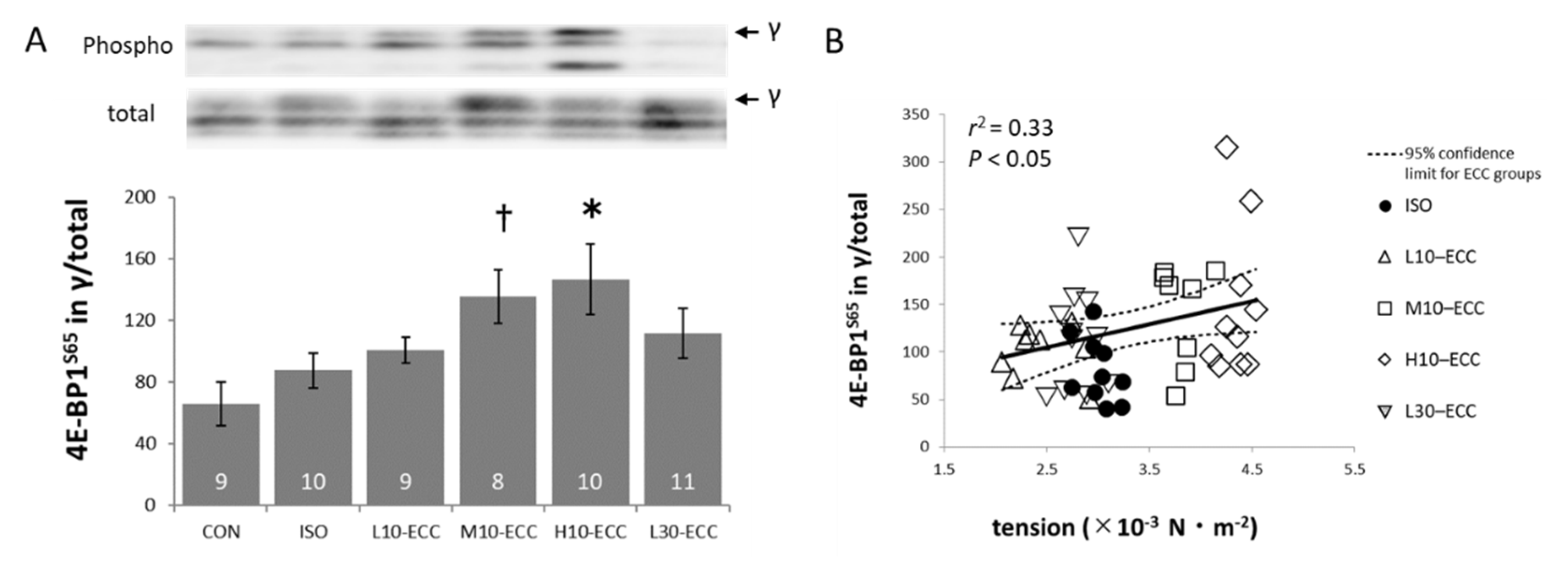
2.3. Signal Proteins
2.4. Myofilament Lattice and Passive Tension at Resting Condition
2.5. Alignment of Myoproteins
3. Discussion
3.1. Muscle Damaging Effects
3.2. Muscle Strengthening Effect
3.3. Sarcomere Structure
3.4. Downstream of the Remodeling Process
3.5. Application to Rehabilitation Science
4. Materials and Methods
4.1. Animals
4.2. Solutions
4.3. In Situ Force Measurement
4.4. ECC Sessions
4.5. Western Blotting
4.6. X-Ray Diffraction Experiment
4.7. Analysis of X-Ray Diffraction Patterns
4.8. Passive Tension Measurement of Mechanically Skinned Fibers
4.9. Electrophoresis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kano, Y.; Masuda, K.; Furukawa, H.; Sudo, M.; Mito, K.; Sakamoto, K. Histological skeletal muscle damage and surface EMG relationships following eccentric contractions. J. Physiol. Sci. 2008, 58, 349–355. [Google Scholar] [CrossRef]
- Gregory, J.E.; Morgan, D.L.; Proske, U. Tendon organs as monitors of muscle damage from eccentric contractions. Exp. Brain Res. 2003, 151, 346–355. [Google Scholar]
- Uchiyama, Y.; Tamaki, T.; Fukuda, H. Relationship between functional deficit and severity of experimental fast-strain injury of rat skeletal muscle. Eur. J. Appl. Physiol. 2001, 85, 1–9. [Google Scholar] [CrossRef]
- Song, H.; Nakazato, K.; Nakajima, H. Effect of increased excursion of the ankle on the severity of acute eccentric contraction-induced strain injury in the gastrocnemius: An in vivo rat study. Am. J. Sports Med. 2004, 32, 1263–1269. [Google Scholar] [CrossRef]
- Tsumiyama, W.; Oki, S.; Takamiya, N.; Umei, N.; Shimizu, M.E.; Ono, T.; Otsuka, A. Induction of muscle hypertrophy in rats through low intensity eccentric contraction. J. Phys. Ther. Sci. 2014, 26, 1623–1625. [Google Scholar] [CrossRef][Green Version]
- Roig, M.; O’Brien, K.; Kirk, G.; Murray, R.; McKinnon, P.; Shadgan, B.; Reid, W.D. The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: A systematic review with meta-analysis. Br. J. Sports Med. 2009, 43, 556–568. [Google Scholar] [CrossRef]
- Chen, T.C.; Tseng, W.C.; Chen, H.L.; Tseng, K.W.; Chou, T.Y.; Huang, Y.C.; Nosaka, K. Striking muscle adaptations induced by volume-dependent repeated bouts of low-intensity eccentric exercise of the elbow flexors. Appl. Physiol. Nutr. Metab. 2021, 46, 897–905. [Google Scholar] [CrossRef]
- Kasuga, N.; Takemori, S. Effects of downhill running incorporated into long-term endurance training on skeletal muscle fiber-type switching and fatigue resistance. J. Phys. Fit. Sports Med. 2014, 3, 353–362. [Google Scholar] [CrossRef][Green Version]
- Douglas, J.; Pearson, S.; Ross, A.; McGuigan, M. Chronic adaptations to eccentric training: A systematic review. Sports Med. 2017, 47, 917–941. [Google Scholar] [CrossRef]
- LaStayo, P.; McDonagh, P.; Lipovic, D.; Napoles, P.; Bartholomew, A.; Esser, K.; Lindstedt, S. Elderly patients and high force resistance exercise–a descriptive report: Can an anabolic, muscle growth response occur without muscle damage or inflammation? J. Geriatr. Phys. Ther. 2007, 30, 128–134. [Google Scholar] [CrossRef]
- Takekura, H.; Fujinami, N.; Nishizawa, T.; Ogasawara, H.; Kasuga, N. Eccentric exercise-induced morphological changes in the membrane systems involved in excitation-contraction coupling in rat skeletal muscle. J. Physiol. 2001, 533, 571–583. [Google Scholar] [CrossRef]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef]
- Hu, M.C.; Lee, D.F.; Xia, W.; Golfman, L.S.; Ou-Yang, F.; Yang, J.Y.; Zou, Y.; Bao, S.; Hanada, N.; Saso, H.; et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 2004, 117, 225–237. [Google Scholar] [CrossRef]
- Deng, K.; Fan, Y.; Liang, Y.; Cai, Y.; Zhang, G.; Deng, M.; Wang, Z.; Lu, J.; Shi, J.; Wang, F.; et al. FTO-mediated demethylation of GADD45B promotes myogenesis through the activation of p38 MAPK pathway. Mol. Ther. Nucleic Acids 2021, 26, 34–48. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in growth, metabolism, and disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Mothe-Satney, I.; Brunn, G.J.; McMahon, L.P.; Capaldo, C.T.; Abraham, R.T.; Lawrence, J.C., Jr. Mammalian target of rapamycin-dependent phosphorylation of PHAS-I in four (S/T)P sites detected by phospho-specific antibodies. J. Biol. Chem. 2000, 275, 33836–33843. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.P. p38β MAPK upregulates atrogin1/MAFbx by specific phosphorylation of C/EBPβ. Skelet. Muscle 2012, 2, 20. [Google Scholar] [CrossRef]
- Ato, S.; Makanae, Y.; Kido, K.; Fujita, S. Contraction mode itself does not determine the level of mTORC1 activity in rat skeletal muscle. Physiol. Rep. 2016, 4, 1–11. [Google Scholar] [CrossRef]
- Martineau, L.C.; Gardiner, P.F. Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension. J. Appl. Physiol. 2001, 91, 693–702. [Google Scholar] [CrossRef]
- Boppart, M.D.; Hirshman, M.F.; Sakamoto, K.; Fielding, R.A.; Goodyear, L.J. Static stretch increases c-Jun NH2-terminal kinase activity and p38 phosphorylation in rat skeletal muscle. Am. J. Physiol. Cell Physiol. 2001, 280, C352–C358. [Google Scholar] [CrossRef]
- Higuchi, H.; Umazume, Y. Lattice shrinkage with increasing resting tension in stretched, single skinned fibers of frog muscle. Biophys. J. 1986, 50, 385–389. [Google Scholar] [CrossRef]
- Nishikawa, K.C.; Monroy, J.A.; Uyeno, T.E.; Yeo, S.H.; Pai, D.K.; Lindstedt, S.L. Is titin a ‘winding filament’? A new twist on muscle contraction. Proc. Biol. Sci. 2012, 279, 981–990. [Google Scholar] [CrossRef]
- Eckels, E.C.; Tapia-Rojo, R.; Rivas-Pardo, J.A.; Fernández, J.M. The work of titin protein folding as a major driver in muscle contraction. Annu. Rev. Physiol. 2018, 80, 327–351. [Google Scholar] [CrossRef]
- Chapman, D.W.; Simpson, J.A.; Iscoe, S.; Robins, T.; Nosaka, K. Changes in serum fast and slow skeletal troponin I concentration following maximal eccentric contractions. J. Sci. Med. Sport. 2013, 16, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Sorichter, S.; Mair, J.; Koller, A.; Gebert, W.; Rama, D.; Calzolari, C.; Artner-Dworzak, E.; Puschendorf, B. Skeletal troponin I as a marker of exercise-induced muscle damage. J. Appl. Physiol. 1997, 83, 1076–1082. [Google Scholar] [CrossRef]
- Takemori, S.; Yamaguchi, M.; Yagi, N. Effects of adenosine diphosphate on the structure of myosin cross-bridges: An X-ray diffraction study on a single skinned frog muscle fibre. J. Muscle Res. Cell Motil. 1995, 16, 571–577. [Google Scholar] [CrossRef]
- Klossner, S.; Li, R.; Ruoss, S.; Durieux, A.C.; Flück, M. Quantitative changes in focal adhesion kinase and its inhibitor, FRNK, drive load-dependent expression of costamere components. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R647–R657. [Google Scholar] [CrossRef][Green Version]
- Roy, R.R.; Meadows, I.D.; Baldwin, K.M.; Edgerton, V.R. Functional significance of compensatory overloaded rat fast muscle. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982, 52, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Mendez, J.; Keys, A. Density and composition of mammalian muscle. Metabolism 1960, 9, 184–188. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Kimura, M.; Li, Z.B.; Ohno, T.; Takemori, S.; Hoh, J.F.; Yagi, N. X-ray diffraction analysis of the effects of myosin regulatory light chain phosphorylation and butanedione monoxime on skinned skeletal muscle fibers. Am. J. Physiol. Cell Physiol. 2016, 310, C692–C700. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Kimura, M.; Ohno, T.; Nakahara, N.; Akiyama, N.; Takemori, S.; Yagi, N. Cross-bridge recruitment capacity of wild-type and hypertrophic cardiomyopathy-related mutant troponin-T evaluated by X-ray diffraction and mechanical study of cardiac skinned fibers. Int. J. Mol. Sci. 2020, 21, 3520. [Google Scholar] [CrossRef]
- Takemori, S. Influence of osmotic swelling on cross section and resting tension in isolated skeletal muscle fibers. Jpn. J. Physiol. 1990, 40, 595–611. [Google Scholar] [CrossRef] [PubMed]
- Yanaga, F.; Morimoto, S.; Ohtsuki, I. Ca2+ sensitization and potentiation of the maximum level of myofibrillar ATPase activity caused by mutations of troponin T found in familial hypertrophic cardiomyopathy. J. Biol. Chem. 1999, 274, 8806–881212. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Hanashima, A.; Kubokawa, K.; Bao, Y.; Tando, Y.; Kohmaru, J.; Nakaya, H.; Maruyama, K.; Kimura, S. Amphioxus connectin exhibits merged structure as invertebrate connectin in I-band region and vertebrate connectin in A-band region. J. Mol. Biol. 2011, 406, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA. Available online: http://imagej.nih.gov/ij/ (accessed on 15 September 2019).



| Muscle Group | |||||||
|---|---|---|---|---|---|---|---|
| CON | ISO | L10–ECC | M10–ECC | H10–ECC | L30–ECC | p | |
| source animals | |||||||
| n | 13 | 12 | 13 | 13 | 12 | 11 | |
| body weight (g) | 157.5 ± 3.0 | 163.6 ± 4.1 | 163.1 ± 2.5 | 158.9 ± 3.3 | 164.0 ± 3.9 | 156.3 ± 2.3 | n.s. |
| plantaris muscles | |||||||
| n | 13 | 12 | 13 | 13 | 12 | 13 * | |
| wet weight (mg) | 147.4 ± 3.6 | 156.1 ± 5.2 | 154.6 ± 2.9 | 148.6 ± 3.4 | 154.4 ± 2.9 | 145.6 ± 3.0 | n.s. |
| cross-sectional area (cm2) | 0.115 ± 0.003 | 0.122 ± 0.004 | 0.121 ± 0.002 | 0.116 ± 0.003 | 0.123 ± 0.004 | 0.114 ± 0.002 | n.s. |
| initial isometric tension (×10−3 N·m−2) | 3.4 ± 0.1 | 3.3 ± 0.1 | 3.3 ± 0.1 | 3.4 ± 0.1 | 3.2 ± 0.1 | 3.4 ± 0.1 | n.s. |
| Krebs-Ringer solution | 137 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM NaH2PO4, 1 g/L NaHCO3, 2 g/L Glucose, 2 mM CaCl2 |
| lysis buffer | 50 mM Tris-HCl (pH 7.5) i, 1 mM EDTA i, 1 mM EGTA i, 0.5% (w/v) Sodium Deoxycholate ii, 0.1% (w/v) SDS iii, 150 mM NaCl, 1% (v/v) NP-40, 1 mM PMSF, 1 mM DTT, protease inhibitor cocktail iv phosphatase inhibitor cocktail v |
| Laemmli sample buffer | 62.5 mM Tris-HCl (pH6.8) i, 0.05% (w/v) Bromophenol blue i, 5% (w/v) SDS iii, 50 mM DTT, 15% (v/v) Glycerol |
| SDS sample solution | 100 mM Tris-HCl (pH 8.0) v, 10% (w/v) SDS, 40 mM DTT, 5 mM EDTA i |
| resting solution | 5.1 mM Na2ATP ii, 10 mM EGTA ii, 5.6 mM Mg-(methanesulfonate)2 i, vi, 75 mM K-(methanesulfonate) vi, vii 20 mM PIPES (pH 7.0) viii, 10 mM creatine phosphate |
| rigor solution | 130.5 mM K-(methanesulfonate) vi, vii, 1.6 mM Mg-(methanesulphonate)2 i, vi, 10 mM EGTA ii 20 mM PIPES (pH 7.0) viii |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirano, K.; Yamauchi, H.; Nakahara, N.; Kinoshita, K.; Yamaguchi, M.; Takemori, S. X-ray Diffraction Analysis to Explore Molecular Traces of Eccentric Contraction on Rat Skeletal Muscle Parallelly Evaluated with Signal Protein Phosphorylation Levels. Int. J. Mol. Sci. 2021, 22, 12644. https://doi.org/10.3390/ijms222312644
Hirano K, Yamauchi H, Nakahara N, Kinoshita K, Yamaguchi M, Takemori S. X-ray Diffraction Analysis to Explore Molecular Traces of Eccentric Contraction on Rat Skeletal Muscle Parallelly Evaluated with Signal Protein Phosphorylation Levels. International Journal of Molecular Sciences. 2021; 22(23):12644. https://doi.org/10.3390/ijms222312644
Chicago/Turabian StyleHirano, Kazuhiro, Hideki Yamauchi, Naoya Nakahara, Kazuo Kinoshita, Maki Yamaguchi, and Shigeru Takemori. 2021. "X-ray Diffraction Analysis to Explore Molecular Traces of Eccentric Contraction on Rat Skeletal Muscle Parallelly Evaluated with Signal Protein Phosphorylation Levels" International Journal of Molecular Sciences 22, no. 23: 12644. https://doi.org/10.3390/ijms222312644
APA StyleHirano, K., Yamauchi, H., Nakahara, N., Kinoshita, K., Yamaguchi, M., & Takemori, S. (2021). X-ray Diffraction Analysis to Explore Molecular Traces of Eccentric Contraction on Rat Skeletal Muscle Parallelly Evaluated with Signal Protein Phosphorylation Levels. International Journal of Molecular Sciences, 22(23), 12644. https://doi.org/10.3390/ijms222312644






