Enhanced Platelet-Rich Plasma (ePRP) Stimulates Wound Healing through Effects on Metabolic Reprogramming in Fibroblasts
Abstract
1. Introduction
2. Results
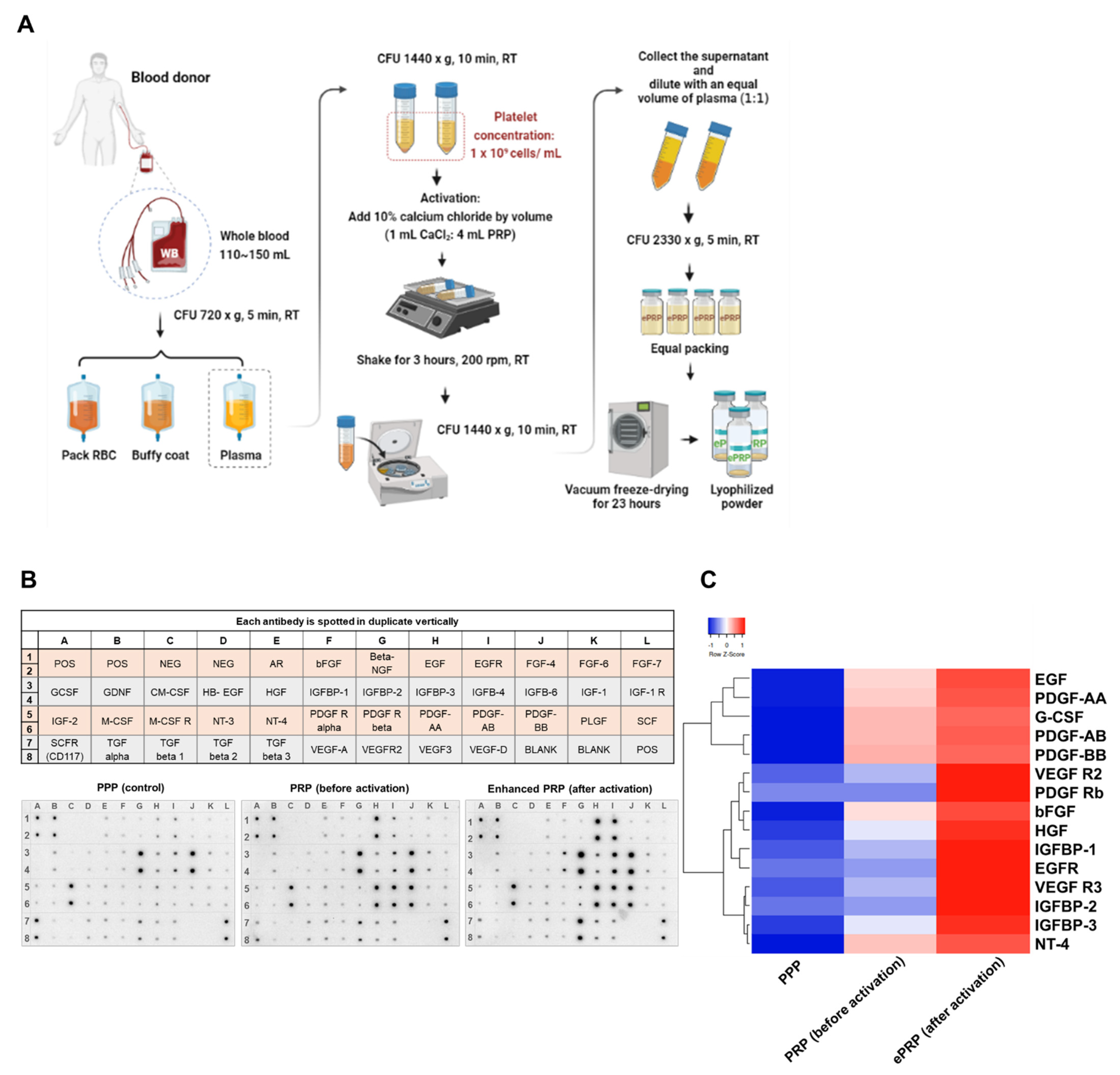
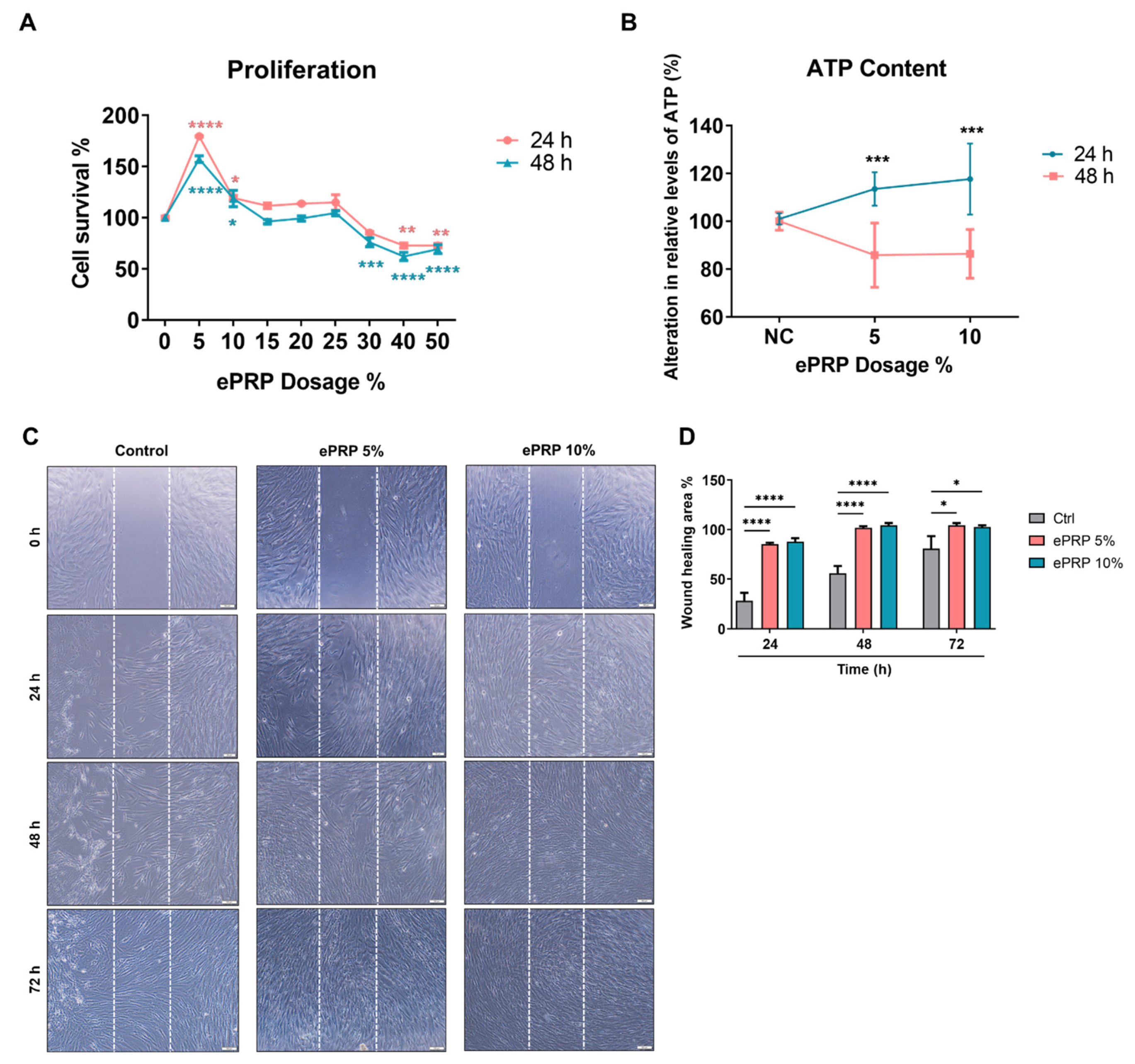
2.1. ePRP Stimulates Wound Healing by Releasing Critical Growth Factors
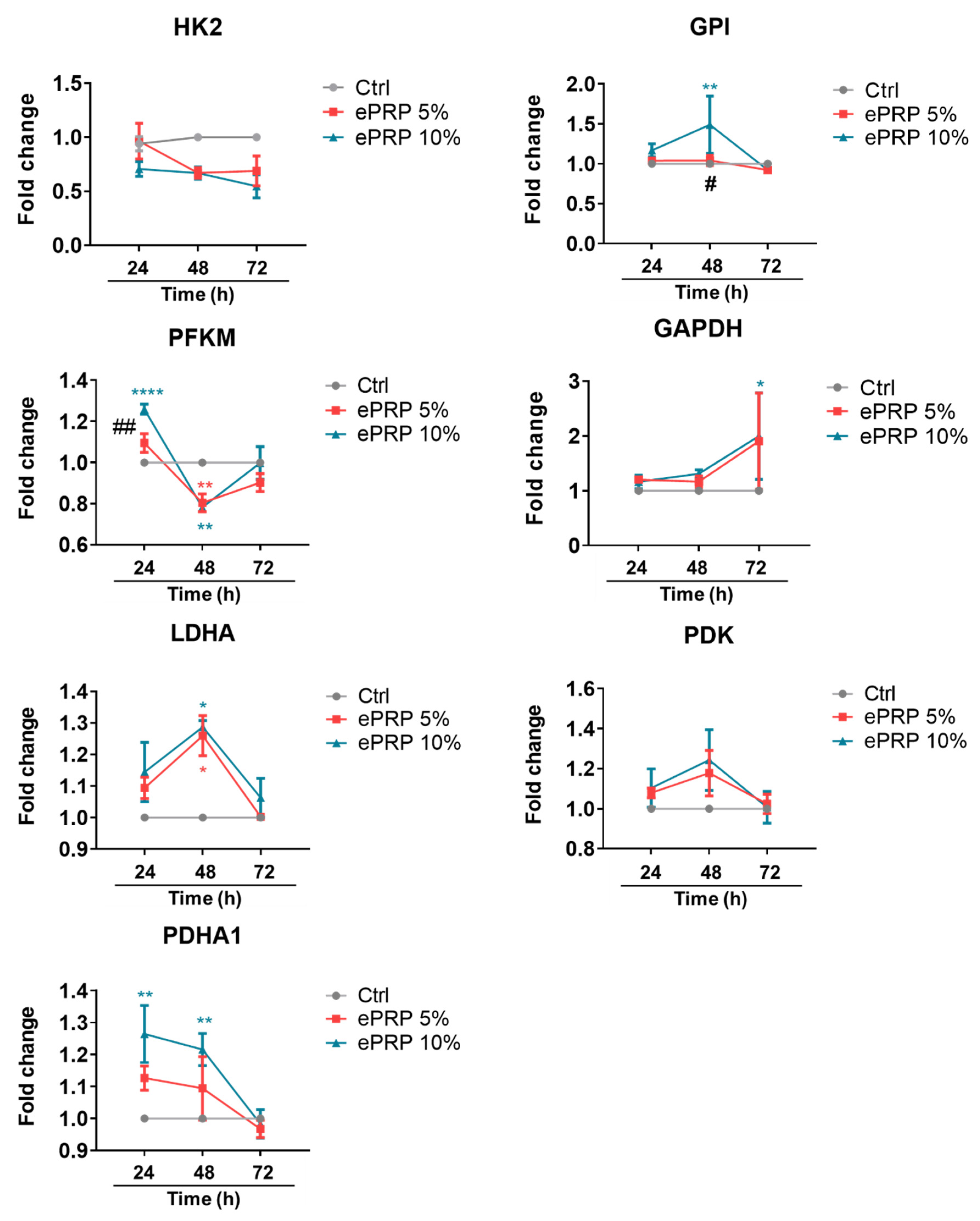
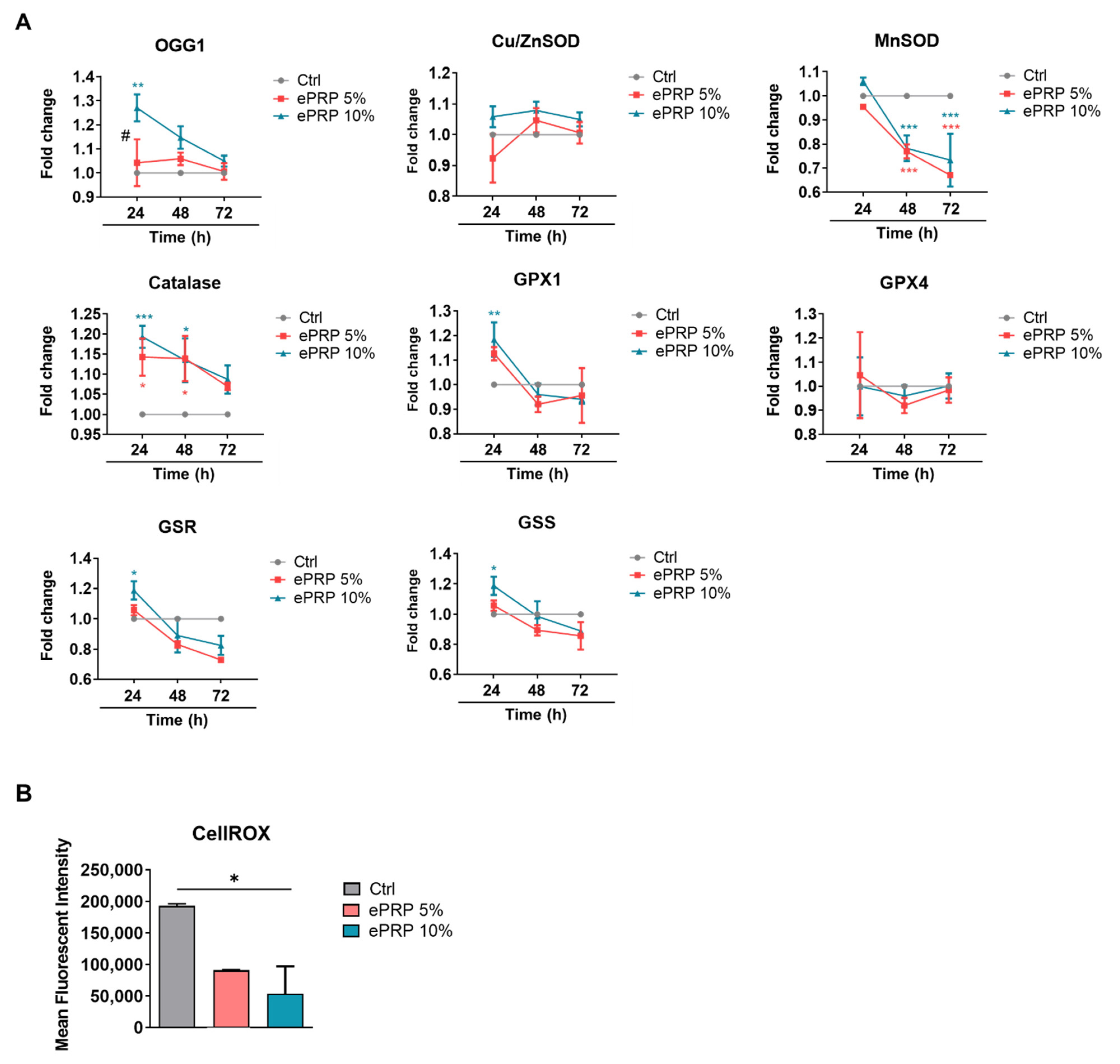
2.2. ePRP Triggers a Metabolic Reprogramming in Fibroblasts
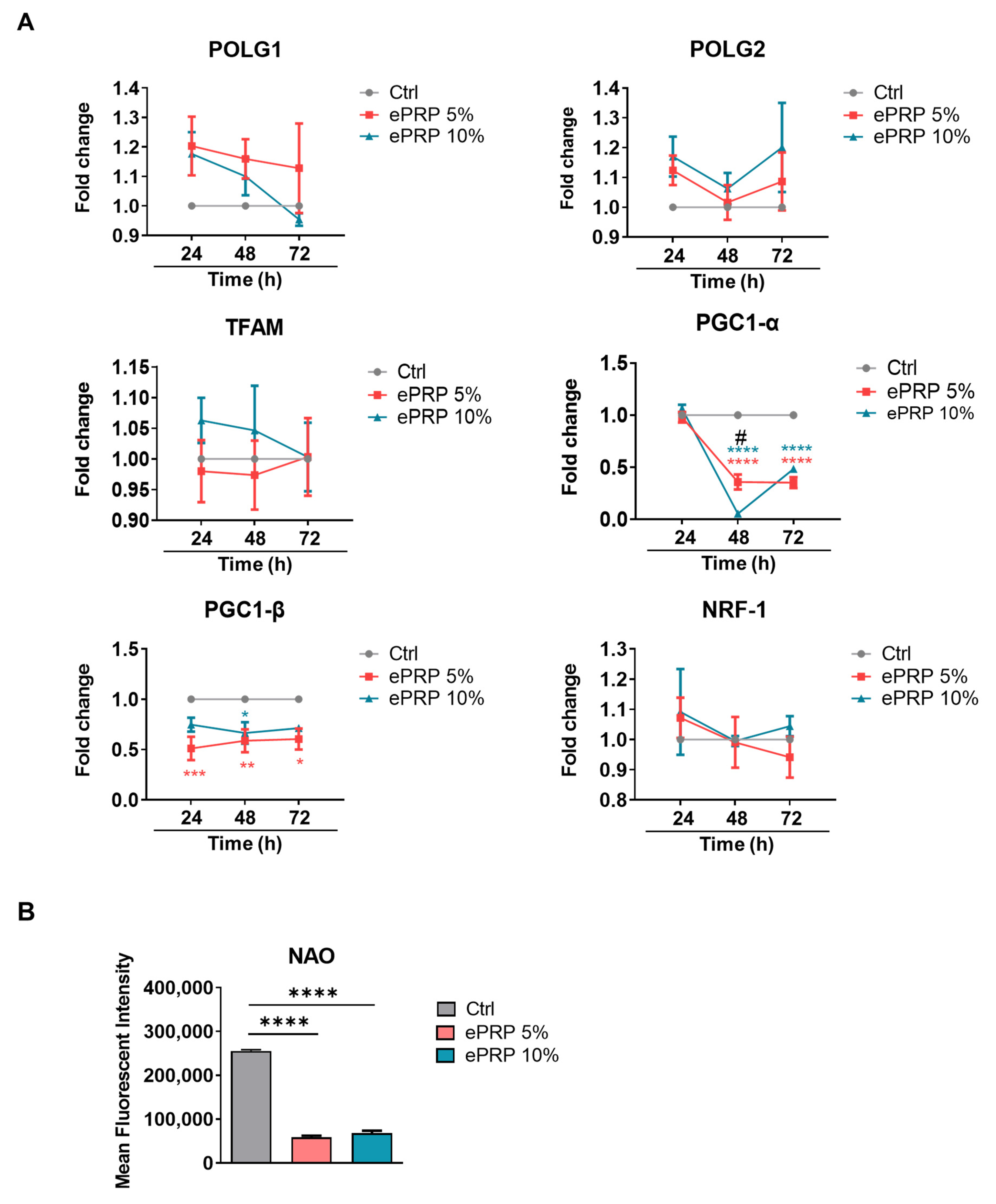
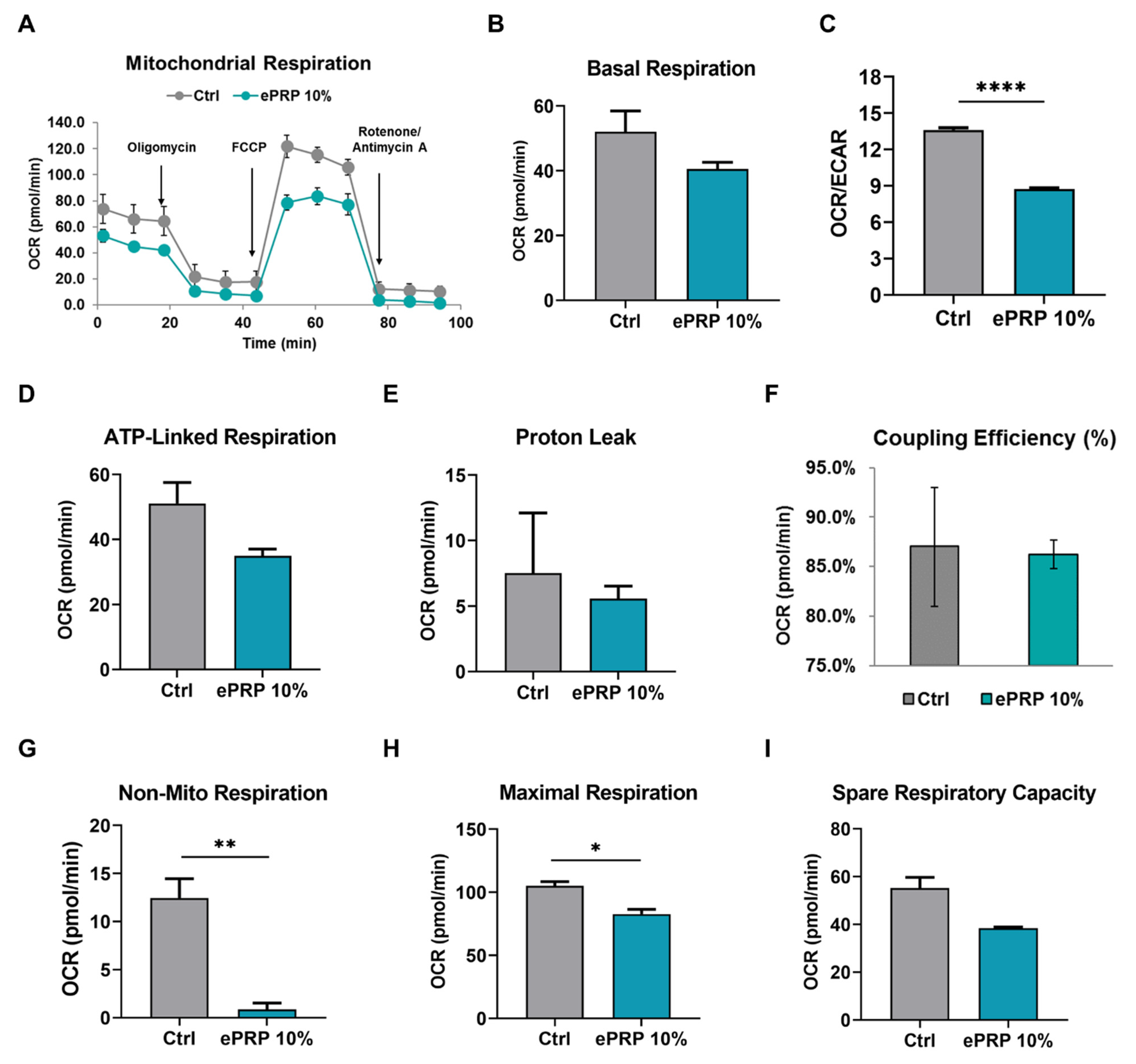
2.3. ePRP Impedes the Mitochondrial Activities in Fibroblasts
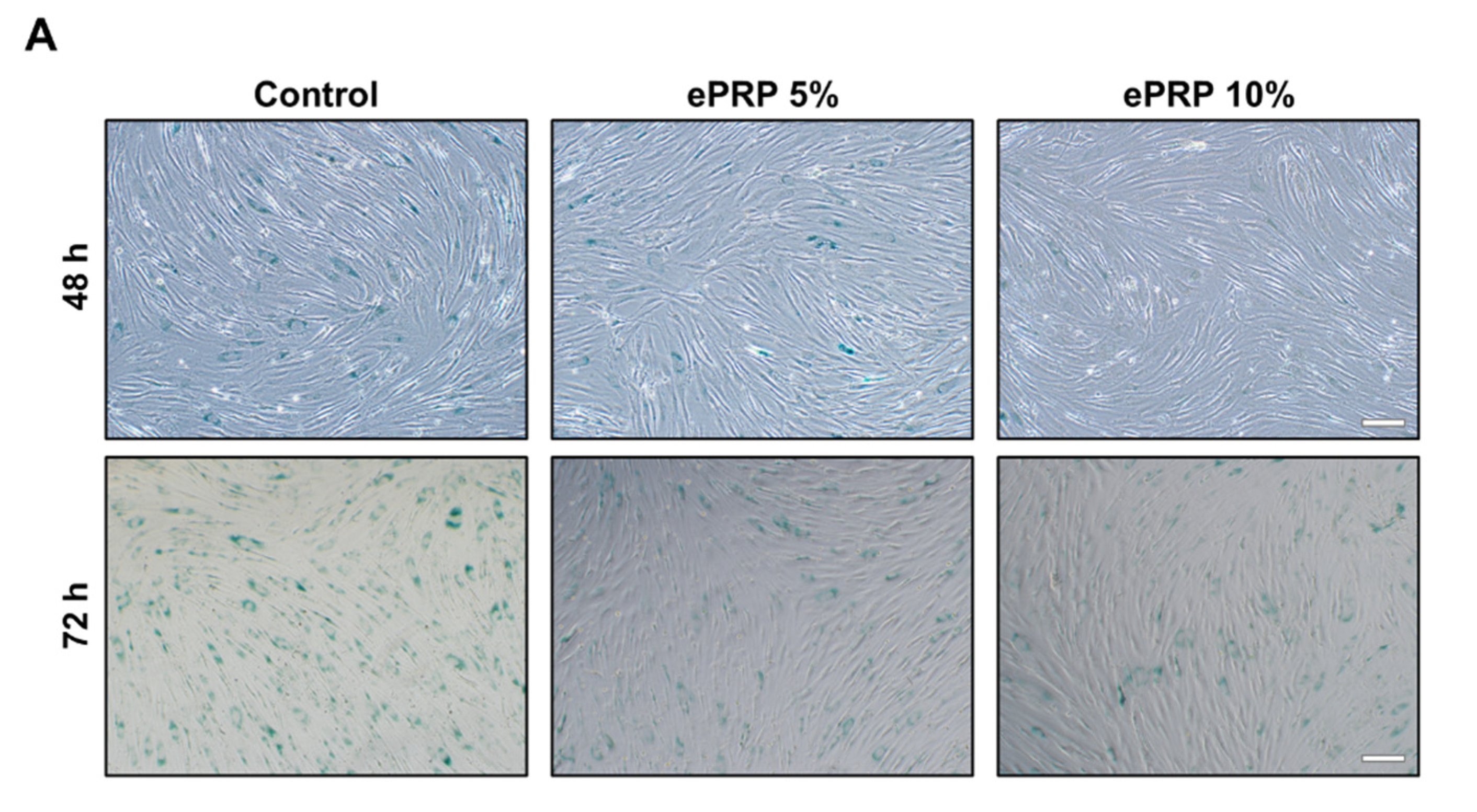
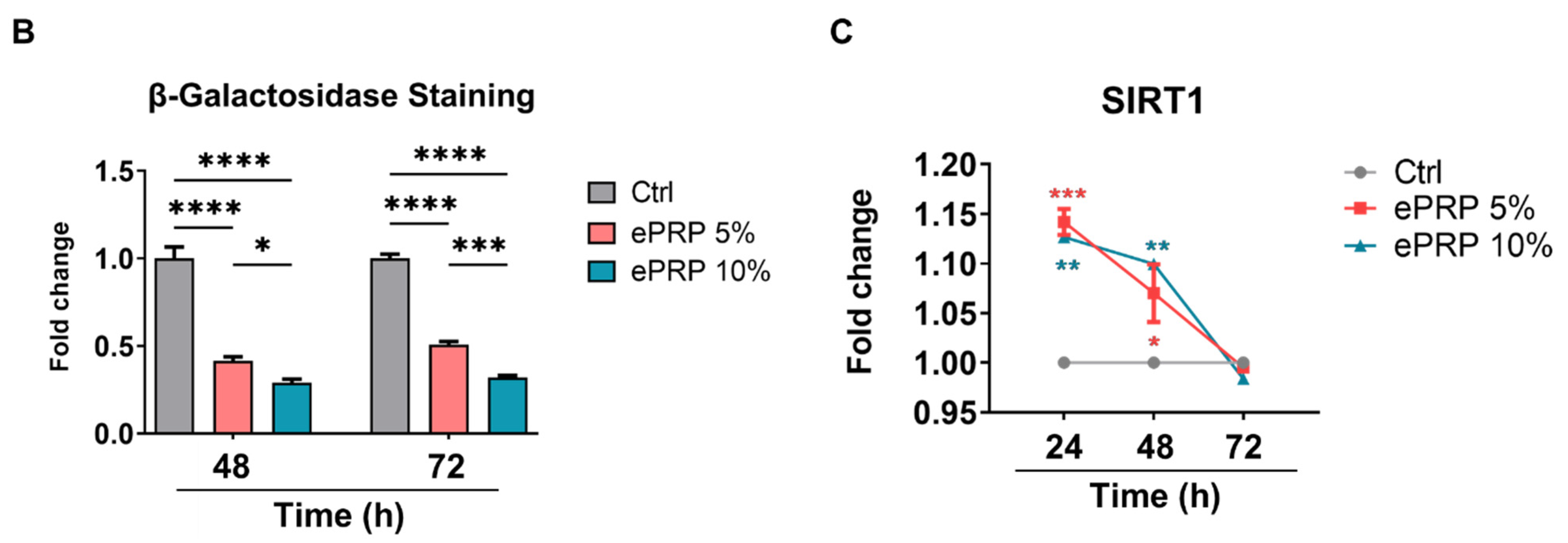
2.4. SIRT1 Activation by ePRP Halts Senescence-Associated Features in Fibroblasts
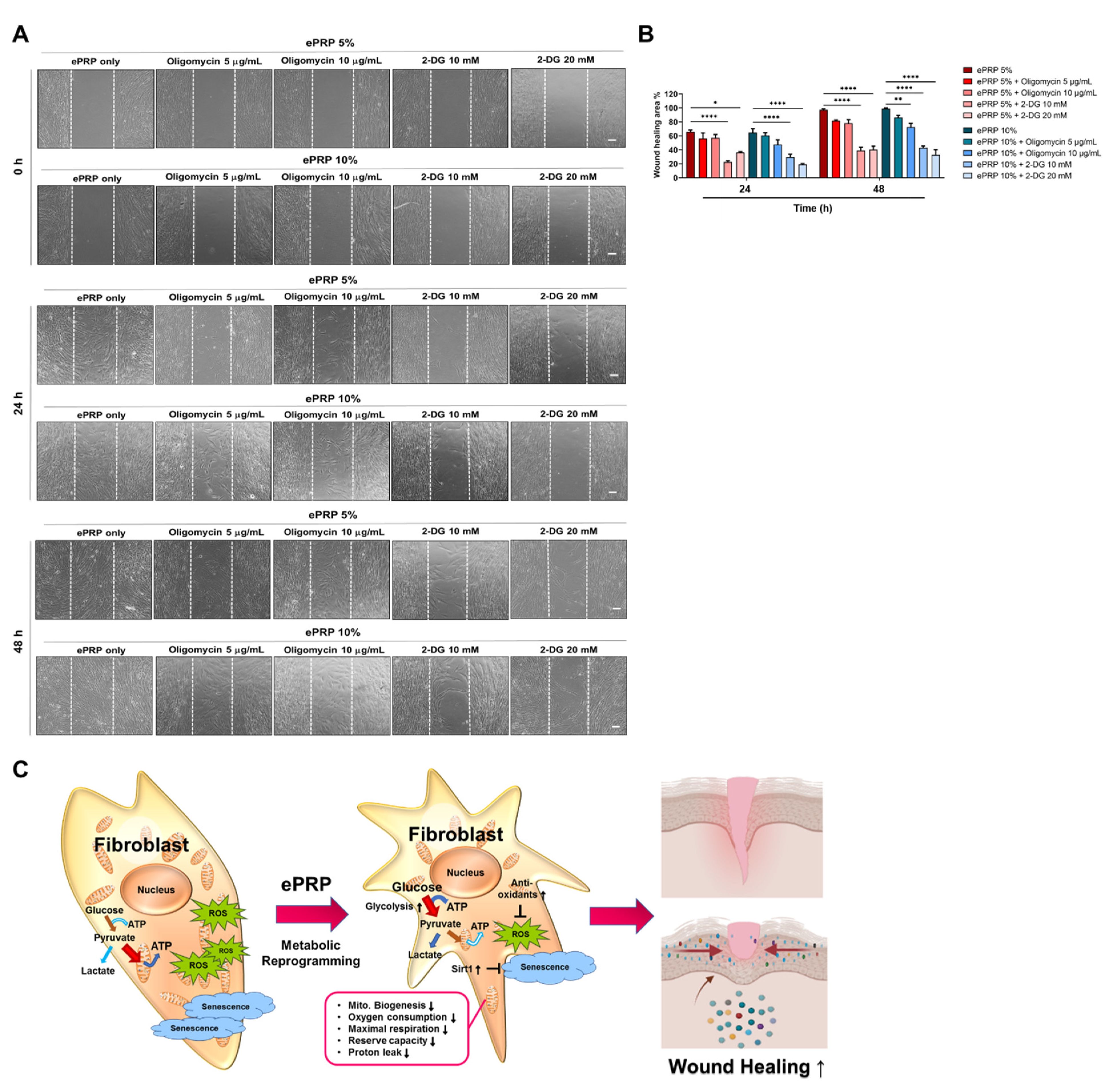
2.5. Glycolytic Inhibition Abolishes the ePRP-Stimulated Wound Healing Capacity of Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Preparation of Enhanced Platelet-Rich Plasma (ePRP)
4.2. Human Growth Factor Cytokine Array
4.3. Cell Culture
4.4. ePRP Treatments
4.5. Cell Proliferation Assay
4.6. Wound Healing Migration Assay
4.7. RNA Isolation and Real-Time RT-PCR
4.8. Measurement of the Intracellular ATP Content
4.9. Flow Cytometry Analysis
4.10. Senescence β-Galactosidase Staining
4.11. Metabolic Flux Assays
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and Skin Aging-From the Perspective of Food Nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef]
- Srivastava, A.; Karlsson, M.; Marionnet, C.; Bernerd, F.; Gueniche, A.; Rawadi, C.E.I.; Ståhle, M.; Sonkoly, E.; Breton, L.; Pivarcsi, A. Identification of chronological and photoageing-associated microRNAs in human skin. Sci. Rep. 2018, 8, 12990. [Google Scholar] [CrossRef]
- Batisse, D.; Bazin, R.; Baldeweck, T.; Querleux, B.; Lévêque, J.L. Influence of age on the wrinkling capacities of skin. Ski. Res. Technol. 2002, 8, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Chien, A.L.; Kang, S. Photoaging. Derm. Clin. 2014, 32, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-B.; Yang, J.-H.; Chung, K.-H. Characterization of the cytokine profile of platelet rich plasma (PRP) and PRP-induced cell proliferation and migration: Upregulation of matrix metalloproteinase-1 and -9 in HaCaT cells. Korean J. Hematol. 2011, 46, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Kassir, M.; Kroumpouzos, G.; Puja, P.; Katsambas, A.; Galadari, H.; Lotti, T.; Abdelmaksoud, A.; Grabbe, S.; Juchems, E.; Goldust, M. Update in minimally invasive periorbital rejuvenation with a focus on platelet-rich plasma: A narrative review. J. Cosmet. Dermatol. 2020, 19, 1057–1062. [Google Scholar] [CrossRef]
- Aust, M.; Pototschnig, H.; Jamchi, S.; Busch, K.-H. Platelet-rich Plasma for Skin Rejuvenation and Treatment of Actinic Elastosis in the Lower Eyelid Area. Cureus 2018, 10, e2999. [Google Scholar] [CrossRef]
- Atiyeh, B.; Oneisi, A.; Ghieh, F. Platelet-Rich Plasma Facial Rejuvenation: Myth or Reality? Aesthetic Plast. Surg. 2021. [Google Scholar] [CrossRef]
- Hersant, B.; SidAhmed-Mezi, M.; Aboud, C.; Niddam, J.; Levy, S.; Mernier, T.; La Padula, S.; Meningaud, J.P. Synergistic Effects of Autologous Platelet-Rich Plasma and Hyaluronic Acid Injections on Facial Skin Rejuvenation. Aesthet. Surg. J. 2021, 41, Np854–Np865. [Google Scholar] [CrossRef]
- Kim, D.H.; Je, Y.J.; Kim, C.D.; Lee, Y.H.; Seo, Y.J.; Lee, J.H.; Lee, Y. Can Platelet-rich Plasma Be Used for Skin Rejuvenation? Evaluation of Effects of Platelet-rich Plasma on Human Dermal Fibroblast. Ann. Derm. 2011, 23, 424–431. [Google Scholar] [CrossRef]
- Cho, E.B.; Park, G.S.; Park, S.S.; Jang, Y.J.; Kim, K.H.; Kim, K.J.; Park, E.J. Effect of platelet-rich plasma on proliferation and migration in human dermal fibroblasts. J. Cosmet. Derm. 2019, 18, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Abuaf, O.K.; Yildiz, H.; Baloglu, H.; Bilgili, M.E.; Simsek, H.A.; Dogan, B. Histologic Evidence of New Collagen Formulation Using Platelet Rich Plasma in Skin Rejuvenation: A Prospective Controlled Clinical Study. Ann. Derm. 2016, 28, 718–724. [Google Scholar] [CrossRef]
- Mihaylova, Z.; Mitev, V.; Stanimirov, P.; Isaeva, A.; Gateva, N.; Ishkitiev, N. Use of platelet concentrates in oral and maxillofacial surgery: An overview. Acta Odontol. Scand. 2017, 75, 1–11. [Google Scholar] [CrossRef]
- Anitua, E.; Fernández-de-Retana, S.; Alkhraisat, M.H. Platelet rich plasma in oral and maxillofacial surgery from the perspective of composition. Platelets 2021, 32, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, L.; Bennardo, F.; Buffone, C.; Giudice, A. Is the application of platelet concentrates effective in the prevention and treatment of medication-related osteonecrosis of the jaw? A systematic review. J. Craniomaxillofac. Surg. 2020, 48, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Bennardo, F.; Liborio, F.; Barone, S.; Antonelli, A.; Buffone, C.; Fortunato, L.; Giudice, A. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: A pilot study. Clin. Oral Investig. 2021, 25, 3747–3755. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, Y.; Antonelli, A.; Barone, S.; Bennardo, F.; Fortunato, L.; Giudice, A. Evaluation of local hemostatic efficacy after dental extractions in patients taking antiplatelet drugs: A randomized clinical trial. Clin. Oral Investig. 2021, 25, 1159–1167. [Google Scholar] [CrossRef]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef]
- Wagener, F.A.; Carels, C.E.; Lundvig, D.M. Targeting the redox balance in inflammatory skin conditions. Int. J. Mol. Sci. 2013, 14, 9126–9167. [Google Scholar] [CrossRef]
- Roberts, L.J., 2nd; Reckelhoff, J.F. Measurement of F(2)-isoprostanes unveils profound oxidative stress in aged rats. Biochem. Biophys. Res. Commun. 2001, 287, 254–256. [Google Scholar] [CrossRef]
- Stadtman, E.R. Protein oxidation and aging. Free Radic. Res. 2006, 40, 1250–1258. [Google Scholar] [CrossRef]
- Sreedhar, A.; Aguilera-Aguirre, L.; Singh, K.K. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wu, Y.; Zhou, L.; Yang, Z.; Zhang, X.; Hu, X.; Yang, J.; Wang, M.; Wang, B.; Luo, G.; et al. Platelet-rich plasma accelerates skin wound healing by promoting re-epithelialization. Burn. Trauma 2020, 8, tkaa028. [Google Scholar] [CrossRef]
- Belebecha, V.; Casagrande, R.; Urbano, M.R.; Crespigio, J.; Martinez, R.M.; Vale, D.L.; de Almeida, S.H.M. Effect of the platelet-rich plasma covering of polypropylene mesh on oxidative stress, inflammation, and adhesions. Int. Urogynecol. J. 2020, 31, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2011, 1813, 1269–1278. [Google Scholar] [CrossRef]
- Dott, W.; Mistry, P.; Wright, J.; Cain, K.; Herbert, K.E. Modulation of mitochondrial bioenergetics in a skeletal muscle cell line model of mitochondrial toxicity. Redox Biol. 2014, 2, 224–233. [Google Scholar] [CrossRef]
- Hill, B.G.; Dranka, B.P.; Zou, L.; Chatham, J.C.; Darley-Usmar, V.M. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem. J. 2009, 424, 99–107. [Google Scholar] [CrossRef]
- Sergiev, P.V.; Dontsova, O.A.; Berezkin, G.V. Theories of aging: An ever-evolving field. Acta Nat. 2015, 7, 9–18. [Google Scholar] [CrossRef]
- Victorelli, S.; Passos, J.F. Reactive Oxygen Species Detection in Senescent Cells. Methods Mol. Biol. 2019, 1896, 21–29. [Google Scholar]
- Petrova, N.V.; Velichko, A.K.; Razin, S.V.; Kantidze, O.L. Small molecule compounds that induce cellular senescence. Aging Cell 2016, 15, 999–1017. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef]
- Hayakawa, T.; Iwai, M.; Aoki, S.; Takimoto, K.; Maruyama, M.; Maruyama, W.; Motoyama, N. SIRT1 Suppresses the Senescence-Associated Secretory Phenotype through Epigenetic Gene Regulation. PLoS ONE 2015, 10, e0116480. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Fozouni, P.; Evjen, G.; Chandra, V.; Jiang, J.; Lu, C.; Nicastri, M.; Bretz, C.; Winkler, J.D.; et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat. Cell Biol. 2020, 22, 1170–1179. [Google Scholar] [CrossRef]
- Pajak, B.; Siwiak, E.; Sołtyka, M.; Priebe, A.; Zieliński, R.; Fokt, I.; Ziemniak, M.; Jaśkiewicz, A.; Borowski, R.; Domoradzki, T.; et al. 2-Deoxy-d-Glucose and Its Analogs: From Diagnostic to Therapeutic Agents. Int. J. Mol. Sci. 2019, 21, 234. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Esposito, M.; Bennardo, F.; Brancaccio, Y.; Buti, J.; Fortunato, L. Dental extractions for patients on oral antiplatelet: A within-person randomised controlled trial comparing haemostatic plugs, advanced-platelet-rich fibrin (A-PRF+) plugs, leukocyte- and platelet-rich fibrin (L-PRF) plugs and suturing alone. Int. J. Oral Implantol. 2019, 12, 77–87. [Google Scholar]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng. Part B Rev. 2016, 23, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Wasterlain, A.S.; Braun, H.J.; Dragoo, J.L. Contents and Formulations of Platelet-Rich Plasma. Oper. Tech. Orthop. 2012, 22, 33–42. [Google Scholar] [CrossRef]
- Di Matteo, B.; Filardo, G.; Kon, E.; Marcacci, M. Platelet-rich plasma: Evidence for the treatment of patellar and Achilles tendinopathy—a systematic review. Musculoskelet. Surg. 2015, 99, 1–9. [Google Scholar] [CrossRef]
- Cavallo, C.; Roffi, A.; Grigolo, B.; Mariani, E.; Pratelli, L.; Merli, G.; Kon, E.; Marcacci, M.; Filardo, G. Platelet-Rich Plasma: The Choice of Activation Method Affects the Release of Bioactive Molecules. BioMed Res. Int. 2016, 2016, 6591717. [Google Scholar] [CrossRef]
- Sumi, S.; Ichihara, K.; Kono, N.; Nonaka, K.; Tarui, S. Insulin and epidermal growth factor stimulate glycolysis in quiescent 3T3 fibroblasts with no changes in key glycolytic enzyme activities. Endocrinol. Jpn. 1984, 31, 117–125. [Google Scholar] [CrossRef]
- Ran, C.; Liu, H.; Hitoshi, Y.; Israel, M.A. Proliferation-Independent Control of Tumor Glycolysis by PDGFR-Mediated AKT Activation. Cancer Res. 2013, 73, 1831–1843. [Google Scholar] [CrossRef]
- Shi, S.; Xu, J.; Zhang, B.; Ji, S.; Xu, W.; Liu, J.; Jin, K.; Liang, D.; Liang, C.; Liu, L.; et al. VEGF Promotes Glycolysis in Pancreatic Cancer via HIF1α Up-Regulation. Curr. Mol. Med. 2016, 16, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Kihira, Y.; Yamano, N.; Izawa-Ishizawa, Y.; Ishizawa, K.; Ikeda, Y.; Tsuchiya, K.; Tamaki, T.; Tomita, S. Basic fibroblast growth factor regulates glucose metabolism through glucose transporter 1 induced by hypoxia-inducible factor-1α in adipocytes. Int. J. Biochem. Cell Biol. 2011, 43, 1602–1611. [Google Scholar] [CrossRef]
- Kaplan, O.; Firon, M.; Vivi, A.; Navon, G.; Tsarfaty, I. HGF/SF activates glycolysis and oxidative phosphorylation in DA3 murine mammary cancer cells. Neoplasia 2000, 2, 365–377. [Google Scholar] [CrossRef][Green Version]
- Kelly, B.; O’Neill, L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Hunt, T.K.; Conolly, W.B.; Aronson, S.B.; Goldstein, P. Anaerobic metabolism and wound healing: An hypothesis for the initiation and cessation of collagen synthesis in wounds. Am. J. Surg. 1978, 135, 328–332. [Google Scholar] [CrossRef]
- Atia, L.; Bi, D.; Sharma, Y.; Mitchel, J.A.; Gweon, B.; Koehler, S.; DeCamp, S.J.; Lan, B.; Kim, J.H.; Hirsch, R.; et al. Geometric constraints during epithelial jamming. Nat. Phys. 2018, 14, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Canovic, E.P.; Iordan, A.L.; Rajendran, K.; Manomohan, G.; Pirentis, A.P.; Smith, M.L.; Butler, J.P.; Fredberg, J.J.; Stamenovic, D. Fluidization, resolidification, and reorientation of the endothelial cell in response to slow tidal stretches. Am. J. Physiol. Cell Physiol. 2012, 303, C368–C375. [Google Scholar] [CrossRef]
- Park, J.-A.; Kim, J.H.; Bi, D.; Mitchel, J.A.; Qazvini, N.T.; Tantisira, K.; Park, C.Y.; McGill, M.; Kim, S.-H.; Gweon, B.; et al. Unjamming and cell shape in the asthmatic airway epithelium. Nat. Mater. 2015, 14, 1040–1048. [Google Scholar] [CrossRef]
- DeCamp, S.J.; Tsuda, V.M.K.; Ferruzzi, J.; Koehler, S.A.; Giblin, J.T.; Roblyer, D.; Zaman, M.H.; Weiss, S.T.; Kılıç, A.; De Marzio, M.; et al. Epithelial layer unjamming shifts energy metabolism toward glycolysis. Sci. Rep. 2020, 10, 18302. [Google Scholar] [CrossRef]
- Sinclair, J.W.; Hoying, D.R.; Bresciani, E.; Nogare, D.D.; Needle, C.D.; Berger, A.; Wu, W.; Bishop, K.; Elkahloun, A.G.; Chitnis, A.; et al. The Warburg effect is necessary to promote glycosylation in the blastema during zebrafish tail regeneration. NPJ Regen. Med. 2021, 6, 55. [Google Scholar] [CrossRef]
- Clayton, D.A. Nuclear-mitochondrial intergenomic communication. Biofactors 1998, 7, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Goffart, S.; Wiesner, R.J. Regulation and co-ordination of nuclear gene expression during mitochondrial biogenesis. Exp. Physiol. 2003, 88, 33–40. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Makrantonaki, E. Clinical aspects and molecular diagnostics of skin aging. Clin. Dermatol. 2011, 29, 3–14. [Google Scholar] [CrossRef]
- Natarajan, V.T.; Ganju, P.; Ramkumar, A.; Grover, R.; Gokhale, R.S. Multifaceted pathways protect human skin from UV radiation. Nat. Chem. Biol. 2014, 10, 542–551. [Google Scholar] [CrossRef]
- Gonzaga, E.R. Role of UV Light in Photodamage, Skin Aging, and Skin Cancer. Am. J. Clin. Dermatol. 2009, 10, 19–24. [Google Scholar] [CrossRef]
- Pleńkowska, J.; Gabig-Cimińska, M.; Mozolewski, P. Oxidative Stress as an Important Contributor to the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2020, 21, 6206. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.; Nfonsam, V.; Calienes, F.; Sligh, J.E.; Jandova, J. Modulation of ROS levels in fibroblasts by altering mitochondria regulates the process of wound healing. Arch. Dermatol. Res. 2016, 308, 239–248. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Defossez, P.A.; Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 2000, 289, 2126–2128. [Google Scholar] [CrossRef] [PubMed]
- Tissenbaum, H.A.; Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 2001, 410, 227–230. [Google Scholar] [CrossRef]
- Rogina, B.; Helfand, S.L. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. USA 2004, 101, 15998–16003. [Google Scholar] [CrossRef]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol 2010, 5, 253–295. [Google Scholar] [CrossRef]
- Huang, J.; Gan, Q.; Han, L.; Li, J.; Zhang, H.; Sun, Y.; Zhang, Z.; Tong, T. SIRT1 Overexpression Antagonizes Cellular Senescence with Activated ERK/S6k1 Signaling in Human Diploid Fibroblasts. PLoS ONE 2008, 3, e1710. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, H.-P.; Cheng, Y.-Y.; Lee, H.-L.; Hsu, T.-Y.; Chang, Y.-T.; Shen, Y.-A. Enhanced Platelet-Rich Plasma (ePRP) Stimulates Wound Healing through Effects on Metabolic Reprogramming in Fibroblasts. Int. J. Mol. Sci. 2021, 22, 12623. https://doi.org/10.3390/ijms222312623
Weng H-P, Cheng Y-Y, Lee H-L, Hsu T-Y, Chang Y-T, Shen Y-A. Enhanced Platelet-Rich Plasma (ePRP) Stimulates Wound Healing through Effects on Metabolic Reprogramming in Fibroblasts. International Journal of Molecular Sciences. 2021; 22(23):12623. https://doi.org/10.3390/ijms222312623
Chicago/Turabian StyleWeng, Hsin-Pei, Yuan-Yang Cheng, Hsin-Lun Lee, Tai-Yi Hsu, Yu-Tang Chang, and Yao-An Shen. 2021. "Enhanced Platelet-Rich Plasma (ePRP) Stimulates Wound Healing through Effects on Metabolic Reprogramming in Fibroblasts" International Journal of Molecular Sciences 22, no. 23: 12623. https://doi.org/10.3390/ijms222312623
APA StyleWeng, H.-P., Cheng, Y.-Y., Lee, H.-L., Hsu, T.-Y., Chang, Y.-T., & Shen, Y.-A. (2021). Enhanced Platelet-Rich Plasma (ePRP) Stimulates Wound Healing through Effects on Metabolic Reprogramming in Fibroblasts. International Journal of Molecular Sciences, 22(23), 12623. https://doi.org/10.3390/ijms222312623





