Pleiotropic Outcomes of Glyphosate Exposure: From Organ Damage to Effects on Inflammation, Cancer, Reproduction and Development
Abstract
:1. Introduction
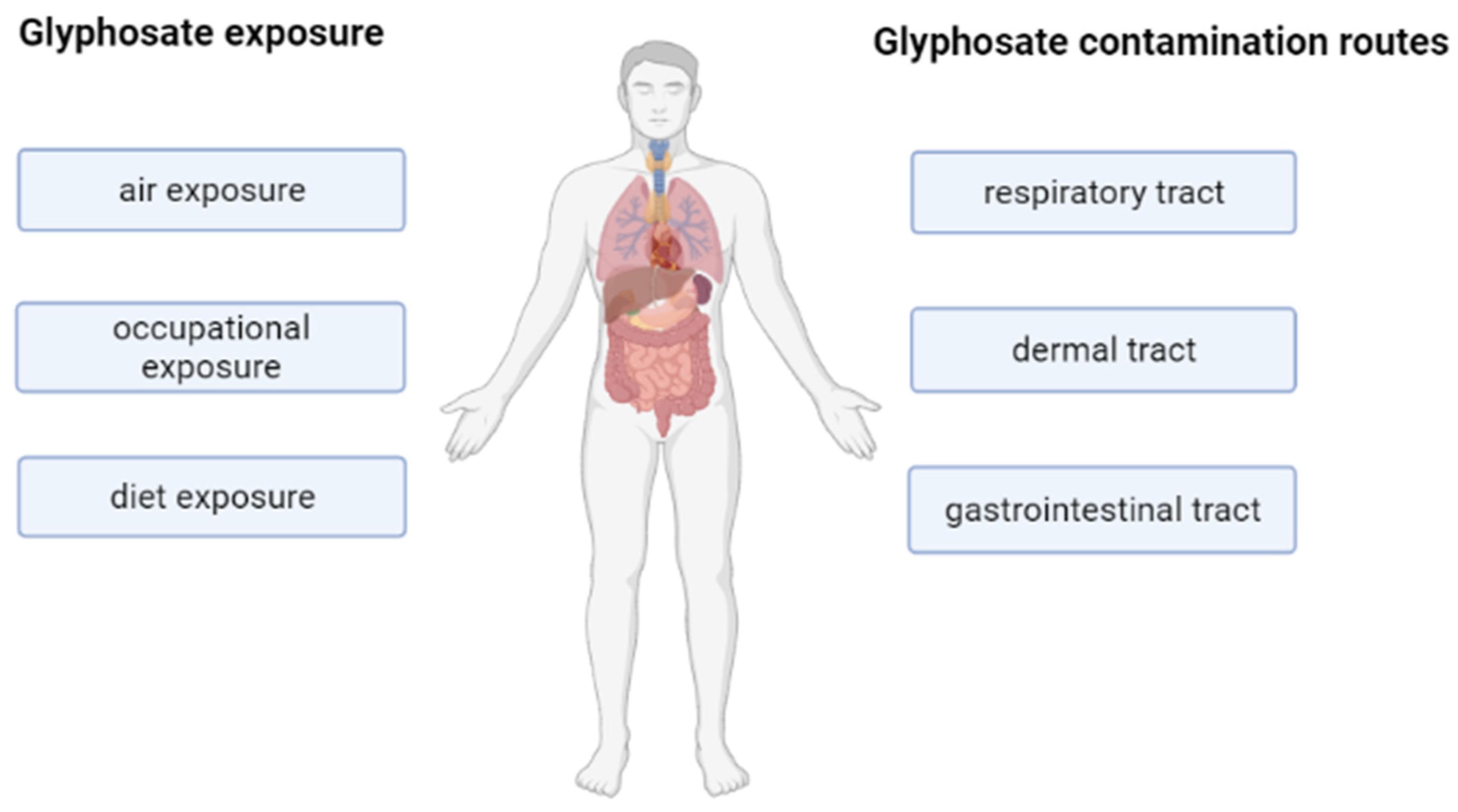
2. Glyphosate Action and Contamination Routes
3. Immunomodulatory and Inflammatory Effects of Glyphosate
3.1. Glyphosate—Induced Effects in Liver, Kidney and Lung
3.2. Glyphosate—Induced Effects in the Intestine
3.3. Glyphosate—Induced Effects in Blood Cells
3.4. Neurodegenerative Glyphosate—Induced Effects
4. Carcinogenic and Mutagenic Effects of Glyphosate
5. Effects of Glyphosate on Reproduction and Development
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Landrigan, P.J.; Belpoggi, F. The Need for Independent Research on the Health Effects of Glyphosate-Based Herbicides. Environ. Health 2018, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and Health Effects of the Herbicide Glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Clements, D.; Dugdale, T.M.; Butler, K.L.; Florentine, S.K.; Sillitoe, J. Herbicide Efficacy for Aquatic Alternanthera Philoxeroides Management in an Early Stage of Invasion: Integrating above-Ground Biomass, below-Ground Biomass and Viable Stem Fragmentation. Weed Res. 2017, 57, 257–266. [Google Scholar] [CrossRef]
- Benbrook, C.M. Trends in Glyphosate Herbicide Use in the United States and Globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some organophosphate insecticides and herbicides. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2017; ISBN 978-92-832-0150-2. [Google Scholar]
- Mink, P.J.; Mandel, J.S.; Sceurman, B.K.; Lundin, J.I. Epidemiologic Studies of Glyphosate and Cancer: A Review. Regul. Toxicol. Pharmacol. 2012, 63, 440–452. [Google Scholar] [CrossRef]
- Kubsad, D.; Nilsson, E.E.; King, S.E.; Sadler-Riggleman, I.; Beck, D.; Skinner, M.K. Assessment of Glyphosate Induced Epigenetic Transgenerational Inheritance of Pathologies and Sperm Epimutations: Generational Toxicology. Sci. Rep. 2019, 9, 6372. [Google Scholar] [CrossRef] [Green Version]
- Gillezeau, C.; van Gerwen, M.; Shaffer, R.M.; Rana, I.; Zhang, L.; Sheppard, L.; Taioli, E. The Evidence of Human Exposure to Glyphosate: A Review. Environ. Health 2019, 18, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesnage, R.; Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Potential Toxic Effects of Glyphosate and Its Commercial Formulations below Regulatory Limits. Food Chem. Toxicol. 2015, 84, 133–153. [Google Scholar] [CrossRef] [Green Version]
- Portier, C.J.; Armstrong, B.K.; Baguley, B.C.; Baur, X.; Belyaev, I.; Bellé, R.; Belpoggi, F.; Biggeri, A.; Bosland, M.C.; Bruzzi, P.; et al. Differences in the Carcinogenic Evaluation of Glyphosate between the International Agency for Research on Cancer (IARC) and the European Food Safety Authority (EFSA). J. Epidemiol. Community Health 2016, 70, 741–745. [Google Scholar] [CrossRef] [Green Version]
- Nagy, K.; Tessema, R.A.; Budnik, L.T.; Ádám, B. Comparative Cyto- and Genotoxicity Assessment of Glyphosate and Glyphosate-Based Herbicides in Human Peripheral White Blood Cells. Environ. Res. 2019, 179, 108851. [Google Scholar] [CrossRef]
- Peillex, C.; Pelletier, M. The Impact and Toxicity of Glyphosate and Glyphosate-Based Herbicides on Health and Immunity. J. Immunotoxicol. 2020, 17, 163–174. [Google Scholar] [CrossRef]
- Andreotti, G.; Koutros, S.; Hofmann, J.N.; Sandler, D.P.; Lubin, J.H.; Lynch, C.F.; Lerro, C.C.; De Roos, A.J.; Parks, C.G.; Alavanja, M.C.; et al. Glyphosate Use and Cancer Incidence in the Agricultural Health Study. JNCI J. Natl. Cancer Inst. 2018, 110, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, K.K.; Miller, G.L.; Kremer, R.J.; Bradley, K.W. Interactions between Glyphosate, Fusarium Infection of Common Waterhemp (Amaranthus Rudis), and Soil Microbial Abundance and Diversity in Soil Collections from Missouri. Weed Sci. 2014, 62, 71–82. [Google Scholar] [CrossRef]
- Noori, J.; Dimaki, M.; Mortensen, J.; Svendsen, W. Detection of Glyphosate in Drinking Water: A Fast and Direct Detection Method without Sample Pretreatment. Sensors 2018, 18, 2961. [Google Scholar] [CrossRef] [Green Version]
- Panzacchi, S.; Mandrioli, D.; Manservisi, F.; Bua, L.; Falcioni, L.; Spinaci, M.; Galeati, G.; Dinelli, G.; Miglio, R.; Mantovani, A.; et al. The Ramazzini Institute 13-Week Study on Glyphosate-Based Herbicides at Human-Equivalent Dose in Sprague Dawley Rats: Study Design and First in-Life Endpoints Evaluation. Environ. Health 2018, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.-E. Major Pesticides Are More Toxic to Human Cells Than Their Declared Active Principles. BioMed Res. Int. 2014, 2014, 179691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benachour, N.; Séralini, G.-E. Glyphosate Formulations Induce Apoptosis and Necrosis in Human Umbilical, Embryonic, and Placental Cells. Chem. Res. Toxicol. 2009, 22, 97–105. [Google Scholar] [CrossRef]
- Chłopecka, M.; Mendel, M.; Dziekan, N.; Karlik, W. The Effect of Glyphosate-Based Herbicide Roundup and Its Co-Formulant, POEA, on the Motoric Activity of Rat Intestine—In Vitro Study. Environ. Toxicol. Pharmacol. 2017, 49, 156–162. [Google Scholar] [CrossRef]
- Arregui, M.C.; Lenardón, A.; Sanchez, D.; Maitre, M.I.; Scotta, R.; Enrique, S. Monitoring Glyphosate Residues in Transgenic Glyphosate-Resistant Soybean: Glyphosate Residues in Transgenic Glyphosate-Resistant Soybean. Pest. Manag. Sci. 2004, 60, 163–166. [Google Scholar] [CrossRef]
- McQueen, H.; Callan, A.C.; Hinwood, A.L. Estimating Maternal and Prenatal Exposure to Glyphosate in the Community Setting. Int. J. Hyg. Environ. Health 2012, 215, 570–576. [Google Scholar] [CrossRef]
- Torretta, V.; Katsoyiannis, I.; Viotti, P.; Rada, E. Critical Review of the Effects of Glyphosate Exposure to the Environment and Humans through the Food Supply Chain. Sustainability 2018, 10, 950. [Google Scholar] [CrossRef] [Green Version]
- Krüger, M.; Schledorn, P.; Schrödl, W.; Hoppe, H.-W.; Lutz, W.; Shehata, A. Detection of Glyphosate Residues in Animals and Humans. J. Environ. Anal. Toxicol. 2014, 4, 1–5. [Google Scholar] [CrossRef]
- Anadón, A.; Martínez-Larrañaga, M.R.; Martínez, M.A.; Castellano, V.J.; Martínez, M.; Martin, M.T.; Nozal, M.J.; Bernal, J.L. Toxicokinetics of Glyphosate and Its Metabolite Aminomethyl Phosphonic Acid in Rats. Toxicol. Lett. 2009, 190, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.M.; Kroes, R.; Munro, I.C. Safety Evaluation and Risk Assessment of the Herbicide Roundup and Its Active Ingredient, Glyphosate, for Humans. Regul. Toxicol. Pharmacol. 2000, 31, 117–165. [Google Scholar] [CrossRef] [Green Version]
- Acquavella, J.F.; Alexander, B.H.; Mandel, J.S.; Gustin, C.; Baker, B.; Chapman, P.; Bleeke, M. Glyphosate Biomonitoring for Farmers and Their Families: Results from the Farm Family Exposure Study. Environ. Health Perspect. 2004, 112, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, M.; Rubaltelli, F.F.; Hammerman, C.; Vilei, M.T.; Leiter, C.; Abramov, A.; Muraca, M. Conjugated Bilirubin in Neonates with Glucose-6-Phosphate Dehydrogenase Deficiency. J. Pediatr. 1996, 128, 695–697. [Google Scholar] [CrossRef]
- Niemann, L.; Sieke, C.; Pfeil, R.; Solecki, R. A Critical Review of Glyphosate Findings in Human Urine Samples and Comparison with the Exposure of Operators and Consumers. J. Verbr. Lebensm. 2015, 10, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Agostini, L.P.; Dettogni, R.S.; dos Reis, R.S.; Stur, E.; dos Santos, E.V.W.; Ventorim, D.P.; Garcia, F.M.; Cardoso, R.C.; Graceli, J.B.; Louro, I.D. Effects of Glyphosate Exposure on Human Health: Insights from Epidemiological and In Vitro Studies. Sci. Total Environ. 2020, 705, 135808. [Google Scholar] [CrossRef]
- Gunatilake, S.; Seneff, S.; Orlando, L. Glyphosate’s Synergistic Toxicity in Combination with Other Factors as a Cause of Chronic Kidney Disease of Unknown Origin. Int. J. Environ. Res. Public Health 2019, 16, 2734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattani, D.; de Liz Oliveira Cavalli, V.L.; Heinz Rieg, C.E.; Domingues, J.T.; Dal-Cim, T.; Tasca, C.I.; Mena Barreto Silva, F.R.; Zamoner, A. Mechanisms Underlying the Neurotoxicity Induced by Glyphosate-Based Herbicide in Immature Rat Hippocampus: Involvement of Glutamate Excitotoxicity. Toxicology 2014, 320, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samsel, A.; Seneff, S. Glyphosate Pathways to Modern Diseases V: Amino Acid Analogue of Glycine in Diverse Proteins. J. Biol. Phys. Chem. 2016, 16, 9–46. [Google Scholar] [CrossRef]
- Antoniou, M.N.; Nicolas, A.; Mesnage, R.; Biserni, M.; Rao, F.V.; Martin, C.V. Glyphosate Does Not Substitute for Glycine in Proteins of Actively Dividing Mammalian Cells. BMC Res. Notes 2019, 12, 494. [Google Scholar] [CrossRef]
- Martínez, M.-A.; Rodríguez, J.-L.; Lopez-Torres, B.; Martínez, M.; Martínez-Larrañaga, M.-R.; Maximiliano, J.-E.; Anadón, A.; Ares, I. Use of Human Neuroblastoma SH-SY5Y Cells to Evaluate Glyphosate-Induced Effects on Oxidative Stress, Neuronal Development and Cell Death Signaling Pathways. Environ. Int. 2020, 135, 105414. [Google Scholar] [CrossRef] [PubMed]
- El-Shenawy, N.S. Oxidative Stress Responses of Rats Exposed to Roundup and Its Active Ingredient Glyphosate. Environ. Toxicol. Pharmacol. 2009, 28, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Arno, M.; Costanzo, M.; Malatesta, M.; Séralini, G.-E.; Antoniou, M.N. Transcriptome Profile Analysis Reflects Rat Liver and Kidney Damage Following Chronic Ultra-Low Dose Roundup Exposure. Environ. Health 2015, 14, 70. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.; Dabhade, P.; Kumarasamy, A. Inflammatory Effects of Subacute Exposure of Roundup in Rat Liver and Adipose Tissue. Dose-Response 2019, 17, 155932581984338. [Google Scholar] [CrossRef] [Green Version]
- Arango Duque, G.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [Green Version]
- Oboki, K.; Ohno, T.; Kajiwara, N.; Arae, K.; Morita, H.; Ishii, A.; Nambu, A.; Abe, T.; Kiyonari, H.; Matsumoto, K.; et al. IL-33 Is a Crucial Amplifier of Innate Rather than Acquired Immunity. Proc. Natl. Acad. Sci. USA 2010, 107, 18581–18586. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Khodoun, M.; Kettleson, E.M.; McKnight, C.; Reponen, T.; Grinshpun, S.A.; Adhikari, A. Glyphosate-Rich Air Samples Induce IL-33, TSLP and Generate IL-13 Dependent Airway Inflammation. Toxicology 2014, 325, 42–51. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA). Conclusion on the Peer Review of the Pesticide Risk Assessment of the Active Substance Glyphosate. EFSA J. 2015, 13, 3973. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Fu, H.; Zhou, R.; Yang, Z.; Bai, G.; Shi, B. Toxic Effects of Glyphosate on Intestinal Morphology, Antioxidant Capacity and Barrier Function in Weaned Piglets. Ecotoxicol. Environ. Saf. 2020, 187, 109846. [Google Scholar] [CrossRef]
- Söderholm, J.D.; Perdue, M.H., II. Stress and Intestinal Barrier Function. Am. J. Physiol.-Gastrointest. Liver Physiol. 2001, 280, G7–G13. [Google Scholar] [CrossRef] [PubMed]
- Ilboudo, S.; Fouche, E.; Rizzati, V.; Toé, A.M.; Gamet-Payrastre, L.; Guissou, P.I. In Vitro Impact of Five Pesticides Alone or in Combination on Human Intestinal Cell Line Caco-2. Toxicol. Rep. 2014, 1, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Velasques, R.R.; Sandrini, J.Z.; da Rosa, C.E. Roundup® in Zebrafish: Effects on Oxidative Status and Gene Expression. Zebrafish 2016, 13, 432–441. [Google Scholar] [CrossRef]
- Mendler, A.; Geier, F.; Haange, S.-B.; Pierzchalski, A.; Krause, J.L.; Nijenhuis, I.; Froment, J.; Jehmlich, N.; Berger, U.; Ackermann, G.; et al. Mucosal-Associated Invariant T-Cell (MAIT) Activation Is Altered by Chlorpyrifos- and Glyphosate-Treated Commensal Gut Bacteria. J. Immunotoxicol. 2020, 17, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Abbas, A.K.; Fausto, N.; Aster, J.C. Robbins e Cotran—Le Basi Patologiche Delle Malattie: Vol. 1 Patologia Generale—Vol. 2 Malattie Degli Organi e Degli Apparati; Elsevier Health Sciences Italy: London, UK, 2011; ISBN 978-88-214-3385-6. [Google Scholar]
- Corsini, E.; Sokooti, M.; Galli, C.L.; Moretto, A.; Colosio, C. Pesticide Induced Immunotoxicity in Humans: A Comprehensive Review of the Existing Evidence. Toxicology 2013, 307, 123–135. [Google Scholar] [CrossRef]
- Mokarizadeh, A.; Faryabi, M.R.; Rezvanfar, M.A.; Abdollahi, M. A Comprehensive Review of Pesticides and the Immune Dysregulation: Mechanisms, Evidence and Consequences. Toxicol. Mech. Methods 2015, 25, 258–278. [Google Scholar] [CrossRef] [PubMed]
- Lioi, M.B.; Scarfi, M.R.; Santoro, A.; Barbieri, R.; Zeni, O.; Salvemini, F.; Di Berardino, D.; Ursini, M.V. Cytogenetic Damage and Induction of Pro-Oxidant State in Human Lymphocytes Exposed In Vitro to Gliphosate, Vinclozolin, Atrazine, and DPX-E9636. Environ. Mol. Mutagen. 1998, 32, 39–46. [Google Scholar] [CrossRef]
- Lioi, M.B.; Scarfi, M.R.; Santoro, A.; Barbieri, R.; Zeni, O.; Di Berardino, D.; Ursini, M.V. Genotoxicity and Oxidative Stress Induced by Pesticide Exposure in Bovine Lymphocyte Cultures In Vitro. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1998, 403, 13–20. [Google Scholar] [CrossRef]
- Jacobsen-Pereira, C.H.; Cardoso, C.C.; Gehlen, T.C.; Regina dos Santos, C.; Santos-Silva, M.C. Immune Response of Brazilian Farmers Exposed to Multiple Pesticides. Ecotoxicol. Environ. Saf. 2020, 202, 110912. [Google Scholar] [CrossRef]
- Barbasz, A.; Kreczmer, B.; Skórka, M.; Czyżowska, A. Toxicity of Pesticides toward Human Immune Cells U-937 and HL-60. J. Environ. Sci. Health Part B 2020, 55, 719–725. [Google Scholar] [CrossRef]
- Cattani, D.; Cesconetto, P.A.; Tavares, M.K.; Parisotto, E.B.; De Oliveira, P.A.; Rieg, C.E.H.; Leite, M.C.; Prediger, R.D.S.; Wendt, N.C.; Razzera, G.; et al. Developmental Exposure to Glyphosate-Based Herbicide and Depressive-like Behavior in Adult Offspring: Implication of Glutamate Excitotoxicity and Oxidative Stress. Toxicology 2017, 387, 67–80. [Google Scholar] [CrossRef]
- Šiviková, K.; Dianovský, J. Cytogenetic Effect of Technical Glyphosate on Cultivated Bovine Peripheral Lymphocytes. Int. J. Hyg. Environ. Health 2006, 209, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Christmann, M.; Kaina, B. Transcriptional Regulation of Human DNA Repair Genes Following Genotoxic Stress: Trigger Mechanisms, Inducible Responses and Genotoxic Adaptation. Nucleic Acids Res. 2013, 41, 8403–8420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woźniak, E.; Sicińska, P.; Michałowicz, J.; Woźniak, K.; Reszka, E.; Huras, B.; Zakrzewski, J.; Bukowska, B. The Mechanism of DNA Damage Induced by Roundup 360 PLUS, Glyphosate and AMPA in Human Peripheral Blood Mononuclear Cells—Genotoxic Risk Assessement. Food Chem. Toxicol. 2018, 120, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Santovito, A.; Ruberto, S.; Gendusa, C.; Cervella, P. In Vitro Evaluation of Genomic Damage Induced by Glyphosate on Human Lymphocytes. Environ. Sci. Pollut. Res. 2018, 25, 34693–34700. [Google Scholar] [CrossRef]
- Basu, S.; Je, G.; Kim, Y.-S. Transcriptional Mutagenesis by 8-OxodG in α-Synuclein Aggregation and the Pathogenesis of Parkinson’s Disease. Exp. Mol. Med. 2015, 47, e179. [Google Scholar] [CrossRef] [Green Version]
- Ladd-Acosta, C.; Feinberg, J.I.; Brown, S.C.; Lurmann, F.W.; Croen, L.A.; Hertz-Picciotto, I.; Newschaffer, C.J.; Feinberg, A.P.; Fallin, M.D.; Volk, H.E. Epigenetic Marks of Prenatal Air Pollution Exposure Found in Multiple Tissues Relevant for Child Health. Environ. Int. 2019, 126, 363–376. [Google Scholar] [CrossRef]
- Gao, X.; Colicino, E.; Shen, J.; Kioumourtzoglou, M.-A.; Just, A.C.; Nwanaji-Enwerem, J.C.; Coull, B.; Lin, X.; Vokonas, P.; Zheng, Y.; et al. Impacts of Air Pollution, Temperature, and Relative Humidity on Leukocyte Distribution: An Epigenetic Perspective. Environ. Int. 2019, 126, 395–405. [Google Scholar] [CrossRef]
- Woźniak, E.; Reszka, E.; Jabłońska, E.; Balcerczyk, A.; Broncel, M.; Bukowska, B. Glyphosate Affects Methylation in the Promoter Regions of Selected Tumor Suppressors as Well as Expression of Major Cell Cycle and Apoptosis Drivers in PBMCs (In Vitro Study). Toxicol. In Vitro 2020, 63, 104736. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Reszka, E.; Woźniak, K.; Jabłońska, E.; Michałowicz, J.; Bukowska, B. DNA Damage and Methylation Induced by Glyphosate in Human Peripheral Blood Mononuclear Cells (In Vitro Study). Food Chem. Toxicol. 2017, 105, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Hardell, L.; Eriksson, M.; Nordström, M. Exposure to Pesticides as Risk Factor for Non-Hodgkin’s Lymphoma and Hairy Cell Leukemia: Pooled Analysis of Two Swedish Case-Control Studies. Leuk. Lymphoma 2002, 43, 1043–1049. [Google Scholar] [CrossRef]
- Gasnier, C.; Dumont, C.; Benachour, N.; Clair, E.; Chagnon, M.-C.; Séralini, G.-E. Glyphosate-Based Herbicides Are Toxic and Endocrine Disruptors in Human Cell Lines. Toxicology 2009, 262, 184–191. [Google Scholar] [CrossRef]
- Koller, V.J.; Fürhacker, M.; Nersesyan, A.; Mišík, M.; Eisenbauer, M.; Knasmueller, S. Cytotoxic and DNA-Damaging Properties of Glyphosate and Roundup in Human-Derived Buccal Epithelial Cells. Arch. Toxicol. 2012, 86, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Mañas, F.; Peralta, L.; Raviolo, J.; Ovando, H.G.; Weyers, A.; Ugnia, L.; Cid, M.G.; Larripa, I.; Gorla, N. Genotoxicity of Glyphosate Assessed by the Comet Assay and Cytogenetic Tests. Environ. Toxicol. Pharmacol. 2009, 28, 37–41. [Google Scholar] [CrossRef]
- Bao, X.; Wu, J.; Xie, Y.; Kim, S.; Michelhaugh, S.; Jiang, J.; Mittal, S.; Sanai, N.; Li, J. Protein Expression and Functional Relevance of Efflux and Uptake Drug Transporters at the Blood–Brain Barrier of Human Brain and Glioblastoma. Clin. Pharmacol. Ther. 2020, 107, 1116–1127. [Google Scholar] [CrossRef]
- Emery, I.F.; Gopalan, A.; Wood, S.; Chow, K.; Battelli, C.; George, J.; Blaszyk, H.; Florman, J.; Yun, K. Expression and Function of ABCG2 and XIAP in Glioblastomas. J. Neurooncol. 2017, 133, 47–57. [Google Scholar] [CrossRef]
- Doğanlar, O.; Doğanlar, Z.B.; Kurtdere, A.K.; Chasan, T.; Ok, E.S. Chronic Exposure of Human Glioblastoma Tumors to Low Concentrations of a Pesticide Mixture Induced Multidrug Resistance against Chemotherapy Agents. Ecotoxicol. Environ. Saf. 2020, 202, 110940. [Google Scholar] [CrossRef]
- De Almeida, L.K.S.; Pletschke, B.I.; Frost, C.L. Moderate Levels of Glyphosate and Its Formulations Vary in Their Cytotoxicity and Genotoxicity in a Whole Blood Model and in Human Cell Lines with Different Estrogen Receptor Status. 3 Biotech 2018, 8, 438. [Google Scholar] [CrossRef]
- Thongprakaisang, S.; Thiantanawat, A.; Rangkadilok, N.; Suriyo, T.; Satayavivad, J. Glyphosate Induces Human Breast Cancer Cells Growth via Estrogen Receptors. Food Chem. Toxicol. 2013, 59, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, Z.R.; Ahammad, M.U.; Benson, A.P. Glyphosate-Based Herbicide Formulations and Reproductive Toxicity in Animals. Vet. Anim. Sci. 2020, 10, 100126. [Google Scholar] [CrossRef] [PubMed]
- Pierantoni, R.; Cobellis, G.; Meccariello, R.; Fasano, S. Evolutionary Aspects of Cellular Communication in the Vertebrate Hypothalamo–Hypophysio–Gonadal Axis. In International Review of Cytology; Elsevier: Amsterdam, The Netherlands, 2002; Volume 218, pp. 69–141, 142e–143e. ISBN 978-0-12-364622-4. [Google Scholar]
- Santoro, A.; Chianese, R.; Troisi, J.; Richards, S.; Nori, S.L.; Fasano, S.; Guida, M.; Plunk, E.; Viggiano, A.; Pierantoni, R.; et al. Neuro-Toxic and Reproductive Effects of BPA. Curr. Neuropharmacol. 2019, 17, 1109–1132. [Google Scholar] [CrossRef]
- Santoro, A.; Mele, E.; Marino, M.; Viggiano, A.; Nori, S.L.; Meccariello, R. The Complex Interplay between Endocannabinoid System and the Estrogen System in Central Nervous System and Periphery. Int. J. Mol. Sci. 2021, 22, 972. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Meccariello, R. Microplastics: A Threat for Male Fertility. Int. J. Environ. Res. Public Health 2021, 18, 2392. [Google Scholar] [CrossRef]
- D’Angelo, S.; Mele, E.; Di Filippo, F.; Viggiano, A.; Meccariello, R. Sirt1 Activity in the Brain: Simultaneous Effects on Energy Homeostasis and Reproduction. Int. J. Environ. Res. Public Health 2021, 18, 1243. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Troisi, J.; Richards, S.; Scafuro, M.; Fasano, S.; Guida, M.; Pierantoni, R.; Meccariello, R. Bisphenol A in Reproduction: Epigenetic Effects. Curr. Med. Chem. 2018, 25, 748–770. [Google Scholar] [CrossRef]
- Ingaramo, P.I.; Varayoud, J.; Milesi, M.M.; Schimpf, M.G.; Muñoz-de-Toro, M.; Luque, E.H. Effects of Neonatal Exposure to a Glyphosate-Based Herbicide on Female Rat Reproduction. Reproduction 2016, 152, 403–415. [Google Scholar] [CrossRef] [Green Version]
- Ingaramo, P.I.; Varayoud, J.; Milesi, M.M.; Guerrero Schimpf, M.; Alarcón, R.; Muñoz-de-Toro, M.; Luque, E.H. Neonatal Exposure to a Glyphosate-Based Herbicide Alters Uterine Decidualization in Rats. Reprod. Toxicol. 2017, 73, 87–95. [Google Scholar] [CrossRef]
- Guerrero Schimpf, M.; Milesi, M.M.; Ingaramo, P.I.; Luque, E.H.; Varayoud, J. Neonatal Exposure to a Glyphosate Based Herbicide Alters the Development of the Rat Uterus. Toxicology 2017, 376, 2–14. [Google Scholar] [CrossRef]
- Ingaramo, P.I.; Guerrero Schimpf, M.; Milesi, M.M.; Luque, E.H.; Varayoud, J. Acute Uterine Effects and Long-Term Reproductive Alterations in Postnatally Exposed Female Rats to a Mixture of Commercial Formulations of Endosulfan and Glyphosate. Food Chem. Toxicol. 2019, 134, 110832. [Google Scholar] [CrossRef]
- Fu, H.; Gao, F.; Wang, X.; Tan, P.; Qiu, S.; Shi, B.; Shan, A. Effects of Glyphosate-Based Herbicide-Contaminated Diets on Reproductive Organ Toxicity and Hypothalamic-Pituitary-Ovarian Axis Hormones in Weaned Piglets. Environ. Pollut. 2021, 272, 115596. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, R.; Rivera, O.E.; Ingaramo, P.I.; Tschopp, M.V.; Dioguardi, G.H.; Milesi, M.M.; Muñoz-de-Toro, M.; Luque, E.H. Neonatal Exposure to a Glyphosate-Based Herbicide Alters the Uterine Differentiation of Prepubertal Ewe Lambs. Environ. Pollut. 2020, 265, 114874. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, R.; Ingaramo, P.I.; Rivera, O.E.; Dioguardi, G.H.; Repetti, M.R.; Demonte, L.D.; Milesi, M.M.; Varayoud, J.; Muñoz-de-Toro, M.; Luque, E.H. Neonatal Exposure to a Glyphosate-Based Herbicide Alters the Histofunctional Differentiation of the Ovaries and Uterus in Lambs. Mol. Cell. Endocrinol. 2019, 482, 45–56. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, N.; Yin, L.; Zhang, W.; Han, F.; Liu, W.; Chen, H.; Cao, J.; Liu, J. A Commercial Roundup® Formulation Induced Male Germ Cell Apoptosis by Promoting the Expression of XAF1 in Adult Mice. Toxicol. Lett. 2018, 296, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.M.; Romano, M.A.; Bernardi, M.M.; Furtado, P.V.; Oliveira, C.A. Prepubertal Exposure to Commercial Formulation of the Herbicide Glyphosate Alters Testosterone Levels and Testicular Morphology. Arch. Toxicol. 2010, 84, 309–317. [Google Scholar] [CrossRef]
- Johansson, H.K.L.; Schwartz, C.L.; Nielsen, L.N.; Boberg, J.; Vinggaard, A.M.; Bahl, M.I.; Svingen, T. Exposure to a Glyphosate-Based Herbicide Formulation, but Not Glyphosate Alone, Has Only Minor Effects on Adult Rat Testis. Reprod. Toxicol. 2018, 82, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avdatek, F.; Birdane, Y.O.; Türkmen, R.; Demirel, H.H. Ameliorative Effect of Resveratrol on Testicular Oxidative Stress, Spermatological Parameters and DNA Damage in Glyphosate-Based Herbicide-Exposed Rats. Andrologia 2018, 50, e13036. [Google Scholar] [CrossRef]
- Dai, P.; Hu, P.; Tang, J.; Li, Y.; Li, C. Effect of Glyphosate on Reproductive Organs in Male Rat. Acta Histochem. 2016, 118, 519–526. [Google Scholar] [CrossRef]
- Mutwedu, V.B.; Nyongesa, A.W.; Azine, P.C.; Chiregereza, D.K.; Ngoumtsop, V.H.; Mugumaarhahama, Y.; Ayagirwe, R.B.B. Growth Performance and Reproductive Function Impairment of Glyphosate-based Herbicide in Male Guinea Pig (Cavia Porcellus). Vet. Med. Sci. 2021, 7, 1047–1055. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Xu, D.-Q.; Feng, X.-Z. The Toxic Effects and Possible Mechanisms of Glyphosate on Mouse Oocytes. Chemosphere 2019, 237, 124435. [Google Scholar] [CrossRef]
- Cao, M.; Wang, Y.; Yang, F.; Li, J.; Qin, X. Melatonin Rescues the Reproductive Toxicity of Low-dose Glyphosate-based Herbicide during Mouse Oocyte Maturation via the GPER Signaling Pathway. J. Pineal Res. 2021, 70, e12718. [Google Scholar] [CrossRef] [PubMed]
- Spinaci, M.; Nerozzi, C.; Tamanini, C.l.; Bucci, D.; Galeati, G. Glyphosate and Its Formulation Roundup Impair Pig Oocyte Maturation. Sci. Rep. 2020, 10, 12007. [Google Scholar] [CrossRef]
- Clair, É.; Mesnage, R.; Travert, C.; Séralini, G.-É. A Glyphosate-Based Herbicide Induces Necrosis and Apoptosis in Mature Rat Testicular Cells In Vitro, and Testosterone Decrease at Lower Levels. Toxicol. In Vitro 2012, 26, 269–279. [Google Scholar] [CrossRef]
- de Liz Oliveira Cavalli, V.L.; Cattani, D.; Heinz Rieg, C.E.; Pierozan, P.; Zanatta, L.; Benedetti Parisotto, E.; Wilhelm Filho, D.; Mena Barreto Silva, F.R.; Pessoa-Pureur, R.; Zamoner, A. Roundup Disrupts Male Reproductive Functions by Triggering Calcium-Mediated Cell Death in Rat Testis and Sertoli Cells. Free Radic. Biol. Med. 2013, 65, 335–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanlaeys, A.; Dubuisson, F.; Seralini, G.-E.; Travert, C. Formulants of Glyphosate-Based Herbicides Have More Deleterious Impact than Glyphosate on TM4 Sertoli Cells. Toxicol. In Vitro 2018, 52, 14–22. [Google Scholar] [CrossRef]
- Walsh, L.P.; McCormick, C.; Martin, C.; Stocco, D.M. Roundup Inhibits Steroidogenesis by Disrupting Steroidogenic Acute Regulatory (StAR) Protein Expression. Environ. Health Perspect. 2000, 108, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Nerozzi, C.; Recuero, S.; Galeati, G.; Bucci, D.; Spinaci, M.; Yeste, M. Effects of Roundup and Its Main Component, Glyphosate, upon Mammalian Sperm Function and Survival. Sci. Rep. 2020, 10, 11026. [Google Scholar] [CrossRef]
- Anifandis, G.; Amiridis, G.; Dafopoulos, K.; Daponte, A.; Dovolou, E.; Gavriil, E.; Gorgogietas, V.; Kachpani, E.; Mamuris, Z.; Messini, C.; et al. The In Vitro Impact of the Herbicide Roundup on Human Sperm Motility and Sperm Mitochondria. Toxics 2017, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Anifandis, G.; Katsanaki, K.; Lagodonti, G.; Messini, C.; Simopoulou, M.; Dafopoulos, K.; Daponte, A. The Effect of Glyphosate on Human Sperm Motility and Sperm DNA Fragmentation. Int. J. Environ. Res. Public Health 2018, 15, 1117. [Google Scholar] [CrossRef] [Green Version]
- Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Toxicity of Formulants and Heavy Metals in Glyphosate-Based Herbicides and Other Pesticides. Toxicol. Rep. 2018, 5, 156–163. [Google Scholar] [CrossRef]
- Richard, S.; Moslemi, S.; Sipahutar, H.; Benachour, N.; Seralini, G.-E. Differential Effects of Glyphosate and Roundup on Human Placental Cells and Aromatase. Environ. Health Perspect. 2005, 113, 716–720. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Yang, X.; Li, X.; Li, H.; Wang, S.; Wu, Z.; Yu, M.; Ma, S.; Tang, S. Low-Dose Roundup Induces Developmental Toxicity in Bovine Preimplantation Embryos In Vitro. Environ. Sci. Pollut. Res. 2020, 27, 16451–16459. [Google Scholar] [CrossRef]
- de Araújo-Ramos, A.T.; Passoni, M.T.; Romano, M.A.; Romano, R.M.; Martino-Andrade, A.J. Controversies on Endocrine and Reproductive Effects of Glyphosate and Glyphosate-Based Herbicides: A Mini-Review. Front. Endocrinol. 2021, 12, 627210. [Google Scholar] [CrossRef]
- Rossetti, M.F.; Canesini, G.; Lorenz, V.; Milesi, M.M.; Varayoud, J.; Ramos, J.G. Epigenetic Changes Associated With Exposure to Glyphosate-Based Herbicides in Mammals. Front. Endocrinol. 2021, 12, 671991. [Google Scholar] [CrossRef]
- Ruuskanen, S.; Rainio, M.J.; Uusitalo, M.; Saikkonen, K.; Helander, M. Effects of Parental Exposure to Glyphosate-Based Herbicides on Embryonic Development and Oxidative Status: A Long-Term Experiment in a Bird Model. Sci. Rep. 2020, 10, 6349. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, B.B.; Nascimento, N.F.; Santos, M.P.; Bertolini, R.M.; Yasui, G.S.; Giaquinto, P.C. Low Concentrations of Glyphosate-Based Herbicide Cause Complete Loss of Sperm Motility of Yellowtail Tetra Fish Astyanax Lacustris: HERBICIDE KILLS A. LACUSTRIS SPERM CELLS. J. Fish. Biol. 2018, 92, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Zebral, Y.D.; Costa, P.G.; de Castro Knopp, B.; Lansini, L.R.; Zafalon-Silva, B.; Bianchini, A.; Robaldo, R.B. Effects of a Glyphosate-Based Herbicide in Pejerrey Odontesthes Humensis Embryonic Development. Chemosphere 2017, 185, 860–867. [Google Scholar] [CrossRef]
- Zebral, Y.D.; Lansini, L.R.; Costa, P.G.; Roza, M.; Bianchini, A.; Robaldo, R.B. A Glyphosate-Based Herbicide Reduces Fertility, Embryonic Upper Thermal Tolerance and Alters Embryonic Diapause of the Threatened Annual Fish Austrolebias Nigrofasciatus. Chemosphere 2018, 196, 260–269. [Google Scholar] [CrossRef] [PubMed]
- García-Espiñeira, M.; Tejeda-Benitez, L.; Olivero-Verbel, J. Toxicity of Atrazine- and Glyphosate-Based Formulations on Caenorhabditis Elegans. Ecotoxicol. Environ. Saf. 2018, 156, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, P.; Saibene, M.; Bacchetta, R.; Mantecca, P.; Colombo, A. A Glyphosate Micro-Emulsion Formulation Displays Teratogenicity in Xenopus Laevis. Aquat. Toxicol. 2018, 195, 103–113. [Google Scholar] [CrossRef]
- de Melo, M.S.; Nazari, E.M.; Joaquim-Justo, C.; Muller, Y.M.R.; Gismondi, E. Effects of Low Glyphosate-Based Herbicide Concentrations on Endocrine-Related Gene Expression in the Decapoda Macrobrachium Potiuna. Environ. Sci. Pollut. Res. 2019, 26, 21535–21545. [Google Scholar] [CrossRef] [PubMed]
- Davico, C.E.; Pereira, A.G.; Nezzi, L.; Jaramillo, M.L.; de Melo, M.S.; Müller, Y.M.R.; Nazari, E.M. Reproductive Toxicity of Roundup WG® Herbicide: Impairments in Ovarian Follicles of Model Organism Danio Rerio. Environ. Sci. Pollut. Res. 2021, 28, 15147–15159. [Google Scholar] [CrossRef] [PubMed]
- Ruuskanen, S.; Rainio, M.J.; Gómez-Gallego, C.; Selenius, O.; Salminen, S.; Collado, M.C.; Saikkonen, K.; Saloniemi, I.; Helander, M. Glyphosate-Based Herbicides Influence Antioxidants, Reproductive Hormones and Gut Microbiome but Not Reproduction: A Long-Term Experiment in an Avian Model. Environ. Pollut. 2020, 266, 115108. [Google Scholar] [CrossRef] [PubMed]
- Teleken, J.L.; Gomes, E.C.Z.; Marmentini, C.; Moi, M.B.; Ribeiro, R.A.; Balbo, S.L.; Amorim, E.M.P.; Bonfleur, M.L. Glyphosate-Based Herbicide Exposure during Pregnancy and Lactation Malprograms the Male Reproductive Morphofunction in F1 Offspring. J. Dev. Orig. Health Dis. 2020, 11, 146–153. [Google Scholar] [CrossRef]
- Pham, T.H.; Derian, L.; Kervarrec, C.; Kernanec, P.-Y.; Jégou, B.; Smagulova, F.; Gely-Pernot, A. Perinatal Exposure to Glyphosate and a Glyphosate-Based Herbicide Affect Spermatogenesis in Mice. Toxicol. Sci. 2019, 169, 260–271. [Google Scholar] [CrossRef]
- Panza, S.B.; Vargas, R.; Balbo, S.L.; Bonfleur, M.L.; Granzotto, D.C.T.; Sant’Ana, D.M.G.; Nogueira-Melo, G.A. Perinatal Exposure to Low Doses of Glyphosate-Based Herbicide Combined with a High-Fat Diet in Adulthood Causes Changes in the Jejunums of Mice. Life Sci. 2021, 275, 119350. [Google Scholar] [CrossRef]
- Dallegrave, E.; Mantese, F.D.; Oliveira, R.T.; Andrade, A.J.M.; Dalsenter, P.R.; Langeloh, A. Pre- and Postnatal Toxicity of the Commercial Glyphosate Formulation in Wistar Rats. Arch. Toxicol. 2007, 81, 665–673. [Google Scholar] [CrossRef]
- Milesi, M.M.; Lorenz, V.; Pacini, G.; Repetti, M.R.; Demonte, L.D.; Varayoud, J.; Luque, E.H. Perinatal Exposure to a Glyphosate-Based Herbicide Impairs Female Reproductive Outcomes and Induces Second-Generation Adverse Effects in Wistar Rats. Arch. Toxicol. 2018, 92, 2629–2643. [Google Scholar] [CrossRef]
- Lorenz, V.; Pacini, G.; Luque, E.H.; Varayoud, J.; Milesi, M.M. Perinatal Exposure to Glyphosate or a Glyphosate-Based Formulation Disrupts Hormonal and Uterine Milieu during the Receptive State in Rats. Food Chem. Toxicol. 2020, 143, 111560. [Google Scholar] [CrossRef]
- Manservisi, F.; Lesseur, C.; Panzacchi, S.; Mandrioli, D.; Falcioni, L.; Bua, L.; Manservigi, M.; Spinaci, M.; Galeati, G.; Mantovani, A.; et al. The Ramazzini Institute 13-Week Pilot Study Glyphosate-Based Herbicides Administered at Human-Equivalent Dose to Sprague Dawley Rats: Effects on Development and Endocrine System. Environ. Health 2019, 18, 15. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, V.; Milesi, M.M.; Schimpf, M.G.; Luque, E.H.; Varayoud, J. Epigenetic Disruption of Estrogen Receptor Alpha Is Induced by a Glyphosate-Based Herbicide in the Preimplantation Uterus of Rats. Mol. Cell. Endocrinol. 2019, 480, 133–141. [Google Scholar] [CrossRef]
- Ait-Bali, Y.; Ba-M’hamed, S.; Gambarotta, G.; Sassoè-Pognetto, M.; Giustetto, M.; Bennis, M. Pre- and Postnatal Exposure to Glyphosate-Based Herbicide Causes Behavioral and Cognitive Impairments in Adult Mice: Evidence of Cortical Ad Hippocampal Dysfunction. Arch. Toxicol. 2020, 94, 1703–1723. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, C.E.; Bartos, M.; Bras, C.; Gumilar, F.; Antonelli, M.C.; Minetti, A. Exposure to a Glyphosate-Based Herbicide during Pregnancy and Lactation Induces Neurobehavioral Alterations in Rat Offspring. NeuroToxicology 2016, 53, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, C.E.; Baier, C.J.; Bartos, M.; Bras, C.; Domínguez, S.; Mónaco, N.; Gumilar, F.; Giménez, M.S.; Minetti, A. Perinatal Glyphosate-Based Herbicide Exposure in Rats Alters Brain Antioxidant Status, Glutamate and Acetylcholine Metabolism and Affects Recognition Memory. Neurotox. Res. 2018, 34, 363–374. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.S.; Laureano-Melo, R.; Herai, R.H.; da Conceição, R.R.; Oliveira, K.C.; da Silva, I.D.C.G.; Dias-da-Silva, M.R.; Romano, R.M.; Romano, M.A.; Maciel, R.M.d.B.; et al. Maternal Glyphosate-Based Herbicide Exposure Alters Antioxidant-Related Genes in the Brain and Serum Metabolites of Male Rat Offspring. NeuroToxicology 2019, 74, 121–131. [Google Scholar] [CrossRef]
- Gomez, A.L.; Altamirano, G.A.; Leturia, J.; Bosquiazzo, V.L.; Muñoz-de-Toro, M.; Kass, L. Male Mammary Gland Development and Methylation Status of Estrogen Receptor Alpha in Wistar Rats Are Modified by the Developmental Exposure to a Glyphosate-Based Herbicide. Mol. Cell. Endocrinol. 2019, 481, 14–25. [Google Scholar] [CrossRef]
- Gomez, A.L.; Altamirano, G.A.; Tschopp, M.V.; Bosquiazzo, V.L.; Muñoz-de-Toro, M.; Kass, L. Exposure to a Glyphosate-Based Herbicide Alters the Expression of Key Regulators of Mammary Gland Development on Pre-Pubertal Male Rats. Toxicology 2020, 439, 152477. [Google Scholar] [CrossRef]
- de Souza, J.S.; Kizys, M.M.L.; da Conceição, R.R.; Glebocki, G.; Romano, R.M.; Ortiga-Carvalho, T.M.; Giannocco, G.; da Silva, I.D.C.G.; Dias da Silva, M.R.; Romano, M.A.; et al. Perinatal Exposure to Glyphosate-Based Herbicide Alters the Thyrotrophic Axis and Causes Thyroid Hormone Homeostasis Imbalance in Male Rats. Toxicology 2017, 377, 25–37. [Google Scholar] [CrossRef]
- Coullery, R.; Pacchioni, A.M.; Rosso, S.B. Exposure to Glyphosate during Pregnancy Induces Neurobehavioral Alterations and Downregulation of Wnt5a-CaMKII Pathway. Reprod. Toxicol. 2020, 96, 390–398. [Google Scholar] [CrossRef]
- Dechartres, J.; Pawluski, J.L.; Gueguen, M.; Jablaoui, A.; Maguin, E.; Rhimi, M.; Charlier, T.D. Glyphosate and Glyphosate-based Herbicide Exposure during the Peripartum Period Affects Maternal Brain Plasticity, Maternal Behaviour and Microbiome. J. Neuroendocrinol. 2019, 31, e12731. [Google Scholar] [CrossRef]
- Smith, C.M.; Vera, M.K.M.; Bhandari, R.K. Developmental and Epigenetic Effects of Roundup and Glyphosate Exposure on Japanese Medaka (Oryzias Latipes). Aquat. Toxicol. 2019, 210, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Makris, S.L. Current Assessment of the Effects of Environmental Chemicals on the Mammary Gland in Guideline Rodent Studies by the U.S. Environmental Protection Agency (U.S. EPA), Organisation for Economic Co-Operation and Development (OECD), and National Toxicology Program (NTP). Environ. Health Perspect. 2011, 119, 1047–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swan, S.H.; Kristensen, D.M. Anogenital Distance: A Marker of Steroidal Endocrine Disruption. In Encyclopedia of Reproduction; Elsevier: Amsterdam, The Netherlands, 2018; pp. 588–593. ISBN 978-0-12-815145-7. [Google Scholar]
- Lesseur, C.; Pirrotte, P.; Pathak, K.V.; Manservisi, F.; Mandrioli, D.; Belpoggi, F.; Panzacchi, S.; Li, Q.; Barrett, E.S.; Nguyen, R.H.N.; et al. Maternal Urinary Levels of Glyphosate during Pregnancy and Anogenital Distance in Newborns in a US Multicenter Pregnancy Cohort. Environ. Pollut. 2021, 280, 117002. [Google Scholar] [CrossRef] [PubMed]




| Experiment | Species/Cell Types | Treatment | Effects | Reference |
|---|---|---|---|---|
| In vivo | Newborn female rats | Subcutaneous injection: 2 mg/kg/day GBH (66% glyphosate in potassium salt) on PND1, 3, 5 and 7 | ↑Number of resorption sites on GD19, associated with altered decidualization response Morphological changes at the implantation site ↓Estrogen and progesterone receptors (ER and PR) ↓COUP-TFII (Nr2f2) and Bmp2 mRNA ↑HOXA10 and Ki67 | [80] |
| In vivo | Newborn female rats | Subcutaneous injection: 2 mg/kg/day GBHs on PND1 to 7 | Disturbed uterine signaling (Wnt5a, β-catenin, Wnt7a, Dkk1 and sFRP4) during gestation | [81] |
| In vivo | Newborn female rats | Subcutaneous injection: 2 mg/kg/day GBH on PND1 to 7 | ↑LE hyperplasia ↑Stromal and myometrial thickness ↑Proliferation and endometrial hyperplasia Altered expression of proteins involved in uterine organogenetic differentiation (i.e., PR and Hoxa10, and ERα) | [82] |
| In vivo | Female Wistar rats (pups) | Subcutaneous injection: Endosulfan (600 μg/kg bw/day), GBHs (2 mg/kg b.w/day) or a mixture (mix) from PND1 to 7 | GBHs and mix: ↑Incidence of luminal epithelial hyperplasia ↑PR and Hoxa10 expression ↑Post-implantation losses during adulthood Endosulfan: Modified ERα and Hoxa10 expression. ↑Pre-implantation losses | [83] |
| In vivo | Female weaned piglets | Glyphosate concentrations 10, 20, and 40 mg/kg into the feed | No significant effect on vulvar size and reproductive organs Altered tissue morphology and ultrastructure in uterus and ovary ↑Oxidative stress in uterus ↑LHRH/GnRH ↑Testosterone ↓FSH | [84] |
| In vivo | Prepubertal female ewe lambs | Oral and subcutaneous exposure to a GBHs (2 mg/kg/day) from PND1 to PND14 | PND45: Altered follicular dynamics ↑Proliferation of granulosa and theca cells ↓FSHR and GDF9 mRNA ↓Proliferation in the uterus | [85] |
| In vivo | Female Friesian ewe lambs | GBHs (2 mg/kg/day) through subcutaneous. injections from PND1 to PND14 | PND45 (uterus): ↓Cell proliferation ↑p27 ↑Insulin-like growth factor binding protein 3 ↓ERα in the LE and GE and in the SS ↓PR expression in the LE ↑PR in the GE and SS ↓Gene expression in the uterus (i.e., Wnt5a in the GE, Wnt7a in the SS, β-catenin in the LE and GE, Hoxa10 in the SS, and Foxa2 in the GE) | [86] |
| In vivo | 8-weeks-old male Kunming mice | Gavage: Roundup, 60, 180, 540 mg/kg | Impaired spermatogenesis, ↓Sperm motility and concentration ↑Sperm deformity rate ↑Apoptosis of germ cells with mechanism involving the over-expression of the X-linked inhibitor of apoptosis-associated factor 1 (XAF1) | [87] |
| In vivo | Prepubertal male Wistar rats | Oral gavage: 5, 50 or 250 mg/kg bw glyphosate-Roundup Transorb from PND23 to PND53 | Dose dependent changes in spermatogenesis progression ↓Seminiferous epithelium height ↓Serum levels of testosterone | [88] |
| In vivo | 4 weeks-old male Sprague-Dawley rats | Oral gavage: two weeks exposure to either glyphosate (2.5 and 25 mg/kg bw/day) or herbicide formulation Glyfonova | Glyfonova: Slight increase in the expression of the steroidogenic genes Cyp11a1 and Cyp17a1 | [89] |
| In vivo | Male Wistar rats (12 weeks old) | Dietary administration: 375 mg/kg/day glyphosate ± 20 mg/kg/day resveratrol | ↓Sperm motility ↓Sperm plasma membrane integrity ↓Glutathione level ↓Superoxide dismutase ↑Abnormal sperm rate ↑Malondialdehyde level ↑DNA damage All the effects were reversed by resveratrol co-administration | [90] |
| In vivo | Male Sprague Dawley rats | Glyphosate 5, 50, 500 mg/kg by gavage | ↓Average daily feed intake at dose of (50 mg/kg dose) ↓Weight of seminal vesicle gland and coagulating gland (500 mg/kg dose). ↓Total sperm count (500 mg/kg dose) No effects on testosterone, estradiol, progesterone and oxidative stress parameters | [91] |
| In vivo | Sexually mature male guinea pigs | Oral exposure: Willosate 186, 280 and 560 mg/kg daily for 60 days | ↓Sperm motility, viability and concentration ↑Sperm morphological alterations | [92] |
| In vitro | Mouse Oocytes | 500 μM Glyphosate | ↓Germinal vesicle breakdown and first polar body extrusion Abnormal spindle morphology and DNA double-strand breaks ↑Oxydative stress ↑Mitochondria aggregation ↓Mitochondria membrane potential ↓Expression levels of autophagy-related genes (lc3, atg14, mTor) and proteins (LC3, Atg12) | [93] |
| In vitro and in vivo | Mouse oocytes | In vitro: 0.00001%, 0.00005%, or 0.00025% GBHs ± melatonin (10 and 100 μM) In vivo: GBHs (0.0005% Roundup solution) daily administered in drinking water for 21 days ± melatonin (0, 0.15, and 1.5 mg/kg bw), once a day through intragastric administration | Impaired oocytes meiotic maturation ↓First polar body extrusion, disorganized spindle morphology, misaligned chromosomes, and ROS production ↑Apoptosis rate ↓Sperm-binding ability and disrupted early embryo cleavage GBHs effects were reversed by in vitro/in vivo melatonin treatment with mechanisms involving the membrane GPER | [94] |
| In vitro | Pig oocytes | 0, 5, 10, 100, 200 and 360 µg/mL Glyphosate or Roundup at the same glyphosate -equivalent doses | Glyphosate: No effect on nuclear maturation and embryo cleavage, impaired oocyte developmental competence in terms of blastocyst rate and cellularity Roundup: more toxic than pure glyphosate, altered steroidogenesis ↑ROS levels | [95] |
| In vitro | Rat isolated testicular cells and co-colture of germ cells-Sertoli cells | Glyphosate and Roundup: 1–10,000 ppm, from 1 to 48 h | Leydig cells: damaged (Roundup, 1–48 h) Sertoli cells: toxic effect (glyphosate alone) Germ cells: necrosis (Roundup, 24–48 h) and apoptosis (high doses) Co-colture assay: apoptosis of Sertoli cells and germ cells (at high doses) ↓Testosterone levels (Roundup and glyphosate 1 ppm) | [96] |
| In vitro | Sertoli cells from PND30 (prepubertal) Wistar rats | Acute Roundup exposure at low doses (36 ppm or 0.036 g/L) for 30 min | Endoplasmic reticulum stress Depletion of antioxidant defences Cell death | [97] |
| In vitro | Immature Sertoli cell line (TM4) | Commercially availableGBHs: Genamin T200 (732 g/L Polyethoxylated tallowamine, 60–80% POE (15) tallowamine (POE-15)); Glyphogan (360 g/L of glyphosate); Roundup Bioforce (360 g/L of glyphosate) | Mitochondrial dysfunction Disruption of cell detoxification systems Lipid droplet accumulation Mortality at sub-agricultural doses Formulants have more deleterious effects than glyphosate | [98] |
| In vitro | MA-10 Leydig cells | Roundup (180 g/L glyphosate) | Inhibition of dibutyryl [(Bu)(2)]cAMP-stimulated progesterone production ↓Activity of Aromatase No effect on the activitiy of 3β-HSD ↓Steroidogenesis by disrupting StAR protein expression | [99] |
| In vitro | Pig semen | 0–360 µg/mL glyphosate or Roundup | Glyphosate: ↓Sperm motility, viability, mitochondrial activity ↓Acrosome integrity Roundup: ↓Sperm motility (≥5 µg/mL glyphosate-equivalent concentration) ↓Mitochondrial activity (25 µg/mL glyphosate-equivalent concentration) ↓Sperm viability and acrosome integrity (≥100 µg/mL glyphosate-equivalent concentration) | [100] |
| In vitro | Human sperm (n = 66 healthy men) | 1 mg/L Roundup | ↓Sperm motility and mitochondrial dysfunction | [101] |
| In vitro | Human sperm (n = 30 healthy men) | 0.36 mg/L glyphosate | ↓Sperm progressive motility (1 h post-treatment) | [102] |
| In vitro | Human cell lines: (JEG3 placental cell lines, HUVEC primary neonate umbilical cord vein, and 293 embryonic kidney HEK293) | GBHs in Roundup formulations | Cell death within 24 h in all cell lines | [18] |
| In vitro | Human cell lines (JEG3 placental cell lines and HEK293) | Glyphosate alone and in 14 ot its formulations | Toxic effects ↓Ativity of Aromatase | [103] |
| In vitro | Human JEG3 placental cell lines | 0.05–2% glyphosate and Roundup (360 g/L glyphosate) | Toxic effects with concentrations lower than those found with agricultural use ↓Aromatase activity | [104] |
| In vitro | Bovine preimplantation embryos | Roundup 0.01~2% (36~7200 ppm, containing 36~7200 mg/L glyphosate) | 0.01~2% Roundup doses are toxic to bovine embryos | [105] |
| In vitro | Bovine preimplantation embryos | Roundup 0, 0.45, 0.9, and 1.8 ppm | ↑Intracellular calcium levels (2-cells embryo) ↑Oxidative stress (2-cells embryos) ↑Apoptosis (bovine blastocysts) | [105] |
| Species | Dams’ Treatment | Exposure Route | Effects on Dams and Litter Size | Effects on F1 (Males) | Effects on F1 (Females) | Reference |
|---|---|---|---|---|---|---|
| Mouse | 0.5% glyphosate-Roundup from GD4 all over lactation period | Drinking water | Reduced bw gain during gestation no effects on litter size | Delayed testicular descent PND150: ↓SPZ in cauda epididymis ↓Epithelial height within the seminiferous epithelium ↑LH in plasma ↑Intratesticular testosterone levels | NA | [117] |
| Mouse | 0.5, 5 and 50 mg/kg/day glyphosate or Roundup 3 Plus from ED10.5 to 20 PND | Drinking water | NA | PND20: Altered testis morphology (glyphosate) PND35: ↓Serum testosterone levels (glyphosate) ↓SPZ, (0.5 mg/kg/day Roundup and 5 mg/kg/day glyphosate) ↓Undifferentiated spermatogonia (5 mg/kg/day glyphosate) 8-month-old animals: ↓testosterone (GBHs) | NA | [118] |
| Mice | 0.5% glyphosate from GD1 until 30 days after birth | Drinking water | NA | ↑Risk of jejunum inflammation and dysfunction in adulthood when combined with a high-fat diet | NA | [119] |
| Wistar rats | 0, 50, 150 or 450 mg/kg glyphosate during pregnancy and lactation | Drinking water | NA | Puberty: ↓Serum testosterone levels Adulthood: ↓Sperm number in epididymis tail ↓Daily sperm production ↑Abnormal sperms ↑Spermatid degeneration | Delay in Vaginal canal-opening | [120] |
| Wistar rat | 2 mg or 200 mg of glyphosate/kg bw/day from GD9 until weaning | Food | NA | NA | F1: No alteration in bw gain or vaginal opening onset ↓Implantation sites F2: Delayed growth ↓Foetal weight and length ↑Incidence of small for gestational age foetuses ↑Placental weight and placental index structural ↑Congenital anomalies like conjoined foetuses and abnormally developed limbs | [121] |
| Rats | GBHs (containing 66.2% of glyphosate potassium salt) or glyphosate (2 mg/kg/day) from GD9 until weaning | Orally | NA | NA | ↓Preimplantation ↑17β-oestradiol serum level ↑ERα in the uterus ↓PR mRNA (glyphosate) ↓Uterine implantation-related genes (i.e., Hoxa10 and Lif) | [122] |
| Sprague Dawley rats | Glyphosate alone and Roundup Bio flow, 1.75 mg/kg bw/day from GD6 up to PND120 | Drinking water | NA | PND4: ↑AGD (all treatments) ADULTS: ↑plasma TSH (glyphosate) ↓DHT (Roundup) ↑BDNF (Roundup) | PND4: ↑AGD (all treatments) ADULTS: Age at first oestrous significantly delayed (Roundup) ↑Serum testosterone (Roundup) | [123] |
| Rats | 350 mg glyphosate/kg bw/day from GD9 until weaning | Food | NA | NA | ↑ERα-O mRNA variant in uterus Epigenetic changes in the Esr1-O promoter (i.e., ↓DNA methylation ↑Histone H4 acetylation ↑Histone H3 lysine 9 trimethylation (H3K9me3) ↓H3K27me3) | [124] |
| Mouse | GBHs (250 or 500 mg/kg) from GD0 to PND21 | Oral gavage | Impaired maternal behaviour fertility and reproduction | Global delay in innate reflexes and a deficit in motor development Hippocampal dysfunctions with behavioural and cognitive impairment | [125] | |
| Wistar rats | 0.65 or 1.30 g/L of glyphosate from GD0, until weaning (PND21) | Drinking water | NA | Neurobehavioral alterations (i.e., early onset of cliff aversion reflex and early auditory canal opening, decrease in locomotor activity and in anxiety levels) | [126] | |
| Wistar rats | 0.65 and 1.30 g/L of pure glyphosate from GD0, until weaning (PND21) | Drinking water | NA | Alterations in brain oxidative stress biomarkers and glutamatergic and cholinergic systems | [127] | |
| Wistar rats | 5 and 50 mg/kg/day Roundup, (per os) from GD18 to PND5 | Drinking water | NA | Altered expression of genes associated with oxidant defence, inflammation and lipid metabolism | [128] | |
| Wistar rats | 1% GBH (0.36% glyphosate) from GD5 until PND15 or PND60 | Drinking water | NA | Oxidative stress and depressive-like behaviour at PND60 Impaired cholinergic and glutamatergic neurotransmission at PND15 and PND60 Altered serum levels of the astrocytic protein S100B at PND15 and PND60 | [54] | |
| Rats | GBH (66.2% glyphosate in potassium salt) 3.5 or 350 mg/kg bw/day GD9 until weaning | Orally exposed through the food | NA | PND21: No differences in mammary gland development or in oestradiol and testosterone levels PND60, GBHs 3.5 mg/kg/day exposed animals: ↑AR protein expression PND60, GBHs 350 mg/kg/day exposed males: ↓Proliferation index and less developed mammary gland ↑PRL serum levels Both exposed groups: ↓ESR1 expression by means of hypermethylation of ESR1 promoter | [129] | |
| Rats | GBH (66.2% glyphosate in potassium salt) 3.5 or 350 mg/kg bw/day from GD9 until weaning | Orally exposed through the food | NA | ↓Proliferation index in GBHs 3.5-exposed animals ↓mRNA levels of ESR1, Ccnd1, Areg, IGF1, EGFR and IGF1R ↓p-Erk1/2 protein | [130] | |
| Wistar rats | 5 mg/kg/day or 50 mg/kg/day Roundup from GD18 to PND5 | Oral gavage | NA | ↓Deiodinases 2 (Dio2) and 3 (Dio3) and TH transporters Slco1c1 and Slc16a2 mRNA within the hypothalamus ↑Dio2, thyroid hormone receptor genes (Thra1 and Thrb1), and Slc16a2 within the pituitary. ↑Thra1 and Thrb1 mRNA in the liver ↑Dio2, Mb, Myh6 and Slc2a4 mRNA expression in the heart | [131] | |
| Wistar rats | 1% Roundup (0.38% glyphosate) from GD5 and up to lactation day 15 | Drinking water | NA | Excitotoxicity and oxidative stress in rat hippocampus | [31] | |
| Wistar rats | Pure glyphosate (24 or 35 mg/kg) every 48 h from ED8 until ED20, every 48 h | Intraperitoneal injections | NA | Dose dependent changes in reflexes development, motor activity and cognitive function, via inhibition of Wnt5a-CaMKII signalling pathway. | [132] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, M.; Mele, E.; Viggiano, A.; Nori, S.L.; Meccariello, R.; Santoro, A. Pleiotropic Outcomes of Glyphosate Exposure: From Organ Damage to Effects on Inflammation, Cancer, Reproduction and Development. Int. J. Mol. Sci. 2021, 22, 12606. https://doi.org/10.3390/ijms222212606
Marino M, Mele E, Viggiano A, Nori SL, Meccariello R, Santoro A. Pleiotropic Outcomes of Glyphosate Exposure: From Organ Damage to Effects on Inflammation, Cancer, Reproduction and Development. International Journal of Molecular Sciences. 2021; 22(22):12606. https://doi.org/10.3390/ijms222212606
Chicago/Turabian StyleMarino, Marianna, Elena Mele, Andrea Viggiano, Stefania Lucia Nori, Rosaria Meccariello, and Antonietta Santoro. 2021. "Pleiotropic Outcomes of Glyphosate Exposure: From Organ Damage to Effects on Inflammation, Cancer, Reproduction and Development" International Journal of Molecular Sciences 22, no. 22: 12606. https://doi.org/10.3390/ijms222212606
APA StyleMarino, M., Mele, E., Viggiano, A., Nori, S. L., Meccariello, R., & Santoro, A. (2021). Pleiotropic Outcomes of Glyphosate Exposure: From Organ Damage to Effects on Inflammation, Cancer, Reproduction and Development. International Journal of Molecular Sciences, 22(22), 12606. https://doi.org/10.3390/ijms222212606








