E-Cigarette Use: Device Market, Study Design, and Emerging Evidence of Biological Consequences
Abstract
:1. Introduction
2. EC Studies: Flavor and Device Market, Populations of Interest, Preclinical Study Design, and Current Challenges
2.1. Variability in Device Design
2.2. Variability in E-Liquid Formulation
2.3. Current Regulatory Proceedings
2.4. ECA Inhalation Study Design
2.5. Human ECA Exposure Studies: Populations of Interest
2.6. Rodent ECA Exposure Studies
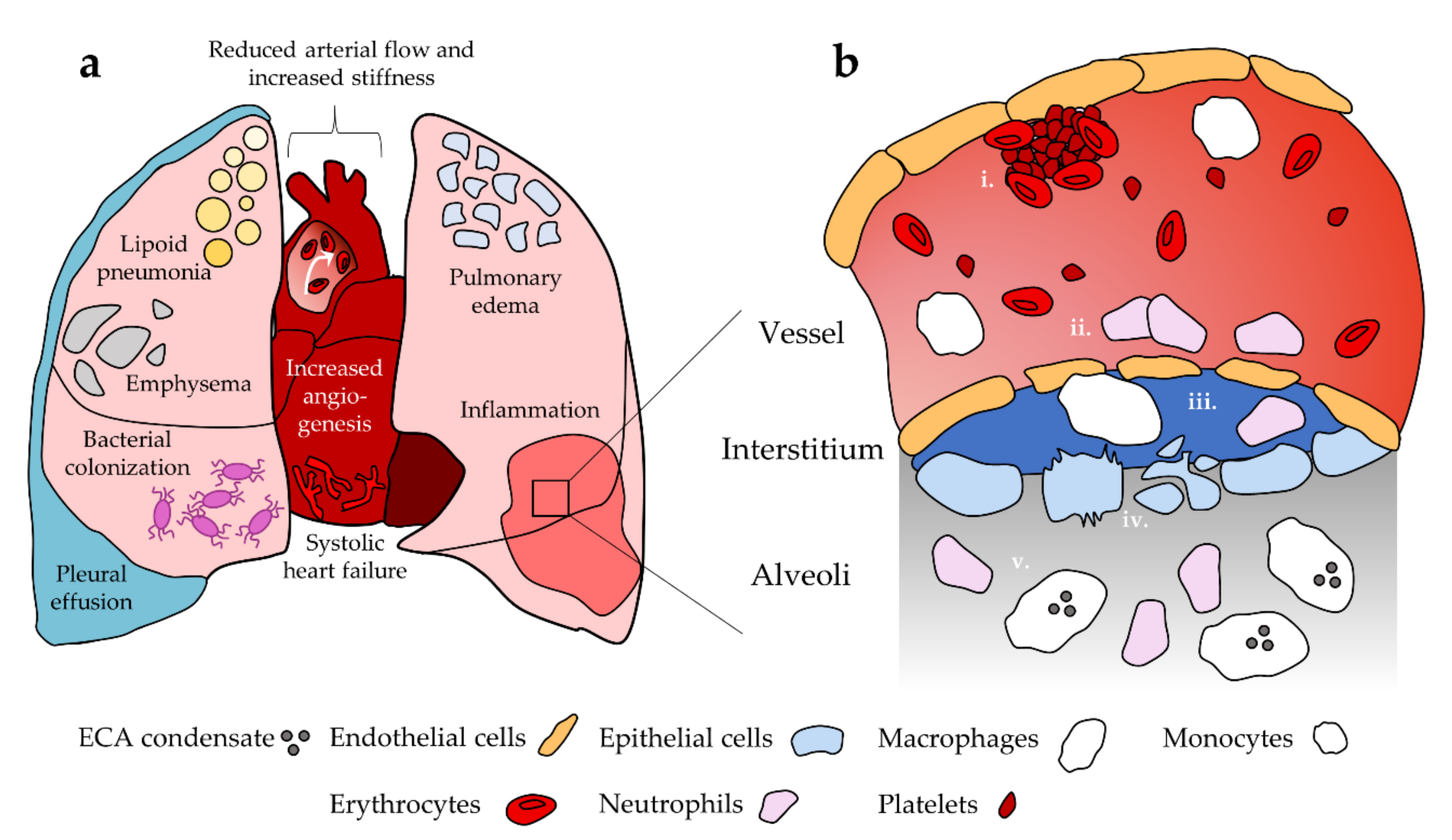
3. Pulmonary Effects of EC Use
3.1. Acute Lung Injury
3.2. Susceptibility to Infection and Chronic Damage Patterns
4. Cardiovascular Effects of EC Use
5. Cellular Effects of EC Use
5.1. Oxidative Stress
5.2. Epithelial Cells
5.3. Endothelial Cells
5.4. Platelets
5.5. Macrophages
5.6. Neutrophils
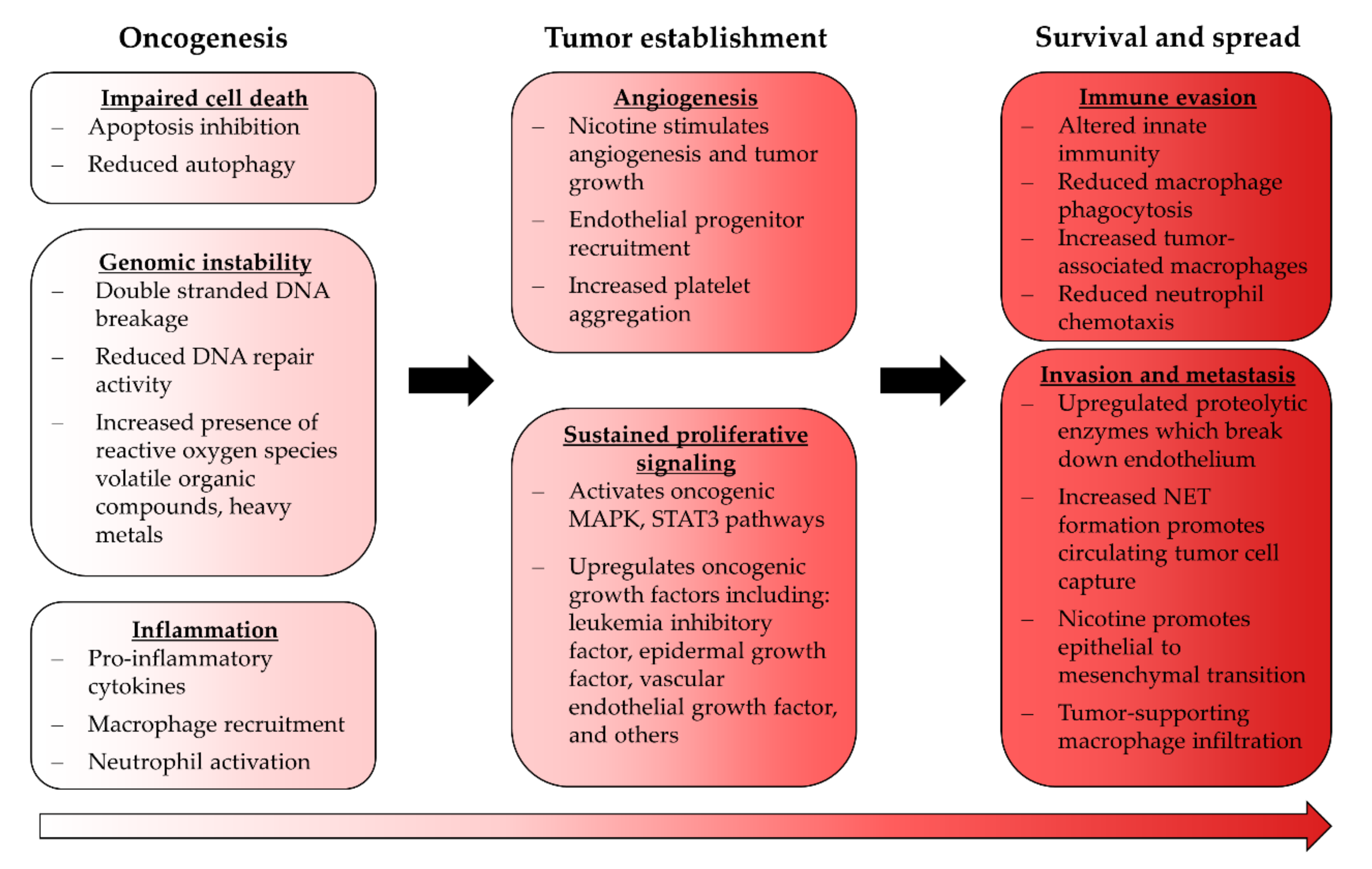
6. Cancer and EC Use
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, H.; Davis, A.H.; Dohack, J.L.; Clark, P.I. E-Cigarettes Use Behavior and Experience of Adults: Qualitative Research Findings to Inform E-Cigarette Use Measure Development. Nicotine Tob. Res. 2016, 19, 190–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helen, G.S.; Ross, K.C.; Dempsey, D.A.; Havel, C.M.; Jacob, P.; Benowitz, N.L. Nicotine Delivery and Vaping Behavior during ad libitum E-cigarette Access. Tob. Regul. Sci. 2016, 2, 363–376. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Cignarella, A.; Diaz, L.V.L.; Balestrini, K.; Holt, G.; Mirsaeidi, M.; Calderon-Candelario, R.; Whitney, P.; Salathe, M.; Campos, M.A. Differences in vaping topography in relation to adherence to exclusive electronic cigarette use in veterans. PLoS ONE 2018, 13, e0195896. [Google Scholar] [CrossRef] [Green Version]
- Miliano, C.; Scott, E.R.; Murdaugh, L.B.; Gnatowski, E.R.; Faunce, C.L.; Anderson, M.S.; Reyes, M.M.; Gregus, A.M.; Buczynski, M.W. Modeling drug exposure in rodents using e-cigarettes and other electronic nicotine delivery systems. J. Neurosci. Methods 2019, 330, 108458. [Google Scholar] [CrossRef]
- Geiss, O.; Bianchi, I.; Barrero-Moreno, J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int. J. Hyg. Environ. Health 2016, 219, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.A.; Olmedo, P.; Navas-Acien, A.; Goessler, W.; Cohen, J.E.; Rule, A.M. E-cigarettes as a source of toxic and potentially carcinogenic metals. Environ. Res. 2016, 152, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Fowles, J.; Barreau, T.; Wu, N. Cancer and Non-Cancer Risk Concerns from Metals in Electronic Cigarette Liquids and Aerosols. Int. J. Environ. Res. Public Health 2020, 17, 2146. [Google Scholar] [CrossRef] [Green Version]
- Farsalinos, K.E.; Romagna, G.; Tsiapras, D.; Kyrzopoulos, S.; Voudris, V. Evaluation of Electronic Cigarette Use (Vaping) Topography and Estimation of Liquid Consumption: Implications for Research Protocol Standards Definition and for Public Health Authorities’ Regulation. Int. J. Environ. Res. Public Health 2013, 10, 2500–2514. [Google Scholar] [CrossRef]
- Yingst, J.M.; Veldheer, S.; Hrabovsky, S.; Nichols, T.T.; Wilson, S.J.; Foulds, J. Factors Associated with Electronic Cigarette Users’ Device Preferences and Transition from First Generation to Advanced Generation Devices. Nicotine Tob. Res. 2015, 17, 1242–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hitchman, S.C.; Brose, L.S.; Brown, J.; Robson, D.; McNeill, A. Associations Between E-Cigarette Type, Frequency of Use, and Quitting Smoking: Findings from a Longitudinal Online Panel Survey in Great Britain. Nicotine Tob. Res. 2015, 17, 1187–1194. [Google Scholar] [CrossRef]
- Wagener, T.L.; Floyd, E.L.; Stepanov, I.; Driskill, L.M.; Frank, S.G.; Meier, E.; Leavens, E.L.; Tackett, A.P.; Molina, N.; Queimado, L. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob. Control 2017, 26, e23–e28. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, Z.T.; Goel, R.; Reilly, S.M.; Foulds, J.; Muscat, J.; Elias, R.J.; Richie, J.P., Jr. Effects of solvent and temperature on free radical formation in electronic cigarette aerosols. Chem. Res. Toxicol. 2018, 31, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Lechasseur, A.; Altmejd, S.; Turgeon, N.; Buonanno, G.; Morawska, L.; Brunet, D.; Duchaine, C.; Morissette, M.C. Variations in coil temperature/power and e-liquid constituents change size and lung deposition of particles emitted by an electronic cigarette. Physiol. Rep. 2019, 7, e14093. [Google Scholar] [CrossRef]
- Jackler, R.K.; Ramamurthi, D. Nicotine arms race: JUUL and the high-nicotine product market. Tob. Control 2019, 28, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Rao, P.; Liu, J.; Springer, M.L. JUUL and Combusted Cigarettes Comparably Impair Endothelial Function. Tob. Regul. Sci. 2020, 6, 30–37. [Google Scholar] [CrossRef]
- Reilly, S.M.; Bitzer, Z.T.; Goel, R.; Trushin, N.; Richie, J.P., Jr. Free radical, carbonyl, and nicotine levels produced by juul electronic cigarettes. Nicotine Tob. Res. 2019, 21, 1274–1278. [Google Scholar] [CrossRef]
- Rastian, B. Quantification of the Transfer Mechanism of Potentially Harmful Heavy Metals to the Inhaled Aerosol Particles Generated by an Electronic Cigarette. Masters’ Thesis, California State University, Fullerton, CA, USA, 2020. [Google Scholar]
- Zhu, S.-H.; Sun, J.Y.; Bonnevie, E.; Cummins, S.E.; Gamst, A.; Yin, L.; Lee, M. Four hundred and sixty brands of e-cigarettes and counting: Implications for product regulation. Tob. Control 2014, 23, iii3–iii9. [Google Scholar] [CrossRef] [Green Version]
- Havermans, A.; Krüsemann, E.J.Z.; Pennings, J.L.; de Graaf, K.; Boesveldt, S.; Talhout, R. Nearly 20 000 e-liquids and 250 unique flavour descriptions: An overview of the Dutch market based on information from manufacturers. Tob. Control 2021, 30, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Raymond, B.H.; Collette-Merrill, K.; Harrison, R.G.; Jarvis, S.; Rasmussen, R.J. The Nicotine Content of a Sample of E-cigarette Liquid Manufactured in the United States. J. Addict. Med. 2018, 12, 127–131. [Google Scholar] [CrossRef]
- Grana, R.; Benowitz, N.; Glantz, S.A. E-cigarettes: A scientific review. Circulation 2014, 129, 1972–1986. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Jabba, S.V.; Dewinter, T.M.; Mendizabal, M.; Anastas, P.T.; Jordt, S.E.; Zimmerman, J.B. Formation of flavorant–propylene Glycol Adducts with Novel Toxicological Properties in Chemically Unstable E-Cigarette Liquids. Nicotine Tob. Res. 2019, 21, 1248–1258. [Google Scholar] [CrossRef]
- Khlystov, A.; Samburova, V. Flavoring Compounds Dominate Toxic Aldehyde Production during E-Cigarette Vaping. Environ. Sci. Technol. 2016, 50, 13080–13085. [Google Scholar] [CrossRef]
- Klager, S.; Vallarino, J.; Macnaughton, P.; Christiani, D.C.; Lu, Q.; Allen, J.G. Flavoring Chemicals and Aldehydes in E-Cigarette Emissions. Environ. Sci. Technol. 2017, 51, 10806–10813. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-W.; Park, S.-H.; Weng, M.-W.; Wang, H.-T.; Huang, W.; Lepor, H.; Wu, X.-R.; Chen, L.C.; Tang, M.-S. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1560–E1569. [Google Scholar] [CrossRef] [Green Version]
- Omaiye, E.E.; McWhirter, K.J.; Luo, W.; Tierney, P.A.; Pankow, J.F.; Talbot, P. High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci. Rep. 2019, 9, 2468. [Google Scholar] [CrossRef] [Green Version]
- Jensen, R.P.; Luo, W.; Pankow, J.F.; Strongin, R.M.; Peyton, D.H. Hidden Formaldehyde in E-Cigarette Aerosols. N. Engl. J. Med. 2015, 372, 392–394. [Google Scholar] [CrossRef] [Green Version]
- Farsalinos, K.E.; Voudris, V.; Poulas, K. E-cigarettes generate high levels of aldehydes only in ‘dry puff’conditions. Addiction 2015, 110, 1352–1356. [Google Scholar] [CrossRef]
- Ogunwale, M.A.; Li, M.; Raju, M.V.R.; Chen, Y.; Nantz, M.H.; Conklin, D.J.; Fu, X.-A. Aldehyde Detection in Electronic Cigarette Aerosols. ACS Omega 2017, 2, 1207–1214. [Google Scholar] [CrossRef]
- Salamanca, J.C.; Meehan-Atrash, J.; Vreeke, S.; Escobedo, J.O.; Peyton, D.H.; Strongin, R.M. E-cigarettes can emit formaldehyde at high levels under conditions that have been reported to be non-averse to users. Sci. Rep. 2018, 8, 7559. [Google Scholar] [CrossRef]
- Lerner, C.A.; Sundar, I.K.; Yao, H.; Gerloff, J.; Ossip, D.J.; McIntosh, S.; Robinson, R.; Rahman, I. Vapors Produced by Electronic Cigarettes and E-Juices with Flavorings Induce Toxicity, Oxidative Stress, and Inflammatory Response in Lung Epithelial Cells and in Mouse Lung. PLoS ONE 2015, 10, e0116732. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, J.; Sundar, I.K.; Freter, R.; Sekera, E.R.; Friedman, A.E.; Robinson, R.; Pagano, T.; Rahman, I. Inflammatory Response and Barrier Dysfunction by Different e-Cigarette Flavoring Chemicals Identified by Gas Chromatography–Mass Spectrometry in e-Liquids and e-Vapors on Human Lung Epithelial Cells and Fibroblasts. Appl. Vitr. Toxicol. 2017, 3, 28–40. [Google Scholar] [CrossRef]
- Jabba, S.V.; Diaz, A.N.; Erythropel, H.C.; Zimmerman, J.B.; Jordt, S.-E. Chemical Adducts of Reactive Flavor Aldehydes Formed in E-Cigarette Liquids Are Cytotoxic and Inhibit Mitochondrial Function in Respiratory Epithelial Cells. Nicotine Tob. Res. 2020, 22, S25–S34. [Google Scholar] [CrossRef] [PubMed]
- Grana, R.A.; Ling, P.M. “Smoking revolution”: A content analysis of electronic cigarette retail websites. Am. J. Prev. Med. 2014, 46, 395–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutzler, C.; Paschke, M.; Kruschinski, S.; Henkler, F.; Hahn, J.; Luch, A. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch. Toxicol. 2014, 88, 1295–1308. [Google Scholar] [CrossRef]
- Tierney, P.A.; Karpinski, C.D.; Brown, J.E.; Luo, W.; Pankow, J.F. Flavour chemicals in electronic cigarette fluids. Tob. Control 2016, 25, e10–e15. [Google Scholar] [CrossRef] [Green Version]
- Kavvalakis, M.P.; Stivaktakis, P.D.; Tzatzarakis, M.N.; Kouretas, D.; Liesivuori, J.; Alegakis, A.K.; Vynias, D.; Tsatsakis, A.M. Multicomponent Analysis of Replacement Liquids of Electronic Cigarettes Using Chromatographic Techniques. J. Anal. Toxicol. 2015, 39, 262–269. [Google Scholar] [CrossRef]
- Goniewicz, M.; Hajek, P.; McRobbie, H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: Regulatory implications. Addiction 2014, 109, 500–507. [Google Scholar] [CrossRef]
- Peace, M.R.; Baird, T.R.; Smith, N.; Wolf, C.E.; Poklis, J.L.; Poklis, A. Concentration of Nicotine and Glycols in 27 Electronic Cigarette Formulations. J. Anal. Toxicol. 2016, 40, 403–407. [Google Scholar] [CrossRef] [Green Version]
- Djordjevic, M.V.; Hoffmann, D.; Hoffmanna, I. Nicotine Regulates Smoking Patterns. Prev. Med. 1997, 26, 435–440. [Google Scholar] [CrossRef]
- Shao, X.M.; Friedman, T.C. Pod-mod vs. conventional e-cigarettes: Nicotine chemistry, pH, and health effects. J. Appl. Physiol. 2020, 128, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Lugg, S.T.; Aldridge, K.; Lewis, K.E.; Bowden, A.; Mahida, R.; Grudzinska, F.; Dosanjh, D.; Parekh, D.; Foronjy, R.; et al. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax 2018, 73, 1161–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, S.; Leigh, N.J.; Vanderbush, T.S.; Choo, E.; Goniewicz, M.L.; Dawkins, L. An exploration into “do-it-yourself”(DIY) e-liquid mixing: Users’ motivations, practices and product laboratory analysis. Addict. Behav. Rep. 2019, 9, 100151. [Google Scholar] [CrossRef]
- Stephenson, J. FDA Orders Many e-Cigarette Products Off the Market, but Delays Decision on Largest Manufacturers. JAMA Health Forum 2021, 2, e213531. [Google Scholar] [CrossRef]
- Kalkhoran, S.; A Glantz, S. E-cigarettes and smoking cessation in real-world and clinical settings: A systematic review and meta-analysis. Lancet Respir. Med. 2016, 4, 116–128. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.J.; Bhadriraju, S.; Glantz, S.A. E-Cigarette Use and Adult Cigarette Smoking Cessation: A Meta-Analysis. Am. J. Public Health 2021, 111, 230–246. [Google Scholar] [CrossRef]
- Watson, N.L.; Mull, K.E.; Bricker, J.B. The association between frequency of e-cigarette use and long-term smoking cessation outcomes among treatment-seeking smokers receiving a behavioral intervention. Drug Alcohol Depend. 2020, 218, 108394. [Google Scholar] [CrossRef]
- Teriba, A.; Mbama, U.; Sharma, S.; Abraham, A.; Ndefo, U.A. Evidence against e-cigarettes for smoking cessation. J. Am. Pharm. Assoc. 2021, 61, e55–e58. [Google Scholar] [CrossRef]
- Barrington-Trimis, J.L.; Gibson, L.; Halpern-Felsher, B.; Harrell, M.B.; Kong, G.; Krishnan-Sarin, S.; Leventhal, A.M.; Loukas, A.; McConnell, R.; Weaver, S.R. Type of E-Cigarette Device Used Among Adolescents and Young Adults: Findings from a Pooled Analysis of Eight Studies of 2166 Vapers. Nicotine Tob. Res. 2018, 20, 271–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, K.; Alzghoul, B.; Innabi, A.; Meena, N. Is vaping a gateway to smoking: A review of the longitudinal studies. Int. J. Adolesc. Med. Health 2016, 30, 20160033. [Google Scholar] [CrossRef]
- Goldenson, N.; Leventhal, A.M.; Stone, M.D.; McConnell, R.S.; Barrington-Trimis, J.L. Associations of Electronic Cigarette Nicotine Concentration with Subsequent Cigarette Smoking and Vaping Levels in Adolescents. JAMA Pediatr. 2017, 171, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Miech, R.; Patrick, M.; O’Malley, P.; Johnston, L. E-cigarette use as a predictor of cigarette smoking: Results from a 1-year follow-up of a national sample of 12th grade students. Tob. Control 2017, 26, e106–e111. [Google Scholar] [CrossRef]
- Levy, D.T.; Warner, K.E.; Cummings, K.M.; Hammond, D.; Kuo, C.; Fong, G.T.; Thrasher, J.F.; Goniewicz, M.; Borland, R. Examining the relationship of vaping to smoking initiation among US youth and young adults: A reality check. Tob. Control 2019, 28, 629–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, X.M.; Lopez, B.; Nathan, D.; Wilson, J.; Bankole, E.; Tumoyan, H.; Munoz, A.; Espinoza-Derout, J.; Hasan, K.M.; Chang, S.; et al. A mouse model for chronic intermittent electronic cigarette exposure exhibits nicotine pharmacokinetics resembling human vapers. J. Neurosci. Methods 2019, 326, 108376. [Google Scholar] [CrossRef]
- Laube, B.L.; Afshar-Mohajer, N.; Koehler, K.; Chen, G.; Lazarus, P.; Collaco, J.M.; McGrath-Morrow, S.A. Acute and chronic in vivo effects of exposure to nicotine and propylene glycol from an E-cigarette on mucociliary clearance in a murine model. Inhal. Toxicol. 2017, 29, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Gordon, T.; Karey, E.; Rebuli, M.E.; Escobar, Y.; Jaspers, I.; Chi Chen, L. E-Cigarette Toxicology. Ann. Rev. Pharmacol. Toxicol. 2021, 62. published online ahead of print. [Google Scholar] [CrossRef]
- Eaton, D.L.; Kwan, L.Y.; Stratton, K.; National Academies of Sciences, Engineering and Medicine. Toxicology of E-Cigarette Constituents. In Public Health Consequences of E-Cigarettes; National Academies Press: Washington, DC, USA, 2018. [Google Scholar]
- Sussan, T.E.; Gajghate, S.; Thimmulappa, R.K.; Ma, J.; Kim, J.-H.; Sudini, K.; Consolini, N.; Cormier, S.; Lomnicki, S.; Hasan, F.; et al. Exposure to Electronic Cigarettes Impairs Pulmonary Anti-Bacterial and Anti-Viral Defenses in a Mouse Model. PLoS ONE 2015, 10, e0116861. [Google Scholar] [CrossRef]
- Qasim, H.; Karim, Z.A.; Silva-Espinoza, J.C.; Khasawneh, F.T.; Rivera, J.O.; Ellis, C.C.; Bauer, S.L.; Almeida, I.C.; Alshbool, F.Z. Short-Term E-Cigarette Exposure Increases the Risk of Thrombogenesis and Enhances Platelet Function in Mice. J. Am. Heart Assoc. 2018, 7, e009264. [Google Scholar] [CrossRef] [Green Version]
- Wright, C. Standardized methods for the regulation of cigarette-smoke constituents. Trends Anal. Chem. 2015, 66, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Besaratinia, A.; Tommasi, S. An opportune and unique research to evaluate the public health impact of electronic cigarettes. Cancer Causes Control 2017, 28, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, Z.T.; Goel, R.; Reilly, S.M.; Bhangu, G.; Trushin, N.; Foulds, J.; Muscat, J.; Richie, J.P., Jr. Emissions of free radicals, carbonyls, and nicotine from the NIDA Standardized Research Electronic Cigarette and comparison to similar commercial devices. Chem. Res. Toxicol. 2018, 32, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Kalininskiy, A.; Bach, C.T.; Nacca, N.E.; Ginsberg, G.; Marraffa, J.; A Navarette, K.; McGraw, M.D.; Croft, D.P. E-cigarette, or vaping, product use associated lung injury (EVALI): Case series and diagnostic approach. Lancet Respir. Med. 2019, 7, 1017–1026. [Google Scholar] [CrossRef]
- Blount, B.C.; Karwowski, M.P.; Shields, P.G.; Morel-Espinosa, M.; Valentin-Blasini, L.; Gardner, M.; Braselton, M.; Brosius, C.R.; Caron, K.T.; Chambers, D.; et al. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N. Engl. J. Med. 2020, 382, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, C.G.; Weiner, D.J.; Nowalk, A.; Larkin, A. Hypersensitivity Pneumonitis and Acute Respiratory Distress Syndrome from E-Cigarette Use. Pediatrics 2018, 141, e20163927. [Google Scholar] [CrossRef] [PubMed]
- Viswam, D.; Trotter, S.; Burge, P.S.; Walters, G.I. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. BMJ Case Rep. 2018, 2018, bcr2018224350. [Google Scholar] [CrossRef]
- Reagan-Steiner, S.; Gary, J.; Matkovic, E.; Ritter, J.M.; Shieh, W.-J.; Martines, R.B.; Werner, A.K.; Lynfield, R.; Holzbauer, S.; Bullock, H.; et al. Pathological findings in suspected cases of e-cigarette, or vaping, product use-associated lung injury (EVALI): A case series. Lancet Respir. Med. 2020, 8, 1219–1232. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Mehrad, M.; Dammert, P.; Arrossi, A.V.; Sarda, R.; Brenner, D.S.; Maldonado, F.; Choi, H.; Ghobrial, M. Lung Biopsy Findings in Severe Pulmonary Illness Associated with E-Cigarette Use (Vaping) A Report of Eight Cases. Am. J. Clin. Pathol. 2020, 153, 30–39. [Google Scholar]
- Baron, S.E.; Haramati, L.B.; Rivera, V.T. Radiological and Clinical Findings in Acute and Chronic Exogenous Lipoid Pneumonia. J. Thorac. Imaging 2003, 18, 217–224. [Google Scholar] [CrossRef]
- Allen, J.N.; Pacht, E.R.; Gadek, J.E.; Davis, W.B. Acute Eosinophilic Pneumonia as a Reversible Cause of Noninfectious Respiratory Failure. N. Engl. J. Med. 1989, 321, 569–574. [Google Scholar] [CrossRef]
- Bhat, T.A.; Kalathil, S.G.; Bogner, P.N.; Blount, B.C.; Goniewicz, M.; Thanavala, Y.M. An Animal Model of Inhaled Vitamin E Acetate and EVALI-like Lung Injury. N. Engl. J. Med. 2020, 382, 1175–1177. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, L.; Suri, R.; Dearing, E.; Mudway, I.; Dove, R.E.; Neill, D.; van Zyl-Smit, R.; Kadioglu, A.; Grigg, J. E-cigarette vapour enhances pneumococcal adherence to airway epithelial cells. Eur. Respir. J. 2018, 51, 1701592. [Google Scholar] [CrossRef] [Green Version]
- Palmer, G.C.; Whiteley, M. Metabolism and Pathogenicity of Pseudomonas aeruginosa Infections in the Lungs of Individuals with Cystic Fibrosis. Microbiol. Spectr. 2015, 3, 185–213. [Google Scholar] [CrossRef]
- Sanou, A.Z.; Ziadeh, C.; Stahlman, S.; Clausen, S.S. Acute Respiratory Infections Among Active Component Service Members Who Use Combustible Tobacco Products and/or E-cigarette/Vaping Products, U.S. Armed Forces, 2018–2019. MSMR 2020, 27, 2–7. [Google Scholar] [PubMed]
- Li, D.; Croft, D.P.; Ossip, D.J.; Xie, Z. The association between statewide vaping prevalence and COVID-19. Prev. Med. Rep. 2020, 20, 101254. [Google Scholar] [CrossRef]
- Lallai, V.; Manca, L.; Fowler, C.D. E-cigarette Vape and Lung ACE2 Expression: Implications for coronavirus vulnerability. Environ. Toxicol. Pharmacol. 2021, 86, 103656. [Google Scholar] [CrossRef] [PubMed]
- Reinikovaite, V.; Rodriguez, I.E.; Karoor, V.; Rau, A.; Trinh, B.B.; Deleyiannis, F.W.-B.; Taraseviciene-Stewart, L. The effects of electronic cigarette vapour on the lung: Direct comparison to tobacco smoke. Eur. Respir. J. 2018, 51, 1701661. [Google Scholar] [CrossRef]
- Olfert, I.M.; DeVallance, E.; Hoskinson, H.; Branyan, K.W.; Clayton, S.; Pitzer, C.R.; Sullivan, D.P.; Breit, M.J.; Wu, Z.; Klinkhachorn, P.; et al. Chronic exposure to electronic cigarettes results in impaired cardiovascular function in mice. J. Appl. Physiol. 2018, 124, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Zafar, I.; Mariappan, N.; Husain, M.; Wei, C.-C.; Vetal, N.; Eltoum, I.A.; Ahmad, A. Acute pulmonary effects of aerosolized nicotine. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L94–L104. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, S.; Vivarelli, F.; Turrini, E.; Fimognari, C.; Burattini, S.; Falcieri, E.; Rocchi, M.B.L.; Cardenia, V.; Rodriguez-Estrada, M.T.; Paolini, M.; et al. The Customizable E-cigarette Resistance Influences Toxicological Outcomes: Lung Degeneration, Inflammation, and Oxidative Stress-Induced in a Rat Model. Toxicol. Sci. 2019, 172, 132–145. [Google Scholar] [CrossRef]
- Garcia-Arcos, I.; Geraghty, P.; Baumlin, N.; Campos, M.; Dabo, A.J.; Jundi, B.; Cummins, N.; Eden, E.; Grosche, A.; Salathe, M.; et al. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax 2016, 71, 1119–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, M.; Atuegwu, N.; Mead, E.; Oncken, C.; Mortensen, E. E-cigarette use is associated with emphysema, chronic bronchitis and COPD. In D22. Cutting Edge Research in Smoking Cessation and E-Cigarettes; American Thoracic Society: San Diego, CA, USA, 2018; p. A6245. [Google Scholar]
- Perez, M.F.; Atuegwu, N.C.; Mead, E.L.; Oncken, C.; Mortensen, E.M. Adult E-Cigarettes Use Associated with a Self-Reported Diagnosis of COPD. Int. J. Environ. Res. Public Health 2019, 16, 3938. [Google Scholar] [CrossRef] [Green Version]
- Bircan, E.; Bezirhan, U.; Porter, A.; Fagan, P.; Orloff, M.S. Correction: Electronic cigarette use and its association with asthma, chronic obstructive pulmonary disease (COPD) and asthma-COPD overlap syndrome among never cigarette smokers. Tob. Induc. Dis. 2021, 19, 23. [Google Scholar] [CrossRef]
- Osei, A.D.; Mirbolouk, M.; Orimoloye, O.A.; Dzaye, O.; Uddin, S.I.; Benjamin, E.J.; Hall, M.E.; DeFilippis, A.P.; Bhatnagar, A.; Biswal, S.S.; et al. Association Between E-Cigarette Use and Chronic Obstructive Pulmonary Disease by Smoking Status: Behavioral Risk Factor Surveillance System 2016 and 2017. Am. J. Prev. Med. 2020, 58, 336–342. [Google Scholar] [CrossRef]
- Shi, H.; Fan, X.; Horton, A.; Haller, S.T.; Kennedy, D.J.; Schiefer, I.T.; Dworkin, L.; Cooper, C.J.; Tian, J. The Effect of Electronic-Cigarette Vaping on Cardiac Function and Angiogenesis in Mice. Sci. Rep. 2019, 9, 4085. [Google Scholar] [CrossRef]
- Caporale, A.; Langham, M.C.; Guo, W.; Johncola, A.; Chatterjee, S.; Wehrli, F.W. Acute Effects of Electronic Cigarette Aerosol Inhalation on Vascular Function Detected at Quantitative MRI. Radiology 2019, 293, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Sciarretta, S.; Violi, F.; Nocella, C.; Loffredo, L.; Perri, L.; Peruzzi, M.; Marullo, A.G.; De Falco, E.; Chimenti, I.; et al. Acute Impact of Tobacco vs Electronic Cigarette Smoking on Oxidative Stress and Vascular Function. Chest 2016, 150, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Derout, J.; Hasan, K.M.; Shao, X.M.; Jordan, M.C.; Sims, C.; Lee, D.L.; Sinha, S.; Simmons, Z.; Mtume, N.; Liu, Y.; et al. Chronic intermittent electronic cigarette exposure induces cardiac dysfunction and atherosclerosis in apolipoprotein-E knockout mice. Am. J. Physiol. Circ. Physiol. 2019, 317, H445–H459. [Google Scholar] [CrossRef]
- Boas, Z.; Gupta, P.; Moheimani, R.S.; Bhetraratana, M.; Yin, F.; Peters, K.M.; Gornbein, J.; Araujo, J.A.; Czernin, J.; Middlekauff, H.R. Activation of the “Splenocardiac Axis” by electronic and tobacco cigarettes in otherwise healthy young adults. Physiol. Rep. 2017, 5, e13393. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, T.; Pena, I.; Temesgen, N.; Glantz, S.A. Association between Electronic Cigarette Use and Myocardial Infarction. Am. J. Prev. Med. 2018, 55, 455–461. [Google Scholar] [CrossRef]
- Schweitzer, K.S.; Hatoum, H.; Brown, M.B.; Gupta, M.; Justice, M.J.; Beteck, B.; Van Demark, M.; Gu, Y.; Presson, R.G., Jr.; Hubbard, W.C. Mechanisms of lung endothelial barrier disruption induced by cigarette smoke: Role of oxidative stress and ceramides. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L836–L846. [Google Scholar] [CrossRef]
- Schweitzer, K.S.; Chen, S.; Law, S.; Van Demark, M.; Poirier, C.; Justice, M.J.; Hubbard, W.C.; Kim, E.S.; Lai, X.; Wang, M.; et al. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am. J. Physiol. Cell. Mol. Physiol. 2015, 309, L175–L187. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Coakley, R.D.; Ghio, A.J.; Muhlebach, M.S.; Esther, C.R., Jr.; Alexis, N.E.; Tarran, R. Chronic e-cigarette use increases neutrophil elastase and matrix metalloprotease levels in the lung. Am. J. Respir. Crit. Care Med. 2019, 200, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Clapp, P.W.; Lavrich, K.; Van Heusden, C.A.; Lazarowski, E.R.; Carson, J.L.; Jaspers, I. Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L470–L486. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.Y.; Fain, M.D.; Jackson, P.L.; Berryhill, T.F.; Wilson, L.S.; Mazur, M.; Barnes, S.J.; Blalock, J.E.; Raju, S.V.; Rowe, S.M. Vaporized E-Cigarette Liquids Induce Ion Transport Dysfunction in Airway Epithelia. Am. J. Respir. Cell Mol. Biol. 2019, 61, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Muthumalage, T.; Lamb, T.; Friedman, M.R.; Rahman, I. E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci. Rep. 2019, 9, 19035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaisar, M.A.; Villalba, H.; Prasad, S.; Liles, T.; Sifat, A.E.; Sajja, R.K.; Abbruscato, T.J.; Cucullo, L. Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: Is Metformin a viable countermeasure? Redox Biol. 2017, 13, 353–362. [Google Scholar] [CrossRef]
- Mobarrez, F.; Antoniewicz, L.; Hedman, L.; Bosson, J.A.; Lundbäck, M. Electronic cigarettes containing nicotine increase endothelial and platelet derived extracellular vesicles in healthy volunteers. Atherosclerosis 2020, 301, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, J.E.M.; Karim, Z.A.; Alarabi, A.; Hernandez, K.R.; Ben Taleb, Z.; Rivera, J.O.; Khasawneh, F.T.; Alshbool, F.Z. The JUUL E-Cigarette Elevates the Risk of Thrombosis and Potentiates Platelet Activation. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 578–586. [Google Scholar] [CrossRef]
- Clapp, P.W.; Pawlak, E.A.; Lackey, J.T.; Keating, J.E.; Reeber, S.L.; Glish, G.L.; Jaspers, I. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am. J. Physiol. Cell. Mol. Physiol. 2017, 313, L278–L292. [Google Scholar] [CrossRef] [PubMed]
- Higham, A.; Rattray, N.; Dewhurst, J.A.; Trivedi, D.; Fowler, S.; Goodacre, R.; Singh, D. Electronic cigarette exposure triggers neutrophil inflammatory responses. Respir. Res. 2016, 17, 56. [Google Scholar] [CrossRef] [Green Version]
- Glynos, C.; Bibli, S.-I.; Katsaounou, P.; Pavlidou, A.; Magkou, C.; Karavana, V.; Topouzis, S.; Kalomenidis, I.; Zakynthinos, S.; Papapetropoulos, A. Comparison of the effects of e-cigarette vapor with cigarette smoke on lung function and inflammation in mice. Am. J. Physiol. Cell. Mol. Physiol. 2018, 315, L662–L672. [Google Scholar] [CrossRef] [Green Version]
- Reidel, B.; Radicioni, G.; Clapp, P.W.; Ford, A.A.; Abdelwahab, S.; Rebuli, M.; Haridass, P.; Alexis, N.E.; Jaspers, I.; Kesimer, M. E-Cigarette Use Causes a Unique Innate Immune Response in the Lung, Involving Increased Neutrophilic Activation and Altered Mucin Secretion. Am. J. Respir. Crit. Care Med. 2018, 197, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Terauchi, K.-I.; Davis, W.H., Jr. Electron spin resonance (ESR) study of cigarette smoke by use of spin trapping techniques. Environ. Health Persp. 1976, 16, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Muthumalage, T.; Prinz, M.; Ansah, K.O.; Gerloff, J.; Sundar, I.K.; Rahman, I. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front. Physiol. 2018, 8, 1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ween, M.P.; Hamon, R.; Macowan, M.; Thredgold, L.; Reynolds, P.N.; Hodge, S.J. Effects of E-cigarette E-liquid components on bronchial epithelial cells: Demonstration of dysfunctional efferocytosis. Respirology 2020, 25, 620–628. [Google Scholar] [CrossRef]
- Anderson, C.; Majeste, A.; Hanus, J.; Wang, S. E-Cigarette Aerosol Exposure Induces Reactive Oxygen Species, DNA Damage, and Cell Death in Vascular Endothelial Cells. Toxicol. Sci. 2016, 154, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Kuntic, M.; Oelze, M.; Steven, S.; Kröller-Schön, S.; Stamm, P.; Kalinovic, S.; Frenis, K.; Vujacic-Mirski, K.; Jimenez, M.T.B.; Kvandova, M.; et al. Short-term e-cigarette vapour exposure causes vascular oxidative stress and dysfunction: Evidence for a close connection to brain damage and a key role of the phagocytic NADPH oxidase (NOX-2). Eur. Heart J. 2019, 41, 2472–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikonomidis, I.; Vlastos, D.; Kourea, K.; Kostelli, G.; Varoudi, M.; Pavlidis, G.; Efentakis, P.; Triantafyllidi, H.; Parissis, J.; Andreadou, I. Electronic cigarette smoking increases arterial stiffness and oxidative stress to a lesser extent than a single conventional cigarette: An acute and chronic study. Circulation 2018, 137, 303–306. [Google Scholar] [CrossRef]
- Hom, S.; Chen, L.; Wang, T.; Ghebrehiwet, B.; Yin, W.; Rubenstein, D.A. Platelet activation, adhesion, inflammation, and aggregation potential are altered in the presence of electronic cigarette extracts of variable nicotine concentrations. Platelets 2016, 27, 694–702. [Google Scholar] [CrossRef]
- Nocella, C.; Biondi-Zoccai, G.; Sciarretta, S.; Peruzzi, M.; Pagano, F.; Loffredo, L.; Pignatelli, P.; Bullen, C.; Frati, G.; Carnevale, R. Impact of Tobacco Versus Electronic Cigarette Smoking on Platelet Function. Am. J. Cardiol. 2018, 122, 1477–1481. [Google Scholar] [CrossRef]
- Li, J.; Huynh, L.; Cornwell, W.D.; Tang, M.-S.; Simborio, H.; Huang, J.; Kosmider, B.; Rogers, T.J.; Zhao, H.; Steinberg, M.B.; et al. Electronic Cigarettes Induce Mitochondrial DNA Damage and Trigger TLR9 (Toll-Like Receptor 9)-Mediated Atherosclerosis. Arter. Thromb. Vasc. Biol. 2021, 41, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Corriden, R.; Moshensky, A.; Bojanowski, C.M.; Meier, A.; Chien, J.; Nelson, R.K.; Alexander, L.E.C. E-cigarette use increases susceptibility to bacterial infection by impairment of human neutrophil chemotaxis, phagocytosis, and NET formation. Am. J. Physiol. Physiol. 2020, 318, C205–C214. [Google Scholar] [CrossRef]
- Wang, Q.; Khan, N.A.; Muthumalage, T.; Lawyer, G.R.; McDonough, S.R.; Chuang, T.; Gong, M.; Sundar, I.K.; Rehan, V.K.; Rahman, I. Dysregulated repair and inflammatory responses by e-cigarette-derived inhaled nicotine and humectant propylene glycol in a sex-dependent manner in mouse lung. FASEB BioAdv. 2019, 1, 609–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hecht, S.S.; Carmella, S.G.; Kotandeniya, D.; Pillsbury, M.E.; Chen, M.; Ransom, B.W.S.; Vogel, R.; Thompson, E.; Murphy, S.E.; Hatsukami, D.K. Evaluation of Toxicant and Carcinogen Metabolites in the Urine of E-Cigarette Users Versus Cigarette Smokers. Nicotine Tob. Res. 2014, 17, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Mravec, B.; Tibensky, M.; Horvathova, L.; Babal, P. E-cigarettes and cancer risk. Cancer Prev. Res. 2020, 13, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klawinski, D.; Hanna, I.; Breslin, N.K.; Katzenstein, H.M.; Indelicato, D.J. Vaping the Venom: Oral Cavity Cancer in a Young Adult with Extensive Electronic Cigarette Use. Pediatrics 2021, 147, e2020022301. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Kitzmiller, J.P.; Bui, T.C. Oral Carcinoma Associated with Chronic Use of Electronic Cigarettes. Otolaryngology 2017, 7, 1–3. [Google Scholar] [CrossRef]
- Borderud, S.P.; Li, Y.; Burkhalter, J.E.; Sheffer, C.E.; Ostroff, J.S. Electronic cigarette use among patients with cancer: Characteristics of electronic cigarette users and their smoking cessation outcomes. Cancer 2014, 120, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
- Canistro, D.; Vivarelli, F.; Cirillo, S.; Marquillas, C.B.; Buschini, A.; Lazzaretti, M.; Marchi, L.; Cardenia, V.; Rodriguez-Estrada, M.T.; Lodovici, M.; et al. E-cigarettes induce toxicological effects that can raise the cancer risk. Sci. Rep. 2017, 7, 2028. [Google Scholar] [CrossRef]
- Thorne, D.; Crooks, I.; Hollings, M.; Seymour, A.; Meredith, C.; Gaca, M. The mutagenic assessment of an electronic-cigarette and reference cigarette smoke using the Ames assay in strains TA98 and TA100. Mutat. Res. Toxicol. Environ. Mutagen. 2016, 812, 29–38. [Google Scholar] [CrossRef]
- Barhdadi, S.; Mertens, B.; Van Bossuyt, M.; Van De Maele, J.; Anthonissen, R.; Canfyn, M.; Courselle, P.; Rogiers, V.; Deconinck, E.; Vanhaecke, T. Identification of flavouring substances of genotoxic concern present in e-cigarette refills. Food Chem. Toxicol. 2021, 147, 111864. [Google Scholar] [CrossRef]
- Bracken-Clarke, D.; Kapoor, D.; Baird, A.M.; Buchanan, P.J.; Gately, K.; Cuffe, S.; Finn, S.P. Vaping and lung cancer—A review of current data and recommendations. Lung Cancer 2021, 153, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Crotty Alexander, L.E.; Drummond, C.A.; Hepokoski, M.; Mathew, D.; Moshensky, A.; Willeford, A.; Das, S.; Singh, P.; Yong, Z.; Lee, J.H.; et al. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R834–R847. [Google Scholar] [CrossRef] [Green Version]
- Merecz-Sadowska, A.; Sitarek, P.; Zielinska-Blizniewska, H.; Malinowska, K.; Zajdel, K.; Zakonnik, L.; Zajdel, R. A Summary of In Vitro and In Vivo Studies Evaluating the Impact of E-Cigarette Exposure on Living Organisms and the Environment. Int. J. Mol. Sci. 2020, 21, 652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, R.; Karantza-Wadsworth, V.; White, E. Role of autophagy in cancer. Nat. Rev. Cancer 2007, 7, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Bodas, M.; Van Westphal, C.; Carpenter-Thompson, R.; Mohanty, D.K.; Vij, N. Nicotine exposure induces bronchial epithelial cell apoptosis and senescence via ROS mediated autophagy-impairment. Free. Radic. Biol. Med. 2016, 97, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Heeschen, C.; Jang, J.J.; Weis, M.; Pathak, A.; Kaji, S.; Hu, R.S.; Tsao, P.S.; Johnson, F.L.; Cooke, J. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat. Med. 2001, 7, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.; Huynh, D.; Le, L.T.T.; Delitto, D.; Yang, L.; Huang, J.; Kang, Y.; Steinberg, M.B.; Li, J.; Zhang, L.; et al. E-cigarette promotes breast carcinoma progression and lung metastasis: Macrophage-tumor cells crosstalk and the role of CCL5 and VCAM-1. Cancer Lett. 2020, 491, 132–145. [Google Scholar] [CrossRef]
- Huynh, D.; Huang, J.; Le, L.T.T.; Liu, D.; Liu, C.; Pham, K.; Wang, H. Electronic cigarettes promotes the lung colonization of human breast cancer in NOD-SCID-Gamma mice. Int. J. Clin. Exp. Pathol. 2020, 13, 2075–2081. [Google Scholar]
- Zahedi, A.; Phandthong, R.; Chaili, A.; Remark, G.; Talbot, P. Epithelial-to-mesenchymal transition of A549 lung cancer cells exposed to electronic cigarettes. Lung Cancer 2018, 122, 224–233. [Google Scholar] [CrossRef] [PubMed]
| Type | Description | Advantages | Disadvantages |
|---|---|---|---|
| Human studies | Studies that examine EC use amongst never-smoker EC users, former-smoker EC users, non-users, or other groups of interest. | Detects evidence of acute and subclinical pathology | Difficult to control for device, e-liquid characteristics, and frequency of use |
| Best physiologic and clinical relevance | Heavily dependent on subject compliance and accurate self-reporting | ||
| Can observe EC use in populations of interest | Difficult to observe long-term outcomes due to relatively new EC popularity | ||
| Whole body or nosecone rodent exposure via nebulizer | Aerosol is generated by nebulizing e-liquid rather than via EC device. | Tight controlled overall ECA delivery | Lack of heating element reduces clinical relevance |
| Highly homogenous individual ECA “puffs” | Homogenous ECA “puffs” do not correspond to actual use | ||
| Enables the addition of labels to track cellular uptake and lung deposition | No standardized ECA exposure paradigm | ||
| Whole body rodent or nosecone exposure via EC | ECA is generated by either a whole EC device or through coil heating (similar to EC) and delivered to the animal in a manner comparable to actual use. | Closely mimics actual ECA delivery | Measuring variations in individual ECA “puffs” requires specialized equipment |
| Realistically heterogenous individual ECA “puffs” | No standardized ECA exposure paradigm | ||
| ECA delivery, device, and e-liquid selection can be tailored to study design | |||
| Cell culture exposure via direct stimulation | E-liquid is added to cell culture media directly. | Precise control of dosage | Difficult to determine physiologically relevant dosages in vitro |
| Rapid analysis of multiple e-liquid formulations on cells of interest | Lack of heating element reduces clinical relevance | ||
| Does not require specialized equipment | Direct cell exposure to e-liquid does not model actual ECA exposure | ||
| Cell culture exposure via ECA | Cells are exposed to ECA generated by EC device. | Recapitulates actual ECA exposure in vitro | Air–liquid interface must be considered to accurately model ECA delivery |
| Direct observation of ECA exposure on cells of interest | Specialized equipment required to expose multiple cultures in parallel | ||
| ECA delivery, device, and e-liquid selection can be tailored to study design |
| Cell Population Impacted | E-Liquid Components | Nicotine Level | Flavoring | E-Liquid Brand | Effects | In Vivo/In Vitro |
|---|---|---|---|---|---|---|
| Epithelial | PG and VG | 16 mg/mL | Tobacco, commercial | Blu | ↑ IL-6, ↑ IL-8 [31] | In vitro |
| N/A | N/A | Acetoin, pentanedione, maltol, OR o-vanillin | N/A | ↑ IL-8 [32] | In vitro | |
| 55% PG, 45% VG | N/A | Cinnamon, commercial | Local | ↓ Ion transport [95] | In vitro | |
| 100% VG | 1.10% | Tobacco, commercial | Johnson Creek | ↓ Ciliary motility, ↓ Mitochondrial respiration [96] | In vitro | |
| 50% PG, 50% VG | 60.9 mg/mL * | Cucumber, commercial | Juul | ↑ IL-8, ↑ IL-15, ↑ IFNγ, ↑ IL-17, ↑ PDGF, ↑ MCP-1, ↓ Membrane resistance [97] | In vitro | |
| 50% PG, 50% VG | 60.9 mg/mL * | Menthol, commercial | Juul | ↑ IL-8, ↑ IL-15, ↑ IL-17, ↑ IL-1β, ↑ IFNγ, ↑ PDGF, ↑ MCP-1, ↑ G-CSF [97] | In vitro | |
| 50% PG, 50% VG | 60.9 mg/mL * | Mango, commercial | Juul | ↑ IL-8, ↑ IL-15, ↑ IL-1β, ↑ IFNγ, ↑ PDGF, ↑ G-CSF, ↑ GM-CSF, ↑ Prostaglandin E2α [97] | In vitro | |
| 50% PG, 50% VG | 60.9 mg/mL * | Coffee, commercial | Juul | ↑ IL-8, ↑ IL-15, ↑ IFNγ, ↑ PDGF, ↑ GM-CSF, ↑ Prostaglandin E2α [97] | In vitro | |
| Endothelial | 50% PG, 50% VG | 24 mg/mL | N/A | N/A | ↑ Angiogenesis, ↑ CD31, ↑ CD34, ↑ Capillary density [86] | In vivo |
| PG and VG | 24 mg/mL | Unspecified | Blu | ↑ ROS, ↓ Membrane resistance, ↑ CD31, ↑ CD54, ↑ CD106 [98] | In vitro, in vivo | |
| 50% PG, 45% VG, 5% ethanol | 19 mg/mL | N/A | Valeo Laboratories | ↑ P-selectin, ↑ Extracellular vesicle secretion [99] | In vivo | |
| Platelets | 30% PG, 70% VG | 18 mg/mL | Menthol, commercial | Absolute Zero | ↑ Granule secretion, ↑ Thrombogenesis, ↓ Occlusion time [59] | In vivo |
| 50% PG, 50% VG | 60.9 mg/mL * | Menthol, commercial | Juul | ↑ CD40, ↑ P-selectin, ↑ Granule secretion, ↑ Thrombogenesis, ↓ Occlusion time [100] | In vivo | |
| 50% PG, 45% VG, 5% ethanol | 19 mg/mL | N/A | Valeo Laboratories | ↑ CD40, ↑ P-selectin, ↑ Extracellular vesicle secretion [99] | In vivo | |
| Macrophages | 50% PG, 50% VG | 60.9 mg/mL * | Cucumber, commercial | Juul | ↑ DNA damage [97] | In vitro |
| 50% PG, 50% VG | 60.9 mg/mL * | Menthol, commercial | Juul | ↑ Prostaglandin E2α, ↑ DNA damage [97] | In vitro | |
| 50% PG, 50% VG | 60.9 mg/mL * | Coffee, commercial | Juul | ↑ IL-8, ↑ DNA damage [42] | In vitro | |
| 50% PG, 50% VG | 36 mg/mL | N/A | American E-liquids Store | ↑ IL-6, ↑ IL-8, ↑ TNFα, ↑ MCP-1, ↑ MMP-9, ↑ ROS, ↑ Necrosis, ↑ Apoptosis [95] | In vitro | |
| 55% PG, 45% VG | N/A | Cinnamon, commercial | Local | ↓ Phagocytosis, ↓ IL-6, ↓ IL-8 [101] | In vitro | |
| 55% PG, 45% VG | N/A | Cola, commercial | Local | --- Phagocytosis, ↑ IL-6 [101] | In vitro | |
| Neutrophils | 30% PG, 70% VG | 18 mg/mL | Menthol, commercial | Absolute Zero | --- Activation [101] | In vivo |
| 55% PG, 45% VG | N/A | Cinnamon, commercial | Local | ↓ Phagocytosis, --- IL-8, ↑ NETosis, ↑ NETosis w/ NET stimuli [101] | In vitro | |
| 55% PG, 45% VG | N/A | Cola, commercial | Local | --- Phagocytosis, ↑ IL-8, --- NETosis, ↑ NETosis w/ NET stimuli [101] | In vitro | |
| PG and VG | 24 mg/mL | Unspecified | VIP | ↑ MMP9, ↑ IL-8, ↑ NE [102] | In vitro |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snoderly, H.T.; Nurkiewicz, T.R.; Bowdridge, E.C.; Bennewitz, M.F. E-Cigarette Use: Device Market, Study Design, and Emerging Evidence of Biological Consequences. Int. J. Mol. Sci. 2021, 22, 12452. https://doi.org/10.3390/ijms222212452
Snoderly HT, Nurkiewicz TR, Bowdridge EC, Bennewitz MF. E-Cigarette Use: Device Market, Study Design, and Emerging Evidence of Biological Consequences. International Journal of Molecular Sciences. 2021; 22(22):12452. https://doi.org/10.3390/ijms222212452
Chicago/Turabian StyleSnoderly, Hunter T., Timothy R. Nurkiewicz, Elizabeth C. Bowdridge, and Margaret F. Bennewitz. 2021. "E-Cigarette Use: Device Market, Study Design, and Emerging Evidence of Biological Consequences" International Journal of Molecular Sciences 22, no. 22: 12452. https://doi.org/10.3390/ijms222212452
APA StyleSnoderly, H. T., Nurkiewicz, T. R., Bowdridge, E. C., & Bennewitz, M. F. (2021). E-Cigarette Use: Device Market, Study Design, and Emerging Evidence of Biological Consequences. International Journal of Molecular Sciences, 22(22), 12452. https://doi.org/10.3390/ijms222212452






