Uric Acid in Inflammation and the Pathogenesis of Atherosclerosis
Abstract
1. Introduction
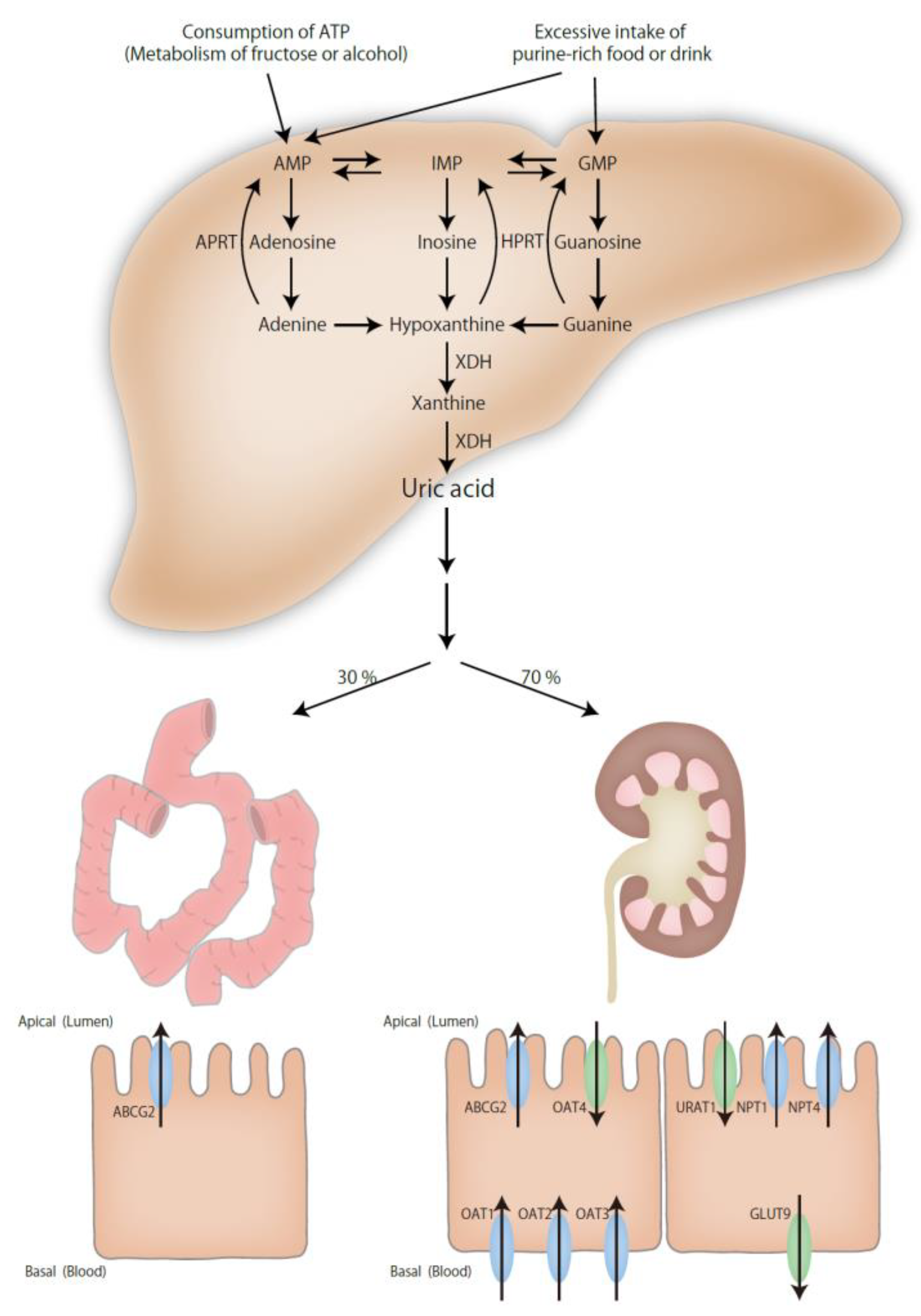
2. Mechanisms of Hyperuricemia
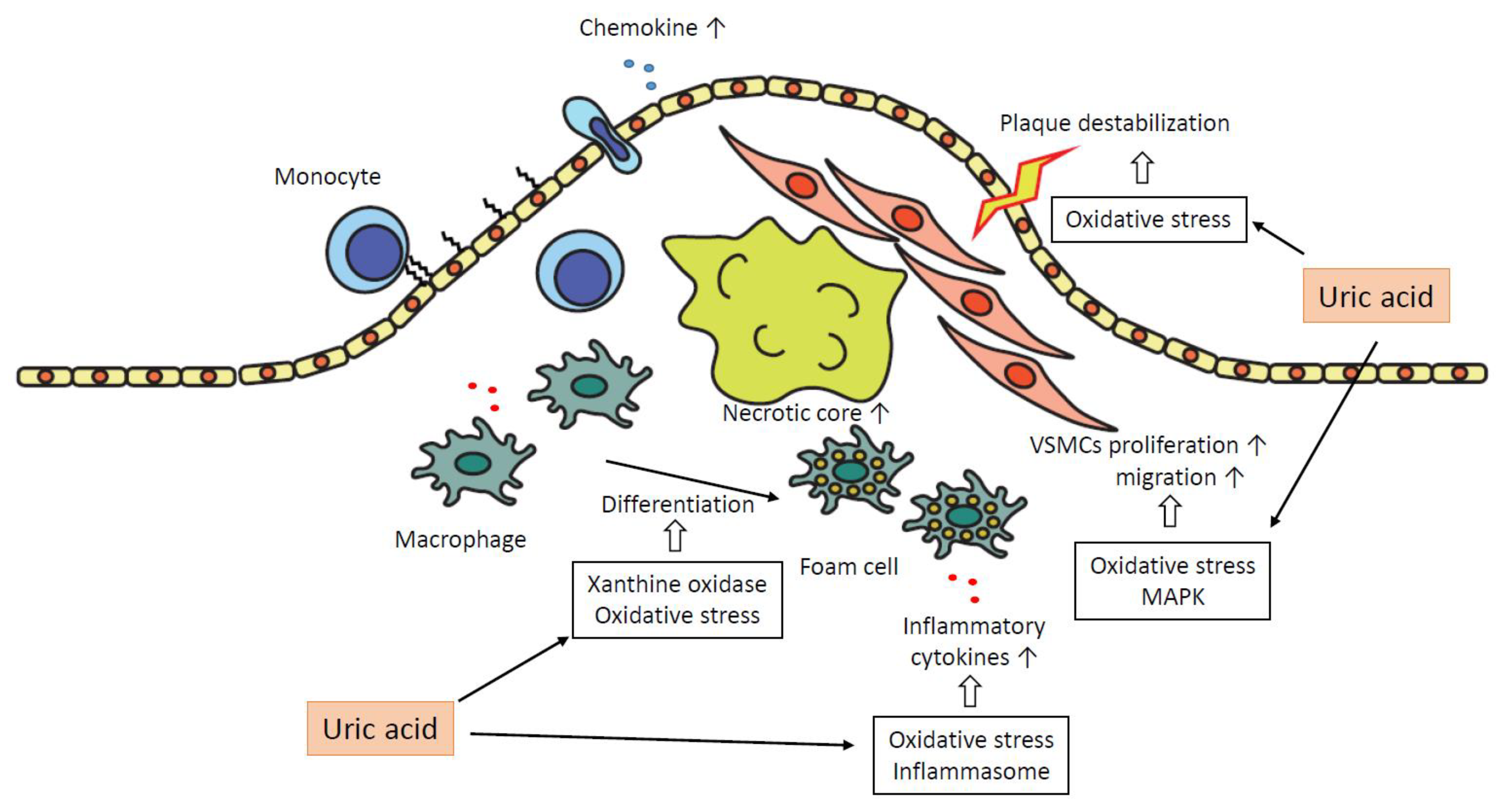
3. Epidemiology of Association between Uric Acid and Atherosclerosis
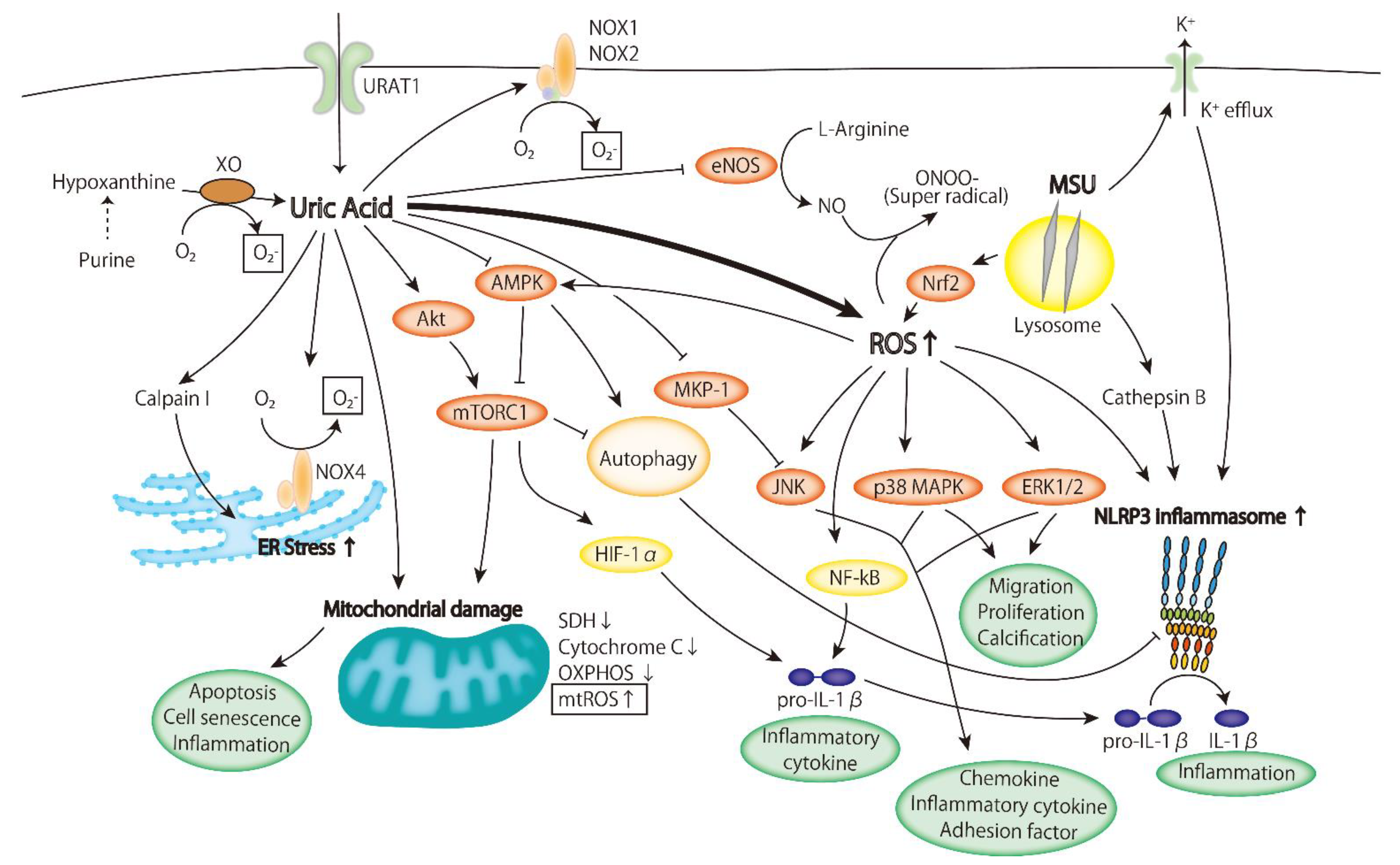
4. The Role of Hyperuricemia in the Pathogenesis of Atherosclerosis
4.1. Oxidative Stress
- ROS are produced due to the increased activity of xanthine oxidase in the metabolic process of uric acid;
- The expression and activity of NADPH oxidase increase;
- Mitochondrial ROS (mtROS) are produced due to mitochondrial injury.
4.1.1. Xanthine Oxidoreductase
4.1.2. NADPH Oxidase
4.1.3. Mitochondrial ROS
4.2. Inflammatory Signaling Pathway
4.2.1. ERK/p38 MAPK Cascade
4.2.2. AMPK
4.2.3. PI3K-Akt Pathway
4.2.4. Inflammasome
4.2.5. NO, HMGB1, RAA, ER Stress, and Mechanism of Sensing UA
5. The Effect of Therapeutic Agents for Gout on Atherosclerosis
| Drugs | Study Design | Control | Participants | Number | Results |
|---|---|---|---|---|---|
| Allopurinol | RCT [107] | Usual therapy | Patientis with CKD (eGFR < 60 mL/min). | 113 | Allopurinol slows down the progression of renal disease and reduces risk of cardiovascular events by 71%. |
| RCT [108] | Placebo | Adults with stage 3 or 4 CKD and no history of gout. | 369 | Allopurinol did not slow the decline in eGFR as compared with placebo. | |
| Meta-analysis: 12 RCTs [123] | Placebo or no treatment | RCTs investigated allopurinol’s effects on endothelial function. Patients with CHF, CKD, or type 2 DM. | CHF; 197 CKD; 183 DM; 170 | Allopurinol had a benefit on endothelial function in patients with CHF and CKD but not in type 2 DM. | |
| Meta-analysis: 9 RCTs [109] | Placebo or control | Patients undergoing CABG, after ACS or CHF. | 850 | Allopurinol was associated with the reduction of odds of periprocedural ACS but not with that of long-term secondary prevention of ACS. | |
| Febuxostat | RCT [116] | Allopurinol | Patients with gout and cardiovascular disease. | 6190 | All-cause and cardiovascular mortality were higher in the febuxostat group than in the allopurinol group (HR for all death, 1.22; HR for cardiovascular death, 1.34). |
| RCT [117] | Allopurinol | Patients were ≥60 y.o., already receiving allopurinol, and had at least one additional cardiovascular risk factor. | 6128 | Febuxostat is non-inferior to allopurinol therapy as the primary cardiovascular endpoint and not associated with an increased risk of death. | |
| Xanthine oxidase inhibitor (XOI) | Meta-analysis: 81 RCTs [119] | Placebo or no treatment | RCTs comparing purine-like or non-purine XOI with placebo or no treatment (control) for a period equal or superior to 28 days in adult patients. | 10,684 (6434 pt·yr) | XOI did not significantly reduce the risk of MACE and death but reduced the risk of TCE and hypertension. |
| Urate-lowering treatment (ULT) | Meta-analysis: 18 RCTs [120] | Placebo or other ULT drugs | RCTs had to report cardiovascular safety of urate-lowering treatment (allopurinol, febuxostat, pegloticase, rasburicase, probenecid, benzbromarone). | 7757 | Any ULT did not demonstrate a significant difference in any cardiovascular death, non-fatal myocardial infarction or non-fatal stroke, or all-cause mortality. |
| Colchcine | RCT [122] | Placebo | Patients suffered from MI within 30 days. | 4745 | The primary endpoint occurred at 5.5% in the colchicine group compared with 7.1% in the placebo group (HR 0.77, 95% CI 0.61–0.96). |
| RCT [89] | Placebo | Patients had any evidence of coronary disease and have been in a clinically stable condition for at least 6 months. | 5522 | A primary endpoint event occurred in 6.8% in the colchicine group and 9.6% in the placebo group (HR 0.69, 95% CI 0.57–0.83). |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Singh, G.; Lingala, B.; Mithal, A. Gout and Hyperuricaemia in the USA: Prevalence and Trends. Rheumatology 2019, 58, 2177–2180. [Google Scholar] [CrossRef] [PubMed]
- Roman, Y.M. The Daniel K. Inouye College of Pharmacy Scripts: Perspectives on the Epidemiology of Gout and Hyperuricemia. Hawaii J. Med. Public Health 2019, 78, 71–76. [Google Scholar]
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef]
- Nuki, G.; Simkin, P.A. A Concise History of Gout and Hyperuricemia and Their Treatment. Arthritis Res. Ther. 2006, 8 (Suppl. S1), S1. [Google Scholar] [CrossRef][Green Version]
- Oshima, Y. Gout. Naika 1966, 17, 341–342. [Google Scholar] [PubMed]
- Sun, H.-L.; Pei, D.; Lue, K.-H.; Chen, Y.-L. Uric Acid Levels Can Predict Metabolic Syndrome and Hypertension in Adolescents: A 10-Year Longitudinal Study. PLoS ONE 2015, 10, e0143786. [Google Scholar] [CrossRef] [PubMed]
- Ohno, I. Relationship between Hyperuricemia and Chronic Kidney Disease. Nucleosides Nucleotides Nucleic Acids 2011, 30, 1039–1044. [Google Scholar] [CrossRef]
- Van Der Schaft, N.; Brahimaj, A.; Wen, K.X.; Franco, O.H.; Dehghan, A. The Association between Serum Uric Acid and the Incidence of Prediabetes and Type 2 Diabetes Mellitus: The Rotterdam Study. PLoS ONE 2017, 12, e0179482. [Google Scholar] [CrossRef]
- Choi, H.K.; Ford, E.S.; Li, C.; Curhan, G. Prevalence of the Metabolic Syndrome in Patients with Gout: The Third National Health and Nutrition Examination Survey. Arthritis Rheumatol. 2007, 57, 109–115. [Google Scholar] [CrossRef]
- Bos, M.J.; Koudstaal, P.J.; Hofman, A.; Witteman, J.C.; Breteler, M.M. Uric Acid Is a Risk Factor for Myocardial Infarction and Stroke: The Rotterdam Study. Stroke 2006, 37, 1503–1507. [Google Scholar] [CrossRef]
- Krishnan, E.; Pandya, B.J.; Chung, L.; Dabbous, O. Hyperuricemia and the Risk for Subclinical Coronary Atherosclerosis—Data from a Prospective Observational Cohort Study. Arthritis Res. Ther. 2011, 13, R66. [Google Scholar] [CrossRef] [PubMed]
- Moriarity, J.T.; Folsom, A.R.; Iribarren, C.; Nieto, F.J.; Rosamond, W.D. Serum Uric Acid and Risk of Coronary Heart Disease: Atherosclerosis Risk in Communities (ARIC) Study. Ann. Epidemiol. 2000, 10, 136–143. [Google Scholar] [CrossRef]
- Culleton, B.F.; Larson, M.G.; Kannel, W.B.; Levy, D. Serum Uric Acid and Risk for Cardiovascular Disease and Death: The Framingham Heart Study. Ann. Intern. Med. 1999, 131, 7–13. [Google Scholar] [CrossRef]
- Álvarez-Lario, B.; Macarrón-Vicente, J. Uric Acid and Evolution. Rheumatology 2010, 49, 2010–2015. [Google Scholar] [CrossRef]
- Joosten, L.A.; Crisan, T.O.; Bjornstad, P.; Johnson, R.J. Asymptomatic Hyperuricemia—A Silent Activator of the Innate Immune System. Nat. Rev. Rheumatol. 2020, 16, 75. [Google Scholar] [CrossRef]
- López-Cruz, R.I.; Crocker, D.E.; Gaxiola-Robles, R.; Bernal, J.A.; Real-Valle, R.A.; Lugo-Lugo, O.; Zenteno-Savín, T. Plasma Hypoxanthine-Guanine Phosphoribosyl Transferase Activity in Bottlenose Dolphins Contributes to Avoiding Accumulation of Non-Recyclable Purines. Front. Physiol. 2016, 7, 213. [Google Scholar] [CrossRef]
- Reginato, A.M.; Mount, D.B.; Yang, I.; Choi, H.K. The Genetics of Hyperuricaemia and Gout. Nat. Rev. Rheumatol. 2012, 8, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, Y.; Bian, H.; Yang, H.; Wang, H.; Meng, X.; Jin, J. Function of Uric Acid Transporters and Their Inhibitors in Hyperuricaemia. Front. Pharmacol. 2021, 12, 1806. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zeng, C. Update on the Epidemiology, Genetics, and Therapeutic Options of Hyperuricemia. Am. J. Transl. Res. 2020, 12, 3167. [Google Scholar]
- Zhang, C.; Li, L.; Zhang, Y.; Zeng, C. Recent Advances in Fructose Intake and Risk of Hyperuricemia. Biomed. Pharmacother. 2020, 131, 110795. [Google Scholar] [CrossRef]
- Perez-Ruiz, F.; Aniel-Quiroga, M.A.; Herrero-Beites, A.M.; Chinchilla, S.P.; Erauskin, G.G.; Merriman, T. Renal Clearance of Uric Acid Is Linked to Insulin Resistance and Lower Excretion of Sodium in Gout Patients. Rheumatol. Int. 2015, 35, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Liu, X.; Jiang, L.; Mao, S.; Yin, X.; Guo, L. Hyperuricemia and Coronary Heart Disease Mortality: A Meta-Analysis of Prospective Cohort Studies. BMC Cardiovasc. Disord. 2016, 16, 207. [Google Scholar] [CrossRef]
- Li, M.; Hu, X.; Fan, Y.; Li, K.; Zhang, X.; Hou, W.; Tang, Z. Hyperuricemia and the Risk for Coronary Heart Disease Morbidity and Mortality a Systematic Review and Dose-Response Meta-Analysis. Sci. Rep. 2016, 6, 19520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Huang, L.; Song, M.; Song, Y. Baseline Serum Uric Acid Level as a Predictor of Cardiovascular Disease Related Mortality and All-Cause Mortality: A Meta-Analysis of Prospective Studies. Atherosclerosis 2013, 231, 61–68. [Google Scholar] [CrossRef]
- Chiang, K.-M.; Tsay, Y.-C.; Vincent Ng, T.-C.; Yang, H.-C.; Huang, Y.-T.; Chen, C.-H.; Pan, W.-H. Is Hyperuricemia, an Early-Onset Metabolic Disorder, Causally Associated with Cardiovascular Disease Events in Han Chinese? J. Clin. Med. 2019, 8, 1202. [Google Scholar] [CrossRef]
- Kleber, M.E.; Delgado, G.; Grammer, T.B.; Silbernagel, G.; Huang, J.; Krämer, B.K.; Ritz, E.; März, W. Uric Acid and Cardiovascular Events: A Mendelian Randomization Study. J. Am. Soc. Nephrol. 2015, 26, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.; Zhao, W.; Rasheed, A.; Ho, W.K.; Malik, R.; Felix, J.F.; Young, R.; Shah, N.; Samuel, M.; Sheikh, N.; et al. Causal Assessment of Serum Urate Levels in Cardiometabolic Diseases Through a Mendelian Randomization Study. J. Am. Coll. Cardiol. 2016, 67, 407–416. [Google Scholar] [CrossRef]
- Lim, S.S.; Yang, Y.L.; Chen, S.C.; Wu, C.H.; Huang, S.S.; Chan, W.L.; Lin, S.J.; Chen, J.W.; Chou, C.Y.; Pan, J.P.; et al. Association of Variability in Uric Acid and Future Clinical Outcomes of Patient with Coronary Artery Disease Undergoing Percutaneous Coronary Intervention. Atherosclerosis 2020, 297, 40–46. [Google Scholar] [CrossRef]
- Sugiura, T.; Dohi, Y.; Takase, H.; Yamashita, S.; Fujii, S.; Ohte, N. Oxidative Stress Is Closely Associated with Increased Arterial Stiffness, Especially in Aged Male Smokers without Previous Cardiovascular Events: A Cross-Sectional Study. J. Atheroscler. Thromb. 2017, 24, 39289. [Google Scholar] [CrossRef]
- Rodrigo, R.; Prat, H.; Passalacqua, W.; Araya, J.; Guichard, C.; Bächler, J.P. Relationship between Oxidative Stress and Essential Hypertension. Hypertens. Res. 2007, 30, 1159–1167. [Google Scholar] [CrossRef]
- Al-Benna, S.; Hamilton, C.A.; McClure, J.D.; Rogers, P.N.; Berg, G.A.; Ford, I.; Delles, C.; Dominiczak, A.F. Low-Density Lipoprotein Cholesterol Determines Oxidative Stress and Endothelial Dysfunction in Saphenous Veins from Patients with Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Motz, E. Is Oxidative Stress the Pathogenic Mechanism Underlying Insulin Resistance, Diabetes, and Cardiovascular Disease? The Common Soil Hypothesis Revisited. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 816–823. [Google Scholar] [CrossRef]
- Sevanian, A.; Davies, K.J.; Hochstein, P. Serum Urate as an Antioxidant for Ascorbic Acid. Am. J. Clin. Nutr. 1991, 54 (Suppl. S6), 1129S–1134S. [Google Scholar] [CrossRef]
- Yu, Z.F.; Bruce-Keller, A.J.; Goodman, Y.; Mattson, M.P. Uric Acid Protects Neurons against Excitotoxic and Metabolic Insults in Cell Culture, and against Focal Ischemic Brain Injury in Vivo. J. Neurosci. Res. 1998, 53, 613–625. [Google Scholar] [CrossRef]
- Shimizu, Y.; Wakabayashi, K.; Totsuka, A.; Hayashi, Y.; Nitta, S.; Hara, K.; Akira, M.; Tomino, Y.; Suzuki, Y. Exercise-Induced Acute Kidney Injury in a Police Officer with Hereditary Renal Hypouricemia. Case Rep. Nephrol. Dial. 2019, 9, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T.; Okamoto, K.; Eger, B.T.; Pai, E.F.; Nishino, T. Mammalian Xanthine Oxidoreductase—Mechanism of Transition from Xanthine Dehydrogenase to Xanthine Oxidase. FEBS J. 2008, 275, 3278–3289. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, S.; Shirakura, T.; Hokama, N.; Kozuka, C.; Yonamine, M.; Namba, T.; Morishima, S.; Nakachi, S.; Nishi, Y.; Ikema, T.; et al. Activity of Xanthine Oxidase in Plasma Correlates with Indices of Insulin Resistance and Liver Dysfunction in Patients with Type 2 Diabetes Mellitus and Metabolic Syndrome: A Pilot Exploratory Study. J. Diabetes Investig. 2019, 10, 94. [Google Scholar] [CrossRef]
- McNally, J.S.; Davis, M.E.; Giddens, D.P.; Saha, A.; Hwang, J.; Dikalov, S.; Jo, H.; Harrison, D.G. Role of Xanthine Oxidoreductase and NAD(P)H Oxidase in Endothelial Superoxide Production in Response to Oscillatory Shear Stress. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2290–H2297. [Google Scholar] [CrossRef]
- Patetsios, P.; Song, M.; Shutze, W.P.; Pappas, C.; Rodino, W.; Ramirez, J.A.; Panetta, T.F. Identification of Uric Acid and Xanthine Oxidase in Atherosclerotic Plaque. Am. J. Cardiol. 2001, 88, 188–191. [Google Scholar] [CrossRef]
- Doehner, W.; Schoene, N.; Rauchhaus, M.; Leyva-Leon, F.; Pavitt, D.V.; Reaveley, D.A.; Schuler, G.; Coats, A.J.S.; Anker, S.D.; Hambrecht, R. Effects of Xanthine Oxidase Inhibition with Allopurinol on Endothelial Function and Peripheral Blood Flow in Hyperuricemic Patients with Chronic Heart Failure: Results from 2 Placebo-Controlled Studies. Circulation 2002, 105, 2619–2624. [Google Scholar] [CrossRef]
- Butler, R.; Morris, A.D.; Belch, J.J.; Hill, A.; Struthers, A.D. Allopurinol Normalizes Endothelial Dysfunction in Type 2 Diabetics with Mild Hypertension. Hypertension 2000, 35, 746–751. [Google Scholar] [CrossRef]
- Guthikonda, S.; Sinkey, C.; Barenz, T.; Haynes, W.G. Xanthine Oxidase Inhibition Reverses Endothelial Dysfunction in Heavy Smokers. Circulation 2003, 107, 416–421. [Google Scholar] [CrossRef]
- El Solh, A.A.; Saliba, R.; Bosinski, T.; Grant BJ, B.; Berbary, E.; Miller, N. Allopurinol Improves Endothelial Function in Sleep Apnoea: A Randomised Controlled Study. Eur. Respir. J. 2006, 27, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Linas, S.L.; Whittenburg, D.; Repine, J.E. Role of Xanthine Oxidase in Ischemia/Reperfusion Injury. Am. J. Physiol. 1990, 258 Pt 2, F711–F716. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N. Role of Xanthine Oxidase and Granulocytes in Ischemia-Reperfusion Injury. Am. J. Physiol.-Heart Circ. Physiol. 1988, 255, H1269–H1275. [Google Scholar] [CrossRef] [PubMed]
- Battelli, M.G.; Polito, L.; Bolognesi, A. Xanthine Oxidoreductase in Atherosclerosis Pathogenesis: Not Only Oxidative Stress. Atherosclerosis 2014, 237, 562–567. [Google Scholar] [CrossRef]
- Kushiyama, A.; Okubo, H.; Sakoda, H.; Kikuchi, T.; Fujishiro, M.; Sato, H.; Kushiyama, S.; Iwashita, M.; Nishimura, F.; Fukushima, T.; et al. Xanthine Oxidoreductase Is Involved in Macrophage Foam Cell Formation and Atherosclerosis Development. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.J.; Tzameli, I.; Pissios, P.; Rovira, I.; Gavrilova, O.; Ohtsubo, T.; Chen, Z.; Finkel, T.; Flier, J.S.; Friedman, J.M. Xanthine Oxidoreductase Is a Regulator of Adipogenesis and PPARgamma Activity. Cell Metab. 2007, 5, 115–128. [Google Scholar] [CrossRef]
- Martinez-Hervas, S.; Real, J.T.; Ivorra, C.; Priego, A.; Chaves, F.J.; Pallardo, F.V.; Viña, J.R.; Redon, J.; Carmena, R.; Ascaso, J.F. Increased Plasma Xanthine Oxidase Activity Is Related to Nuclear Factor Kappa Beta Activation and Inflammatory Markers in Familial Combined Hyperlipidemia. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 734–739. [Google Scholar] [CrossRef]
- Ives, A.; Nomura, J.; Martinon, F.; Roger, T.; LeRoy, D.; Miner, J.N.; Simon, G.; Busso, N.; So, A. Xanthine Oxidoreductase Regulates Macrophage IL1β Secretion upon NLRP3 Inflammasome Activation. Nat. Commun. 2015, 6, 6555. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Grechko, A.V.; Orekhova, V.A.; Khotina, V.; Ivanova, E.A.; Orekhov, A.N. Biomedicines NADPH Oxidases and Their Role in Atherosclerosis. Biomedicines 2020, 8, 206. [Google Scholar] [CrossRef]
- Sautin, Y.Y.; Nakagawa, T.; Zharikov, S.; Johnson, R.J. Adverse Effects of the Classic Antioxidant Uric Acid in Adipocytes: NADPH Oxidase-Mediated Oxidative/Nitrosative Stress. Am. J. Physiol. Cell Physiol. 2007, 293, C584–C596. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.H.; Liu, J.C.; Lin, J.W.; Chen, C.H.; Wu, C.H.; Cheng, T.H. Uric Acid Stimulates Endothelin-1 Gene Expression Associated with NADPH Oxidase in Human Aortic Smooth Muscle Cells. Acta Pharmacol. Sin. 2008, 29, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Verzola, D.; Ratto, E.; Villaggio, B.; Parodi, E.L.; Pontremoli, R.; Garibotto, G.; Viazzi, F. Uric Acid Promotes Apoptosis in Human Proximal Tubule Cells by Oxidative Stress and the Activation of NADPH Oxidase NOX 4. PLoS ONE 2014, 9, e115210. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Shin, H.-S.; Choi, H.S.; Park, J.-W.; Jo, I.; Oh, E.-S.; Lee, K.-Y.; Lee, B.-H.; Johnson, R.J.; Kang, D.-H. Uric Acid Induces Fat Accumulation via Generation of Endoplasmic Reticulum Stress and SREBP-1c Activation in Hepatocytes. Lab. Investig. 2014, 94, 1114–1125. [Google Scholar] [CrossRef]
- Yu, E.P.K.; Reinhold, J.; Yu, H.; Starks, L.; Uryga, A.K.; Foote, K.; Finigan, A.; Figg, N.; Pung, Y.-F.; Logan, A.; et al. Mitochondrial Respiration Is Reduced in Atherosclerosis, Promoting Necrotic Core Formation and Reducing Relative Fibrous Cap Thickness. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Cai, G.; Xia, Y.; Chen, J.; Wu, P.; Wang, Z.; Li, G.; Wei, D. Mitochondrial Dysfunction in Atherosclerosis. DNA Cell Biol. 2019, 38, 597–606. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Y.; Peng, T.; Li, L.; Zhu, W.; Liu, F.; Liu, S.; An, X.; Luo, R.; Cheng, J.; et al. Mitochondrial ROS-Induced Lysosomal Dysfunction Impairs Autophagic Flux and Contributes to M1 Macrophage Polarization in a Diabetic Condition. Clin. Sci. 2019, 133, 1759–1777. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Y.; Cheng, S.; Sun, J.L.; Yao, H.; Ma, L. Effect of Uric Acid on Mitochondrial Function and Oxidative Stress in Hepatocytes. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Sánchez-Lozada, L.G.; Lanaspa, M.A.; Cristóbal-García, M.; García-Arroyo, F.; Soto, V.; Cruz-Robles, D.; Nakagawa, T.; Yu, M.A.; Kang, D.-H.; Johnson, R.J. Uric Acid-Induced Endothelial Dysfunction Is Associated with Mitochondrial Alterations and Decreased Intracellular ATP Concentrations. Nephron. Exp. Nephrol. 2012, 121, e71–e78. [Google Scholar] [CrossRef]
- Kimura, Y.; Yanagida, T.; Onda, A.; Tsukui, D.; Hosoyamada, M.; Kono, H. Soluble Uric Acid Promotes Atherosclerosis via AMPK (AMP-Activated Protein Kinase)-Mediated Inflammation. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Hu, L.; Wang, Y.; Ying, G.; Ma, C.; Wei, J. The Rho Kinase Signaling Pathway Participates in Tubular Mitochondrial Oxidative Injury and Apoptosis in Uric Acid Nephropathy. J. Int. Med. Res. 2021, 49. [Google Scholar] [CrossRef]
- Muslin, A.J. MAPK Signalling in Cardiovascular Health and Disease: Molecular Mechanisms and Therapeutic Targets. Clin. Sci. 2008, 115, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Reustle, A.; Torzewski, M. Role of P38 MAPK in Atherosclerosis and Aortic Valve Sclerosis. Int. J. Mol. Sci. 2018, 19, 3761. [Google Scholar] [CrossRef] [PubMed]
- Kırça, M.; Oğuz, N.; Çetin, A.; Uzuner, F.; Yeşilkaya, A. Uric Acid Stimulates Proliferative Pathways in Vascular Smooth Muscle Cells through the Activation of P38 MAPK, P44/42 MAPK and PDGFRβ. J. Recept. Signal Transduct. 2016, 37, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shen, Y.; Chen, Y.; Zhang, G.; Cheng, J.; Wang, W. High Uric Acid Inhibits Cardiomyocyte Viability Through the ERK/P38 Pathway via Oxidative Stress. Cell. Physiol. Biochem. 2018, 45, 1156–1164. [Google Scholar] [CrossRef]
- Kanellis, J.; Watanabe, S.; Li, J.H.; Kang, D.H.; Li, P.; Nakagawa, T.; Wamsley, A.; Sheikh-Hamad, D.; Lan, H.Y.; Feng, L.; et al. Uric Acid Stimulates Monocyte Chemoattractant Protein-1 Production in Vascular Smooth Muscle Cells Via Mitogen-Activated Protein Kinase and Cyclooxygenase-2. Hypertension 2003, 41, 1287–1293. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, K.; Jia, Z.; Xu, T.; Xu, Q.; Zhang, C.; Liu, J.; Chen, R.; Du, Z.; Sun, J. Zurampic Protects Pancreatic β-Cells from High Uric Acid Induced-Damage by Inhibiting URAT1 and Inactivating the ROS/AMPK/ERK Pathways. Cell. Physiol. Biochem. 2018, 47, 1074–1083. [Google Scholar] [CrossRef]
- Nomura, J.; Busso, N.; Ives, A.; Tsujimoto, S.; Tamura, M.; So, A.; Yamanaka, Y. Febuxostat, an Inhibitor of Xanthine Oxidase, Suppresses Lipopolysaccharide-Induced MCP-1 Production via MAPK Phosphatase-1-Mediated Inactivation of JNK. PLoS ONE 2013, 8, e75527. [Google Scholar] [CrossRef]
- Sag, D.; Carling, D.; Stout, R.D.; Suttles, J. AMP-Activated Protein Kinase Promotes Macrophage Polarization to an Anti-Inflammatory Functional Phenotype. J. Immunol. 2008, 181, 8633. [Google Scholar] [CrossRef]
- Cordero, M.D.; Williams, M.R.; Ryffel, B. AMP-Activated Protein Kinase Regulation of the NLRP3 Inflammasome during Aging Implication of AMPK in Aging. Trends Endocrinol. Metab. 2018, 29, 8–17. [Google Scholar] [CrossRef]
- Ma, A.; Wang, J.; Yang, L.; An, Y.; Zhu, H. AMPK Activation Enhances the Anti-Atherogenic Effects of High Density Lipoproteins in ApoE -/- Mice. J. Lipid Res. 2017, 58, 1536–1547. [Google Scholar] [CrossRef]
- Vasamsetti, S.B.; Karnewar, S.; Kanugula, A.K.; Thatipalli, A.R.; Kumar, J.M.; Kotamraju, S. Metformin Inhibits Monocyte-to-Macrophage Differentiation via AMPK-Mediated Inhibition of STAT3 Activation: Potential Role in Atherosclerosis. Diabetes 2015, 64, 2028–2041. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, J.; Ma, Q.; Liu, Z.; Sudhahar, V.; Cao, Y.; Wang, L.; Zeng, X.; Zhou, Y.; Zhang, M.; et al. PRKAA1/AMPKα1-Driven Glycolysis in Endothelial Cells Exposed to Disturbed Flow Protects against Atherosclerosis. Nat. Commun. 2018, 9, 4667. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Cicerchi, C.; Garcia, G.; Li, N.; Roncal-Jimenez, C.A.; Rivard, C.J.; Hunter, B.; Andrés-Hernando, A.; Ishimoto, T.; Sánchez-Lozada, L.G.; et al. Counteracting Roles of AMP Deaminase and AMP Kinase in the Development of Fatty Liver. PLoS ONE 2012, 7, e48801. [Google Scholar] [CrossRef] [PubMed]
- Cicerchi, C.; Li, N.; Kratzer, J.; Garcia, G.; Roncal-Jimenez, C.A.; Tanabe, K.; Hunter, B.; Rivard, C.J.; Sautin, Y.Y.; Gaucher, E.A.; et al. Uric Acid-Dependent Inhibition of AMP Kinase Induces Hepatic Glucose Production in Diabetes and Starvation: Evolutionary Implications of the Uricase Loss in Hominids. FASEB J. 2014, 28, 3339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yamamoto, T.; Hisatome, I.; Li, Y.; Cheng, W.; Sun, N.; Cai, B.; Huang, T.; Zhu, Y.; Li, Z.; et al. Uric acid induces oxidative stress and growth inhibition by activating adenosine monophosphate-activated protein kinase and extracellular signal-regulated kinase signal pathways in pancreatic β cells. Mol Cell Endocrinol. 2013, 375, 89. [Google Scholar] [CrossRef]
- Luo, C.; Lian, X.; Hong, L.; Zou, J.; Li, Z.; Zhu, Y.; Huang, T.; Zhang, Y.; Hu, Y.; Yuan, H.; et al. High Uric Acid Activates the ROS-AMPK Pathway, Impairs CD68 Expression and Inhibits OxLDL-Induced Foam-Cell Formation in a Human Monocytic Cell Line, THP-1. Cell. Physiol. Biochem. 2016, 40, 538–548. [Google Scholar] [CrossRef]
- García-Arroyo, F.E.; Monroy-Sánchez, F.; Muñoz-Jiménez, I.; Gonzaga, G.; Andrés-Hernando, A.; Zazueta, C.; Juárez-Rojas, J.G.; Lanaspa, M.A.; Johnson, R.J.; Sánchez-Lozada, L.G. Allopurinol Prevents the Lipogenic Response Induced by an Acute Oral Fructose Challenge in Short-Term Fructose Fed Rats. Biomolecules 2019, 9, 601. [Google Scholar] [CrossRef]
- Zhao, Y.; Qian, Y.; Sun, Z.; Shen, X.; Cai, Y.; Li, L.; Wang, Z. Role of PI3K in the Progression and Regression of Atherosclerosis. Front. Pharmacol. 2021, 12, 263. [Google Scholar] [CrossRef]
- Fernández-Hernando, C.; Ackah, E.; Yu, J.; Suárez, Y.; Murata, T.; Iwakiri, Y.; Prendergast, J.; Miao, R.Q.; Birnbaum, M.J.; Sessa, W.C. Loss of Akt1 Leads to Severe Atherosclerosis and Occlusive Coronary Artery Disease. Cell Metab. 2007, 6, 446. [Google Scholar] [CrossRef] [PubMed]
- Crişan, T.O.; Cleophas, M.C.P.; Novakovic, B.; Erler, K.; van de Veerdonk, F.L.; Stunnenberg, H.G.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A.B. Uric Acid Priming in Human Monocytes Is Driven by the AKT-PRAS40 Autophagy Pathway. Proc. Natl. Acad. Sci. USA 2017, 114, 5485–5490. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, H.; Lu, J.; Xie, D.; Wang, Q.; Huang, T.; Xin, H.; Hisatome, I.; Yamamoto, T.; Wang, W.; et al. High Uric Acid Promotes Dysfunction in Pancreatic β Cells by Blocking IRS2/AKT Signalling. Mol. Cell. Endocrinol. 2021, 520, 111070. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent Advances in the Mechanisms of NLRP3 Inflammasome Activation and Its Inhibitors. Cell Death Dis. 2019, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nat. Med. 2015, 21, 677. [Google Scholar] [CrossRef]
- So, A.K.; Martinon, F. Inflammation in Gout: Mechanisms and Therapeutic Targets. Nat. Rev. Rheumatol. 2017, 13, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Jhang, J.-J.; Cheng, Y.-T.; Ho, C.-Y.; Yen, G.-C. Monosodium Urate Crystals Trigger Nrf2- and Heme Oxygenase-1-Dependent Inflammation in THP-1 Cells. Cell. Mol. Immunol. 2015, 12, 424–434. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef]
- Klauser, A.S.; Halpern, E.J.; Strobl, S.; Gruber, J.; Feuchtner, G.; Bellmann-Weiler, R.; Weiss, G.; Stofferin, H.; Jaschke, W. Dual-Energy Computed Tomography Detection of Cardiovascular Monosodium Urate Deposits in Patients with Gout. JAMA Cardiol. 2019, 4, 1019–1028. [Google Scholar] [CrossRef]
- Abdellatif, W.; Chow, B.; Nicolaou, S. THU0598 ROLE OF DUAL-ENERGY CT AS A SCREENING TOOL FOR CORONARY GOUT. Ann. Rheum. Dis. 2019, 78 (Suppl. S2), 590–592. [Google Scholar] [CrossRef]
- Yokose, C.; Eide, S.; Simeone, F.; Shojania, K.; Nicolaou, S.; Becce, F.; Choi, H.K. Frequently Encountered Artifacts in Novel Application of Dual-Energy CT to Vascular Imaging: A Pilot Study—ACR Meeting Abstracts. Arthritis Rheumatol. 2019, 71 (Suppl. S10). Available online: https://acrabstracts.org/abstract/frequently-encountered-artifacts-in-novel-application-of-dual-energy-ct-to-vascular-imaging-a-pilot-study/ (accessed on 11 November 2021).
- Andrés, M.; Quintanilla, M.-A.; Sivera, F.; Sánchez-Payá, J.; Pascual, E.; Vela, P.; Ruiz-Nodar, J.-M. Silent Monosodium Urate Crystal Deposits Are Associated with Severe Coronary Calcification in Asymptomatic Hyperuricemia: An Exploratory Study. Arthritis Rheumatol. 2016, 68, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.T.; Forni, M.F.; Correa-Costa, M.; Ramos, R.N.; Barbuto, J.A.; Branco, P.; Castoldi, A.; Hiyane, M.I.; Davanso, M.R.; Latz, E.; et al. Soluble Uric Acid Activates the NLRP3 Inflammasome. Sci. Rep. 2017, 7, 39884. [Google Scholar] [CrossRef]
- Cavalcanti, N.G.; Marques, C.D.L.; Lins, T.; Pereira, M.C.; Rêgo, M.J.B.D.M.; Duarte, A.L.B.P.; Pitta, I.D.R.; Pitta, M.G.D.R. Cytokine Profile in Gout: Inflammation Driven by IL-6 and IL-18? Immunol. Investig. 2016, 45, 383–395. [Google Scholar] [CrossRef]
- Choe, J.-Y.; Choi, C.-H.; Park, K.-Y.; Kim, S.-K. High-Mobility Group Box 1 Is Responsible for Monosodium Urate Crystal-Induced Inflammation in Human U937 Macrophages. Biochem. Biophys. Res. Commun. 2018, 503, 3248–3255. [Google Scholar] [CrossRef]
- Ruggiero, C.; Cherubini, A.; Ble, A.; Bos, A.J.; Maggio, M.; Dixit, V.D.; Lauretani, F.; Bandinelli, S.; Senin, U.; Ferrucci, L. Uric Acid and Inflammatory Markers. Eur. Heart J. 2006, 27, 1174–1181. [Google Scholar] [CrossRef]
- Mishima, M.; Hamada, T.; Maharani, N.; Ikeda, N.; Onohara, T.; Notsu, T.; Ninomiya, H.; Miyazaki, S.; Mizuta, E.; Sugihara, S.; et al. Effects of Uric Acid on the NO Production of HUVECs and Its Restoration by Urate Lowering Agents. Drug Res. 2016, 66, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Duan, X.-M.; Liu, Y.; Yu, J.; Tang, Y.-L.; Liu, Z.-L.; Jiang, S.; Zhang, C.-P.; Liu, J.-Y.; Xu, J.-X. Uric Acid Induces Endothelial Dysfunction by Activating the HMGB1/RAGE Signaling Pathway. Biomed. Res. Int. 2017, 2017, 4391920. [Google Scholar] [CrossRef]
- Rabadi, M.M.; Kuo, M.-C.; Ghaly, T.; Rabadi, S.M.; Weber, M.; Goligorsky, M.S.; Ratliff, B.B. Interaction between Uric Acid and HMGB1 Translocation and Release from Endothelial Cells. Am. J. Physiol. Renal Physiol. 2012, 302, F730–F741. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gu, J.; Lv, H.; Li, H.; Cheng, Y.; Liu, Y.; Jiang, Y. Uric Acid Induced Inflammatory Responses in Endothelial Cells via Up-Regulating(pro)Renin Receptor. Biomed. Pharmacother. 2019, 109, 1163–1170. [Google Scholar] [CrossRef]
- Yu, M.A.; Sánchez-Lozada, L.G.; Johnson, R.J.; Kang, D.H. Oxidative Stress with an Activation of the Renin-Angiotensin System in Human Vascular Endothelial Cells as a Novel Mechanism of Uric Acid-Induced Endothelial Dysfunction. J. Hypertens. 2010, 28, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Chen, K.; He, L.; Li, S.; Huang, D.; Li, J. Uric Acid Induces Cardiomyocyte Apoptosis via Activation of Calpain-1 and Endoplasmic Reticulum Stress. Cell. Physiol. Biochem. 2018, 45, 2122–2135. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.T.; Davanso, M.R.; Mendes, D.; de Souza, T.A.; de Brito, A.F.; Cruz, M.C.; Hiyane, M.I.; de Lima, D.S.; Nunes, V.; de Fátima Giarola, J.; et al. Sensing Soluble Uric Acid by Naip1-Nlrp3 Platform. Cell Death Dis. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- FitzGerald, J.D.; Dalbeth, N.; Mikuls, T.; Brignardello-Petersen, R.; Guyatt, G.; Abeles, A.M.; Gelber, A.C.; Harrold, L.R.; Khanna, D.; King, C.; et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res. 2020, 72, 744–760. [Google Scholar] [CrossRef]
- Kohagura, K.; Sato, Y.; Taniguchi, A.; Masuda, I.; Moriwaki, Y.; Yamamoto, T. Japanese Society of Gout and Uric & Nucleic Acids 2019 Guidelines for Management of Hyperuricemia and Gout 3rd Edition. Gout Uric Nucleic Acids 2020, 44, S1–S40. [Google Scholar] [CrossRef]
- Goicoechea, M.; de Vinuesa, S.G.; Verdalles, U.; Ruiz-Caro, C.; Ampuero, J.; Rincón, A.; Arroyo, D.; Luño, J. Effect of Allopurinol in Chronic Kidney Disease Progression and Cardiovascular Risk. Clin. J. Am. Soc. Nephrol. 2010, 5, 1388–1393. [Google Scholar] [CrossRef]
- Badve, S.V.; Pascoe, E.M.; Tiku, A.; Boudville, N.; Brown, F.G.; Cass, A.; Clarke, P.; Dalbeth, N.; Day, R.O.; Zoysa, J.R.; et al. Effects of Allopurinol on the Progression of Chronic Kidney Disease. N. Engl. J. Med. 2020, 382, 2504–2513. [Google Scholar] [CrossRef]
- Ullah, W.; Khanal, S.; Khan, R.; Basyal, B.; Munir, S.; Minalyan, A.; Chadi Alraies, M.; Fischman, D.L. Efficacy of Allopurinol in Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Cardiol. Res. 2020, 11, 226–232. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, C.; Xu, Z.; Shen, J.; Zhang, X.; Du, H.; Zhang, K.; Zhang, D. Clinical Study on Efficacy of Allopurinol in Patients with Acute Coronary Syndrome and Its Functional Mechanism. Hell. J. Cardiol. 2017, 58, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.S.; Pottegård, A.; Lindegaard, H.M.; Hallas, J. Effect of Allopurinol on Cardiovascular Outcomes in Hyperuricemic Patients: A Cohort Study. Am. J. Med. 2016, 129, 299–306.e2. [Google Scholar] [CrossRef]
- Grimaldi-Bensouda, L.; Alpérovitch, A.; Aubrun, E.; Danchin, N.; Rossignol, M.; Abenhaim, L.; Richette, P.; The PGRx MI Group. Impact of Allopurinol on Risk of Myocardial Infarction. Ann. Rheum. Dis. 2015, 74, 836–842. [Google Scholar] [CrossRef]
- Lytvyn, Y.; Har, R.; Locke, A.; Lai, V.; Fong, D.; Advani, A.; Perkins, B.A.; Cherney, D.Z.I. Renal and Vascular Effects of Uric Acid Lowering in Normouricemic Patients with Uncomplicated Type 1 Diabetes. Diabetes 2017, 66, 1939–1949. [Google Scholar] [CrossRef] [PubMed]
- Nakata, T.; Ikeda, S.; Koga, S.; Yonekura, T.; Tsuneto, A.; Doi, Y.; Fukae, S.; Minami, T.; Kawano, H.; Maemura, K. Randomized, Open-Label, Cross-Over Comparison of the Effects of Benzbromarone and Febuxostat on Endothelial Function in Patients with Hyperuricemia. Int. Heart J. 2020, 61, 20–114. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Taguchi, I.; Teragawa, H.; Ishizaka, N.; Kanzaki, Y.; Tomiyama, H.; Sata, M.; Sezai, A.; Eguchi, K.; Kato, T.; et al. Febuxostat Does Not Delay Progression of Carotid Atherosclerosis in Patients with Asymptomatic Hyperuricemia: A Randomized, Controlled Trial. PLoS Med. 2020, 17, e1003095. [Google Scholar] [CrossRef]
- White, W.B.; Saag, K.G.; Becker, M.A.; Borer, J.S.; Gorelick, P.B.; Whelton, A.; Hunt, B.; Castillo, M.; Gunawardhana, L. Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout. N. Engl. J. Med. 2018, 378, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, I.S.; Ford, I.; Nuki, G.; Hallas, J.; Hawkey, C.J.; Webster, J.; Ralston, S.H.; Walters, M.; Robertson, M.; De Caterina, R.; et al. Long-Term Cardiovascular Safety of Febuxostat Compared with Allopurinol in Patients with Gout (FAST): A Multicentre, Prospective, Randomised, Open-Label, Non-Inferiority Trial. Lancet 2020, 396, 1745–1757. [Google Scholar] [CrossRef]
- Kim, S.C.; Neogi, T.; Kang, E.H.; Liu, J.; Desai, R.J.; Zhang, M.A.; Solomon, D.H. Cardiovascular Risks of Probenecid Versus Allopurinol in Older Patients with Gout. J. Am. Coll. Cardiol. 2018, 71, 994–1004. [Google Scholar] [CrossRef]
- Bredemeier, M.; Lopes, L.M.; Eisenreich, M.A.; Hickmann, S.; Bongiorno, G.K.; d’Avila, R.; Morsch, A.L.B.; Stein, F.D.S.; Campos, G.G.D. Xanthine Oxidase Inhibitors for Prevention of Cardiovascular Events: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMC Cardiovasc. Disord. 2018, 18, 24. [Google Scholar] [CrossRef]
- Zhang, T.; Pope, J.E. Cardiovascular Effects of Urate-Lowering Therapies in Patients with Chronic Gout: A Systematic Review and Meta-Analysis. Rheumatology 2017, 56, 1144–1153. [Google Scholar] [CrossRef]
- Mackenzie, I.S.; Ford, I.; Walker, A.; Hawkey, C.; Begg, A.; Avery, A.; Taggar, J.; Wei, L.; Struthers, A.D.; MacDonald, T.M.; et al. Multicentre, Prospective, Randomised, Open-Label, Blinded End Point Trial of the Efficacy of Allopurinol Therapy in Improving Cardiovascular Outcomes in Patients with Ischaemic Heart Disease: Protocol of the ALL-HEART Study. BMJ Open 2016, 6, e013774. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Alem, M.M. Allopurinol and Endothelial Function: A Systematic Review with Meta-Analysis of Randomized Controlled Trials. Cardiovasc. Ther. 2018, 36, e12432. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, Y.; Tsukui, D.; Kono, H. Uric Acid in Inflammation and the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 12394. https://doi.org/10.3390/ijms222212394
Kimura Y, Tsukui D, Kono H. Uric Acid in Inflammation and the Pathogenesis of Atherosclerosis. International Journal of Molecular Sciences. 2021; 22(22):12394. https://doi.org/10.3390/ijms222212394
Chicago/Turabian StyleKimura, Yoshitaka, Daisuke Tsukui, and Hajime Kono. 2021. "Uric Acid in Inflammation and the Pathogenesis of Atherosclerosis" International Journal of Molecular Sciences 22, no. 22: 12394. https://doi.org/10.3390/ijms222212394
APA StyleKimura, Y., Tsukui, D., & Kono, H. (2021). Uric Acid in Inflammation and the Pathogenesis of Atherosclerosis. International Journal of Molecular Sciences, 22(22), 12394. https://doi.org/10.3390/ijms222212394







