Janus-Faced Molecules against Plant Pathogenic Fungi
Abstract
:1. Introduction
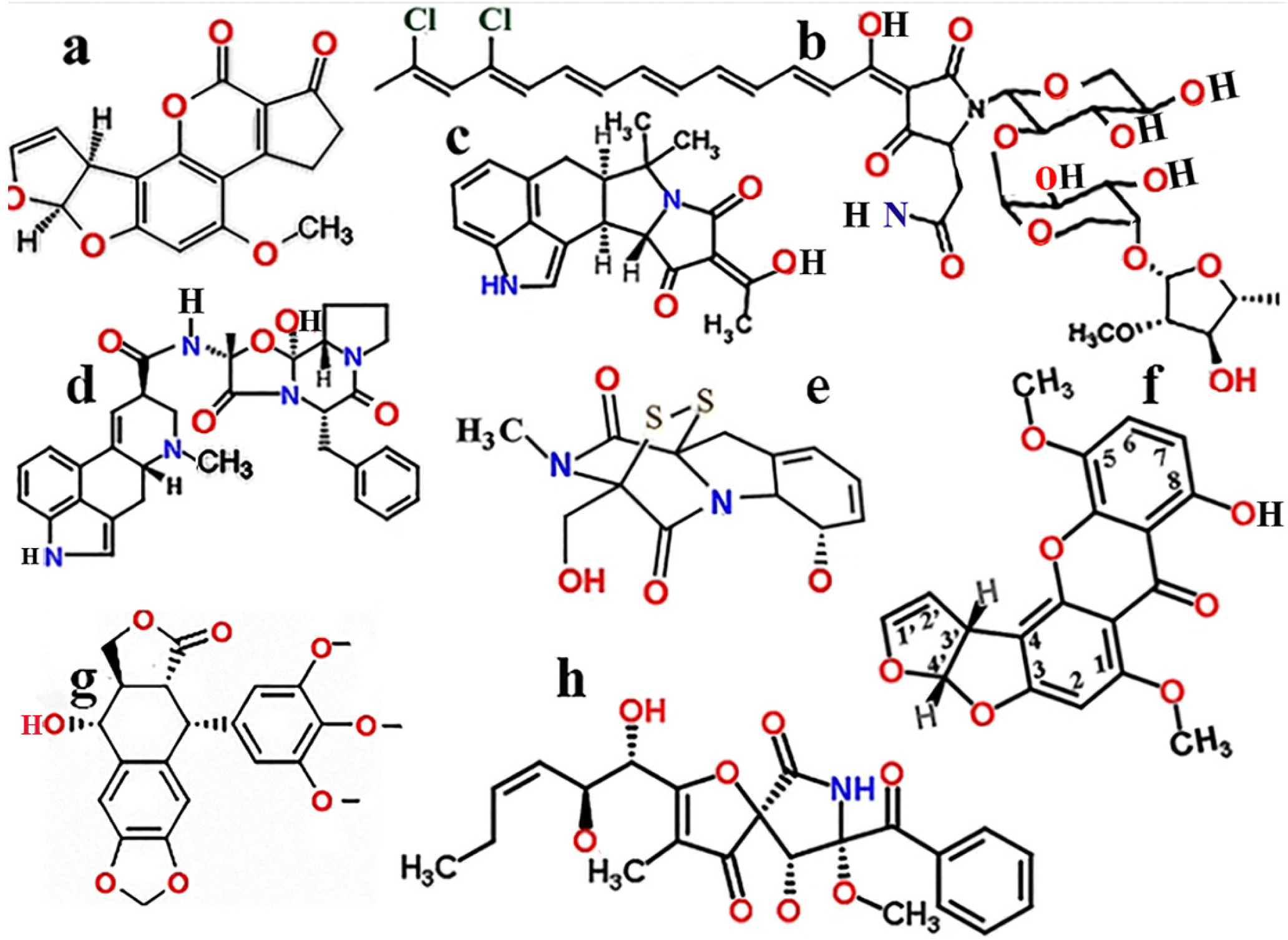
1.1. Aspergillus Toxins
1.2. Structural and Toxic Similarities of Aspergillus Mycotoxins
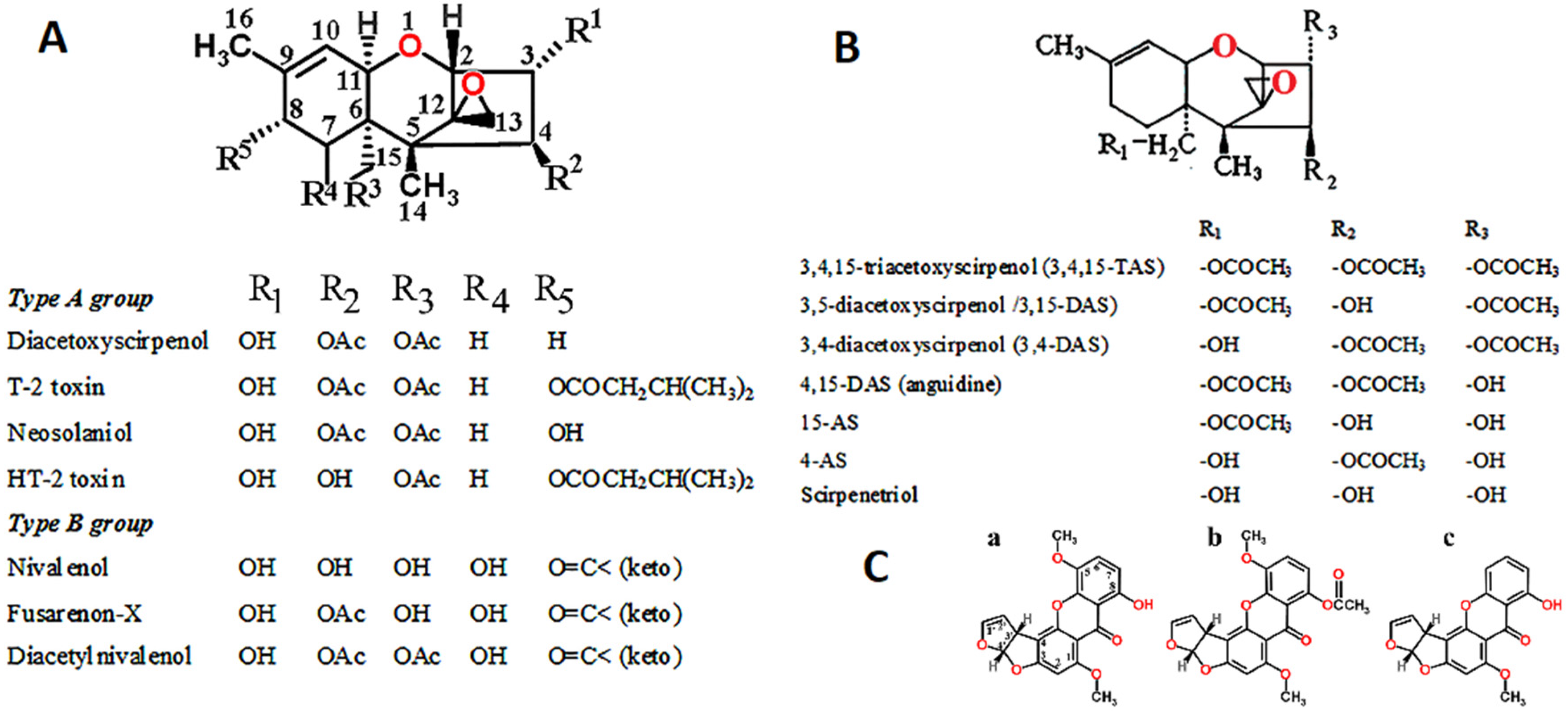
1.3. Fusarium Toxins
1.4. Cryptococcus Toxins
1.5. Toxins Inhibiting the Growth of Aspergillus Species
2. Animal Models to Test Mycoses Caused by Plant Pathogenic Fungi
Detection of Serum Beta (1-3)-D-Glucan
3. Treatment of Plant Pathogenic Fungal Infections
3.1. Therapy of Invasive Plant Mycoses
Aspergillosis and Cryptococcosis
- -
- Infection with conidial spores of Aspergillus species should be avoided.
- -
- Filtered air should be introduced into the air-conditioning systems in bone and marrow transplant units.
- -
- Invasive aspergillosis can be effectively treated with azoles, especially with voriconazole.
- -
- Due to the relatively fast-developing resistance, the combined therapy of voriconazole with amphotericin B or in combination with other fungal agents is recommended.
- -
- Double and triple antifungal combinations against clinical isolates of Aspergillus fumigatus and A. terreus are recommended. Combinations of caspofungin with either amphotericin B or voriconazole were additive for all the isolates, and antagonism was not observed. In contrast, the interaction between voriconazole and fluorocytosine was not synergistic; rather, antagonism was noted for 93% of the isolates [116].
- -
- Prophylactic measures should be taken to prevent the infection of the personnel.
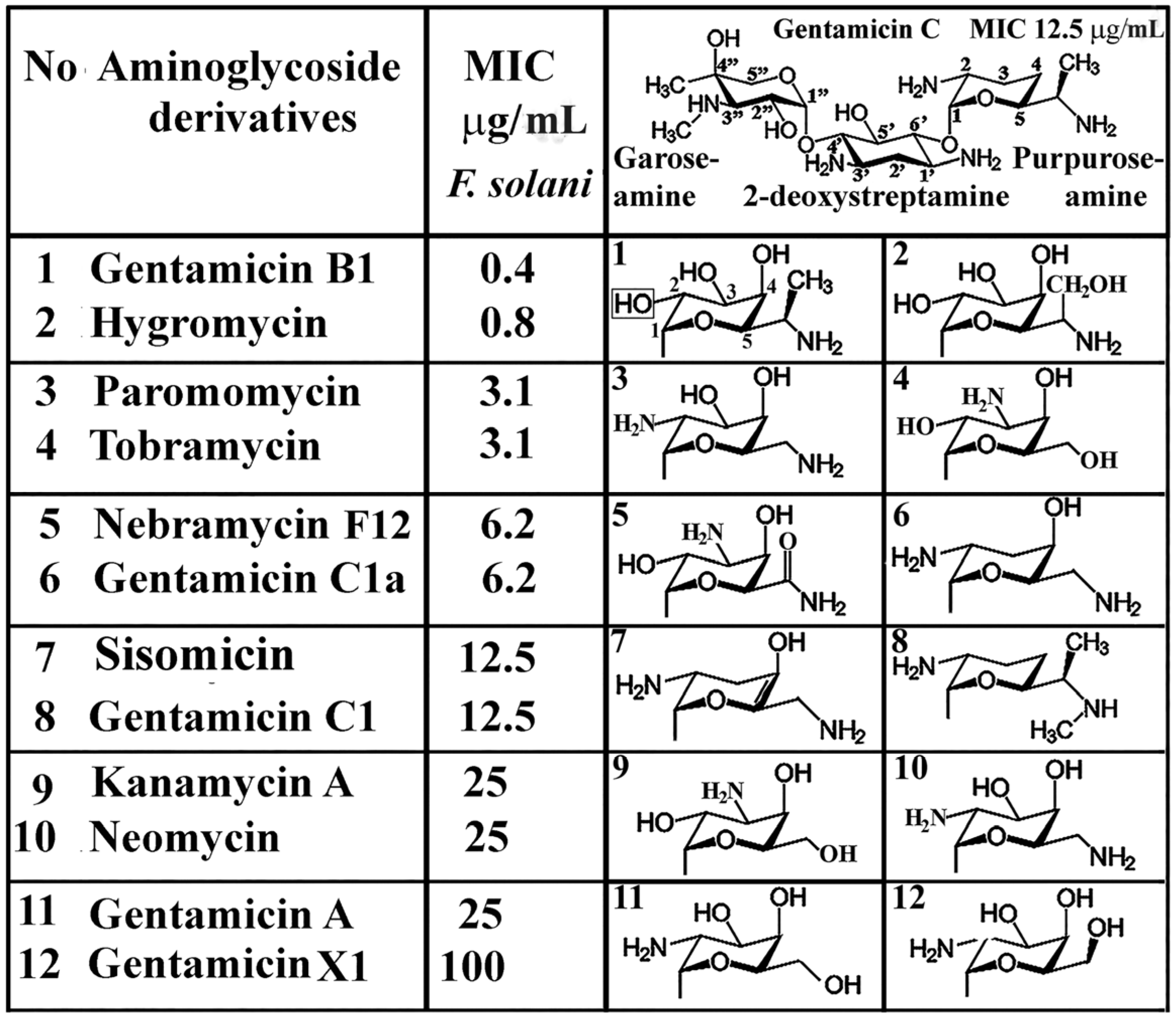
4. Functional Groups of Aminoglycosides against Plant Pathogenic Fungi
5. Discussion
6. Conclusions
- -
- Cytotoxicity is attributable to the presence of multiple hydroxyl groups. In poly-hydroxylated benzenes, toxicity is associated with hydrophobicity and the probability of radical formation [123];
- -
- -
- Mutagenicity and genotoxicity could be contributed by the simultaneous presence of keto, hydroxy, and cumulative presence of carboxy groups, although the genotoxicity and mutagenicity of ketamines are doubted [124];
- -
- -
- Ring structures are not aromatic and do not contribute to genotoxicity. Examples are most of the vitamins, nucleic acids, enzymes, coenzymes, hormones, and alkaloids containing N-based heterocycles as scaffolds;
- -
- Polyhydroxy and carbonyl (=CO) groups contribute to the oxidation state but are biodegradable;
- -
- Poly(hydroxy acids) are prepared by the self-condensation polymerization of hydroxy acids. They are biodegradable and have the potential to be chemically recycled [127];
- -
- Gentamicins are less toxic than one would expect from their oxygen-containing substituents.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hammond, N.G.L. The Oxford Classical Dictionary, 2nd ed.; Hammond, N.G.L., Scullard, H.H., Eds.; Clarendon Press: Oxford, UK, 1970; ISBN 978-0198691174. [Google Scholar]
- Gupta, N.; Verma, K.; Nalla, S.; Kulshreshtha, A.; Lall, R.; Prasad, S. Free Radicals as a Double-Edged Sword: The Cancer Preventive and Therapeutic Roles of Curcumin. Molecules 2020, 25, 5390. [Google Scholar] [CrossRef]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M.G. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef]
- A Receptor-Mediated Pathway for Cholesterol Homeostasis, Nobel lecture, December 9, 1985 by Michael Brown and Joseph Goldstein. Available online: http://nobelprize.virtual.museum/nobel_prizes/medicine/laureates/1985/brown-goldstein-lecture.pdf (accessed on 5 November 2021).
- Shivaji, A.; Thadke, V.; Hridya, M.; Dinithi, J.; Perera, R.; Gil, R.R.; Mukherjee, A.; Ly, D.H. Shape selective bifacial recognition of double helical DNA. Commun. Chem. 2018, 1, 79. [Google Scholar] [CrossRef] [Green Version]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Novotna, E. Ochratoxin A: Developmental and reproductive toxicity—An overview. Birth Defects Res. B Dev. Reprod. Toxicol. 2013, 98, 493–502. [Google Scholar] [CrossRef]
- Wang, J.S.; Groopman, J.D. DNA damage by mycotoxins. Mutat. Res. 1999, 424, 167–181. [Google Scholar] [CrossRef]
- Dornetshuber, R.; Heffeter, P.; Kamyar, M.R.; Peterbauer, T.; Berger, W.; LemmensGruber, R. Enniatin exerts p53-dependent cytostatic and p53-independent cytotoxic activities against human cancer cells. Chem. Res. Toxicol. 2007, 20, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Behm, C.; Degen, G.H.; Föllmann, W. The Fusarium toxin enniatin B exerts no genotoxic activity, but pronounced cytotoxicity in vitro. Mol. Nutr. Food Res. 2009, 53, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Tedjiotsop Feudjio, F.; Dornetshuber, R.; Lemmens, M.; Hoffmann, O.R.; LemmensGruber, R.; Berger, W. Beauvericin and enniatin: Emerging toxins and/or remedies? World Mycotox. J. 2010, 3, 415–430. [Google Scholar] [CrossRef]
- Yoon, H.J.; Choi, H.Y.; Kim, Y.K.; Song, Y.J.; Ki, M. Prevalence of fungal infections using National Health Insurance data from 2009–2013, South Korea. Epidemiol. Health 2014, 36, E2014017. [Google Scholar] [CrossRef] [Green Version]
- Hoenigl, M. Invasive Fungal Disease complicating COVID-19: When it rains it pours. Clin. Infect. Dis. 2021, 73, e1645–e1648. Available online: https://ncbi.nlm.nih.gov/pmc/articles/pmc7499555 (accessed on 5 November 2021). [CrossRef]
- Van de Veerdonk, F.L.; Brüggemann, R.J.; Vos, S.; De Hertogh, G.; Wauters, J.; Reijers, M.H.E.; Netea, M.; Shouten, J.A.; Verwei, P. COVID-19-Associated Aspergillus Tracheobronchitis: The interplay between viral tropism, host defence, and fungal invasion. Lancet Respir. Med. 2021, 9, 795–802. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis external icon. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Rogers, T.R.; Talento, A.F. COVID-19 associated invasive pulmonary aspergillosis: Diagnostic and therapeutic challenges external icon. J. Fungi 2020, 6, 115. [Google Scholar] [CrossRef]
- Verweij, P.E.; Gangneu, J.; Bassetti, M.; Bruggemann, R.J.M.; Cornely, O.A.; Koehler, P.; Hoenigl, M.; Lass-Flörl, C.; van de Veerdonk, F.L.; Chakrabarti, A. Diagnosing COVID-19-associated pulmonary aspergillosis external icon. Lancet Microbe 2020, 1, E53–E55. [Google Scholar] [CrossRef]
- Dellière, S.; Dugnon, E.; Fodil, S.; Voicu, S.; Collet, M.; Oillic, P.; Salmona, M.; Depret, F.; Ghelfenstein-Ferreira, T.; Plaud, B. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: A French multicentric retrospective cohortexternal icon. Clin. Microbiol. Infect. 2020, 27, 790.e1–790.e5. [Google Scholar] [CrossRef]
- Benedetti, M.F.; Alava, K.H.; Sagardia, J.; Cadena, R.C.; Laplume, D.; Capece, P.; Posse, G.; Nusblat, A.D.; Cuestas, M.L. COVID-19 associated pulmonary aspergillosis in ICU patients: Report of five cases from Argentina. Med. Mycol. Case Rep. 2020, 31, 24–28. [Google Scholar] [CrossRef]
- Marr, K.A.; Platt, A.; Tornheim, J.A.; Zhang, S.X.; Datta, K.; Cardozo, C.; Garcia-Vidal, C. Aspergillosis complicating severe coronavirus disease. Emerg. Infect. Dis. 2021, 27, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Schoental, R. Trichothecenes, zearalenone, and other carcinogenic metabolites of Fusarium and related microfungi. In Advances in Cancer Research; Klein, G., Weinhouse, S., Eds.; Academic Press: Cambridge, MA, USA, 1985; Volume 45, pp. 217–290. [Google Scholar]
- Sulaiman, M.R.; Chye, F.Y.; Hamid, A.A.; Yatim, A.M. The occurrence of aflatoxins in raw shelled peanut samples from three districts of Perak, Malaysia. Electron J. Environ. Food Chem. 2007, 2045–2052. Available online: http://eprints.ums.edu.my/3769/ (accessed on 5 November 2021).
- Palacios, C.F.; Spichler Moffarah, A. Diagnosis of pneumonia due to ivasive moulds. Diagnostics 2021, 11, 1226. Available online: https://doi.org/10.3390/diagnostics11071226 (accessed on 5 November 2021). [CrossRef]
- Al-Hatmi, A.M.S.; Bonifaz, A.; Ranque, S.; Sybren de Hoog, G.; Verweij, P.E.; Meis, J.F. Current antifungal treatment of fusariosis. Int. J. Antimicrob. Agents 2018, 51, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Banfalvi, G. Retrospective evaluation of in vitro effect of gentamicin B1 against Fusarium species. Appl. Microbiol. Biotechnol. 2018, 102, 10353–10359. [Google Scholar] [CrossRef]
- Banfalvi, G. Antifungal activity of gentamicin B1 against systemic plant mycoses. Molecules 2020, 25, 2401. [Google Scholar] [CrossRef]
- Baradaran-Heravi, A.; Niesser, J.; Balgi, A.D.; Choi, K.; Zimmerman, C.; South, A.P.; Anderson, H.J.; Strynadka, N.C.; Bally, M.B.; Roberge, M. Gentamicin B1 is a minor gentamicin component with major nonsense mutation suppression activity. Proc. Natl. Acad. Sci. USA 2017, 114, 3479–3484. [Google Scholar] [CrossRef] [Green Version]
- Bidou, L.; Bugaud, O.; Belakhov, V.; Baasov, T.; Namy, O. Characterization of new-generation aminoglycoside promoting premature termination codon readthrough in cancer cells. RNA Biol. 2017, 14, 378–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baradaran-Heravi, A.; Niesser, J.; Balgi, A.D.; Choi, K.; Zimmerman, C.; South, A.P.; Anderson, H.J.; Strynadka, N.C.; Bally, M.B.; Roberge, M. Retraction for Baradaran-Heravi et al., Gentamicin B1 is a minor gentamicin component with major nonsense mutation suppression activity. Proc. Nat. Acad. Sci. USA 2018, 115, E1185. [Google Scholar] [CrossRef] [Green Version]
- Gadó, I.; Bérdy, J.; Koczka, I.; Horváth, I.; Járay, M.; Zlatos, G. Gentamicin Antibiotics. Hungarian Patent 1975, 30 December 1973. [Google Scholar]
- Bérdy, J.; Pauncz, J.K.; Vajna, Z.M.; Horváth, G.; Gyimesi, J.; Koczka, I. Metabolites of gentamicin-producing Micromonospora species I. Isolation and identification of metabolites. J. Antibiot. 1977, 30, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Van de Veerdonk, F.L.; Gresnigt, M.S.; Romani, L.; Netea, M.G.; Latgé, J.-P. Aspergillus fumigatus morphology and dynamic host interactions. Nat. Rev. Microbiol. 2017, 15, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Pócsi, I.; Király, G.; Bánfalvi, G. Antineoplastic potential of mycotoxins. Acta Microbiol. Immunol. 2018, 65, 267–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pócsi, I.; Giacometti, F.; Ambrus, Á.; Logrieco, A.F. Editorial: Aspergillus-derived mycotoxins in the feed and food chain. Front Microbiol. 2020, 11, 606108. [Google Scholar] [CrossRef] [PubMed]
- Ráduly, Z.; Szabó, L.; Madar, A.; Pócsi, I.; Csernoch, L. Toxicological and medical aspects of Aspergillus-derived mycotoxins entering the feed and food chain. Front Microbiol. 2020, 10, 2908. [Google Scholar] [CrossRef] [Green Version]
- Peles, F.; Sipos, P.; Győri, Z.; Pfliegler, W.P.; Giacometti, F.; Serraino, A.; Pagliuca, G.; Gazzotti, T.; Pócsi, I. Adverse effects, transformation and channeling of aflatoxins into food raw materials in livestock. Front Microbiol. 2019, 10, 2861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norlia, M.; Jinap, S.; Nor-Khaizura, M.A.R.; Radu, S.; Samsudin, N.I.P.; Azri, F.A. Aspergillus section Flavi and aflatoxins: Occurrence, detection, and identification in raw peanuts and peanut-based products along the supply chain. Front Microbiol. 2019, 10, 2602. [Google Scholar] [CrossRef] [Green Version]
- Miklós, G.; Angeli, C.; Ambrus, Á.; Nagy, A.; Kardos, V.; Zentai, A.; Kerekes, K.; Farkas, Z.; Józwiak, Á.; Bartók, T. Detection of aflatoxins in different matrices and food-chain positions. Front Microbiol. 2021, 12, 669714. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Yue, X.; Mwakinyali, S.E.; Zhang, W.; Zhang, Q.; Peiwu, L. Small molecular contaminant and microorganism can be simultaneously detected based on nanobody-phage: Using carcinogen aflatoxin and its main fungal Aspergillus section Flavi spp. in stored maize for demonstration. Front Microbiol. 2019, 10, 3023. [Google Scholar] [CrossRef] [PubMed]
- Serraino, A.; Bonilauri, P.; Kerekes, K.; Farkas, Z.; Giacometti, F.; Canever, A.; Zambrini, A.V.; Ambrus, Á. Occurence of aflatoxin M1 in raw milk marketed in Italy: Exposure assessment and risk characterization. Front Microbiol. 2019, 10, 2516. [Google Scholar] [CrossRef]
- Pfliegler, W.P.; Pócsi, I.; Győri, Z.; Pusztahelyi, T. Aspergilli and their mycotoxins: Metabolic interactions with plants and the soil biota. Front Microbiol. 2019, 10, 2921. [Google Scholar] [CrossRef] [Green Version]
- Soni, P.; Gangurde, S.S.; Ortega-Beltran, A.; Kumar, R.; Parmar, S.; Hari, K.P.; Sudini, H.K.; Lei, Y.; NI, X.; Huai, D.; et al. Functional biology and molecular mechanisms of host-pathogen interactions for aflatoxin contamination in groundnut (Arachis hypogaea L.) and Maize (Zea mays L.). Front Microbiol. 2020, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.H.; Carbone, I.; Luis, J.M.; Payne, G.A.; Bowen, K.L.; Hagan, A.K.; RoKemerait, R.; Heiniger, R.; Ojiambo, P.S. Biocontrol strains differentially shift the genetic structure of indigenous soil populations of Aspergillus flavus. Front Microbiol. 2019, 10, 1738. [Google Scholar] [CrossRef] [Green Version]
- Agbetiameh, D.; Ortega-Beltran, A.; Awuah, R.T.; Atehnkeng, J.; Islam, M.-S.; Callicott, KA.; Cotty, P.J.; Bandyopadhyay, R. Potential of atoxigenic Aspergillus flavus vegetative compatibility groups associated with maize and groundnut in Ghana as biocontrol agents for aflatoxin management. Front Microbiol. 2019, 10, 2069. [Google Scholar] [CrossRef]
- Shenge, K.C.; Adhikari, B.N.; Akande, A.; Callicott, K.A.; Atehnkeng, J.; Ortega-Beltran, A.; Kumar, P.L.; Bandyopadhyay, R.; Cotty, P. Monitoring Aspergillus flavus genotypes in a multi-genotype aflatoxin biocontrol product with quantitative pyrosequencing. Front Microbiol. 2019, 10, 2529. [Google Scholar] [CrossRef]
- Lagogianni, C.S.; Tsitsigiannis, D.I. Effective biopesticides and biostimulants to reduce aflatoxins in maize fields: A Corrigendum on: Effective biopesticides and biostimulants to reduce aflatoxins in maize fields. Front Microbiol. 2020, 11, 29. [Google Scholar] [CrossRef]
- Ren, Y.; Jin, J.; Zheng, M.; Yang, Q.; Xing, F. Ethanol Inhibits aflatoxin B1 biosynthesis in Aspergillus flavus by up-regulating oxidative stress-related genes. Front Microbiol. 2020, 10, 2946. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhang, H.; Wang, Y.; Liu, F.; Li, E.; Ma, J.; Yang, B.; Zhang, C.; Li, L.; Liu, Y. Requirement of LaeA, VeA, and VelB on asexual development, ochratoxin A biosynthesis, and fungal virulence in Aspergillus ochraceus. Front. Microbiol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Barda, O.; Maor, U.; Sadhasivam, S.; Bi, Y.; Zakin, V.; Prusky, D.; Sionov, E. The pH-Responsive Transcription Factor PacC Governs Pathogenicity and Ochratoxin A Biosynthesis in Aspergillus carbonarius. Front. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Paciolla, C.; Logrieco, A.F.; Mulè, G. Plant Bioactive Compounds in Pre- and Postharvest Management for Aflatoxins Reduction. Front Microbiol. 2020, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Yang, K.; Chen, L.; Liu, M.; He, X.; Li, X.; Liu, Y.; Tian, J. Cinnamaldehyde, a promising natural preservative against Aspergillus flavus. Front Microbiol. 2019, 10, 2895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haidukowski, M.; Casamassima, E.; Cimmarusti, M.T.; Branà, M.T.; Longobardi, F.; Acquafredda, P.; Logrieco, A.; Altomar, C. Aflatoxin B1—Adsorbing capability of Pleurotus eryngii mycelium: Efficiency and modeling of the process. Front Microbiol. 2019, 10, 1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pangga, I.B.; Salvacion, A.R.; Cumagun, C.J.R. Climate change and plant diseases caused by mycotoxigenic fungi: Implications for food security. In Climate Change and Mycotoxins; DeGruyter: Berlin, Germany, 2015; pp. 1–28. [Google Scholar]
- Moretti, A.T.; Logrieco, A.F.; Susca, A. Mycotoxin: An underhand food problem. In Mycotoxigenic Fungi Methods and Protocols; Moretti, A., Susca, A., Eds.; Humana Press: New York, NY, USA, 2017; pp. 3–12. [Google Scholar]
- Sweeney, M.J.; Dobson, A.D. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998, 43, 141–158. [Google Scholar] [CrossRef]
- Pashin, Y.V.; Bakhitova, L.M. Mutagenic and carcinogenic properties of polycyclic aromatic hydrocarbons. Environ. Health Perspect. 1979, 30, 185–189. [Google Scholar] [CrossRef]
- Pitot, H.C.; Dragan, Y.P. The multistage nature of chemically induced hepatocarcinogenesis in the rat. Drug Met. Rev. 1994, 26, 209–220. [Google Scholar] [CrossRef]
- Jacob, J. The significance of polycyclic aromatic hydrocarbons as environmental carcinogens. Pure Appl. Chem. 1996, 88, 301–308. [Google Scholar] [CrossRef]
- Gerhards, N.; Neubauer, L.; Tudzynski, P.; Li, S.M. Biosynthetic pathways of ergot alkaloids. Toxins 2014, 6, 3281–3295. [Google Scholar] [CrossRef]
- Eadie, M.J. Convulsive ergotism: Epidemics of the serotonin syndrome? Lancet Neurol. 2003, 2, 429–434. [Google Scholar] [CrossRef]
- Plenge-Tellechea, F.; Soler, F.; Fernandez-Belda, F. On the inhibition mechanism of sarcoplasmic or endoplasmic reticulum Ca2+-ATPases by cyclopiazonic acid. J. Biol. Chem. 1997, 272, 2794–2800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiya, S.; Grundmann, A.; Li, X.; Li, S.M.; Turner, G. Identification of a hybrid PKS/NRPS required for pseurotin A biosynthesis in the human pathogen. Aspergillus fumigatus. ChemBioChem 2007, 8, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Voödisch, M.; Scherlach, K.; Winkler, R.; Hertweck, C.; Braun, H.P.; Roth, M.; Haas, H.; Werner, E.R.; Brakhage, A.A.; Kniemeyer, O. Analysis of the Aspergillus fumigatus proteome reveals metabolic changes and the activation of the pseurotin A biosynthesis gene cluster in response to hypoxia. J. Proteome Res. 2011, 10, 2508–2524. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Hormazabal, E.; Rodriguez, J.A.; Theoduloz, C. Cycloaspeptide A and pseurotin A from the endophytic fungus Penicillium janczewskii. Zeitschrift Naturforsch. 2008, 63, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Ninomiya, T.; Akabane, H.; Kushida, N.; Tsujiuchi, G.; Ohyama, M.; Gomi, S.; Shito, K.; Murata, T. Pseurotin A and its analogues as inhibitors of immunoglobuline E production. Bioorg. Med. Chem. Letts. 2009, 19, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Vašíček, O.; Fedr, R.; Skoroplyas, S.; Chalupa, D.; Sklenář, M.; Tharra, P.R.; Švenda, J.; Kubala, L. Natural pseurotins and analogs thereof inhibit activation of B-cells and differentiation into the plasma cells. Phytomedicine 2020, 69, 153–194. [Google Scholar] [CrossRef]
- Rubanova, D.; Dadova, P.; Vasicek, O.; Kubala, L. Pseurotin D inhibits the activation of human lymphocytes. Int. J. Mol. Sci. 2021, 2, 1938. [Google Scholar] [CrossRef]
- Vasicek, O.; Rubanova, D.; Chytkova, B.; Kubala, L. Natural pseurotins inhibit proliferation and inflammatory responses through the inactivation of STAT signaling pathways in macrophages. Food Chem. Toxicol. 2020, 141, 111348. [Google Scholar] [CrossRef]
- Abdelwahed, K.S.; Siddique, A.B.; Mohyeldin, M.M.; Qusa, M.H.; Goda, A.A.; Sitanshu, S.S.; Ayoub, N.M.; King, J.A.; Jois, S.D.; El Sayed, K. Pseurotin A as a novel suppressor of hormone-dependent breast cancer progression and recurrence by inhibiting PCSK9 secretion and interaction with LDL receptor. Pharmacology 2020, 158, 104847. [Google Scholar] [CrossRef]
- Chen, L.C.; Blank, E.S.; Casadevall, A. Extracellular proteinase activity of Cryptococcus neoformans. Clin. Diagnost. Lab. Immunol. 1996, 3, 570–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradner, W.T.; Bush, J.A.; Myllymaki, R.W.; Nettleton, D.E. Jr.; O’herron, F.A. Fermentation, isolation, and antitumour activity of sterigmatocystins. Antimicrob. Agents Chemother. 1975, 8, 159–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adpressa, D.A.; Loesgen, S. Bioprospecting chemical diversity and bioactivity in a marine derived Aspergillus terreus. Chem. Biodiv. 2016, 13, 253–259. [Google Scholar] [CrossRef]
- Kuiper-Goodman, T.; Scott, P.M. Risk assessment of the mycotoxin ochratoxin. Biomed. Environ. Sci. 1989, 2, 179–248. [Google Scholar]
- Boorman, G.A.; McDonald, M.R.; Imoto, S.; Persing, R. Renal lesions induced by ochratoxin A exposure in the F344 rat. Toxicol Pathol. 1992, 20, 236–245. [Google Scholar] [CrossRef]
- Chen, K.; Qiu, P.; Yuan, Y.; Zheng, L.; He, J.; Wang, C.; Guo, Q.; Kenny, J.; Liu, Q.; Zhao, J.; et al. Pseurotin A inhibits osteoclastogenesis and prevents ovariectomized-induced bone loss by suppressing reactive oxygen species. Theranostics 2019, 9, 1634–1650. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Pons, S.; Pinson-Gadais, L.; Picot, A.; Marchegay, G.; Bonnin-Verdal, M.N.; Ducos, C.; Barreau, C.; Roucolle, J.; Sehabigue, P.; et al. Chlorogenic acid and maize ear rot resistance: A dynamic study investigating Fusarium graminearum development, deoxynivalenol production, and phenolic acid accumulation. Mol. Plant-Microb. Interact. 2012, 25, 1605–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shank, R.A.; Foroud, N.A.; Hazendonk, P.; Eudes, F.; Blackwell, B.A. Current and future experimental strategies for structural analysis of trichothecene mycotoxins—A prospectus. Toxins 2011, 3, 1518–1553. [Google Scholar] [CrossRef] [Green Version]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef]
- Hofgaard, I.S.; Aamot, H.U.; Torp, T.; Jestoi, M.; Lattanzio, V.M.T.; Klemsdal, S.S.; Waalwijk, C.; Van der Lee, T.; Brodal, G. Associations between Fusarium species and mycotoxins in oats and spring wheat from farmers’ fields in Norway over a six-year period. World Mycotoxin J. 2016, 9, 365–378. [Google Scholar] [CrossRef]
- Gab-Allah, M.A.; Kim, B. Accurate determination of type B trichothecenes and conjugated deoxynivalenol in grains by isotope dilution–liquid chromatography tandem mass spectrometry. Food Control 2021, 121, 107557. [Google Scholar] [CrossRef]
- Normand, A.-C.; Imbert, S.; Brun., S.; Al-Hatmi, A.M.S; Chryssanthou, E.; Cassaing, S.; Schuttler., C.; Hasseine, L.; Caroline Mahinc, C.; Costa, D.; et al. Clinical origin and species distribution of Fusarium spp. Isolates identified by molecular sequencing and mass spectrometry: A European multicenter hospital prospective study. J. Fungi 2021, 25, 246. [Google Scholar] [CrossRef] [PubMed]
- Claridge, C.A.; Schmitz., H. Antitumor activity of some microbial and chemical transformation products of anguidine (4,15-diacetoxyscirpene-3-ol). Cancer Chemother. Pharmacol. 1979, 2, 81–82. [Google Scholar] [CrossRef]
- Jakšić, D.; Puel, O.; Canlet, C.; Kopjar, N.; Kosalec, I.; Šegvić Klarić, M. Cytotoxicity and genotoxicity of versicolorins and 5-methoxysterigmatocystin in A549 cells. Archives Toxicol. 2012, 6, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.; Wolf, J.M.; Casadevall, A. Virulence-associated enzymes of Cryptococcus neoformans. Eukaryot. Cell 2015, 14, 1173–1185. [Google Scholar] [CrossRef] [Green Version]
- Baldissera, M.D.; Souza, C.F.; Zeppenfeld, C.C.; Garzon, L.R.; Descovi, S.N.; Da Silva, A.S.; Stefani, L.M.; Baldisserotto, B. Urinergic signalling displays a pro-inflammatory profile in spleen and splenic lymphocytes of Rhamdia quelen fed with a diet contaminated by fungal mycotoxin: Involvement on disease pathogenesis. Microb Pathog. 2018, 123, 449–453. [Google Scholar] [CrossRef]
- Matsunaga, S.; Fusetani, N.; Kato, Y.; Hirota, H. Aurantosides A and B: Cytotoxic tetramic acid glycosides from the marine sponge Theonella sp. J. Am. Chem. Soc. 1991, 113, 9690–9692. [Google Scholar] [CrossRef]
- Sata, N.U.; Matsunaga, S.; Fusetani, N.; van Soest, R.W. Aurantosides D, E and F: New antifungal tetramic acid glycosides from the marine sponge Siliquariaspongia japonica. J. Nat. Prod. 1999, 62, 969–971. [Google Scholar] [CrossRef]
- Lewis, R.E.; Wiederhold, N.P. Murine model of invasive aspergillosis. In Antifungal Agents; Humana Press: Totowa, NU, USA, 2005; pp. 129–142. [Google Scholar] [CrossRef]
- Herbst, S.; Shah, A.; Carby, M.; Chusney, G.; Kikkeri, N.; Dorling, A.; Bignell, E.; Shaunak, S.; Armstrong-James, D. A new and clinically relevant murine model of solid-organ transplant aspergillosis. Dis. Models Mech. 2013, 6, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Banfalvi, G. Improved and adopted murine models to combat pulmonary aspergillosis. Appl. Microbiol. Biotechnol. 2018, 102, 6865–6875. [Google Scholar] [CrossRef]
- Ford, S.; Friedman, L. Experimental study of the pathogenicity of aspergilli for mice. J. Bacteriol. 1967, 94, 928–933. [Google Scholar] [CrossRef] [Green Version]
- Hanson, L.H.; Clemons, K.V.; Denning, D.W.; Stevens, D.A. Efficacy of oral saperconazole in systemic murine aspergillosis. J. Med. Vet. Mycol. 1995, 33, 311–317. [Google Scholar] [CrossRef]
- Graybill, J.R.; Kaster, S.R.; Drutz, D.J. Treatment of experimental murine aspergillosis with BAY n7133. J. Infect. Dis. 1983, 148, 898–906. [Google Scholar] [CrossRef]
- Williams, D.M.; Weiner, M.H.; Drutz, D.J. Immunologic studies of disseminated infection with Aspergillus fumigatus in the nude mouse. J Infect Dis. 1981, 143, 726–733. [Google Scholar] [CrossRef]
- Sheppard, D.C.; Rieg, G.; Chiang, L.Y.; Filler, S.G.; Edwards, J.E., Jr.; Ibrahim, A.S. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 2004, 48, 1908–1911. [Google Scholar] [CrossRef] [Green Version]
- Steinbach, W.J.; Benjamin, D.K., Jr.; Trasi, S.A.; Miller, J.L.; Schell, W.A.; Zaas, A.K.; Foster, W.M.; Perfect, J.R. Value of an inhalational model of invasive aspergillosis. Med. Mycol. 2004, 42, 417–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephens-Romero, S.D.; Mednick, A.J.; Feldmesser, M. The pathogenesis of fatal outcome in murine pulmonary aspergillosis depends on the neutrophil depletion strategy. Infect Immun. 2005, 73, 114–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szigeti, Z.M.; Talas, L.; Palicz, Z.; Szentesi, P.; Hargitai, Z.; Csernoch, L.; Balla, J.; Pocsi, I.; Banfalvi, G.; Szeman-Nagy, G. Murine model to follow hyphal development in invasive pulmonary aspergillosis. Appl. Microbiol. Biotechnol. 2018, 102, 2817–2825. [Google Scholar] [CrossRef] [PubMed]
- Pizer, S.M.; Johnston, R.E.; Rogers, D.C.; Beard, D.V. Effective presentation of medical images on an electronic display station. Radiographics 1987, 7, 1267–1274. [Google Scholar] [CrossRef] [Green Version]
- Obayashi, T. (1→3)-β-D-glucanemia in deep mycosis. Mediat. Inflamm. 1997, 6, 271–273. [Google Scholar] [CrossRef]
- Barbedo, J.G.A. Digital image processing techniques for detecting, quantifying and classifying plant diseases. Springer Plus 2013, 2, 660. [Google Scholar] [CrossRef] [Green Version]
- Nagy, G.; Hennig, G.W.; Petrenyi, K.; Kovacs, L.; Pocsi, I.; Dombradi, V.; Banfalvi, G. Time-lapse video microscopy and image analysis of adherence and growth patterns of Candida albicans strains. Appl. Microbiol. Biotechnol. 2014, 98, 5185–5194. [Google Scholar] [CrossRef]
- Rafferty, P.; Biggs, B.–A.; Crompton, G.K.; Grant, I.W. What happens to patients with pulmonary aspergilloma? Analysis of 23 cases. Thorax 1983, 38, 579–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, J.; Wannemuehler, K.; Marr, K.A.; Hadley, S.; Kontoyiannis, D.P.; Walsh, T.J.; Fridkin, S.K.; Pappas, P.G.; Warnock, D.W. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: Interim results of a prospective multicenter surveillance program. Med. Mycol. 2005, 43, 9–58. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, C.A. Pulmonary and invasive fungal infections. Semin. Respir. Crit. Care Med. 2015, 36, 639–640. [Google Scholar] [CrossRef]
- Pham, C.D.; Ahn, S.; Turner, L.A.; Wohrle, R.; Lockhart, S.R. Development and validation of benomyl birdseed agar for the isolation of Cryptococcus neoformans and Cryptococcus gattii from environmental samples. Med. Mycol. 2014, 52, 417–421. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Chamilos, G.; Lewis, R.E.; Wiederhold, N.P.; Raad, I.I.; Samonis, G.; Kontoyiannis, D.P. Pentamidine is active in a neutropenic murine model of acute invasive pulmonary fusariosis. Antimicrob Agents Chemother. 2006, 50, 294–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hata, K.; Horii, T.; Miyazaki, M.; Watanabe, N.A.; Okubo, M.; Sonoda, J.; Nakamoto, K.; Tanaka, K.; Shirotori, S.M.; Urai, N.; et al. Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis. Antimicrob. Agents Chemother. 2011, 55, 4543–4551. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.J.; Muhammed, M.; Kasperkovitz, P.V.; Vyas, J.M.; Mylonakis, E. Fusarium pathogenesis investigated using Galleria mellonella as a heterologous host. Fungal Biol. 2011, 115, 1279–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Velasco, G.Y.; Prados-Rosales, R.C.; Ortíz-Urquiza, A.; Quesada-Moraga, E.; Di Pietro, A. Galleria mellonella as model host for the trans-kingdom pathogen Fusarium oxysporum. Fungal Genet. Biol. 2011, 48, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, K.; Di Pietro, A.; Gow, N.A.; MacCallum, D. Murine model for Fusarium oxysporum invasive fusariosis reveals organ-specific structures for dissemination and long-term persistence. PLoS ONE 2014, 9, e89920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, G.M.; Márquez, J.; Treviño-Rangel, R.D.J.; Palma-Nicolás, J.P.; Garza-González, E.; Ceceñas, L.A.; González, J.G. Murine model of disseminated fusariosis: Evaluation of the fungal burden by traditional CFU and quantitative PCR. Mycopathologia 2013, 176, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Li, Z.; Liu, S.; Xie, Y.; He, S.; Deng, X.; Yang, B.; Liu, H.; Chen, G.; et al. A novel murine model of Fusarium solani keratitis utilizing fluorescent labeled fungi. Exp Eye Res. 2013, 110, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, H.; Yue, J.; Liu, S.; Li, Z.; Wang, L. Antimicrobial efficacy of corneal cross-linking in vitro and in vivo for Fusarium solani: A potential new treatment for fungal keratitis. BMC Ophthalmol. 2018, 18, 65. [Google Scholar] [CrossRef]
- Li, Y.; Fang, X.; Zhou, X.; Geng, S.; Wang, Y.; Yang, X. Pathogenicity of Conidiobolus coronatus and Fusarium solani in mouse models. Mycoses 2017, 60, 394–401. [Google Scholar] [CrossRef]
- Muraosa, Y.; Schreiber, A.Z.; Trabasso, P.; Matsuzawa, T.; Taguchi, H.; Moretti, M.L.; Mikami, Y.; Kamei, K. Development of cycling probe-based real-time PCR system to detect Fusarium species and Fusarium solani species complex (FSSC). Int. J. Med. Microbiol. 2014, 304, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Merz, W.G.; Karp, J.E.; Hoagland, M.; Goheen, M.J.; Junkins, J.M.; Hood, A.F. Diagnosis and successful treatment of fusariosis in the compromised host. J. Infect. Dis. 1988, 158, 1046–1055. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Najvar, L.K.; Bocanegra, R.; Graybill, J.R.; Patterson, T.F. Efficacy of posaconazole as treatment and prophylaxis against Fusarium solani. Antimicrobial Agents Chemother. 2010, 54, 1055–1059. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Zhou, T.; Young, C.; Boland, G.J.; Scott, P.M. Chemical and biological transformations for detoxification of trichothecene mycotoxins in human and animal food chains: A review. Trend Food Sci. Technol. 2010, 21, 67–76. [Google Scholar] [CrossRef]
- Pinton, P.; Oswald, I.P. Effect of Deoxynivalenol and other type B trichothecenes on the intestine: A review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef] [PubMed]
- Janik, E.; Niemcewicz, M.; Ceremuga, M.; Stela, M.; Saluk-Bijak, J.; Siadkowski, A.; Bijak, M. Molecular aspects of mycotoxins-a serious problem for human health. Int. J. Mol. Sci. 2020, 21, 8187. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, M.E.B.; Freire, F.D.C.O.; Maia, F.E.F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Chandrika, N.T.; Garneau-Tsodikova, S. A review of patients (2011–2015) towards combating resistance to and toxicity of aminoglycosides. MedChemComm 2016, 7, 50–68. [Google Scholar] [CrossRef] [Green Version]
- Wargo, K.A.; Edwards, J.D. Aminoglycoside-induced nephrotoxicity. J. Pharm. Pract. 2014, 27, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Netzeva, T.I.; Aptula, A.O.; Chaudary, S.H.; Duffy, J.C.; Schultz, T.W.; Schüürmann, G.; Cronin, M.T.D. Structure-activity relationships for the toxicity of substituted poly-hydroxylated benzenes to Tetrahymena pyriformis: Influence of free radical formation. QSAR Combinat Sci. 2003, 22, 575–582. [Google Scholar] [CrossRef]
- Cavalcanti, B.C.; de Andrade Neto, J.B.; de Sousa Silva, A.A.; Barreto, F.S.; de Oliveira Ferreira, J.R.; Da Silva, C.; Aires do Nascimento, F.B.S.; Gurgel do Amaral Valente Sa, L.; Iury FerreiraMagalhães, H.; Vitoriano Nobre Júniora, H.; et al. Evaluation of genotoxicity and mutagenicity of ketamine on human peripheral blood leukocytes and in Salmonerlla typhimurium. Toxicol. Vitro 2020, 62, 104718. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.O.; Loureiro-Bracarense, A.-P.; Oswald, I.P. Mycotoxins and oxidative stress: Where are we? World Mycotox J. 2018, 11, 113–133. [Google Scholar] [CrossRef]
- Gabirondo, E.; Sangroniz, A.; Etxeberria, A.; Torres-Giner, S.; Sardon, H. Poly(hydroxy acids) derived from the self-condensation of hydroxy acids: From polymerization to end-of-life options. Polymer Chem. 2020, 30, 4861–4874. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banfalvi, G. Janus-Faced Molecules against Plant Pathogenic Fungi. Int. J. Mol. Sci. 2021, 22, 12323. https://doi.org/10.3390/ijms222212323
Banfalvi G. Janus-Faced Molecules against Plant Pathogenic Fungi. International Journal of Molecular Sciences. 2021; 22(22):12323. https://doi.org/10.3390/ijms222212323
Chicago/Turabian StyleBanfalvi, Gaspar. 2021. "Janus-Faced Molecules against Plant Pathogenic Fungi" International Journal of Molecular Sciences 22, no. 22: 12323. https://doi.org/10.3390/ijms222212323
APA StyleBanfalvi, G. (2021). Janus-Faced Molecules against Plant Pathogenic Fungi. International Journal of Molecular Sciences, 22(22), 12323. https://doi.org/10.3390/ijms222212323





