Metals in ALS TDP-43 Pathology
Abstract
:1. Introduction
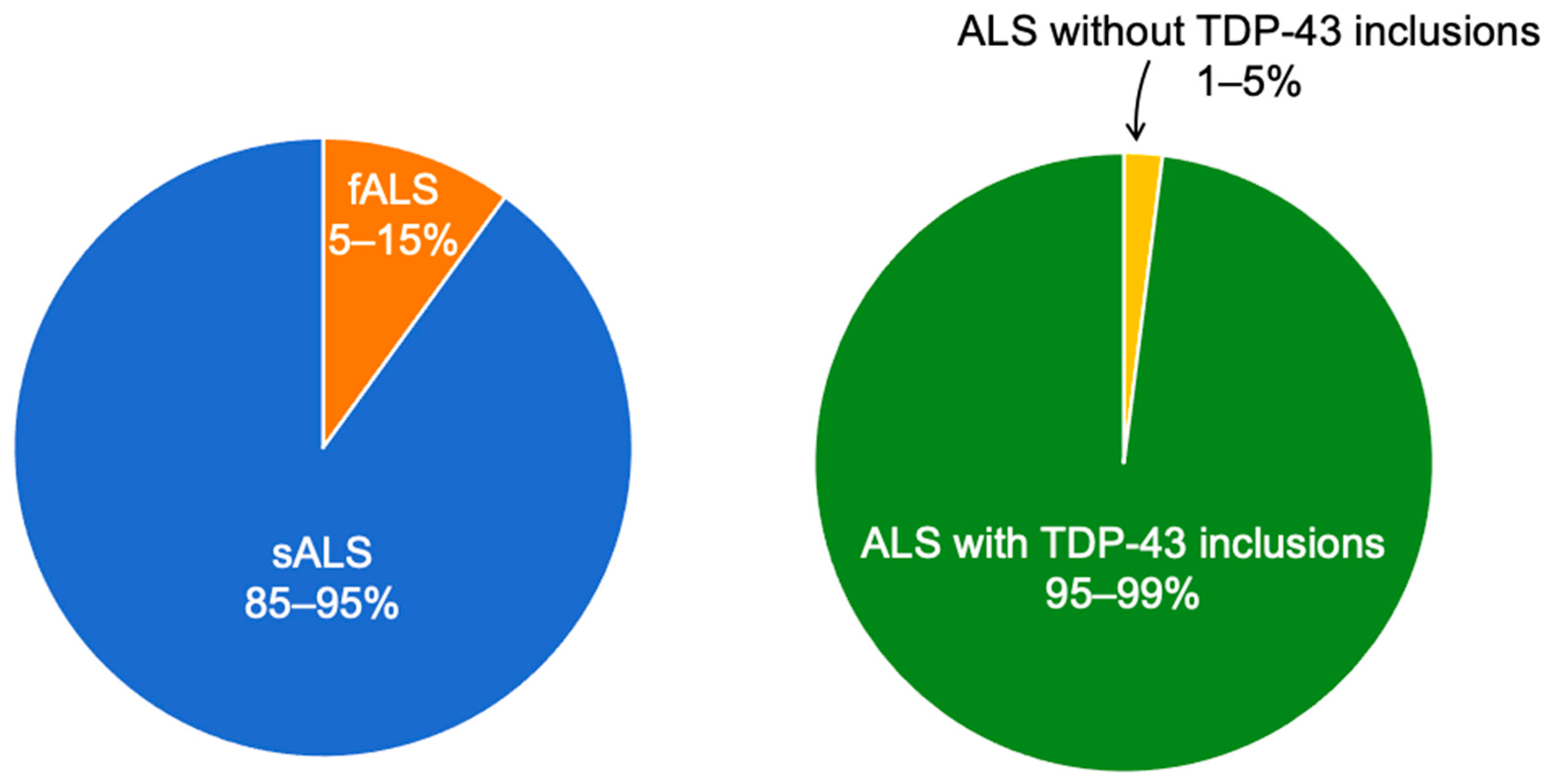
2. ALS and Metal Exposure
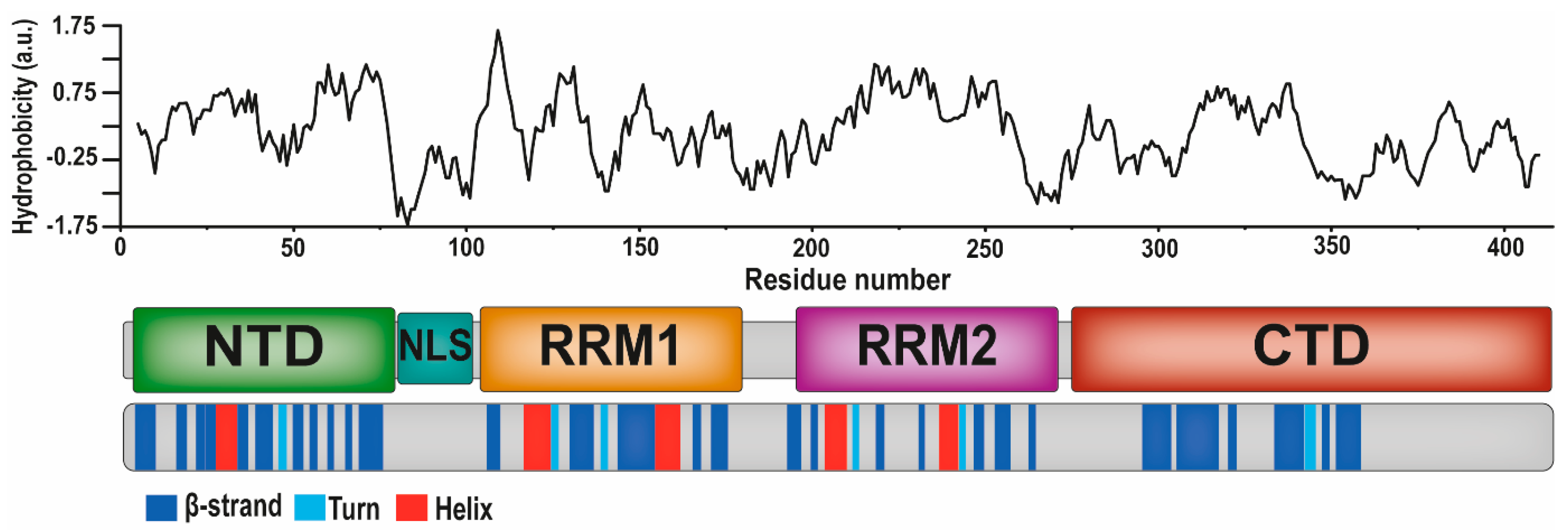
3. The TDP-43 Protein and Its Aggregation
4. Lead and TDP-43
5. Mercury and TDP-43
6. Zinc and TDP-43
7. Other Metals and TDP-43
8. Metal-Induced TDP-43 Aggregation: A Possible Pathological Mechanism in ALS?
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- Al-Chalabi, A.; Hardiman, O.; Kiernan, M.C.; Chio, A.; Rix-Brooks, B.; van den Berg, L.H. Amyotrophic lateral sclerosis: Moving towards a new classification system. Lancet Neurol. 2016, 15, 1182–1194. [Google Scholar] [CrossRef]
- Forman, M.S.; Trojanowski, J.Q.; Lee, V.M. TDP-43: A novel neurodegenerative proteinopathy. Curr. Opin. Neurobiol. 2007, 17, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Ingre, C.; Roos, P.M.; Piehl, F.; Kamel, F.; Fang, F. Risk factors for amyotrophic lateral sclerosis. Clin. Epidemiol. 2015, 7, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Wang, L.; Yan, T.; Perry, G.; Wang, X. TDP-43 proteinopathy and mitochondrial abnormalities in neurodegeneration. Mol. Cell Neurosci. 2019, 100, 103396. [Google Scholar] [CrossRef] [PubMed]
- Strong, M.J.; Kesavapany, S.; Pant, H.C. The pathobiology of amyotrophic lateral sclerosis: A proteinopathy? J. Neuropathol. Exp. Neurol. 2005, 64, 649–664. [Google Scholar] [CrossRef]
- Mackenzie, I.R.; Bigio, E.H.; Ince, P.G.; Geser, F.; Neumann, M.; Cairns, N.J.; Kwong, L.K.; Forman, M.S.; Ravits, J.; Stewart, H.; et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann. Neurol. 2007, 61, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.M.; Freeman, W.M.; Randazzo, W.T.; Stephens, H.E.; Beard, J.L.; Simmons, Z.; Connor, J.R. A CSF biomarker panel for identification of patients with amyotrophic lateral sclerosis. Neurology 2009, 72, 14–19. [Google Scholar] [CrossRef]
- Bereman, M.S.; Beri, J.; Enders, J.R.; Nash, T. Machine Learning Reveals Protein Signatures in CSF and Plasma Fluids of Clinical Value for ALS. Sci. Rep. 2018, 8, 16334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramström, M.; Ivonin, I.; Johansson, A.; Askmark, H.; Markides, K.E.; Zubarev, R.; Håkansson, P.; Aquilonius, S.M.; Bergquist, J. Cerebrospinal fluid protein patterns in neurodegenerative disease revealed by liquid chromatography-Fourier transform ion cyclotron resonance mass spectrometry. Proteomics 2004, 4, 4010–4018. [Google Scholar] [CrossRef] [PubMed]
- Reijn, T.S.; Abdo, W.F.; Schelhaas, H.J.; Verbeek, M.M. CSF neurofilament protein analysis in the differential diagnosis of ALS. J. Neurol. 2009, 256, 615–619. [Google Scholar] [CrossRef]
- Katzeff, J.S.; Bright, F.; Lo, K.; Kril, J.J.; Connolly, A.; Crossett, B.; Ittner, L.M.; Kassiou, M.; Loy, C.T.; Hodges, J.R.; et al. Altered serum protein levels in frontotemporal dementia and amyotrophic lateral sclerosis indicate calcium and immunity dysregulation. Sci. Rep. 2020, 10, 13741. [Google Scholar] [CrossRef] [PubMed]
- McAlary, L.; Chew, Y.L.; Lum, J.S.; Geraghty, N.J.; Yerbury, J.J.; Cashman, N.R. Amyotrophic Lateral Sclerosis: Proteins, Proteostasis, Prions, and Promises. Front. Cell. Neurosci. 2020, 14, 581907. [Google Scholar] [CrossRef]
- Mulligan, V.K.; Kerman, A.; Laister, R.C.; Sharda, P.R.; Arslan, P.E.; Chakrabartty, A. Early steps in oxidation-induced SOD1 misfolding: Implications for non-amyloid protein aggregation in familial ALS. J. Mol. Biol. 2012, 421, 631–652. [Google Scholar] [CrossRef]
- Parakh, S.; Atkin, J.D. Protein folding alterations in amyotrophic lateral sclerosis. Brain Res. 2016, 1648, 633–649. [Google Scholar] [CrossRef]
- Blokhuis, A.M.; Groen, E.J.; Koppers, M.; van den Berg, L.H.; Pasterkamp, R.J. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013, 125, 777–794. [Google Scholar] [CrossRef] [Green Version]
- Mori, K.; Weng, S.M.; Arzberger, T.; May, S.; Rentzsch, K.; Kremmer, E.; Schmid, B.; Kretzschmar, H.A.; Cruts, M.; Van Broeckhoven, C.; et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science 2013, 339, 1335–1338. [Google Scholar] [CrossRef]
- Ticozzi, N.; Ratti, A.; Silani, V. Protein aggregation and defective RNA metabolism as mechanisms for motor neuron damage. CNS Neurol. Disord. Drug Targets 2010, 9, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Zhou, J.; Li, Y.; Wu, K.; Chen, Z.; Luo, Z.; Zhang, X.; Liang, Y.; Esteban, M.A.; Zhou, Y.; et al. TDP-43 aggregation induced by oxidative stress causes global mitochondrial imbalance in ALS. Nat. Struct. Mol. Biol. 2021, 28, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Aschner, M.; Ghersi-Egea, J.F. Brain barrier systems: A new frontier in metal neurotoxicological research. Toxicol. Appl. Pharmacol. 2003, 192, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Roos, P.M. Ultraclean paired sampling for metal analysis in neurodegenerative disorders. J. Trace Elem. Med. Biol. 2019, 52, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; O′Regan, J.P.; Deng, H.X.; et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993, 362, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Chia, R.; Chio, A.; Traynor, B.J. Novel genes associated with amyotrophic lateral sclerosis: Diagnostic and clinical implications. Lancet Neurol. 2018, 17, 94–102. [Google Scholar] [CrossRef]
- Kim, G.; Gautier, O.; Tassoni-Tsuchida, E.; Ma, X.R.; Gitler, A.D. ALS Genetics: Gains, Losses, and Implications for Future Therapies. Neuron 2020, 108, 822–842. [Google Scholar] [CrossRef]
- Nonaka, T.; Hasegawa, M. TDP-43 Prions. Cold Spring Harb. Perspect. Med. 2018, 8, a024463. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Wang, L.; Huntley, M.L.; Perry, G.; Wang, X. Pathomechanisms of TDP-43 in neurodegeneration. J. Neurochem. 2018, 146, 7–20. [Google Scholar] [CrossRef]
- Ratti, A.; Buratti, E. Physiological functions and pathobiology of TDP-43 and FUS/TLS proteins. J. Neurochem. 2016, 138, 95–111. [Google Scholar] [CrossRef]
- Arai, T.; Hasegawa, M.; Akiyama, H.; Ikeda, K.; Nonaka, T.; Mori, H.; Mann, D.; Tsuchiya, K.; Yoshida, M.; Hashizume, Y.; et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006, 351, 602–611. [Google Scholar] [CrossRef]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreedharan, J.; Blair, I.P.; Tripathi, V.B.; Hu, X.; Vance, C.; Rogelj, B.; Ackerley, S.; Durnall, J.C.; Williams, K.L.; Buratti, E.; et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 2008, 319, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Berning, B.A.; Walker, A.K. The Pathobiology of TDP-43 C-Terminal Fragments in ALS and FTLD. Front. Neurosci. 2019, 13, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Görß, D.; Kilimann, I.; Dyrba, M.; Nitsch, S.; Krause, B.; Teipel, S. LATE: Nicht jede Demenz ist Alzheimer—Diskussion einer neuen Krankheitsentität am Fallbeispiel. Nervenarzt 2021, 92, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Fuller-Thomson, E.; Deng, Z. Could Lifetime Lead Exposure Play a Role in Limbic-predominant Age-related TDP-43 Encephalopathy (LATE)? J. Alzheimer’s Dis. 2020, 73, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Hergesheimer, R.C.; Chami, A.A.; de Assis, D.R.; Vourc′h, P.; Andres, C.R.; Corcia, P.; Lanznaster, D.; Blasco, H. The debated toxic role of aggregated TDP-43 in amyotrophic lateral sclerosis: A resolution in sight? Brain 2019, 142, 1176–1194. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Bharathi, V.; Sivalingam, V.; Girdhar, A.; Patel, B.K. Molecular Mechanisms of TDP-43 Misfolding and Pathology in Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2019, 12, 25. [Google Scholar] [CrossRef]
- Winton, M.J.; Igaz, L.M.; Wong, M.M.; Kwong, L.K.; Trojanowski, J.Q.; Lee, V.M. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. J. Biol. Chem. 2008, 283, 13302–13309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, Y.; Yamada, S. A novel hypothesis on metal dyshomeostasis and mitochondrial dysfunction in amyotrophic lateral sclerosis: Potential pathogenetic mechanism and therapeutic implications. Eur. J. Pharmacol. 2021, 892, 173737. [Google Scholar] [CrossRef] [PubMed]
- Bozzo, F.; Mirra, A.; Carri, M.T. Oxidative stress and mitochondrial damage in the pathogenesis of ALS: New perspectives. Neurosci. Lett. 2017, 636, 3–8. [Google Scholar] [CrossRef]
- Ash, P.E.A.; Dhawan, U.; Boudeau, S.; Lei, S.; Carlomagno, Y.; Knobel, M.; Al Mohanna, L.F.A.; Boomhower, S.R.; Newland, M.C.; Sherr, D.H.; et al. Heavy Metal Neurotoxicants Induce ALS-Linked TDP-43 Pathology. Toxicol. Sci. 2019, 167, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer′s-Association. Alzheimer′s disease facts and figures. Alzheimer′s Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef] [PubMed]
- Melo, F.; Caballero, L.; Zamorano, E.; Ventura, N.; Navarro, C.; Doll, I.; Zamorano, P.; Cornejo, A. The Cytotoxic Effect of alpha-Synuclein Aggregates. ChemPhysChem 2021, 22, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Rocha Cabrero, F.; Morrison, E.H. Lewy Bodies. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Saa, P.; Harris, D.A.; Cervenakova, L. Mechanisms of prion-induced neurodegeneration. Expert Rev. Mol. Med. 2016, 18, e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prusiner, S.B. Nobel Lecture: Prions. Proc. Natl. Acad. Sci. USA 1998, 95, 13363–13383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, F.; Tenreiro, S.; Miller-Fleming, L.; Outeiro, T.F. Visualization of cell-to-cell transmission of mutant huntingtin oligomers. PLoS Curr. 2011, 3, RRN1210. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Blum, T.B.; Farrell, D.P.; DiMaio, F.; Abrahams, J.P.; Luo, J. Cryo-electron Microscopy Imaging of Alzheimer’s Amyloid-beta 42 Oligomer Displayed on a Functionally and Structurally Relevant Scaffold. Angew. Chem. Int. Ed. Engl. 2021, 60, 18680–18687. [Google Scholar] [CrossRef]
- Luo, J.; Wärmländer, S.K.; Gräslund, A.; Abrahams, J.P. Alzheimer peptides aggregate into transient nanoglobules that nucleate fibrils. Biochemistry 2014, 53, 6302–6308. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, H.A.; Ahsan, W.; Ibrahim, A.M.M.; Khubrani, R.A.Y.; Haddadi, Z.A.A.; Safhi, A.Y.F.; Shubayr, N.; Al Bratty, M.; Najmi, A. Investigation of bovine serum albumin aggregation upon exposure to silver(I) and copper(II) metal ions using Zetasizer. Open Chem. 2021, 19, 987–997. [Google Scholar] [CrossRef]
- Wallin, C.; Jarvet, J.; Biverstål, H.; Wärmländer, S.; Danielsson, J.; Gräslund, A.; Abelein, A. Metal ion coordination delays amyloid-beta peptide self-assembly by forming an aggregation-inert complex. J. Biol. Chem. 2020, 295, 7224–7234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berntsson, E.; Paul, S.; Vosough, F.; Sholts, S.B.; Jarvet, J.; Roos, P.M.; Barth, A.; Gräslund, A.; Wärmländer, S. Lithium ions display weak interaction with amyloid-beta (Abeta) peptides and have minor effects on their aggregation. Acta Biochim. Pol. 2021, 68, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Jucker, M. The amyloid state of proteins in human diseases. Cell 2012, 148, 1188–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abelein, A.; Abrahams, J.P.; Danielsson, J.; Gräslund, A.; Jarvet, J.; Luo, J.; Tiiman, A.; Wärmländer, S.K. The hairpin conformation of the amyloid beta peptide is an important structural motif along the aggregation pathway. J. Biol. Inorg. Chem. 2014, 19, 623–634. [Google Scholar] [CrossRef]
- Sinha, M.S.; Ansell-Schultz, A.; Civitelli, L.; Hildesjo, C.; Larsson, M.; Lannfelt, L.; Ingelsson, M.; Hallbeck, M. Alzheimer′s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018, 136, 41–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armen, R.S.; DeMarco, M.L.; Alonso, D.O.; Daggett, V. Pauling and Corey’s alpha-pleated sheet structure may define the prefibrillar amyloidogenic intermediate in amyloid disease. Proc. Natl. Acad. Sci. USA 2004, 101, 11622–11627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wärmländer, S.K.T.S.; Österlund, N.; Wallin, C.; Wu, J.; Luo, J.; Tiiman, A.; Jarvet, J.; Gräslund, A. Metal binding to the amyloid-beta peptides in the presence of biomembranes: Potential mechanisms of cell toxicity. J. Biol. Inorg. Chem. 2019, 24, 1189–1196. [Google Scholar] [CrossRef] [Green Version]
- Owen, M.C.; Gnutt, D.; Gao, M.; Wärmländer, S.K.T.S.; Jarvet, J.; Gräslund, A.; Winter, R.; Ebbinghaus, S.; Strodel, B. Effects of in vivo conditions on amyloid aggregation. Chem. Soc. Rev. 2019, 48, 3946–3996. [Google Scholar] [CrossRef]
- Wallin, C.; Sholts, S.B.; Österlund, N.; Luo, J.; Jarvet, J.; Roos, P.M.; Ilag, L.; Gräslund, A.; Wärmländer, S.K.T.S. Alzheimer’s disease and cigarette smoke components: Effects of nicotine, PAHs, and Cd(II), Cr(III), Pb(II), Pb(IV) ions on amyloid-beta peptide aggregation. Sci. Rep. 2017, 7, 14423. [Google Scholar] [CrossRef] [Green Version]
- Wärmländer, S.; Tiiman, A.; Abelein, A.; Luo, J.; Jarvet, J.; Söderberg, K.L.; Danielsson, J.; Gräslund, A. Biophysical studies of the amyloid beta-peptide: Interactions with metal ions and small molecules. ChemBioChem 2013, 14, 1692–1704. [Google Scholar] [CrossRef]
- Caragounis, A.; Price, K.A.; Soon, C.P.; Filiz, G.; Masters, C.L.; Li, Q.X.; Crouch, P.J.; White, A.R. Zinc induces depletion and aggregation of endogenous TDP-43. Free Radic. Biol. Med. 2010, 48, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Warmlander, S.K.; Graslund, A.; Abrahams, J.P. Cross-interactions between the Alzheimer Disease Amyloid-beta Peptide and Other Amyloid Proteins: A Further Aspect of the Amyloid Cascade Hypothesis. J. Biol. Chem. 2016, 291, 16485–16493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, P.; Rodriguez-Muela, N.; Klim, J.R.; de Boer, A.S.; Agrawal, S.; Sandoe, J.; Lopes, C.S.; Ogliari, K.S.; Williams, L.A.; Shear, M.; et al. Reactive Astrocytes Promote ALS-like Degeneration and Intracellular Protein Aggregation in Human Motor Neurons by Disrupting Autophagy through TGF-beta1. Stem. Cell Rep. 2017, 9, 667–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, T.N.; Lim, N.K.; Grubman, A.; Li, Q.X.; Volitakis, I.; White, A.R.; Crouch, P.J. Increased metal content in the TDP-43(A315T) transgenic mouse model of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Front. Aging Neurosci. 2014, 6, 15. [Google Scholar] [CrossRef]
- Tokuda, E.; Okawa, E.; Watanabe, S.; Ono, S.; Marklund, S.L. Dysregulation of intracellular copper homeostasis is common to transgenic mice expressing human mutant superoxide dismutase-1s regardless of their copper-binding abilities. Neurobiol. Dis. 2013, 54, 308–319. [Google Scholar] [CrossRef]
- Felmus, M.T.; Patten, B.M.; Swanke, L. Antecedent events in amyotrophic lateral sclerosis. Neurology 1976, 26, 167–172. [Google Scholar] [CrossRef]
- Roos, P.M.; Vesterberg, O.; Syversen, T.; Flaten, T.P.; Nordberg, M. Metal concentrations in cerebrospinal fluid and blood plasma from patients with amyotrophic lateral sclerosis. Biol. Trace Elem. Res. 2013, 151, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Sutedja, N.A.; Veldink, J.H.; Fischer, K.; Kromhout, H.; Heederik, D.; Huisman, M.H.; Wokke, J.H.; van den Berg, L.H. Exposure to chemicals and metals and risk of amyotrophic lateral sclerosis: A systematic review. Amyotroph. Lateral Scler. 2009, 10, 302–309. [Google Scholar] [CrossRef]
- Roos, P.M.; Vesterberg, O.; Nordberg, M. Metals in motor neuron diseases. Exp. Biol. Med. 2006, 231, 1481–1487. [Google Scholar] [CrossRef]
- Roos, P.M. Studies on Metals in Motor Neuron Disease; Karolinska Institutet: Solna, Sweden, 2013. [Google Scholar]
- Peters, T.L.; Kamel, F.; Lundholm, C.; Feychting, M.; Weibull, C.E.; Sandler, D.P.; Wiebert, P.; Sparen, P.; Ye, W.; Fang, F. Occupational exposures and the risk of amyotrophic lateral sclerosis. Occup. Environ. Med. 2017, 74, 87–92. [Google Scholar] [CrossRef]
- Hamilton, A. Lead Poisoning in Potteries, Tile Works, and Porcelain Enameled Sanitary Ware Factories; Bureau of Labor: Washington, DC, USA, 1912.
- OSHA. Safety and Health Topics Lead; United States Department of Labor: Washington, DC, USA, 2021.
- Bar-Sela, S.; Reingold, S.; Richter, E.D. Amyotrophic lateral sclerosis in a battery-factory worker exposed to cadmium. Int. J. Occup. Environ. Health 2001, 7, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Bachmeyer, C.; Bagur, E.; Lenglet, T.; Maier-Redelsperger, M.; Lecomte, I. Lead poisoning mimicking amyotrophic lateral sclerosis: An adverse effect of rituals. Am. J. Med. 2012, 125, e5–e6. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.S.; Kim, E.A.; Lee, S.W.; Kim, M.K.; Kang, S.K. A case of amyotrophic lateral sclerosis in electronic parts manufacturing worker exposed to lead. Neurotoxicology 2007, 28, 324–327. [Google Scholar] [CrossRef]
- Roos, E.; Wärmländer, S.; Meyer, J.; Sholts, S.B.; Jarvet, J.; Gräslund, A.; Roos, P.M. Amyotrophic Lateral Sclerosis After Exposure to Manganese from Traditional Medicine Procedures in Kenya. Biol. Trace Elem. Res. 2021, 199, 3618–3624. [Google Scholar] [CrossRef]
- Voss, H. Progressive bulbar paralysis and amyotrophic lateral sclerosis from chronic manganese poisoning. Arch. Gewerbepathol. Gewerbehyg. 1939, 9, 464–476. [Google Scholar]
- Bowman, A.B.; Kwakye, G.F.; Herrero Hernandez, E.; Aschner, M. Role of manganese in neurodegenerative diseases. J. Trace Elem. Med. Biol. 2011, 25, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Adams, C.R.; Ziegler, D.K.; Lin, J.T. Mercury intoxication simulating amyotrophic lateral sclerosis. JAMA 1983, 250, 642–643. [Google Scholar] [CrossRef]
- Hyser, C.L.; Kissel, J.T.; Mendell, J.R. Three cases of amyotrophic lateral sclerosis in a common occupational environment. J. Neurol. 1987, 234, 443–444. [Google Scholar] [CrossRef]
- Kantarjian, A.D. A syndrome clinically resembling amyotrophic lateral sclerosis following chronic mercurialism. Neurology 1961, 11, 639–644. [Google Scholar] [CrossRef]
- Tanndag, T.; Ince, D.; Tekin, S.; Nourikhalichi, K.; Aktan, S. ALS-like syndrome due to aluminium intoxication. Electroencephalogr. Clin. Neurophysiol. Electromyogr. Mot. Control. 1995, 97, S229. [Google Scholar] [CrossRef]
- Chancellor, A.M.; Slattery, J.M.; Fraser, H.; Warlow, C.P. Risk factors for motor neuron disease: A case-control study based on patients from the Scottish Motor Neuron Disease Register. J. Neurol. Neurosurg. Psychiatry 1993, 56, 1200–1206. [Google Scholar] [CrossRef] [Green Version]
- Currier, R.D.; Haerer, A.F. Amyotrophic lateral sclerosis and metallic toxins. Arch. Environ. Health 1968, 17, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Chio, A.; Meineri, P.; Tribolo, A.; Schiffer, D. Risk factors in motor neuron disease: A case-control study. Neuroepidemiology 1991, 10, 174–184. [Google Scholar] [CrossRef]
- Johnson, F.O.; Atchison, W.D. The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology 2009, 30, 761–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangelsdorf, I.; Walach, H.; Mutter, J. Healing of Amyotrophic Lateral Sclerosis: A Case Report. Complement. Med. Res. 2017, 24, 175–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, F.; Kwee, L.C.; Allen, K.D.; Umbach, D.M.; Ye, W.; Watson, M.; Keller, J.; Oddone, E.Z.; Sandler, D.P.; Schmidt, S.; et al. Association between blood lead and the risk of amyotrophic lateral sclerosis. Am. J. Epidemiol. 2010, 171, 1126–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garzillo, E.M.; Lamberti, M.; Genovese, G.; Pedata, P.; Feola, D.; Sannolo, N.; Daniele, L.; Trojsi, F.; Monsurro, M.R.; Miraglia, N. Blood lead, manganese, and aluminum levels in a regional Italian cohort of ALS patients: Does aluminum have an influence? J. Occup. Environ. Med. 2014, 56, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Solovyev, N.; Mandrioli, J.; Crespi, C.M.; Bonvicini, F.; Arcolin, E.; Georgoulopoulou, E.; Michalke, B. Cerebrospinal fluid of newly diagnosed amyotrophic lateral sclerosis patients exhibits abnormal levels of selenium species including elevated selenite. Neurotoxicology 2013, 38, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheykhansari, S.; Kozielski, K.; Bill, J.; Sitti, M.; Gemmati, D.; Zamboni, P.; Singh, A.V. Redox metals homeostasis in multiple sclerosis and amyotrophic lateral sclerosis: A review. Cell Death Dis. 2018, 9, 348. [Google Scholar] [CrossRef]
- Le Gall, L.; Anakor, E.; Connolly, O.; Vijayakumar, U.G.; Duddy, W.J.; Duguez, S. Molecular and Cellular Mechanisms Affected in ALS. J. Pers. Med. 2020, 10, 101. [Google Scholar] [CrossRef]
- Arvidson, B. A review of axonal transport of metals. Toxicology 1994, 88, 1–14. [Google Scholar] [CrossRef]
- Tjalve, H.; Henriksson, J. Uptake of metals in the brain via olfactory pathways. Neurotoxicology 1999, 20, 181–195. [Google Scholar] [PubMed]
- Nordberg, G.; Fowler, B.A.; Nordberg, M. Handbook on the Toxicology of Metals, 4th ed.; Academic Press: London, UK, 2015. [Google Scholar]
- Milton, B.; Krewski, D.; Mattison, D.R.; Karyakina, N.A.; Ramoju, S.; Shilnikova, N.; Birkett, N.; Farrell, P.J.; McGough, D. Modeling U-shaped dose-response curves for manganese using categorical regression. Neurotoxicology 2017, 58, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Shiina, Y.; Arima, K.; Tabunoki, H.; Satoh, J. TDP-43 dimerizes in human cells in culture. Cell. Mol. Neurobiol. 2010, 30, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Caulfield, T.; Xu, Y.F.; Gendron, T.F.; Hubbard, J.; Stetler, C.; Sasaguri, H.; Whitelaw, E.C.; Cai, S.; Lee, W.C.; et al. The dual functions of the extreme N-terminus of TDP-43 in regulating its biological activity and inclusion formation. Hum. Mol. Genet. 2013, 22, 3112–3122. [Google Scholar] [CrossRef] [PubMed]
- Francois-Moutal, L.; Perez-Miller, S.; Scott, D.D.; Miranda, V.G.; Mollasalehi, N.; Khanna, M. Structural Insights Into TDP-43 and Effects of Post-translational Modifications. Front. Mol. Neurosci. 2019, 12, 301. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [CrossRef]
- Abraham, D.J.; Leo, A.J. Extension of the fragment method to calculate amino acid zwitterion and side chain partition coefficients. Proteins 1987, 2, 130–152. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.C.; Lee, M.H.; Chen, T.C.; Huang, J.R. Interactions between the Intrinsically Disordered Regions of hnRNP-A2 and TDP-43 Accelerate TDP-43′s Conformational Transition. Int. J. Mol. Sci. 2020, 21, 5930. [Google Scholar] [CrossRef] [PubMed]
- Mompean, M.; Romano, V.; Pantoja-Uceda, D.; Stuani, C.; Baralle, F.E.; Buratti, E.; Laurents, D.V. The TDP-43 N-terminal domain structure at high resolution. FEBS J. 2016, 283, 1242–1260. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.H.; Doudeva, L.G.; Wang, Y.T.; Shen, C.K.; Yuan, H.S. Structural insights into TDP-43 in nucleic-acid binding and domain interactions. Nucleic Acids Res. 2009, 37, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Lukavsky, P.J.; Daujotyte, D.; Tollervey, J.R.; Ule, J.; Stuani, C.; Buratti, E.; Baralle, F.E.; Damberger, F.F.; Allain, F.H. Molecular basis of UG-rich RNA recognition by the human splicing factor TDP-43. Nat. Struct. Mol. Biol. 2013, 20, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Afroz, T.; Hock, E.M.; Ernst, P.; Foglieni, C.; Jambeau, M.; Gilhespy, L.A.B.; Laferriere, F.; Maniecka, Z.; Pluckthun, A.; Mittl, P.; et al. Functional and dynamic polymerization of the ALS-linked protein TDP-43 antagonizes its pathologic aggregation. Nat. Commun. 2017, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.K.; Wu, T.H.; Wu, C.Y.; Chiang, M.H.; Toh, E.K.; Hsu, Y.C.; Lin, K.F.; Liao, Y.H.; Huang, T.H.; Huang, J.J. The N-terminus of TDP-43 promotes its oligomerization and enhances DNA binding affinity. Biochem. Biophys. Res. Commun. 2012, 425, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Shodai, A.; Morimura, T.; Ido, A.; Uchida, T.; Ayaki, T.; Takahashi, R.; Kitazawa, S.; Suzuki, S.; Shirouzu, M.; Kigawa, T.; et al. Aberrant assembly of RNA recognition motif 1 links to pathogenic conversion of TAR DNA-binding protein of 43 kDa (TDP-43). J. Biol. Chem. 2013, 288, 14886–14905. [Google Scholar] [CrossRef] [Green Version]
- Saini, A.; Chauhan, V.S. Delineation of the core aggregation sequences of TDP-43 C-terminal fragment. ChemBioChem 2011, 12, 2495–2501. [Google Scholar] [CrossRef]
- Yang, C.; Tan, W.; Whittle, C.; Qiu, L.; Cao, L.; Akbarian, S.; Xu, Z. The C-terminal TDP-43 fragments have a high aggregation propensity and harm neurons by a dominant-negative mechanism. PLoS ONE 2010, 5, e15878. [Google Scholar] [CrossRef]
- Jiang, L.L.; Che, M.X.; Zhao, J.; Zhou, C.J.; Xie, M.Y.; Li, H.Y.; He, J.H.; Hu, H.Y. Structural transformation of the amyloidogenic core region of TDP-43 protein initiates its aggregation and cytoplasmic inclusion. J. Biol. Chem. 2013, 288, 19614–19624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos, P.M.; Dencker, L. Mercury in the spinal cord after inhalation of mercury. Basic Clin. Pharmacol. Toxicol. 2012, 111, 126–132. [Google Scholar] [CrossRef]
- Pamphlett, R.; Waley, P. Uptake of inorganic mercury by the human brain. Acta Neuropathol. 1996, 92, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Schionning, J.D.; Eide, R.; Moller-Madsen, B.; Ernst, E. Detection of mercury in rat spinal cord and dorsal root ganglia after exposure to mercury vapor. Exp. Mol. Pathol. 1993, 58, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, R. Atrophy of large myelinated motor axons and declining muscle grip strength following mercury vapor inhalation in mice. Inhal. Toxicol. 2006, 18, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Wakabayashi, K.; Kakita, A.; Ikuta, F.; Takahashi, H. Selective involvement of large motor neurons in the spinal cord of rats treated with methylmercury. J. Neurol. Sci. 1998, 156, 12–17. [Google Scholar] [CrossRef]
- Schwarz, S.; Husstedt, I.; Bertram, H.P.; Kuchelmeister, K. Amyotrophic lateral sclerosis after accidental injection of mercury. J. Neurol. Neurosurg. Psychiatry 1996, 60, 698. [Google Scholar] [CrossRef] [Green Version]
- Brown, I.A. Chronic mercurialism; a cause of the clinical syndrome of amyotrophic lateral sclerosis. AMA Arch. Neurol. Psychiatry 1954, 72, 674–681. [Google Scholar] [CrossRef]
- Barber, T.E. Inorganic mercury intoxication reminiscent of amyotrophic lateral sclerosis. J. Occup. Med. 1978, 20, 667–669. [Google Scholar] [PubMed]
- Praline, J.; Guennoc, A.M.; Limousin, N.; Hallak, H.; de Toffol, B.; Corcia, P. ALS and mercury intoxication: A relationship? Clin. Neurol. Neurosurg. 2007, 109, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bhardwaj, V.; Tiwari, J.N.; Patel, D.K.; Singh, D.S.; Singh, R.L.; Kalita, J.; Misra, U.K.; Babu, G.N. Mercury exposure in sporadic amyotrophic lateral sclerosis patients from Ganga plain region in India: A retrospective study. Toxicol. Environ. Chem. 2010, 92, 373–381. [Google Scholar] [CrossRef]
- Pamphlett, R.; Kum Jew, S. Inorganic mercury within motor neurons does not cause the TDP-43 changes seen in sporadic ALS. Toxicol. Lett. 2011, 201, 58–61. [Google Scholar] [CrossRef]
- Wallin, C.; Friedemann, M.; Sholts, S.B.; Noormagi, A.; Svantesson, T.; Jarvet, J.; Roos, P.M.; Palumaa, P.; Gräslund, A.; Wärmländer, S.K.T.S. Mercury and Alzheimer’s Disease: Hg(II) Ions Display Specific Binding to the Amyloid-beta Peptide and Hinder Its Fibrillization. Biomolecules 2019, 10, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Wu, H.; Zhao, J. Multifunctional roles of zinc in Alzheimer′s disease. Neurotoxicology 2020, 80, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.O.; Popov, I.A.; Nikolaev, E.N.; Archakov, A.I.; Makarov, A.A.; Kozin, S.A. Isomerization of the Asp7 residue results in zinc-induced oligomerization of Alzheimer′s disease amyloid beta(1–16) peptide. ChemBioChem 2008, 9, 1564–1567. [Google Scholar] [CrossRef]
- Garnier, C.; Devred, F.; Byrne, D.; Puppo, R.; Roman, A.Y.; Malesinski, S.; Golovin, A.V.; Lebrun, R.; Ninkina, N.N.; Tsvetkov, P.O. Zinc binding to RNA recognition motif of TDP-43 induces the formation of amyloid-like aggregates. Sci. Rep. 2017, 7, 6812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gade Malmos, K.; Blancas-Mejia, L.M.; Weber, B.; Buchner, J.; Ramirez-Alvarado, M.; Naiki, H.; Otzen, D. ThT 101: A primer on the use of thioflavin T to investigate amyloid formation. Amyloid 2017, 24, 1–16. [Google Scholar] [CrossRef]
- Golovin, A.V.; Devred, F.; Yatoui, D.; Roman, A.Y.; Zalevsky, A.O.; Puppo, R.; Lebrun, R.; Guerlesquin, F.; Tsvetkov, P.O. Zinc Binds to RRM2 Peptide of TDP-43. Int. J. Mol. Sci. 2020, 21, 9080. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82–83, 969–974. [Google Scholar] [CrossRef]
- Meloni, G.; Faller, P.; Vasak, M. Redox silencing of copper in metal-linked neurodegenerative disorders: Reaction of Zn7metallothionein-3 with Cu2+ ions. J. Biol. Chem. 2007, 282, 16068–16078. [Google Scholar] [CrossRef] [Green Version]
- Parker, S.J.; Meyerowitz, J.; James, J.L.; Liddell, J.R.; Nonaka, T.; Hasegawa, M.; Kanninen, K.M.; Lim, S.; Paterson, B.M.; Donnelly, P.S.; et al. Inhibition of TDP-43 accumulation by bis(thiosemicarbazonato)-copper complexes. PLoS ONE 2012, 7, e42277. [Google Scholar] [CrossRef] [Green Version]
- Mitani, T.T.; Beck, G.; Kido, K.; Yamashita, R.; Yonenobu, Y.; Ogawa, T.; Saeki, C.; Okuno, T.; Nagano, S.; Morii, E.; et al. Amyotrophic lateral sclerosis with speech apraxia, predominant upper motor neuron signs, and prominent iron accumulation in the frontal operculum and precentral gyrus. Neuropathology 2021, 41, 324–331. [Google Scholar] [CrossRef]
- Roos, P.M.; Lierhagen, S.; Flaten, T.P.; Syversen, T.; Vesterberg, O.; Nordberg, M. Manganese in cerebrospinal fluid and blood plasma of patients with amyotrophic lateral sclerosis. Exp. Biol. Med. 2012, 237, 803–810. [Google Scholar] [CrossRef]
- Luo, J.; Warmlander, S.K.; Graslund, A.; Abrahams, J.P. Human lysozyme inhibits the in vitro aggregation of Abeta peptides, which in vivo are associated with Alzheimer’s disease. Chem. Commun. 2013, 49, 6507–6509. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Warmlander, S.K.; Graslund, A.; Abrahams, J.P. Non-chaperone proteins can inhibit aggregation and cytotoxicity of Alzheimer amyloid beta peptide. J. Biol. Chem. 2014, 289, 27766–27775. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Warmlander, S.K.; Graslund, A.; Abrahams, J.P. Reciprocal Molecular Interactions between the Abeta Peptide Linked to Alzheimer′s Disease and Insulin Linked to Diabetes Mellitus Type II. ACS Chem. Neurosci. 2016, 7, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Wallin, C.; Hiruma, Y.; Warmlander, S.; Huvent, I.; Jarvet, J.; Abrahams, J.P.; Graslund, A.; Lippens, G.; Luo, J. The Neuronal Tau Protein Blocks in Vitro Fibrillation of the Amyloid-beta (Abeta) Peptide at the Oligomeric Stage. J. Am. Chem. Soc. 2018, 140, 8138–8146. [Google Scholar] [CrossRef]
- Wallin, C.; Kulkarni, Y.S.; Abelein, A.; Jarvet, J.; Liao, Q.; Strodel, B.; Olsson, L.; Luo, J.; Abrahams, J.P.; Sholts, S.B.; et al. Characterization of Mn(II) ion binding to the amyloid-beta peptide in Alzheimer′s disease. J. Trace Elem. Med. Biol. 2016, 38, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Gielnik, M.; Pietralik, Z.; Zhukov, I.; Szymanska, A.; Kwiateke, W.M.; Kozak, M. PrP (58–93) peptide from unstructured N-terminal domain of human prion protein forms amyloid-like fibrillar structures in the presence of Zn2+ ions. RSC Adv. 2019, 9, 22211–22219. [Google Scholar] [CrossRef] [Green Version]
- Gielnik, M.; Taube, M.; Zhukova, L.; Zhukov, I.; Wärmländer, S.K.T.S.; Svedružić, Ž.; Kwiatek, W.M.; Gräslund, A.; Kozak, M. Zn(II) binding causes interdomain changes in the structure and flexibility of the human prion protein. Sci. Rep. 2021, 11, 21703. [Google Scholar] [CrossRef] [PubMed]
- Abelein, A.; Gräslund, A.; Danielsson, J. Zinc as chaperone-mimicking agent for retardation of amyloid beta peptide fibril formation. Proc. Natl. Acad. Sci. USA 2015, 112, 5407–5412. [Google Scholar] [CrossRef] [Green Version]
- Faller, P.; Hureau, C. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-beta peptide. Dalton Trans. 2009, 7, 1080–1094. [Google Scholar] [CrossRef]
- Robinson, J.L.; Geser, F.; Stieber, A.; Umoh, M.; Kwong, L.K.; Van Deerlin, V.M.; Lee, V.M.; Trojanowski, J.Q. TDP-43 skeins show properties of amyloid in a subset of ALS cases. Acta Neuropathol. 2013, 125, 121–131. [Google Scholar] [CrossRef]
- White, A.R.; Aschner, M.; Costa, L.G.; Bush, A.I. Biometals in Neurodegenerative Diseases: Mechanisms and Therapeutics; Elsevier Science & Technology: San Diego, CA, USA, 2017. [Google Scholar]
| Metal | CSF | TDP-43 | Study Model | Ref. |
|---|---|---|---|---|
| Al | ↑ | [68,71] | ||
| Cd | ↑ | [68,71] | ||
| Co | ↑ | [68,71] | ||
| Cu | ↑ | – | In vitro | [62,68,71] |
| Fe | – | – | In vitro | [62,68,71] |
| Hg | – | – | In vivo | [68,71,124] |
| MeHg | ↑ | In vitro, In vivo | [42,68,71] | |
| Mn | ↑ | – | In vitro | [42,68,71] |
| Pb | ↑ | ↑ | In vitro | [42,68,71] |
| U | ↑ | [68,71] | ||
| V | ↑ | [68,71] | ||
| Zn | ↑ | ↑ | In vitro | [62,68,71] |
| ↑ | In vitro | [68,71,128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koski, L.; Ronnevi, C.; Berntsson, E.; Wärmländer, S.K.T.S.; Roos, P.M. Metals in ALS TDP-43 Pathology. Int. J. Mol. Sci. 2021, 22, 12193. https://doi.org/10.3390/ijms222212193
Koski L, Ronnevi C, Berntsson E, Wärmländer SKTS, Roos PM. Metals in ALS TDP-43 Pathology. International Journal of Molecular Sciences. 2021; 22(22):12193. https://doi.org/10.3390/ijms222212193
Chicago/Turabian StyleKoski, Lassi, Cecilia Ronnevi, Elina Berntsson, Sebastian K. T. S. Wärmländer, and Per M. Roos. 2021. "Metals in ALS TDP-43 Pathology" International Journal of Molecular Sciences 22, no. 22: 12193. https://doi.org/10.3390/ijms222212193
APA StyleKoski, L., Ronnevi, C., Berntsson, E., Wärmländer, S. K. T. S., & Roos, P. M. (2021). Metals in ALS TDP-43 Pathology. International Journal of Molecular Sciences, 22(22), 12193. https://doi.org/10.3390/ijms222212193






