Sulfur Induces Resistance against Canker Caused by Pseudomonas syringae pv. actinidae via Phenolic Components Increase and Morphological Structure Modification in the Kiwifruit Stems
Abstract
:1. Introduction
2. Results
2.1. Disease Severity and Protection Percentage after Sulfur Application
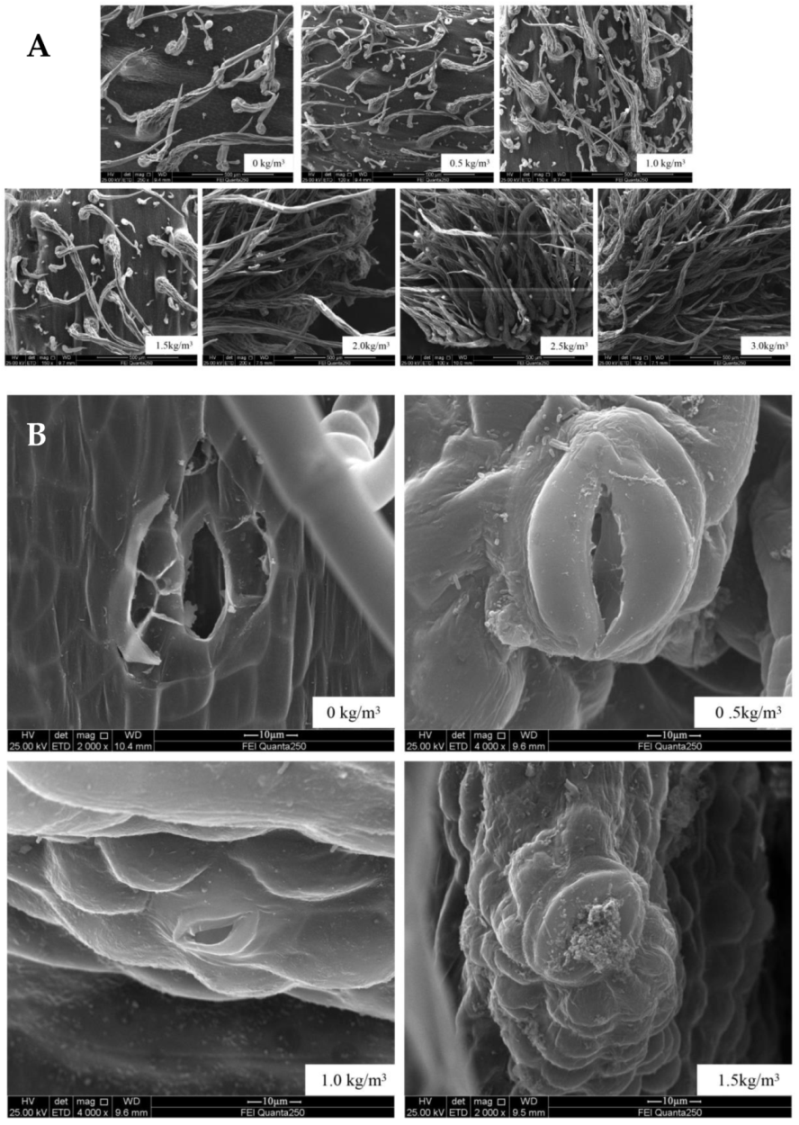
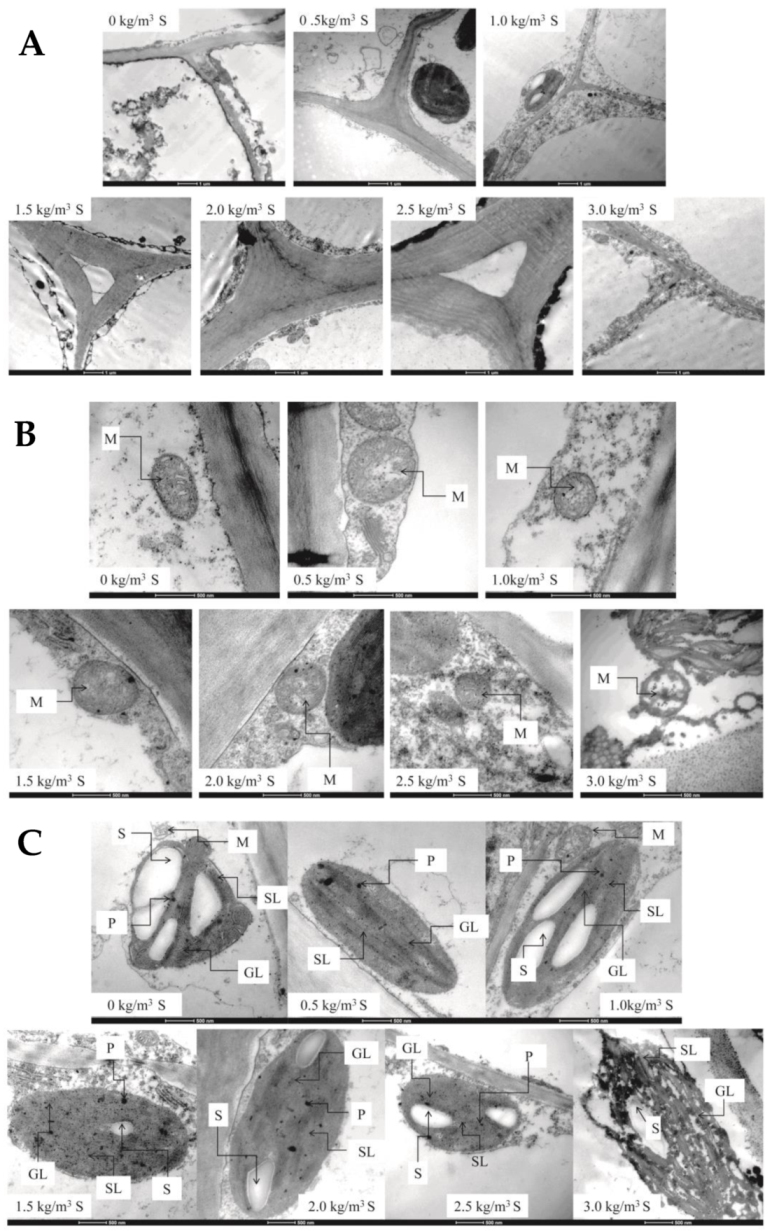
2.2. Changes in Morphological Structure of Kiwifruit Stem after Sulfur Application
2.2.1. External Morphological Structure of the Stem
2.2.2. Ultrastructure of Stems
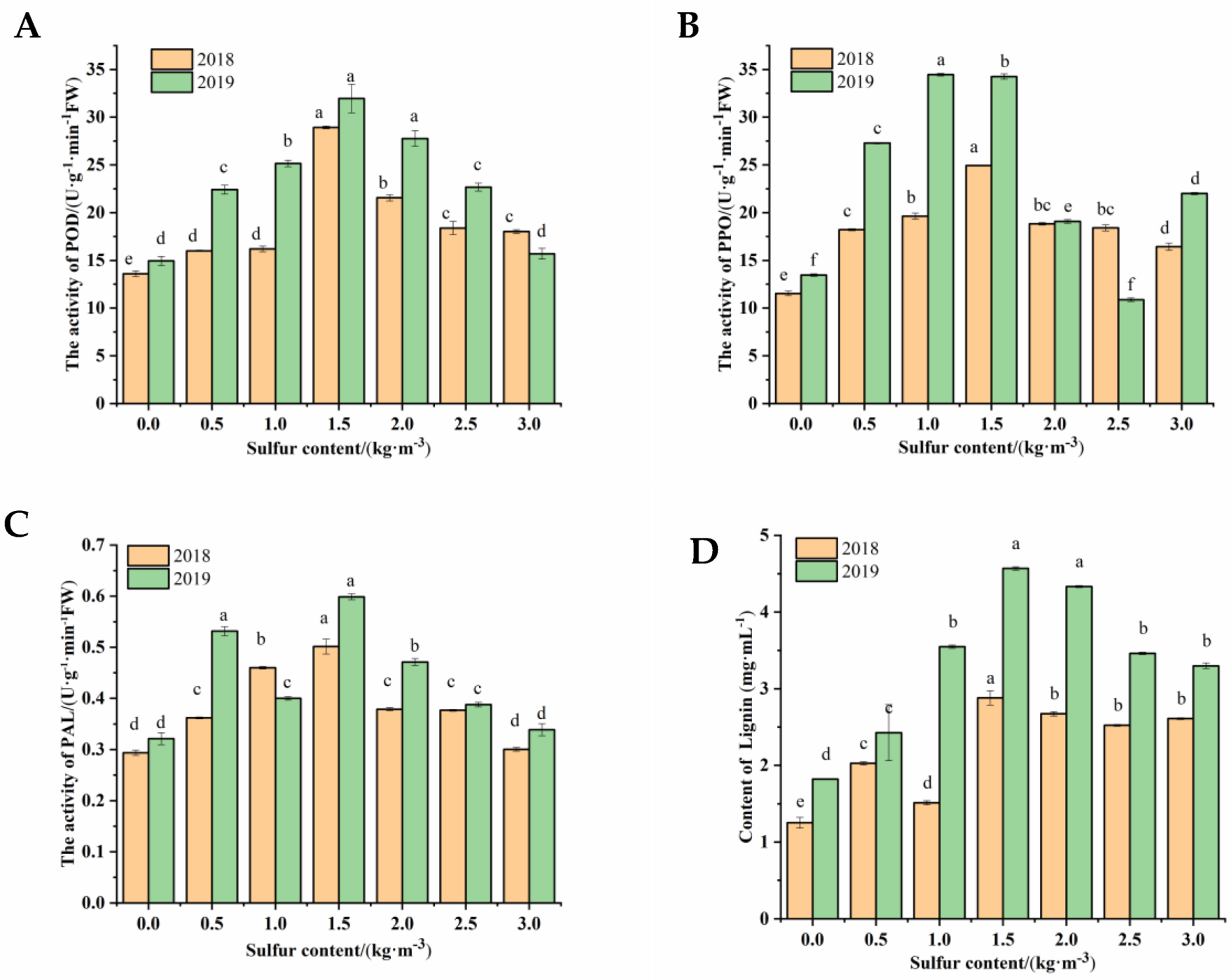
2.3. Effects of Sulfur Treatment on Phenolic Components in Kiwifruit Stems
2.3.1. The Activities of POD, PPO, and PAL
2.3.2. Content of Lignin
2.4. Correlation Analysis between Protection Efficiency and Phenolic Components in Kiwifruit Stems
3. Discussion
4. Materials and Methods
4.1. Kiwifruit Plants
4.2. Fertilizers and Reagents
4.3. Instruments and Equipment
4.4. Field Trials
4.5. Investigation on the Occurrence of Disease
4.6. Observation on the Morphological Structure of Stems
4.7. Determination of Phenolic Component Activity in Stems
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vanneste, J.L. The scientific, economic, and social impacts of the New Zealand outbreak of bacterial canker of kiwifruit (Pseudomonas syringae pv. actinidiae). Annu. Rev. Phytopathol. 2017, 55, 377–399. [Google Scholar] [CrossRef]
- Froud, K.J.; Beresford, R.M.; Cogger, N.C. Impact of kiwifruit bacterial canker on productivity of ‘Hayward’ kiwifruit using observational data and multivariable analysis. Plant Pathol. 2018, 67, 671–681. [Google Scholar] [CrossRef]
- Fujikawa, T.; Sawada, H. Draft genome sequences of nine Japanese strains of the kiwifruit bacterial canker pathogen Pseudomonas syringae pv. actinidiae biovar 3. Microbiol. Resour. Announc. 2020, 9, e01007. [Google Scholar] [CrossRef]
- Walters, D.; Walsh, D.; Newton, A.; Lyon, G. Induced resistance for plant disease control: Maximizing the efficacy of resistance elicitors. Phytopathology 2005, 95, 1368–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sequeira, L Mechanisms of induced resistance in plants. Annu. Rev. Microbiol. 1983, 37, 51–79. [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [Green Version]
- Niranjan Raj, S.N.; Lavanya, S.N.; Amruthesh, K.N.; Niranjana, S.R.; Reddy, M.S.; Shetty, H.S. Histo-chemical changes induced by PGPR during induction of resistance in pearl millet against downy mildew disease. Biol. Control. 2012, 60, 90–102. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, J.; Tian, J.; Li, N.; Jia, L.; Shen, W.; Cui, J. Enhanced anthocyanin accumulation of immature radish microgreens by hydrogen-rich water under short wavelength light. Sci. Hortic. 2019, 247, 75–85. [Google Scholar] [CrossRef]
- Figueira, E.P.P.; Kuhn, O.J.; Martinazzo-Portz, T.; Stangarlin, J.R.; Pereira, M.D.P.; Lampugnani, C. Histo-chemical changes induced by Trichoderma spp. and potassium phosphite in common bean (Phaseolus vulgaris) in response to the attack by Colletotrichum lindemuthianum. Semin-Cienc. Agrar. 2020, 41, 811. [Google Scholar] [CrossRef]
- Liu, D.; Li, K.; Hu, J.; Wang, W.; Liu, X.; Gao, Z. Biocontrol and action mechanism of bacillus amyloliquefaciens and bacillus subtilis in soybean phytophthora blight. Int. J. Mol. Sci. 2019, 20, 2908. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Qiu, L.; Liu, X.; Zhang, Q.; Zhuansun, X.; Fahima, T.; Krugman, T.; Sun, Q.; Xie, C. Glycerol-induced powdery mildew resistance in wheat by regulating plant fatty acid metabolism, plant hormones cross-talk, and pathogenesis-related genes. Int. J. Mol. Sci. 2020, 21, 673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, A.E.D.; Lopes, M.M.A.; Moreira, A.D.R.; Macedo, J.J.N.; Moura, C.F.H.; Zocolo, G.J.; Miranda, M.R.A.; Silva, E.O. Induction of postharvest resistance in melon using pulsed light as abiotic stressor. Sci. Hortic. 2019, 246, 921–927. [Google Scholar] [CrossRef]
- Xue, H.; Sun, Y.; Li, L.; Yang, B.; Zhang, R.; Long, H. Acetylsalicylic acid (ASA) induced fusarium rot resistance and suppressed neosolaniol production by elevation of ROS metabolism in muskmelon fruit. Sci. Hortic. 2020, 265, 109264. [Google Scholar] [CrossRef]
- Bloem, E.; Haneklaus, S.; Schnug, E. Milestones in plant sulfur research on sulfur-induced-resistance (SIR) in Europe. Front. Plant Sci. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dubuis, P.H.; Marazzi, C.; Städler, E.; Mauch, F. Sulphur deficiency causes a reduction in antimicrobial potential and leads to increased disease susceptibility of oilseed rape. J. Phytopathol. 2005, 153, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Klikocka, H.; Haneklaus, S.; Bloem, E.; Schnug, E. Influence of Sulfur fertilization on infection of potato tubers with Rhizoctonia solani and Streptomyces scabies. J. Plant Nutr. 2005, 28, 819–833. [Google Scholar] [CrossRef]
- Bloem, E.; Haneklaus, S.; Salac, I.; Wickenhäuser, F.; Schnug, E. Facts and fiction about sulfur metabolism in relation to plant-pathogen interactions. Plant Biol. 2007, 9, 596–607. [Google Scholar] [CrossRef]
- Haneklaus, S.; Bloem, E.; Schnug, E. Disease control by sulphur induced resistance. In Disease Control in Crops: Biological and Environmentally; Walters, D., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2009; pp. 221–236. [Google Scholar]
- Fu, X.; Li, C.; Zhou, X.; Liu, S.; Wu, F. Physiological response and sulfur metabolism of the V. dahliae-infected tomato plants in tomato/potato onion companion cropping. Sci. Rep. 2016, 6, 232–426. [Google Scholar] [CrossRef] [Green Version]
- Bollig, K.; Specht, A.; Myint, S.S.; Zahn, M.; Horst, W.J. Sulfur supply impairs spread of Verticillium dahliae in tomato. Eur. J. Plant Pathol. 2013, 135, 81–96. [Google Scholar] [CrossRef]
- Long, Y.; Yin, X.; Wang, M.; Wu, X.; Tian, X.L.; Li, M. Effects of sulfur on kiwifruit canker caused by Pseudomonas syringae pv. actinidae. Bangladesh J. Bot. 2017, 46, 1183–1192. [Google Scholar]
- Yin, X.; Wang, M.; Long, Y.; Tian, X.; Li, X.; Xu, C.; Wang, Y. Effects of sulfur treatment on chloroplast ultrastructure and fruit quality in kiwifruit (Actinidia deliciosa). J. Fruit Sci. 2017, 34, 454–463. (In Chinese) [Google Scholar]
- Kopriva, S.; Malagoli, M.; Takahashi, H. Sulfur nutrition: Impacts on plant development, metabolism, and stress responses. J. Exp. Bot. 2019, 70, 4069–4073. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.; Uslu, V.V.; Rajab, H.; Ahmad, N.; Waadt, R.; Geiger, D.; Malagoli, M.; Xiang, C.; Hedrich, R.; Rennenberg, H.; et al. Sulfate is incorporated into cysteine to trigger ABA production and stomatal closure. Plant Cell 2018, 30, 2973–2987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossini, F.; Provenzano, M.D.; Sestili, F.; Roberto, R. Synergistic effect of sulfur and nitrogen in the organic and mineral fertilization of durum wheat: Grain yield and quality traits in the mediterranean environment. Agronomy 2018, 8, 189. [Google Scholar] [CrossRef] [Green Version]
- Ibaez, T.B.; Santos, L.F.D.M.; Marcoslapaz, A.D.; Ribeiro, I.V.; Heinrichs, R. Sulfur modulates yield and storage proteins in soybean grains. Sci. Agric. 2019, 78, 2021. [Google Scholar]
- Singh, A.K.; Singh, K.M.; Bharati, R.C.; Pedapati, A. Potential of residual sulfur and zinc nutrition in improving powdery mildew (Erysiphe trifolii) disease tolerance of lentil (Lens culunaris L.). Commun. Soil. Sci. Plant Anal. 2014, 45, 2807–2818. [Google Scholar] [CrossRef]
- Pavlista, A.D. Early-Season applications of sulfur fertilizers increase potato yield and reduce tuber defects. Agron. J. 2005, 97, 599–603. [Google Scholar] [CrossRef]
- Ostaszewska, M.; Juszczuk, I.M.; Kodziejek, I.; Rychter, A.M. Long-term sulphur starvation of Arabidopsis thaliana modifies mitochondrial ultrastructure and activity and changes tissue energy and redox status. J. Plant Physiol. 2014, 171, 549–558. [Google Scholar] [CrossRef]
- Christopher, A.; Sarkar, D.; Shetty, K. Elicitation of stress-induced phenolic metabolites for antimicrobial applications against foodborne human bacterial pathogens. Antibiotics. 2021, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Deenamo, N.; Kuyyogsuy, A.; Khompatara, K.; Chanwun, T.; Ekchaweng, K.; Churngchow, N. Salicylic acid induces resistance in rubber tree against Phytophthora palmivora. Int. J. Mol. Sci. 2018, 19, 1883. [Google Scholar] [CrossRef] [Green Version]
- Vega, D.D.; Holden, N.; Hedley, P.E.; Morris, J.; Luna, E.; Newton, A. Chitosan primes plant defence mechanisms against Botrytis cinerea, including expression of Avr9/Cf-9 rapidly elicited genes. Plant Cell Environ. 2020, 44, 290–303. [Google Scholar] [CrossRef]
- Yin, L.; Gan, X.; Zan, N.; Zhang, A.; Ren, X.; Li, M.; Xie, D.; Hu, D.; Song, B. Induced resistance mechanism of novel curcumin analogs bearing a quinazoline moiety to plant virus. Int. J. Mol. Sci. 2018, 19, 4065. [Google Scholar] [CrossRef] [Green Version]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jia, H.; Gong, P.; Sadeghnezhad, E.; Pang, Q.; Dong, T.; Li, T.; Jin, H.; Fang, J. Chitosan induces jsmonic acid production leading to resistance of ripened fruit against Botrytis cinerea infectioan. Food Chem. 2020, 337, 127772. [Google Scholar]
- Meshram, S.; Patel, J.S.; Yadav, S.K.; Kumar, G.; Singh, D.P.; Singh, H.B.; Sarma, B.K. Trichoderma mediate early and enhanced lignifications in chickpea during Fusarium oxysporum f. sp. ciceris infection. J. Basic Microb. 2019, 59, 74–86. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Xiao, S.; Wang, X.; Ao, C.; Zhang, X.; Zhu, L. GhWRKY1-like enhances cotton resistance to Verticillium dahliae via an increase in defense-induced lignification and S monolignol content. Plant Sci. 2021, 305, 110833. [Google Scholar] [CrossRef]
- Dixon, R.A.; Barros, J. Lignin biosynthesis: Old roads revisited and new roads explored. Open Biol. 2019, 9, 190215. [Google Scholar] [CrossRef] [Green Version]
- Bhaskara Reddy, M.V.; Arul, J.; Angers, P.; Couture, L. Chitosan treatment of wheat seeds induces resistance to Fusarium graminearum and improves seed quality. J. Agric. Food Chem. 1999, 47, 1208–1216. [Google Scholar] [CrossRef]
- Lee, M.; Jeon, H.S.; Kim, S.H.; Chung, J.H.; Roppolo, D.; Lee, H.; Cho, H.J.; Tobimatsu, Y.; Ralph, J.; Park, O. Lignin-based barrier restricts pathogens to the infection site and confers resistance in plants. EMBO J. 2019, 38, e101948. [Google Scholar] [CrossRef] [PubMed]

| Correlation Coefficient | Protection Efficiency/% | POD Activity /U·g−1 min−1 FW | PPO Activity /U·g−1 min−1 FW | PAL Activity /U·g−1 min−1 FW | Lignin Content /mg·mL−1 |
|---|---|---|---|---|---|
| Protection efficiency/% | 1 | ||||
| POD activity/U·g−1 min−1 FW | 0.35 | 1 | |||
| PPO activity/U·g−1 min−1 FW | 0.71 * | 0.56 | 1 | ||
| PAL activity/U·g−1 min−1 FW | 0.51 | 0.6 | 0.83 * | 1 | |
| Lignin content/mg·mL−1 | 0.88 ** | 0.39 | 0.81 * | 0.52 | 1 |
| Number | Treatment |
|---|---|
| S0.5 | 0.5 kg/m3 Sulfur powder * + 10 kg Organic fertilizer |
| S1.0 | 1.0 kg/m3 Sulfur powder + 10 kg Organic fertilizer |
| S1.5 | 1.5 kg/m3 Sulfur powder + 10 kg Organic fertilizer |
| S2.0 | 2.0 kg/m3 Sulfur powder + 10 kg Organic fertilizer |
| S2.5 | 2.5 kg/m3 Sulfur powder + 10 kg Organic fertilizer |
| S3.0 | 3 kg/m3 Sulfur powder + 10 kg Organic fertilizer |
| S0 | 10 kg Organic fertilizer, CK |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, G.; Yang, S.; Yin, X.; Long, Y.; Ma, Y.; Li, R.; Wang, G. Sulfur Induces Resistance against Canker Caused by Pseudomonas syringae pv. actinidae via Phenolic Components Increase and Morphological Structure Modification in the Kiwifruit Stems. Int. J. Mol. Sci. 2021, 22, 12185. https://doi.org/10.3390/ijms222212185
Gu G, Yang S, Yin X, Long Y, Ma Y, Li R, Wang G. Sulfur Induces Resistance against Canker Caused by Pseudomonas syringae pv. actinidae via Phenolic Components Increase and Morphological Structure Modification in the Kiwifruit Stems. International Journal of Molecular Sciences. 2021; 22(22):12185. https://doi.org/10.3390/ijms222212185
Chicago/Turabian StyleGu, Guifei, Sen Yang, Xianhui Yin, Youhua Long, Yue Ma, Rongyu Li, and Guoli Wang. 2021. "Sulfur Induces Resistance against Canker Caused by Pseudomonas syringae pv. actinidae via Phenolic Components Increase and Morphological Structure Modification in the Kiwifruit Stems" International Journal of Molecular Sciences 22, no. 22: 12185. https://doi.org/10.3390/ijms222212185
APA StyleGu, G., Yang, S., Yin, X., Long, Y., Ma, Y., Li, R., & Wang, G. (2021). Sulfur Induces Resistance against Canker Caused by Pseudomonas syringae pv. actinidae via Phenolic Components Increase and Morphological Structure Modification in the Kiwifruit Stems. International Journal of Molecular Sciences, 22(22), 12185. https://doi.org/10.3390/ijms222212185





