Molecular and Metabolic Mechanism of Low-Intensity Pulsed Ultrasound Improving Muscle Atrophy in Hindlimb Unloading Rats
Abstract
:1. Introduction
2. Result
2.1. Body Weight and Muscle Weight
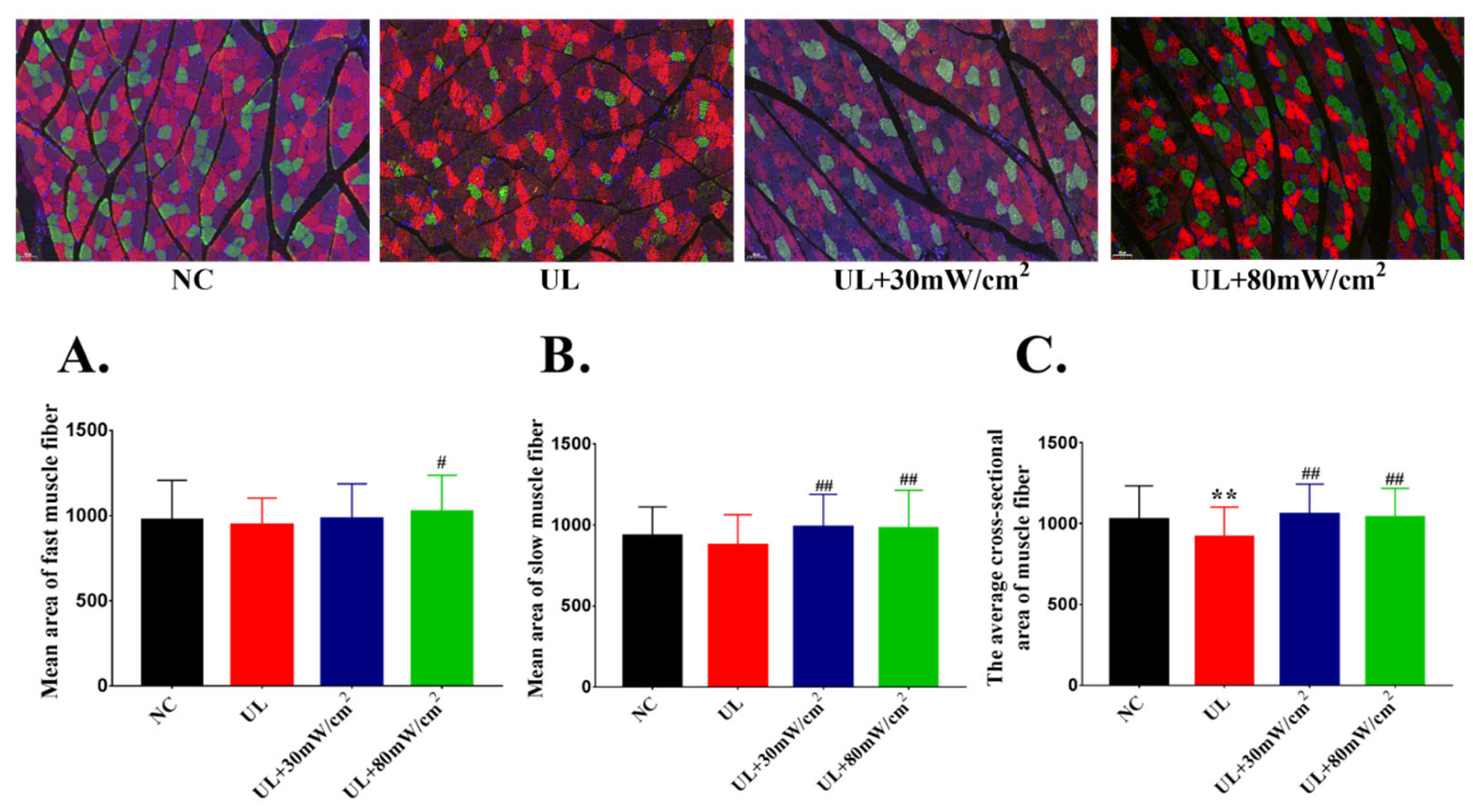
2.2. Mean Cross Sectional Area of Muscle Fiber
2.3. Muscle Tension
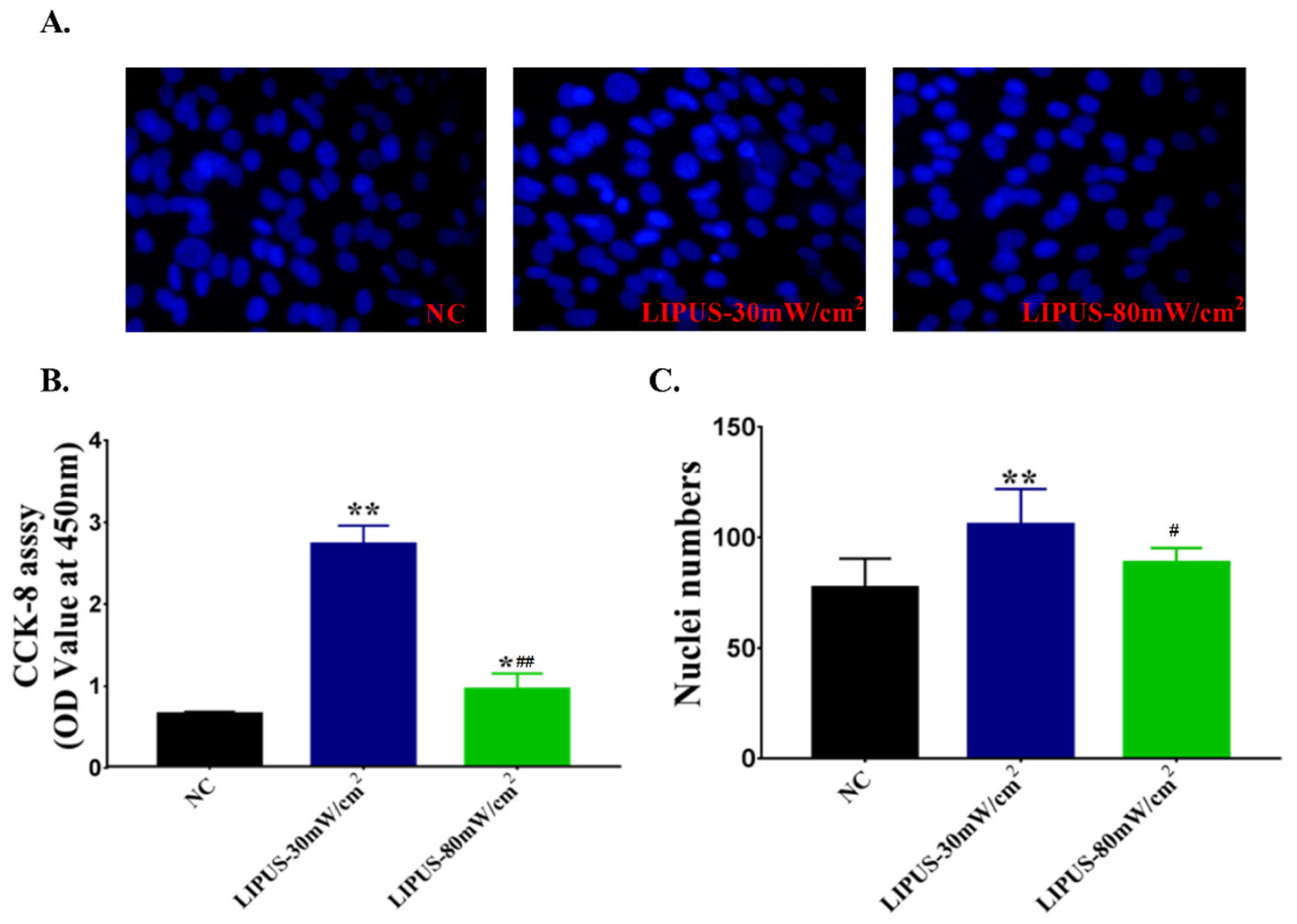
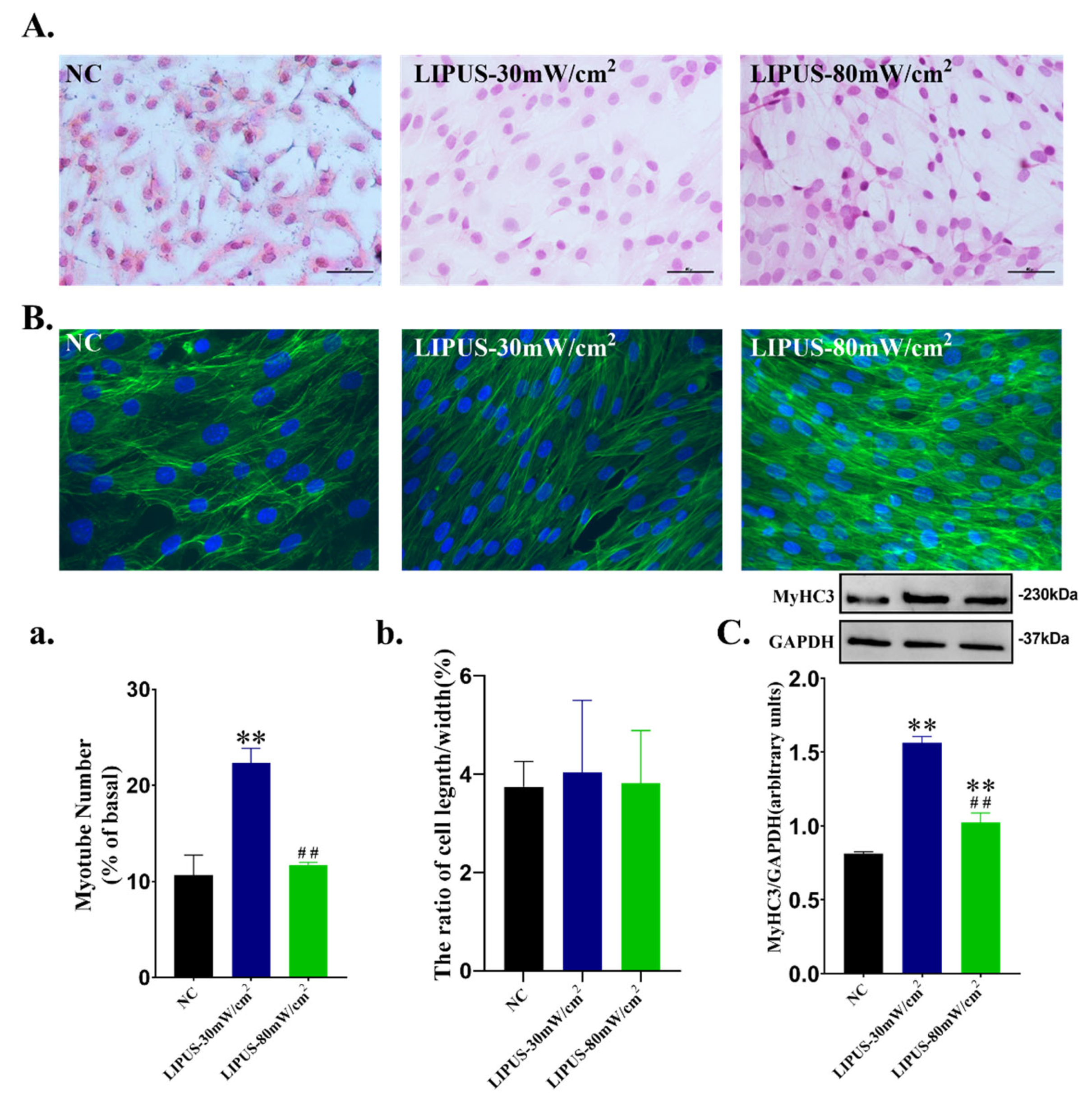
2.4. Cell Proliferation and Differentiation
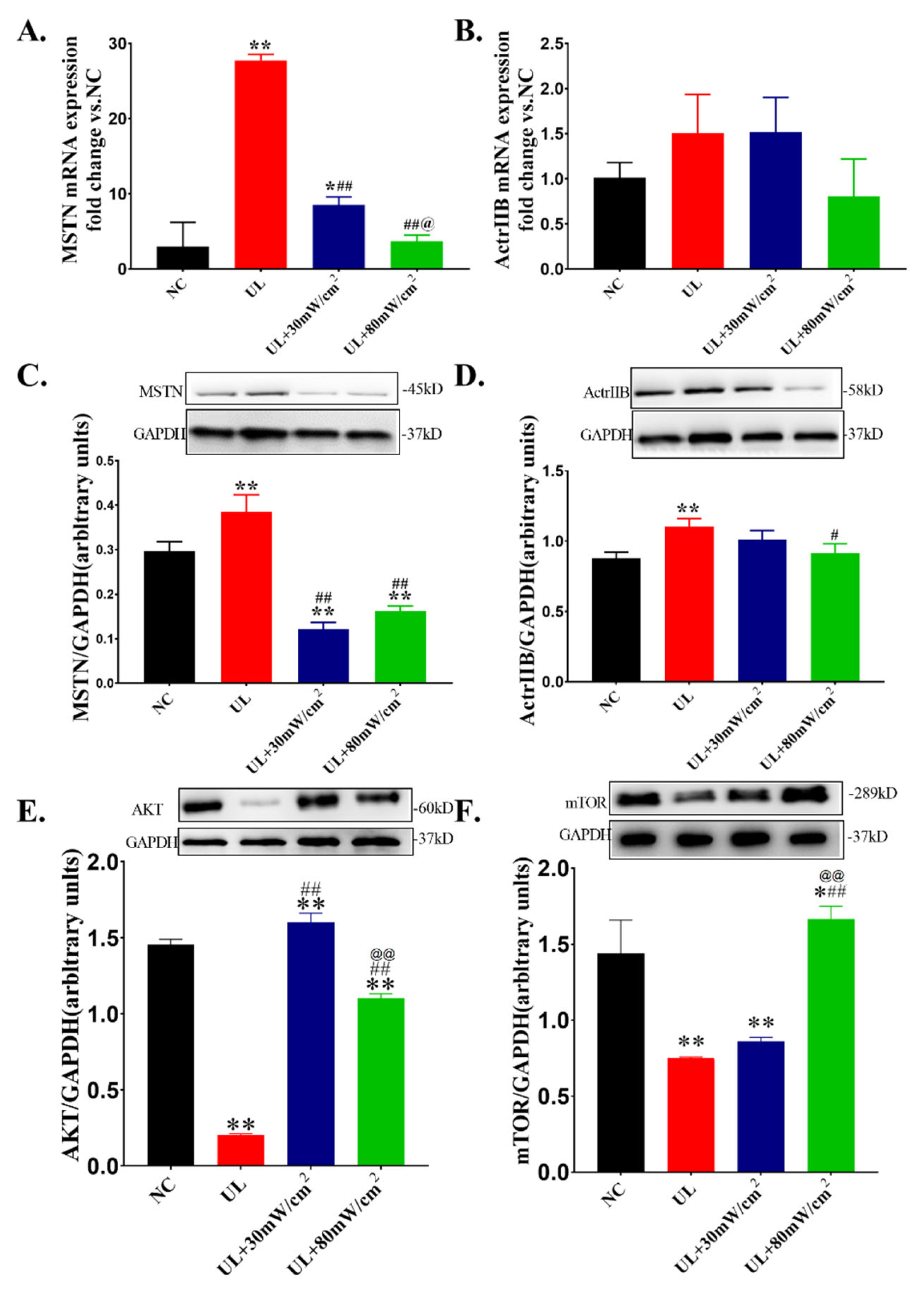
2.5. The Expressions of AKT, mTOR and MSTN and Its ReceptorActrIIB
2.6. GC-MS Analysis of Metabolic Profiling
3. Discussion
4. Method
4.1. Animals
4.2. Animal Modeling and Grouping
4.3. LIPUS Irradiation
4.4. Sample Preparation
4.5. Fast and Slow Muscle Immunofluorescence
4.6. Muscle Tension In Vivo
4.7. Cell Culture and Differentiation
4.8. CCK-8 Detection
4.9. DAPI Staining
4.10. HE Staining
4.11. Microfilament Immunofluorescence Staining
4.12. Western Blot
4.13. RNA Isolation and RT-PCR
4.14. Metabonomicsan Analysis of Differential Metabolites and Pathways
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitts, R.H.; Riley, D.R.; Widrick, J.J. Physiology of a Microgravity Environment Invited Review: Microgravity and skeletal muscle. J. Appl. Physiol. 2000, 89, 823–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitts, R.H.; Riley, D.R.; Widrick, J.J. Functional and structural adaptations of skeletal muscle to microgravity. J. Exp. Biol. 2001, 204 Pt 18, 3201–3208. [Google Scholar] [CrossRef]
- Mulavara, A.P.; Peters, B.T.; Miller, C.A.; Kofman, I.S.; Reschke, M.F.; Taylor, L.C.; Lawrence, E.L.; Wood, S.J.; Laurie, S.S.; Lee, S.M.C.; et al. Physiological and Functional Alterations after Spaceflight and Bed Rest. Med. Sci. Sports Exerc. 2018, 50, 1961–1980. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.S.; Kimball, K.T.; Mulavara, A.P.; Bloomberg, J.J.; Paloski, W.H. Posturography and locomotor tests of dynamic balance after long-duration spaceflight. J. Vestib. Res. 2012, 22, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Tesch, P.A.; Berg, H.E.; Bring, D.; Evans, H.J.; Leblanc, A.D. Effects of 17-day spaceflight on knee extensor muscle function and size. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 93, 463–468. [Google Scholar] [CrossRef]
- Shiba, N.; Matsuse, H.; Takano, Y.; Yoshimitsu, K.; Omoto, M.; Hashida, R.; Tagawa, Y.; Inada, T.; Yamada, S.; Ohshima, H. Electrically Stimulated Antagonist Muscle Contraction Increased Muscle Mass and Bone Mineral Density of One Astronaut-Initial Verification on the International Space Station. PLoS ONE 2015, 10, e0134736. [Google Scholar]
- FFitts, R.H.; Trappe, S.W.; Costill, D.L.; Gallagher, P.; Creer, A.C.; Colloton, P.A.; Peters, J.R.; Romatowski, J.G.; Bain, J.L.; Riley, D.A. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J. Physiol. 2010, 588 Pt 18, 3567–3592. [Google Scholar] [CrossRef]
- Bloomfield, S.A. Changes in musculoskeletal structure and function with prolonged bed rest. Med. Sci. Sports Exerc. 1997, 29, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.E. Daily activity inventory and progress record for those with atypical movement. Am. J. Occup. Ther. 1950, 4, 261–272. [Google Scholar]
- Ali, S.; Garcia, J.M. Sarcopenia, Cachexia and Aging: Diagnosis, Mechanisms and Therapeutic Options—A Mini-Review. Gerontology 2014, 60, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, E.; Zoli, M.; Gonzalez-Freire, M.; Salive, M.E.; Studenski, S.A.; Ferrucci, L. Aging and Multimorbidity: New Tasks, Priorities, and Frontiers for Integrated Gerontological and Clinical Research. J. Am. Med. Dir. Assoc. 2015, 16, 640–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, W.K.; Williams, J.; Atherton, P.J.; Larvin, M.; Lund, J.N.; Narici, M. Sarcopenia, Dynapenia, and the Impact of Advancing Age on Human Skeletal Muscle Size and Strength; a Quantitative Review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schofer, M.D.; Block, J.E.; Aigner, J.; Schmelz, A. Improved healing response in delayed unions of the tibia with low-intensity pulsed ultrasound: Results of a randomized sham-controlled trial. BMC Musculoskelet Disord. 2010, 11, 229. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Li, N.; Jian, W.; Kang, Y.; Yin, B.; Sun, S.; Guo, J.; Sun, L.; Ta, D. Low-intensity pulsed ultrasound prevents muscle atrophy induced by type 1 diabetes in rats. Skelet. Muscle 2017, 7, 29. [Google Scholar] [CrossRef]
- Puts, R.; Rikeit, P.; Ruschke, K.; Kadow-Romacker, A.; Hwang, S.; Jenderka, K.-V.; Knaus, P.; Raum, K. Activation of Mechanosensitive Transcription Factors in Murine C2C12 Mesenchymal Precursors by Focused Low-Intensity Pulsed Ultrasound (FLIPUS). IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2016, 63, 1505–1513. [Google Scholar] [CrossRef]
- Salgarella, A.R.; Cafarelli, A.; Ricotti, L.; Capineri, L.; Dario, P.; Menciassi, A. Optimal Ultrasound Exposure Conditions for Maximizing C2C12 Muscle Cell Proliferation and Differentiation. Ultrasound Med. Biol. 2017, 43, 1452–1465. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, J.; Zhao, X.; Li, N.; Jian, W.; Sun, S.; Guo, J.; Sun, L.; Ta, D. Low-Intensity Pulsed Ultrasound Promotes Exercise-Induced Muscle Hypertrophy. Ultrasound Med. Biol. 2017, 43, 1411–1420. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holloszy, J.O.; Chen, M.; Cartee, G.; Young, J.C. Skeletal muscle atrophy in old rats: Differential changes in the three fiber types. Mech. Ageing Dev. 1991, 60, 199–213. [Google Scholar] [CrossRef]
- Baldwin, K.M.; Herrick, R.E.; McCue, S.A. Substrate oxidation capacity in rodent skeletal muscle: Effects of exposure to zero gravity. J. Appl. Physiol. 1993, 75, 2466–2470. [Google Scholar] [CrossRef]
- Henriksen, E.J.; Tischler, M.E. Glucose uptake in rat soleus: Effect of acute unloading and subsequent reloading. J. Appl. Physiol. 1988, 64, 1428–1432. [Google Scholar] [CrossRef] [PubMed]
- Ritz, P.; Acheson, K.J.; Gachon, P.; Vico, L.; Bernard, J.J.; Alexandre, C.; Beaufrère, B. Energy and substrate metabolism during a 42-day bed-rest in a head-down tilt position in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 78, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D. ging skeletal muscle: Response to exercise. Exerc. Sport Sci. Rev. 1994, 22, 91–120. [Google Scholar] [CrossRef]
- Blanc, S.; Normand, S.; Ritz, P.; Pachiaudi, C.; Vico, L.; Gharib, C.; Gauquelin-Koch, G. Energy and water metabolism, body composition, and hormonal changes induced by 42 days of enforced inactivity and simulated weightlessness. J. Clin. Endocrinol. Metab. 1998, 83, 4289–4297. [Google Scholar] [CrossRef] [PubMed]
- Lane, H.W. Energy requirements for space flight. J. Nutr. 1992, 122, 13–18. [Google Scholar] [CrossRef]
- Musacchia, X.J.; Steffen, J.M.; Fell, R.D.; Dombrowski, M.J.; Oganov, V.W.; Ilyina-Kakueva, E.I. Skeletal muscle atrophy in response to 14 days of weightlessness: Vastus medialis. J. Appl. Physiol. 1992, 73 (Suppl. S2), S44–S50. [Google Scholar] [CrossRef]
- Senoo, N.; Miyoshi, N.; Goto-Inoue, N.; Minami, K.; Yoshimura, R.; Morita, A.; Sawada, N.; Matsuda, J.; Ogawa, Y.; Setou, M.; et al. PGC-1α-mediated changes in phospholipid profiles of exercise-trained skeletal muscle. J. Lipid Res. 2015, 56, 2286–2296. [Google Scholar] [CrossRef] [Green Version]
- Der-Torossian, H.; Wysong, A.; Shadfar, S.; Willis, M.S.; McDunn, J.; Couch, M.E. Metabolic derangements in the gastrocnemius and the effect of Compound A therapy in a murine model of cancer cachexia. J. Cachex-Sarcopenia Muscle 2013, 4, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.C.; Kulp, S.K.; Lai, I.L.; Hsu, E.C.; He, W.A.; Frankhouser, D.E.; Yan, P.S.; Mo, X.; Bloomston, M.; Lesinski, G.B.; et al. Preclinical Investigation of the Novel Histone Deacetylase Inhibitor AR-42 in the Treatment of Cancer-Induced Cachexia. J. Natl. Cancer Inst. 2015, 107, djv274. [Google Scholar] [CrossRef] [Green Version]
- Mirzoev, T.M. Skeletal Muscle Recovery from Disuse Atrophy: Protein Turnover Signaling and Strategies for Accelerating Muscle Regrowth. Int. J. Mol. Sci. 2020, 21, 7940. [Google Scholar] [CrossRef]
- Ikeda, K.; Takayama, T.; Suzuki, N.; Shimada, K.; Otsuka, K.; Ito, K. Effects of low-intensity pulsed ultrasound on the differentiation of C2C12 cells. Life Sci. 2006, 79, 1936–1943. [Google Scholar] [CrossRef]
- Anderson, J.E.; Zhu, A.; Mizuno, T.M. Nitric oxide treatment attenuates muscle atrophy during hind limb suspension in mice. Free. Radic. Biol. Med. 2018, 115, 458–470. [Google Scholar] [CrossRef]
- Rossignoli, C.P.; Dechandt, C.R.; Souza, A.O.; Sampaio, I.H.; Vicentini, T.M.; Teodoro, B.G.; Neto, M.P.C.; Ferrari, G.D.; Couto-Lima, C.A.; Alberici, L.C. Effects of intermittent dietary supplementation with conjugated linoleic acid and fish oil (EPA/DHA) on body metabolism and mitochondrial energetics in mice. J. Nutr. Biochem. 2018, 60, 16–23. [Google Scholar] [CrossRef]
- Leblanc, A.; Lin, C.; Shackelford, L.; Sinitsyn, V.; Evans, H.; Belichenko, O.; Schenkman, B.; Kozlovskaya, I.; Oganov, V.; Bakulin, A.; et al. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. J. Appl. Physiol. 2000, 89, 2158–2164. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, T.; Tsuzuki, T.; Chang, S.-W.; Kakigi, R.; Sugiura, T.; Naito, H. Exercise preconditioning attenuates hind limb unloading-induced gastrocnemius muscle atrophy possibly via the HDAC4/Gadd45 axis in old rats. Exp. Gerontol. 2019, 122, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Sandonà, D.; Desaphy, J.-F.; Camerino, G.M.; Bianchini, E.; Ciciliot, S.; Danieli, D.; Dobrowolny, G.; Furlan, S.; Germinario, E.; Goto, K.; et al. Adaptation of Mouse Skeletal Muscle to Long-Term Microgravity in the MDS Mission. PLoS ONE 2012, 7, e33232. [Google Scholar] [CrossRef] [Green Version]
- Campione, M.; Ausoni, S.; Guezennec, C.Y.; Schiaffino, S. Myosin and troponin changes in rat soleus muscle after hindlimb suspension. J. Appl. Physiol. 1993, 74, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Voet, N.B.M.; Van Der Kooi, E.L.; Riphagen, I.; Lindeman, E.; Van Engelen, B.G.M.; Geurts, A.C.H. Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst. Rev. 2013, 12, CD003907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imashiro, C.; Hirano, M.; Morikura, T.; Fukuma, Y.; Ohnuma, K.; Kurashina, Y.; Miyata, S.; Takemura, K. Detachment of cell sheets from clinically ubiquitous cell culture vessels by ultrasonic vibration. Sci. Rep. 2020, 10, 9468. [Google Scholar] [CrossRef]
- Wolfson, R.L.; Sabatini, D.M. The Dawn of the Age of Amino Acid Sensors for the mTORC1 Pathway. Cell Metab. 2017, 26, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Abrunhosa, V.M.; Soares, C.P.; Possidonio, A.C.B.; Alvarenga, A.V.; Costa-Felix, R.; Costa, M.L.; Mermelstein, C. Induction of Skeletal Muscle Differentiation In Vitro by Therapeutic Ultrasound. Ultrasound Med. Biol. 2014, 40, 504–512. [Google Scholar] [CrossRef]
- McPherron, C.A.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lee, S.-J. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA 1997, 94, 12457–12461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Q.; Yuan, L.; Deng, J.; Chen, M.; Wang, Y.; Zeng, J.; Liangxue, L.; Lai, L. Efficient Generation of Myostatin Gene Mutated Rabbit by CRISPR/Cas9. Sci. Rep. 2016, 6, 25029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, H.; Cao, Y.; Qiu, B.; Zhou, Z.; Deng, R.; Chen, Z.; Li, R.; Li, X.; Wei, Q.; Xia, X.; et al. Establishment and phenotypic analysis of an Mstn knockout rat. Biochem. Biophys. Res. Commun. 2016, 477, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Schuelke, M.; Wagner, K.R.; Stolz, L.E.; Hübner, C.; Riebel, T.; Kömen, W.; Braun, T.; Tobin, J.F.; Lee, S.-J. Myostatin Mutation Associated with Gross Muscle Hypertrophy in a Child. New Engl. J. Med. 2004, 350, 2682–2688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bataille, S.; Chauveau, P.; Fouque, D.; Aparicio, M.; Koppe, L. Myostatin and muscle atrophy during chronic kidney disease. Nephrol. Dial. Transplant. 2020. [CrossRef]
- Kobayashi, M.; Kasamatsu, S.; Shinozaki, S.; Yasuhara, S.; Kaneki, M. Myostatin deficiency not only prevents muscle wasting but also improves survival in septic mice. Am. J. Physiol. Metab. 2021, 320, E150–E159. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lehar, A.; Meir, J.U.; Koch, C.; Morgan, A.; Warren, L.E.; Rydzik, R.; Youngstrom, D.W.; Chandok, H.; George, J.; et al. Targeting myostatin/activin A protects against skeletal muscle and bone loss during spaceflight. Proc. Natl. Acad. Sci. USA 2020, 117, 23942–23951. [Google Scholar] [CrossRef]
- Artaza, J.N.; Bhasin, S.; Mallidis, C.; Taylor, W.; Ma, K.; Gonzalez-Cadavid, N.F. Endogenous expression and localization of myostatin and its relation to myosin heavy chain distribution in C2C12 skeletal muscle cells. J. Cell Physiol. 2002, 190, 170–179. [Google Scholar] [CrossRef]
- Latres, E.; Pangilinan, J.; Miloscio, L.; Bauerlein, R.; Na, E.; Potocky, T.B.; Huang, Y.; Eckersdorff, M.; Rafique, A.; Mastaitis, J.; et al. Myostatin blockade with a fully human monoclonal antibody induces muscle hypertrophy and reverses muscle atrophy in young and aged mice. Skelet. Muscle 2015, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Marzuca-Nassr, G.N.; Fortes, M.; Guimarães-Ferreira, L.; Murata, G.; Vitzel, K.; Vasconcelos, D.; Bassit, R.; Curi, R. Short-term creatine supplementation changes protein metabolism signaling in hindlimb suspension. Braz. J. Med. Biol. Res. 2019, 52, e8391. [Google Scholar] [CrossRef]
- Tang, L.; An, S.; Zhang, Z.; Fan, X.; Guo, J.; Sun, L.; Ta, D. MSTN is a key mediator for low-intensity pulsed ultrasound preventing bone loss in hindlimb-suspended rats. Bone 2021, 143, 115610. [Google Scholar] [CrossRef]
- Tang, L.; Kang, Y.; Sun, S.; Zhao, T.; Cao, W.; Fan, X.; Guo, J.; Sun, L.; Ta, D. Inhibition of MSTN signal pathway may participate in LIPUS preventing bone loss in ovariectomized rats. J. Bone Miner. Metab. 2020, 38, 14–26. [Google Scholar] [CrossRef]
- Han, C.; Mu, J.; Kim, J.K. Insulin Resistance and a Diabetes Mellitus-Like Syndrome in Mice Lacking the Protein Kinase Akt2 (PKBβ). Science 2001, 292, 1728–1731. [Google Scholar]
- Chen, W.S.; Xu, P.-Z.; Gottlob, K.; Chen, M.-L.; Sokol, K.; Shiyanova, T.; Roninson, I.; Weng, W.; Suzuki, R.; Tobe, K.; et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the akt1 gene. Genes Dev. 2001, 15, 2203–2208. [Google Scholar] [CrossRef] [Green Version]
- Halseth, A.E.; Bracy, D.P.; Wasserman, D.H. Functional limitations to glucose uptake in muscles comprised of different fiber types. Am. J. Physiol. Metab. 2001, 280, E994–E999. [Google Scholar] [CrossRef]
- He, J.; Kelley, D.E. Muscle glycogen content in type 2 diabetes mellitus. Am. J. Physiol. Metab. 2004, 287, E1002–E1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, K.-M.V.; Gonzalez, M.; Poueymirou, W.T.; Kline, W.O.; Na, E.; Zlotchenko, E.; Stitt, T.N.; Economides, A.; Yancopoulos, G.D.; Glass, D.J. Conditional Activation of Akt in Adult Skeletal Muscle Induces Rapid Hypertrophy. Mol. Cell. Biol. 2004, 24, 9295–9304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajduch, E.; Alessi, D.R.; Hemmings, B.A.; Hundal, H.S. Constitutive activation of protein kinase B alpha by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes 1998, 47, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.H.; Frost, R.A. Sepsis-induced suppression of skeletal muscle translation initiation mediated by tumor necrosis factor α. Metabolism 2007, 56, 49–57. [Google Scholar] [CrossRef]
- Hribal, M.L.; Nakae, J.; Kitamura, T.; Shutter, J.R.; Accili, D. Regulation of insulin-like growth factor–dependent myoblast differentiation by Foxo forkhead transcription factors. J. Cell Biol. 2003, 162, 535–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Kumar, Y.; Sevak, J.K.; Kumar, S.; Kumar, N.; Gopinath, S.D. Metabolomic analysis of primary human skeletal muscle cells during myogenic progression. Sci. Rep. 2020, 10, 11824. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Vernus, B.; Chelh, I.; Cassar-Malek, I.; Gabillard, J.-C.; Sassi, A.H.; Seiliez, I.; Picard, B.; Bonnieu, A. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell. Mol. Life Sci. 2014, 71, 4361–4371. [Google Scholar] [CrossRef]
- Braidy, N.; Guillemin, G.J.; Mansour, H.; Chan-Ling, T.; Grant, R. Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. FEBS J. 2011, 278, 4425–4434. [Google Scholar] [CrossRef]
- Caballero, B.; Gleason, R.E.; Wurtman, R.J. Plasma amino acid concentrations in healthy elderly men and women. Am. J. Clin. Nutr. 1991, 53, 1249–1252. [Google Scholar] [CrossRef]
- Dukes, A.; Davis, C.; El Refaey, M.; Upadhyay, S.; Mork, S.; Arounleut, P.; Johnson, M.H.; Hill, W.D.; Isales, C.M.; Hamrick, M.W. The aromatic amino acid tryptophan stimulates skeletal muscle IGF1/p70s6k/mTor signaling in vivo and the expression of myogenic genes in vitro. Nutrition 2015, 31, 1018–1024. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.M.; Moon, J.S.; Yoon, J.S.; Won, K.C.; Lee, H.W. Low alanine aminotransferase levels predict low muscle strength in older patients with diabetes: A nationwide cross-sectional study in Korea. Geriatr. Gerontol. Int. 2020, 20, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Ives, S.J.; Zaleski, K.S.; Slocum, C.; Escudero, D.; Sheridan, C.; Legesse, S.; Vidal, K.; Lagalwar, S.; Reynolds, T.H. The effect of succinic acid on the metabolic profile in high-fat diet-induced obesity and insulin resistance. Physiol. Rep. 2020, 8, e14630. [Google Scholar] [CrossRef]
- Corpeleijn, W.E.; Riedijk, M.A.; Zhou, Y.; Schierbeek, H.; Huang, Y.; Chen, C.; van Goudoever, J.B. Almost all enteral aspartate is taken up in first-pass metabolism in enterally fed preterm infants. Clin. Nutr. 2010, 29, 341–346. [Google Scholar] [CrossRef]
- Sullivan, L.; Gui, D.Y.; Hosios, A.M.; Bush, L.N.; Freinkman, E.; Heiden, M.G.V. Supporting Aspartate Biosynthesis Is an Essential Function of Respiration in Proliferating Cells. Cell 2015, 162, 552–563. [Google Scholar] [CrossRef] [Green Version]
- Lancha, A.H.; Poortmans, J.R.; Pereira, L.O. The effect of 5 days of aspartate and asparagine supplementation on glucose transport activity in rat muscle. Cell Biochem. Funct. 2009, 27, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Lancha, A.; Recco, M.; Abdalla, D.; Curi, R. Effect of aspartate, asparagine, and carnitine supplementation in the diet on metabolism of skeletal muscle during a moderate exercise. Physiol. Behav. 1995, 57, 367–371. [Google Scholar] [CrossRef]
- Giesbertz, P.; Padberg, I.; Rein, D.; Ecker, J.; Höfle, A.S.; Spanier, B.; Daniel, H. Metabolite profiling in plasma and tissues of ob/ob and db/db mice identifies novel markers of obesity and type 2 diabetes. Diabetologia 2015, 58, 2133–2143. [Google Scholar] [CrossRef] [Green Version]
- Ham, D.J.; Murphy, K.; Chee, A.; Lynch, G.; Koopman, R. Glycine administration attenuates skeletal muscle wasting in a mouse model of cancer cachexia. Clin. Nutr. 2014, 33, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Caldow, M.K.; Ham, D.J.; Trieu, J.; Chung, J.D.; Lynch, G.S.; Koopman, R. Glycine Protects Muscle Cells from Wasting in vitro via mTORC1 Signaling. Front. Nutr. 2019, 6, 172. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.-C.; Kim, D.G.; Lee, W.; Sim, H.; Choi, Y.-J.; Lee, B.-H. Activation of SIRT1 by l-serine increases fatty acid oxidation and reverses insulin resistance in C2C12 myotubes (l-serine activates SIRT1 in C2C12 myotubes). Cell Biol. Toxicol. 2019, 35, 457–470. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Lu, Y.; Karagounis, L.G.; Ng, T.P.; Carre, C.; Narang, V.; Wong, G.; Tan, C.T.Y.; Nyunt, M.S.Z.; Gao, Q.; Abel, B.; et al. Systemic and Metabolic Signature of Sarcopenia in Community-Dwelling Older Adults. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2019, 75, 309–317. [Google Scholar] [CrossRef]
- Caron, A.Z.; Drouin, G.; Desrosiers, J.; Trensz, F.; Grenier, G. A novel hindlimb immobilization procedure for studying skeletal muscle atrophy and recovery in mouse. J. Appl. Physiol. 2009, 106, 2049–2059. [Google Scholar] [CrossRef] [Green Version]


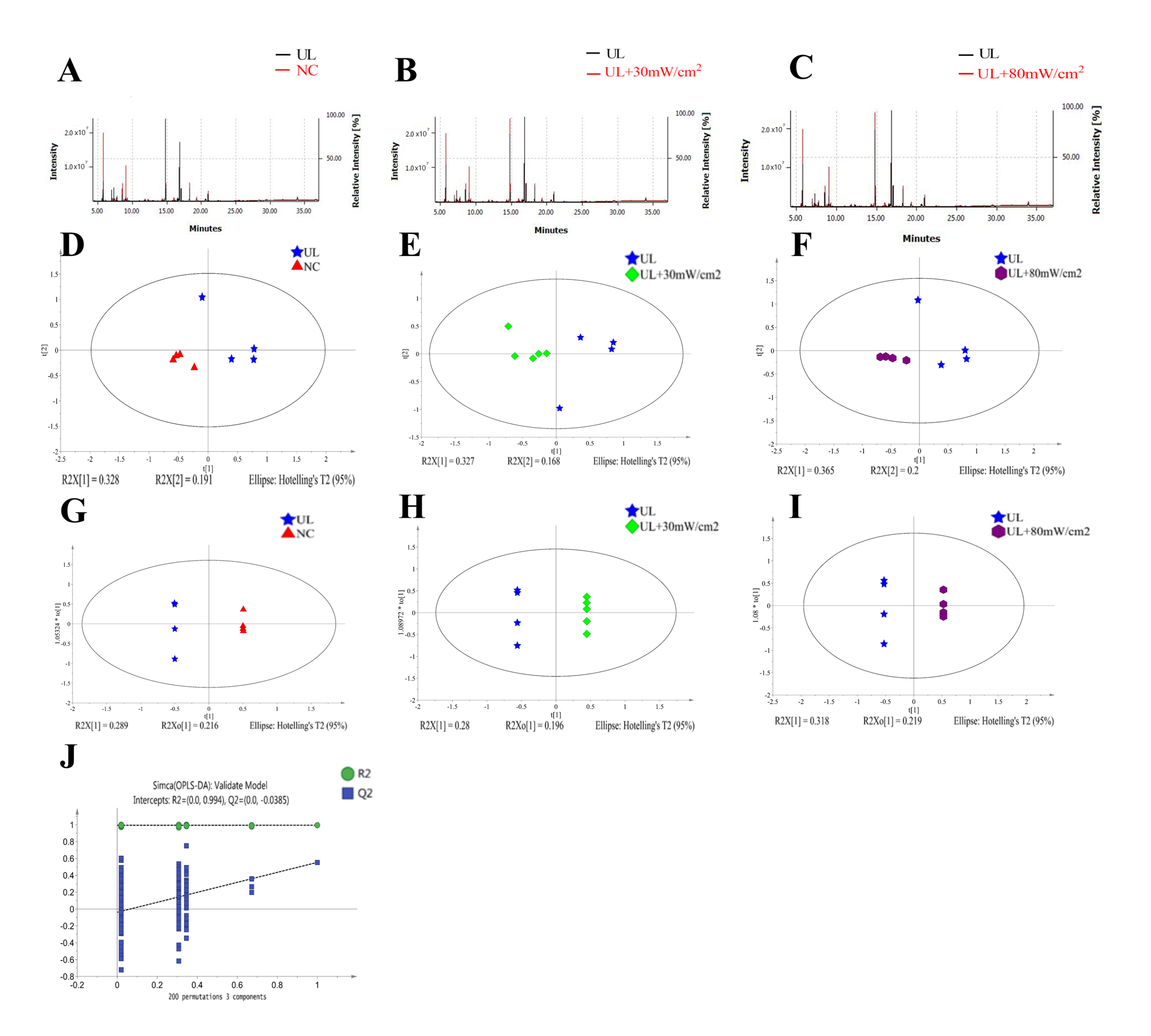
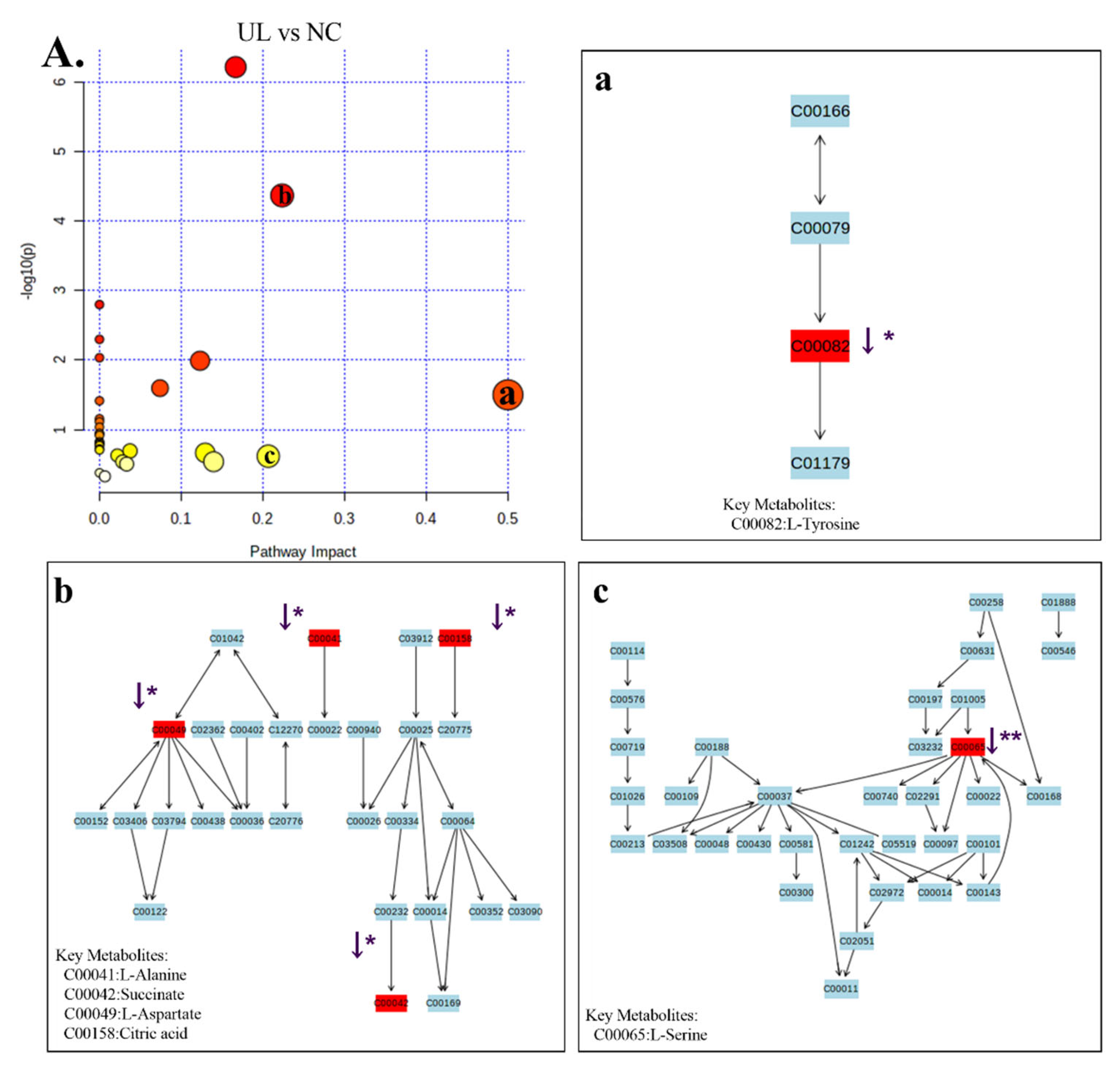
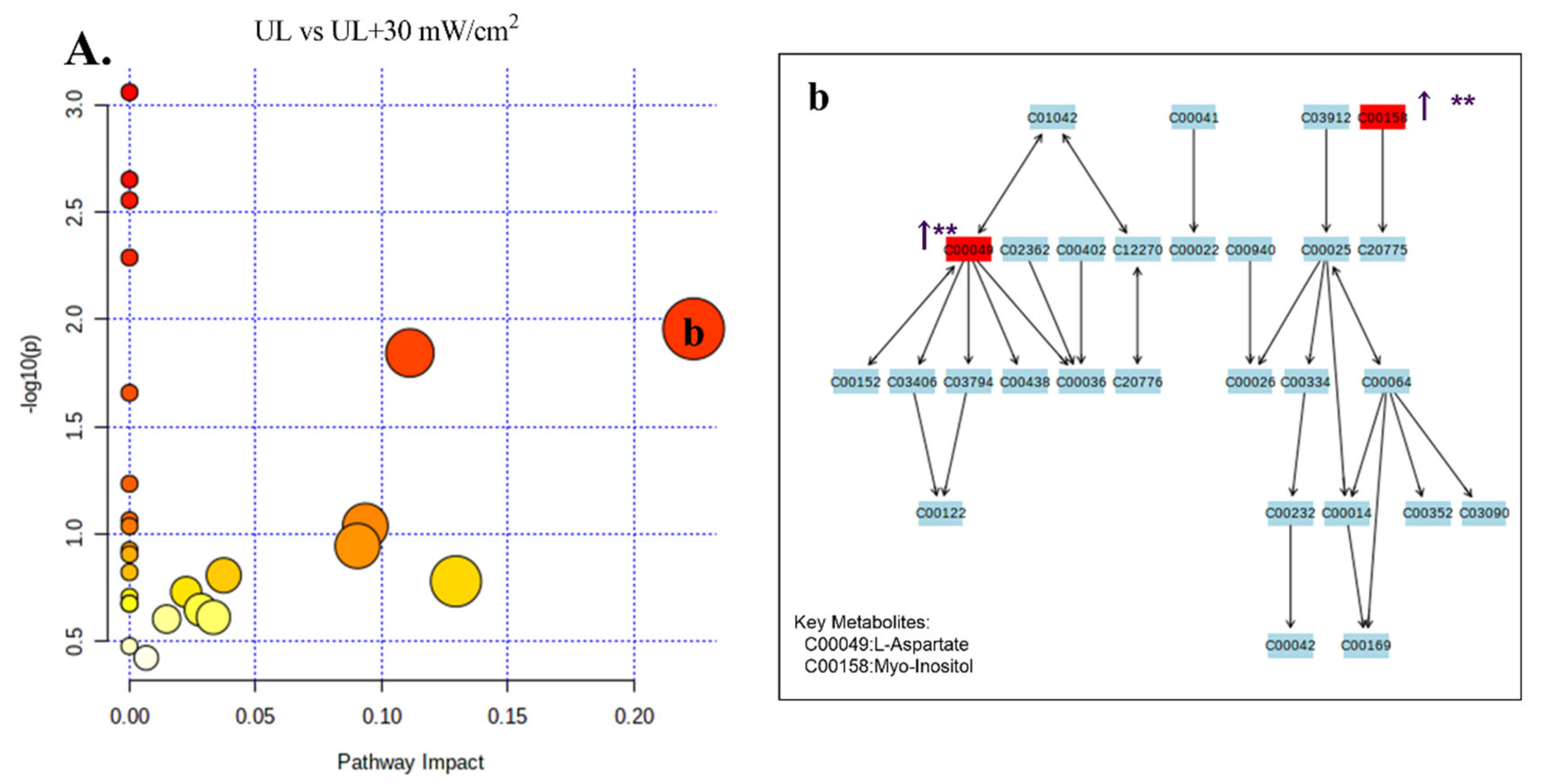
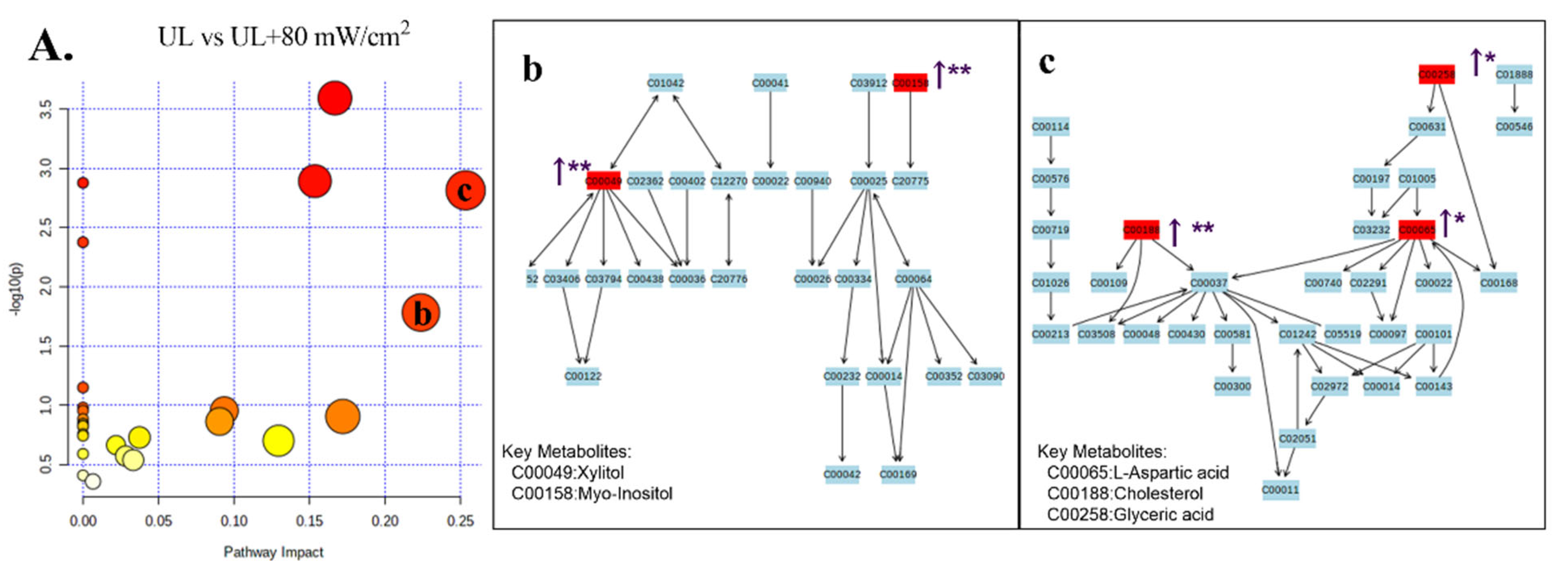
| Comparison | No. | RT | m/z | KEGG | Formula | Metabolite | Trend | Related Pathway |
|---|---|---|---|---|---|---|---|---|
| UL vs. NC | 1 | 6.387 | 147.050 | C00041 | C3H7NO2 | Alanine | ↓ * | Alanine, aspartate and glutamate metabolism; ABC transporters |
| 2 | 9.490 | 149.050 | C00042 | C4H6O4 | Succinic acid | ↓ * | TCA cycle; oxidative phosphorylation; alanine, aspartate and glutamate metabolism | |
| 3 | 10.985 | 130.050 | C00049 | C4H7NO4 | L-Aspartic acid | ↓ * | Alanine, aspartate and glutamate metabolism; serine and threonine metabolism; ABC transporters | |
| 4 | 8.720 | 132.050 | C00065 | C3H7NO3 | L-Serine | ↓ ** | Glycine, serine and threonine metabolism; sphingolipid metabolism | |
| 5 | 16.436 | 179.050 | C00082 | C9H11NO3 | Tyrosine | ↓ * | Phenylalanine, tyrosine and tryptophan biosynthesis; phenylalanine metabolism | |
| 6 | 8.495 | 189.050 | C00086 | CH4N2O | Urea | ↓ ** | Arginine and proline metabolism | |
| 7 | 7.168 | 86.150 | C00123 | C6H13NO2 | L-Leucine | ↓ ** | valine, leucine and isoleucine degradation; valine, leucine and isoleucine biosynthesis | |
| 8 | 19.307 | 147.050 | C00137 | C6H12O6 | Myo-Inositol | ↓ * | Galactose metabolism | |
| 9 | 15.779 | 73.050 | C00158 | C6H8O7 | Citric acid | ↓ * | TCA cycle | |
| 10 | 6.112 | 72.150 | C00183 | C5H11NO2 | L-Valine | ↓ ** | ABC transporters; Protein digestion and absorption | |
| 11 | 5.748 | 106.050 | C00186 | C3H6O3 | Lactic acid | ↓ ** | Gluconeogenesis; Pyruvate metabolism | |
| 12 | 33.925 | 329.350 | C00187 | C27H46O | Cholesterol | ↓ * | Steroid biosynthesis; Steroid hormone biosynthesis | |
| UL vs. UL + 30 mW/cm2 | 1 | 10.985 | 130.050 | C00049 | C4H7NO4 | L-Aspartic acid | ↑ ** | Alanine, aspartate and glutamate metabolism; arginine and proline metabolism |
| 2 | 8.509 | 73.050 | C00086 | CH4N2O | Urea | ↑ * | Arginine and proline metabolism | |
| 3 | 86.150 | 7.168 | C00123 | C6H13NO2 | L-Leucine | ↑ ** | Valine, leucine and isoleucine degradation; valine, leucine and isoleucine biosynthesis | |
| 4 | 226.150 | 19.338 | C00137 | C6H12O6 | Myo-Inositol | ↑ ** | Galactose metabolism | |
| 5 | 73.050 | 15.779 | C00158 | C6H8O7 | Citric acid | ↑ ** | TCA cycle | |
| 6 | 6.112 | 72.150 | C00183 | C5H11NO2 | L-Valine | ↑ ** | ABC transporters; protein digestion and absorption | |
| 7 | 33.920 | 129.050 | C00187 | C27H46O | Cholesterol | ↑ * | Steroid biosynthesis; steroid hormone biosynthesis | |
| 8 | 18.290 | 154.150 | C00249 | C16H32O2 | Palmitic Acid | ↑ *** | Fatty acid biosynthesis; fatty acid degradation | |
| 9 | 9.837 | 89.050 | C00258 | C3H6O4 | Glyceric acid | ↓ * | Glycine, serine and threonine metabolism | |
| 10 | 14.597 | 73.050 | C00379 | C5H12O5 | Xylitol | ↑ * | Pentose and glucuronate interconversions | |
| UL vs. UL + 80 mW/cm2 | 1 | 14.597 | 73.050 | C00049 | C4H7NO4 | Xylitol | ↑ * | Alanine, aspartate and glutamate metabolism; arginine and proline metabolism |
| 2 | 10.985 | 130.05 | C00065 | C3H7NO3 | L-Aspartic acid | ↑ * | Glycine, serine and threonine metabolism; sphingolipid metabolism | |
| 3 | 8.711 | 116.05 | C00086 | CH4N2O | L-Serine | ↑ * | Arginine and proline metabolism | |
| 4 | 8.506 | 137.05 | C00123 | C6H13NO2 | Urea | ↑ *** | Valine, leucine and isoleucine degradation; valine, leucine and isoleucine biosynthesis | |
| 5 | 7.168 | 86.15 | C00137 | C6H12O6 | L-Leucine | ↑ * | Galactose metabolism | |
| 6 | 19.307 | 217.15 | C00158 | C6H8O7 | Myo-Inositol | ↑ * | TCA cycle | |
| 7 | 15.779 | 73.05 | C00186 | C3H6O3 | Citric acid | ↑ * | Gluconeogenesis; pyruvate metabolism | |
| 8 | 5.742 | 6 | C00187 | C27H46O | Lactic acid | ↑ * | Steroid biosynthesis; steroid hormone biosynthesis | |
| 9 | 33.92 | 129.05 | C00188 | C4H9NO3 | Cholesterol | ↑ * | Valine, leucine and isoleucine biosynthesis; aminoacyl-tRNA biosynthesis | |
| 10 | 9.245 | 117.05 | C00249 | C16H32O2 | L-Threonine | ↑ * | Fatty acid biosynthesis; fatty acid degradation | |
| 11 | 9.807 | 133.050 | C00258 | C3H6O4 | Glyceric acid | ↑ * | Glycine, serine and threonine metabolism | |
| 12 | 18.287 | 133.05 | C00249 | C16H32O2 | Palmitic Acid | ↑ ** | Fatty acid metabolism | |
| 13 | 6.116 | 55.15 | C00183 | C5H11NO2 | L-Valine | ↑ * | ABC transporters; Protein digestion and absorption |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; An, S.; Zhang, Z.; Zhou, Y.; Yu, Y.; Ma, Z.; Fan, X.; Tang, L.; Guo, J. Molecular and Metabolic Mechanism of Low-Intensity Pulsed Ultrasound Improving Muscle Atrophy in Hindlimb Unloading Rats. Int. J. Mol. Sci. 2021, 22, 12112. https://doi.org/10.3390/ijms222212112
Sun L, An S, Zhang Z, Zhou Y, Yu Y, Ma Z, Fan X, Tang L, Guo J. Molecular and Metabolic Mechanism of Low-Intensity Pulsed Ultrasound Improving Muscle Atrophy in Hindlimb Unloading Rats. International Journal of Molecular Sciences. 2021; 22(22):12112. https://doi.org/10.3390/ijms222212112
Chicago/Turabian StyleSun, Lijun, Shasha An, Zhihao Zhang, Yaling Zhou, Yanan Yu, Zhanke Ma, Xiushan Fan, Liang Tang, and Jianzhong Guo. 2021. "Molecular and Metabolic Mechanism of Low-Intensity Pulsed Ultrasound Improving Muscle Atrophy in Hindlimb Unloading Rats" International Journal of Molecular Sciences 22, no. 22: 12112. https://doi.org/10.3390/ijms222212112
APA StyleSun, L., An, S., Zhang, Z., Zhou, Y., Yu, Y., Ma, Z., Fan, X., Tang, L., & Guo, J. (2021). Molecular and Metabolic Mechanism of Low-Intensity Pulsed Ultrasound Improving Muscle Atrophy in Hindlimb Unloading Rats. International Journal of Molecular Sciences, 22(22), 12112. https://doi.org/10.3390/ijms222212112





