Anaesthesia-Induced Transcriptomic Changes in the Context of Renal Ischemia Uncovered by the Use of a Novel Clamping Device
Abstract
1. Introduction
2. Results
2.1. Absence of a Commercially Available Device Suitable to Induce Multiple Episodes of IRI in Mice
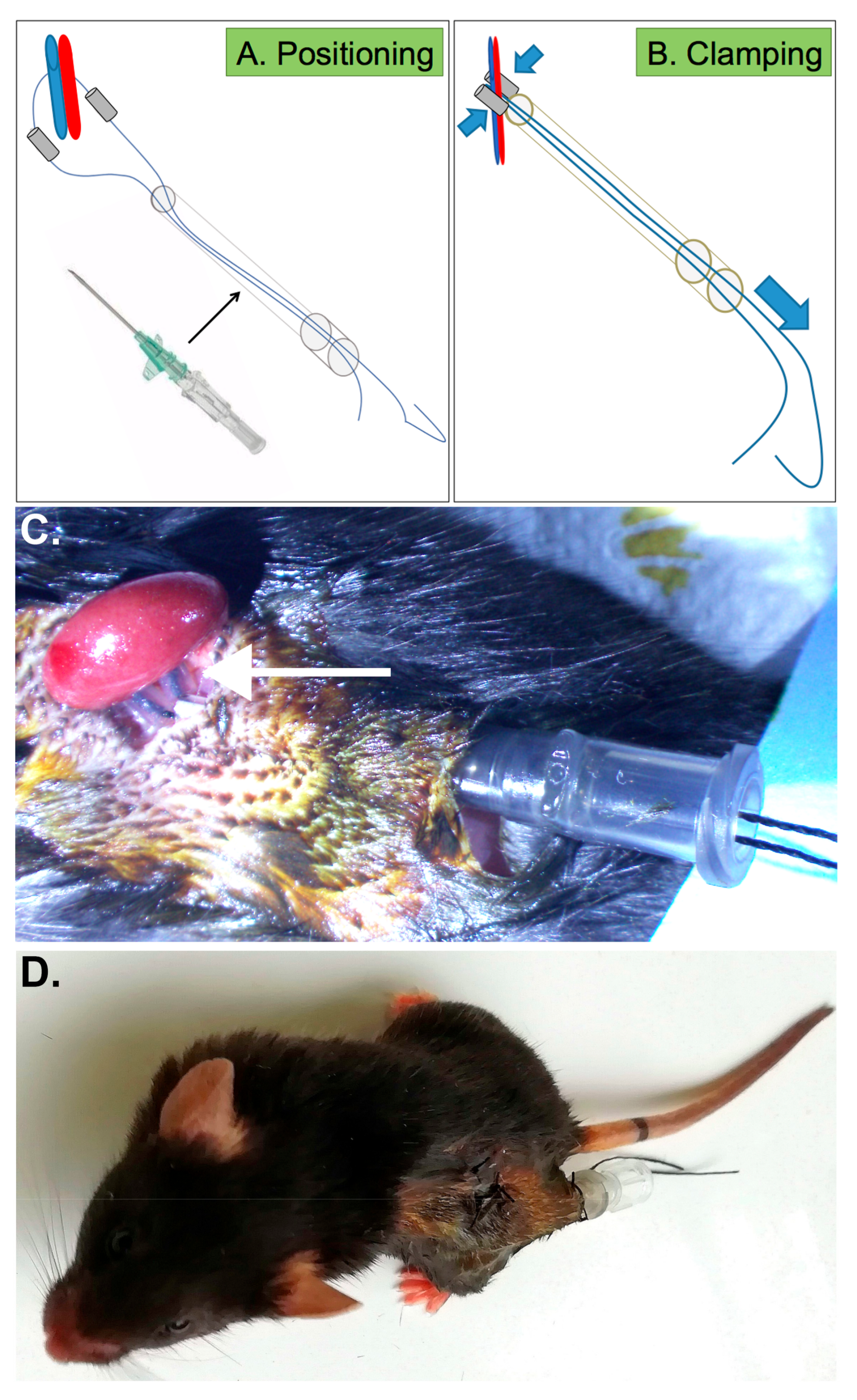
2.2. Generation of New Device Allowing Repeated Renal Ischemia in Conscious Mice
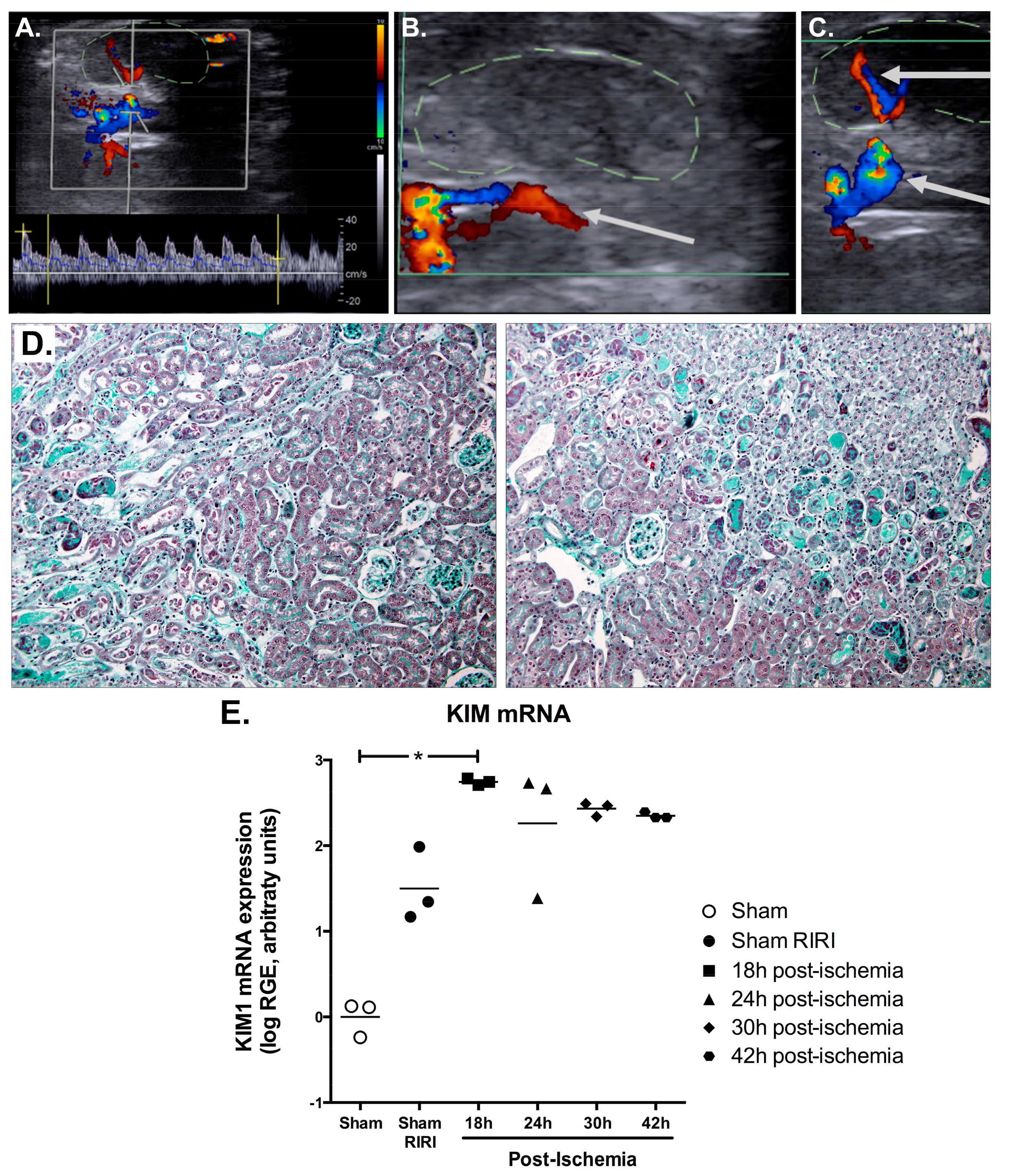
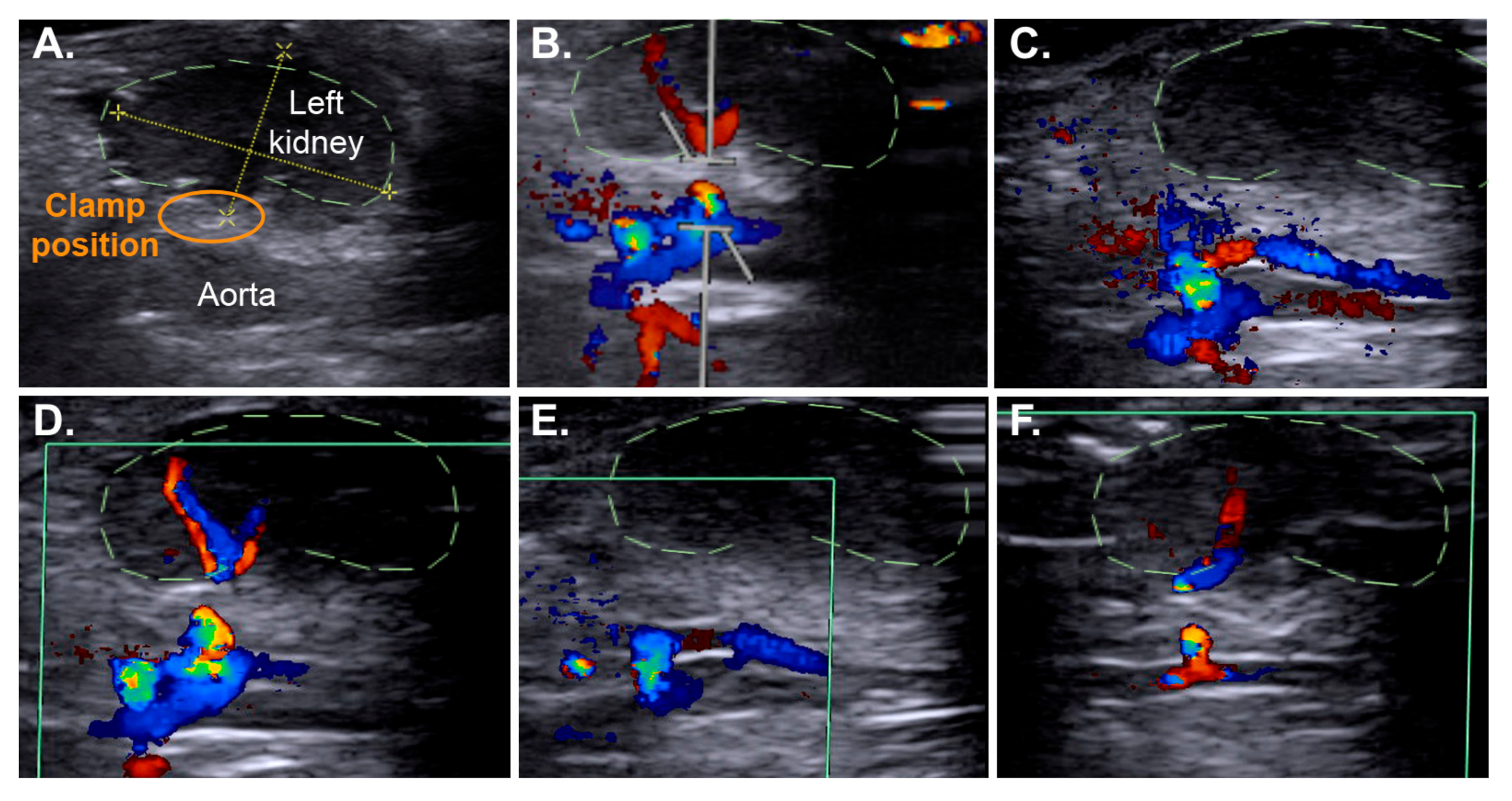
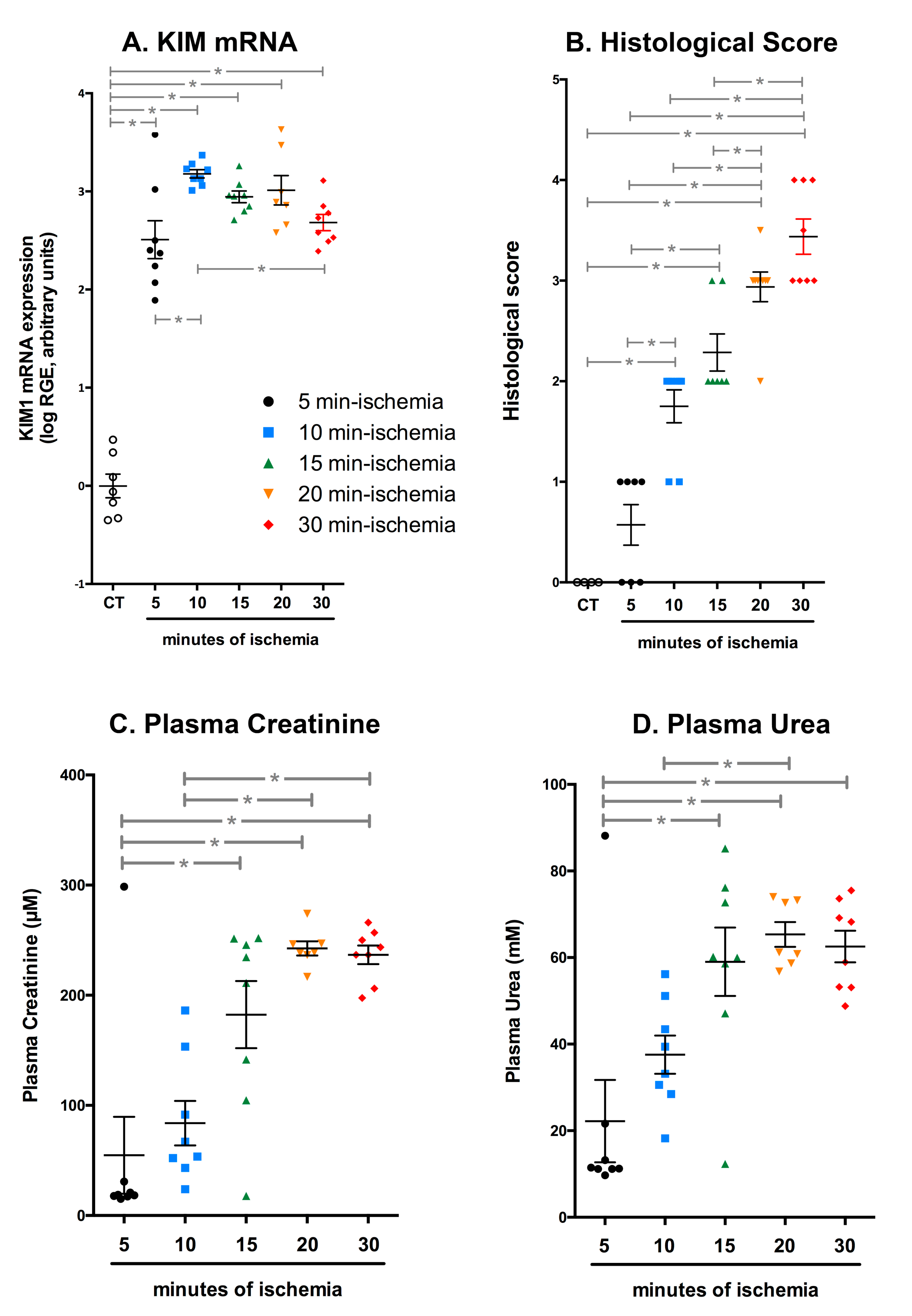
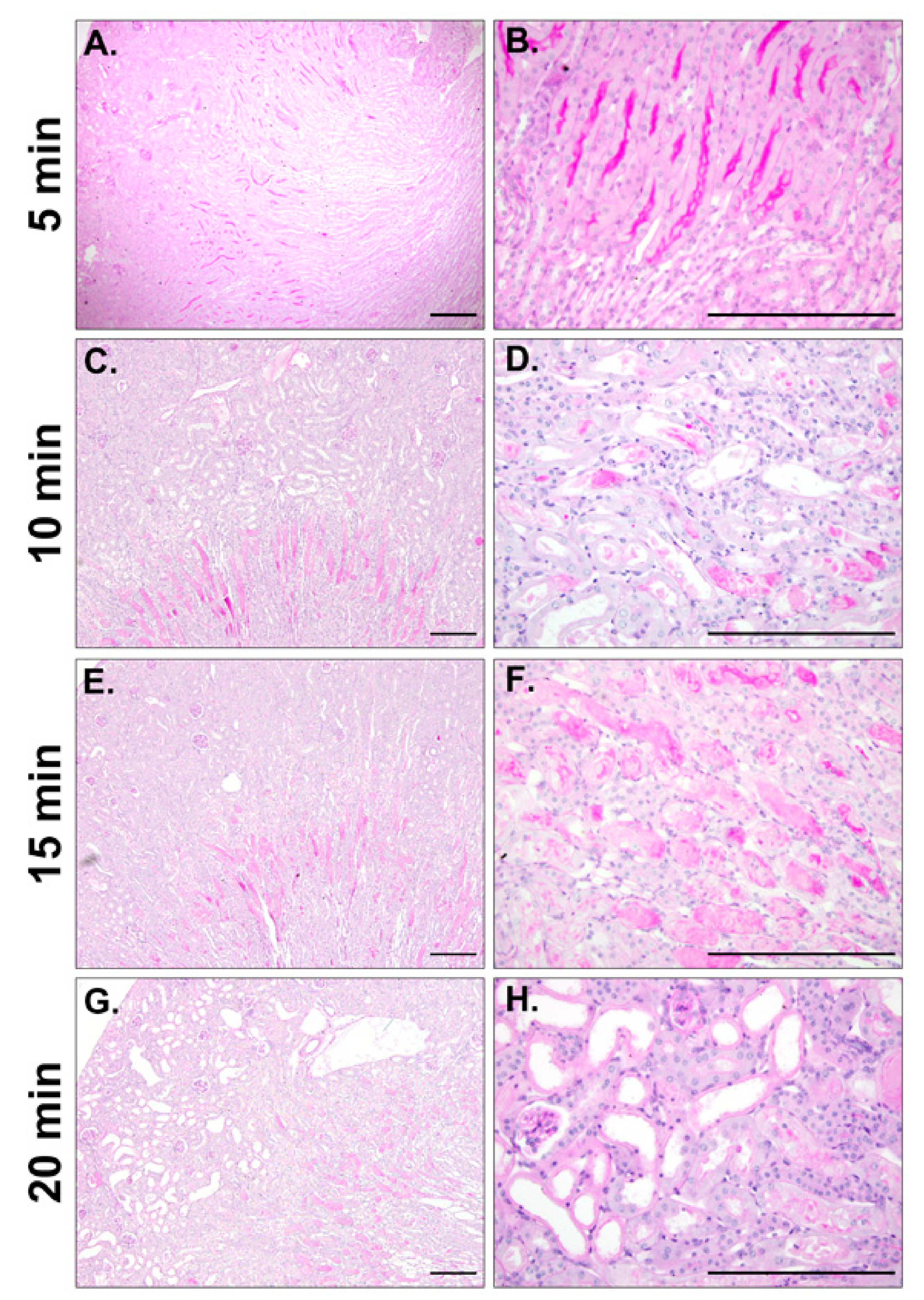
2.3. Efficient IRI Using the RIRI Clamp
2.4. The RIRI Clamp Can Be Used to Induce Ischemia in a Large Number of Mice in a Short Period of Time
2.5. Multiple Episodes of IRI Can Be Induced with the RIRI Clamp
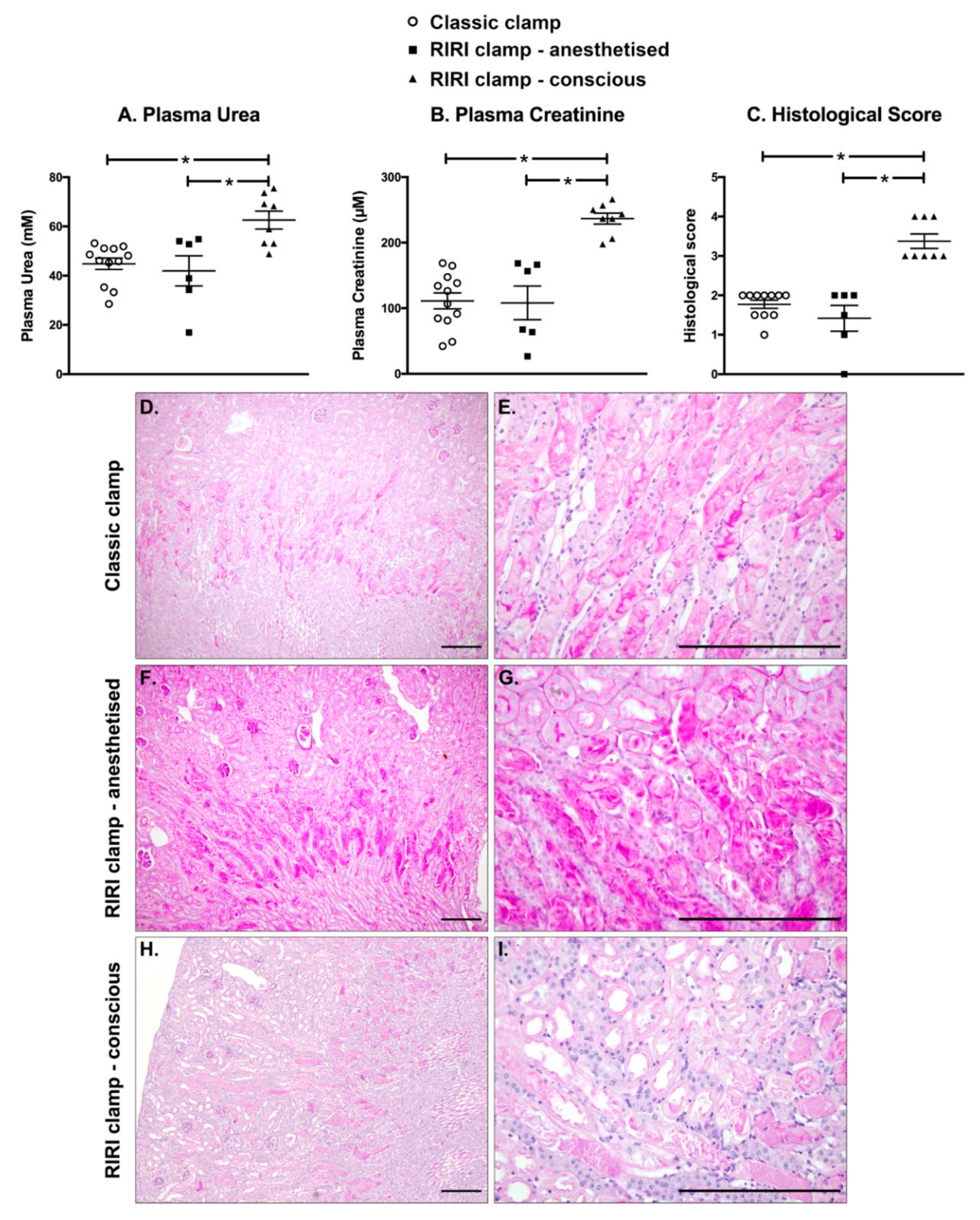
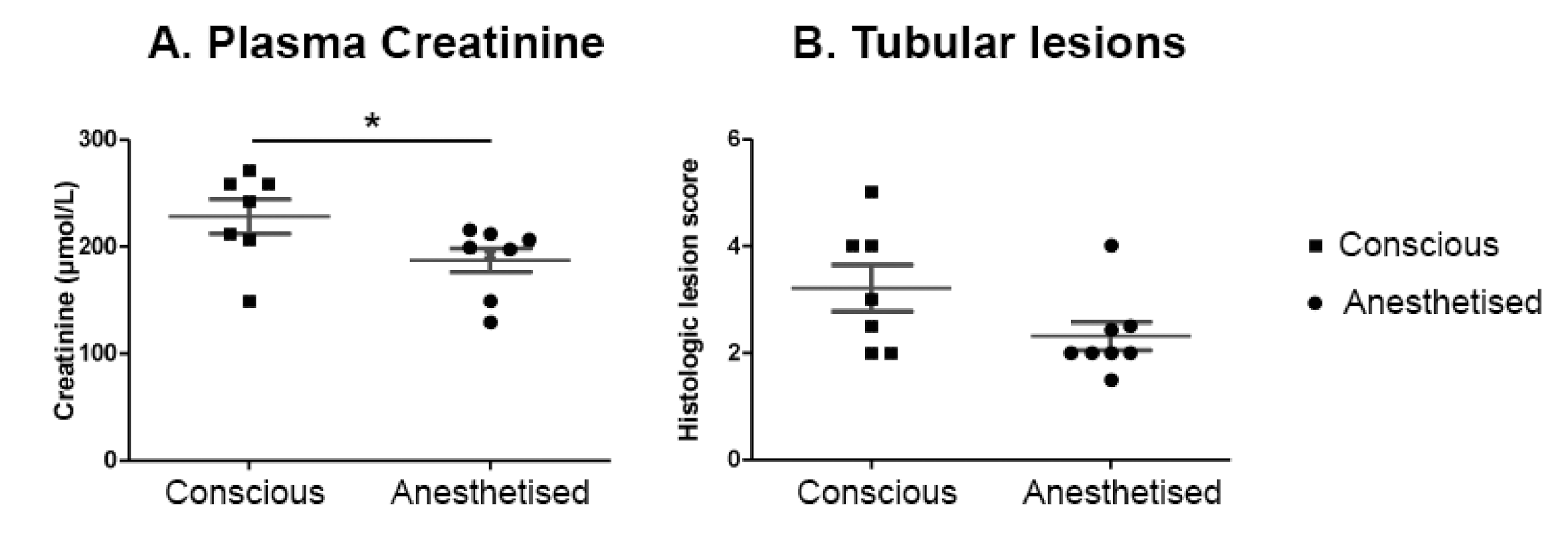
2.6. IRI Induced in Conscious Animals Is More Severe Than in Anaesthetised Ones
2.7. Characterisation of the Protective Effect of Anaesthesia at the Transcriptomic Level
2.7.1. Anaesthesia Protects from IRI Independently of Hypothermia
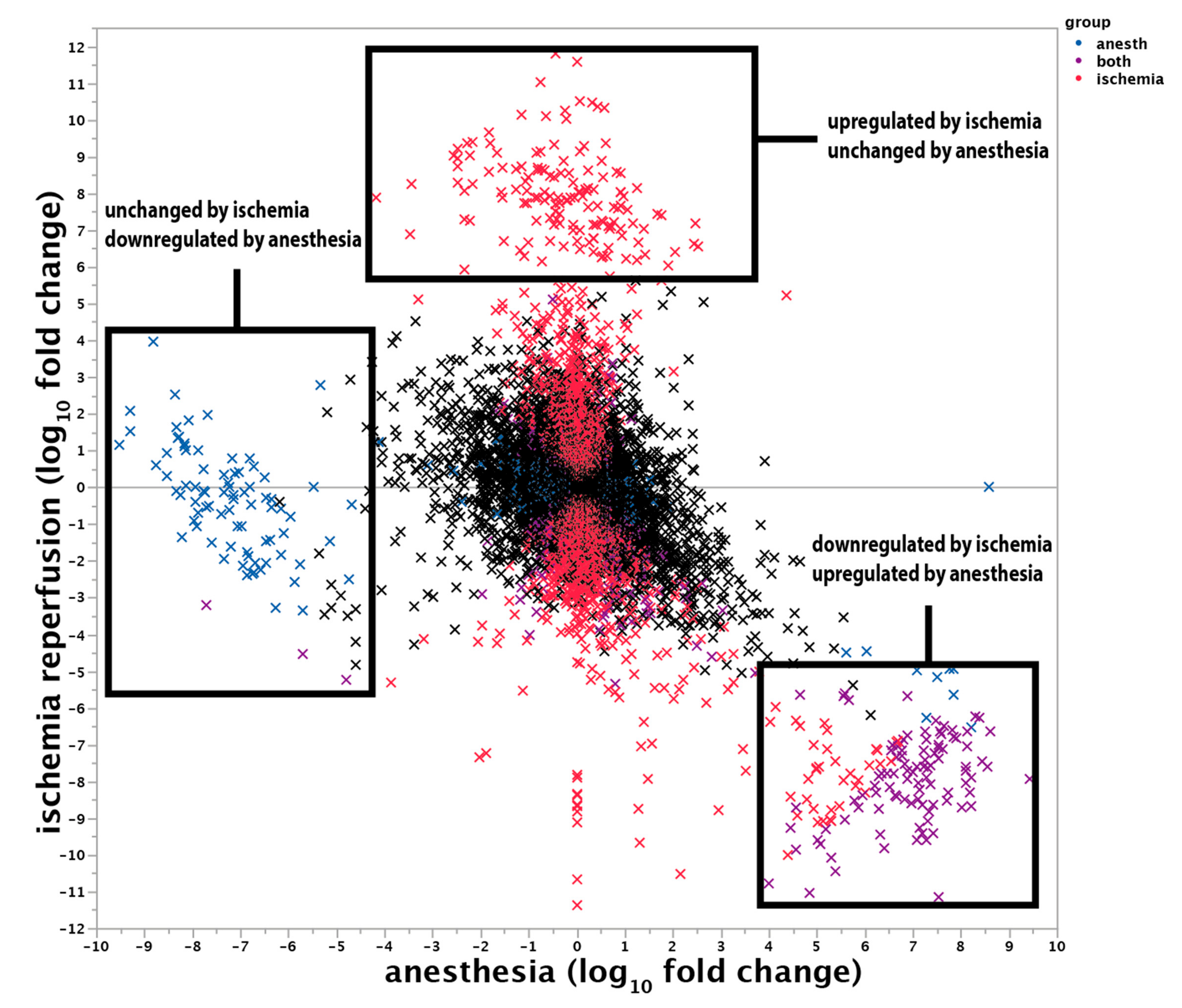
2.7.2. Transcriptional Changes Induced by the Anaesthesia
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Testing of the Vascular Occluder Inflatable Device (InterFocus® Ref. 18080-02)
4.3. Nephrectomy and Positioning of the RIRI Clamp
4.4. Induction of Ischemia with the RIRI Device
4.5. Renal Ischemia-Reperfusion Injury Using the Non-Traumatic Surgical Clamp
4.6. Non-Invasive Ultrasound Assessment of Renal Hemodynamic
4.7. Tissue Collection
4.8. Histological Analysis
4.9. RNA Extraction and RT-qPCR
4.10. Statistical Analysis
4.11. RNA Extraction and Bulk RNA Sequencing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute kidney injury. Lancet Lond. Engl. 2012, 380, 756–766. [Google Scholar] [CrossRef]
- Peters-Sengers, H.; Houtzager, J.H.E.; Idu, M.M.; Heemskerk, M.B.A.; van Heurn, E.L.W.; van der Heide, J.J.H.; Kers, J.; Berger, S.P.; van Gulik, T.M.; Bemelman, F.J. Impact of Cold Ischemia Time on Outcomes of Deceased Donor Kidney Transplantation: An Analysis of a National Registry. Transplant. Direct 2019, 5, e448. [Google Scholar] [CrossRef]
- Summers, D.M.; Johnson, R.J.; Hudson, A.; Collett, D.; Watson, C.J.; Bradley, J.A. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: A cohort study. Lancet Lond. Engl. 2013, 381, 727–734. [Google Scholar] [CrossRef]
- Heyman, S.N.; Rosenberger, C.; Rosen, S.S. Experimental ischemia-reperfusion: Biases and myths-the proximal vs. distal hypoxic tubular injury debate revisited. Kidney Int. 2010, 77, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Thakar, C.V.; Christianson, A.; Himmelfarb, J.; Leonard, A.C. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin. J. Am. Soc. Nephrol. CJASN 2011, 6, 2567–2572. [Google Scholar] [CrossRef]
- Le Clef, N.; Verhulst, A.; D’Haese, C.; Vervaet, B.A. Unilateral Renal Ischemia-Reperfusion as a Robust Model for Acute to Chronic Kidney Injury in Mice. PLoS ONE 2016, 11, e0152153. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Preconditioning and postconditioning: Underlying mechanisms and clinical application. Atherosclerosis 2009, 204, 334–341. [Google Scholar] [CrossRef]
- Crowley, L.E.; McIntyre, C.W. Remote ischaemic conditioning-therapeutic opportunities in renal medicine. Nat. Rev. Nephrol. 2013, 9, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Zager, R.A.; Altschuld, R. Body temperature: An important determinant of severity of ischemic renal injury. Am. J. Physiol.-Ren. Physiol. 1986, 251, F87–F93. [Google Scholar] [CrossRef]
- De Rosa, S.; Antonelli, M.; Ronco, C. Hypothermia and kidney: A focus on ischaemia–reperfusion injury. Nephrol. Dial. Transplant. 2017, 32, 241–247. [Google Scholar] [CrossRef]
- Jaber, S.M.; Hankenson, F.C.; Heng, K.; McKinstry-Wu, A.; Kelz, M.B.; Marx, J.O. Dose regimens, variability, and complications associated with using repeat-bolus dosing to extend a surgical plane of anaesthesia in laboratory mice. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 684–691. [Google Scholar] [PubMed]
- Iguchi, N.; Kosaka, J.; Booth, L.C.; Iguchi, Y.; Evans, R.G.; Bellomo, R.; May, C.N.; Lankadeva, Y.R. Renal perfusion, oxygenation, and sympathetic nerve activity during volatile or intravenous general anaesthesia in sheep. Br. J. Anaesth. 2019, 122, 342–349. [Google Scholar] [CrossRef]
- Motayagheni, N.; Phan, S.; Eshraghi, C.; Nozari, A.; Atala, A. A Review of Anesthetic Effects on Renal Function: Potential Organ Protection. Am. J. Nephrol. 2017, 46, 380–389. [Google Scholar] [CrossRef] [PubMed]
- du Pré, B.; van Veen, T.; Crnko, S.; Vos, M.; Deddens, J.; Doevendans, P.; van Laake, L. Variation within Variation: Comparison of 24-h Rhythm in Rodent Infarct Size between Ischemia Reperfusion and Permanent Ligation. Int. J. Mol. Sci. 2017, 18, 1670. [Google Scholar] [CrossRef]
- Bartman, C.M.; Eckle, T. Circadian-Hypoxia Link and its Potential for Treatment of Cardiovascular Disease. Curr. Pharm. Des. 2019, 25, 1075–1090. [Google Scholar] [CrossRef]
- Rabinovich-Nikitin, I.; Lieberman, B.; Martino, T.A.; Kirshenbaum, L.A. Circadian-Regulated Cell Death in Cardiovascular Diseases. Circulation 2019, 139, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Thosar, S.S.; Butler, M.P.; Shea, S.A. Role of the circadian system in cardiovascular disease. J. Clin. Investig. 2018, 128, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Burioka, N.; Koyanagi, S.; Fukuoka, Y.; Okazaki, F.; Fujioka, T.; Kusunose, N.; Endo, M.; Suyama, H.; Chikumi, H.; Ohdo, S.; et al. Influence of intermittent hypoxia on the signal transduction pathways to inflammatory response and circadian clock regulation. Life Sci. 2009, 85, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Zager, R.A.; Gmur, D.J.; Bredl, C.R.; Eng, M.J. Degree and time sequence of hypothermic protection against experimental ischemic acute renal failure. Circ. Res. 1989, 65, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Pelkey, T.J.; Frank, R.S.; Stanley, J.J.; Frank, T.S.; Zelenock, G.B.; D’Alecy, L.G. Minimal physiologic temperature variations during renal ischemia alter functional and morphologic outcome. J. Vasc. Surg. 1992, 15, 619–625. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, S.; Yamamoto, M.; Nakamura, J.; Mii, A.; Yamamoto, S.; Takahashi, M.; Kaneko, K.; Uchino, E.; Sato, Y.; Fukuma, S.; et al. Spatiotemporal ATP Dynamics during AKI Predict Renal Prognosis. J. Am. Soc. Nephrol. 2020, 31, 2855–2869. [Google Scholar] [CrossRef]
- Niemann, C.U.; Feiner, J.; Swain, S.; Bunting, S.; Friedman, M.; Crutchfield, M.; Broglio, K.; Hirose, R.; Roberts, J.P.; Malinoski, D. Therapeutic Hypothermia in Deceased Organ Donors and Kidney-Graft Function. N. Engl. J. Med. 2015, 373, 405–414. [Google Scholar] [CrossRef]
- Macedo, E.; Mehta, R.L. Prerenal failure: From old concepts to new paradigms. Curr. Opin. Crit. Care 2009, 15, 467–473. [Google Scholar] [CrossRef]
- Robertson, L.T.; Treviño-Villarreal, J.H.; Mejia, P.; Grondin, Y.; Harputlugil, E.; Hine, C.; Vargas, D.; Zheng, H.; Ozaki, C.K.; Kristal, B.S.; et al. Protein and Calorie Restriction Contribute Additively to Protection from Renal Ischemia Reperfusion Injury Partly via Leptin Reduction in Male Mice. J. Nutr. 2015, 145, 1717–1727. [Google Scholar] [CrossRef]
- Darreau, C.; Martino, F.; Saint-Martin, M.; Jacquier, S.; Hamel, J.F.; Nay, M.A.; Terzi, N.; Ledoux, G.; Roche-Campo, F.; Camous, L.; et al. Use, timing and factors associated with tracheal intubation in septic shock: A prospective multicentric observational study. Ann. Intensive Care 2020, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Hajibandeh, S.; Hajibandeh, S.; Antoniou, S.A.; Torella, F.; Antoniou, G.A. Meta-analysis and trial sequential analysis of local vs. general anaesthesia for carotid endarterectomy. Anaesthesia 2018, 73, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Zager, R.A. “Biologic memory” in response to acute kidney injury: Cytoresistance, toll-like receptor hyper-responsiveness and the onset of progressive renal disease. Nephrol. Dial. Transplant. 2013, 28, 1985–1993. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, J.; Wang, L.; Jiang, S.; Fu, L.; Buggs, J.; Liu, R. New mouse model of chronic kidney disease transitioned from ischemic acute kidney injury. Am. J. Physiol. Renal Physiol. 2019, 317, F286–F295. [Google Scholar] [CrossRef]
- Ichimura, T.; Asseldonk EJ, P.V.; Humphreys, B.D.; Gunaratnam, L.; Duffield, J.S.; Bonventre, J.V. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Investig. 2008, 118, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Domrachez, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acid Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verney, C.; Legouis, D.; Placier, S.; Migeon, T.; Bonnin, P.; Buob, D.; Hadchouel, J.; Galichon, P. Anaesthesia-Induced Transcriptomic Changes in the Context of Renal Ischemia Uncovered by the Use of a Novel Clamping Device. Int. J. Mol. Sci. 2021, 22, 9840. https://doi.org/10.3390/ijms22189840
Verney C, Legouis D, Placier S, Migeon T, Bonnin P, Buob D, Hadchouel J, Galichon P. Anaesthesia-Induced Transcriptomic Changes in the Context of Renal Ischemia Uncovered by the Use of a Novel Clamping Device. International Journal of Molecular Sciences. 2021; 22(18):9840. https://doi.org/10.3390/ijms22189840
Chicago/Turabian StyleVerney, Charles, David Legouis, Sandrine Placier, Tiffany Migeon, Philippe Bonnin, David Buob, Juliette Hadchouel, and Pierre Galichon. 2021. "Anaesthesia-Induced Transcriptomic Changes in the Context of Renal Ischemia Uncovered by the Use of a Novel Clamping Device" International Journal of Molecular Sciences 22, no. 18: 9840. https://doi.org/10.3390/ijms22189840
APA StyleVerney, C., Legouis, D., Placier, S., Migeon, T., Bonnin, P., Buob, D., Hadchouel, J., & Galichon, P. (2021). Anaesthesia-Induced Transcriptomic Changes in the Context of Renal Ischemia Uncovered by the Use of a Novel Clamping Device. International Journal of Molecular Sciences, 22(18), 9840. https://doi.org/10.3390/ijms22189840





