Rhizosphere Microbiomes of Potato Cultivated under Bacillus subtilis Treatment Influence the Quality of Potato Tubers
Abstract
:1. Introduction
2. Results
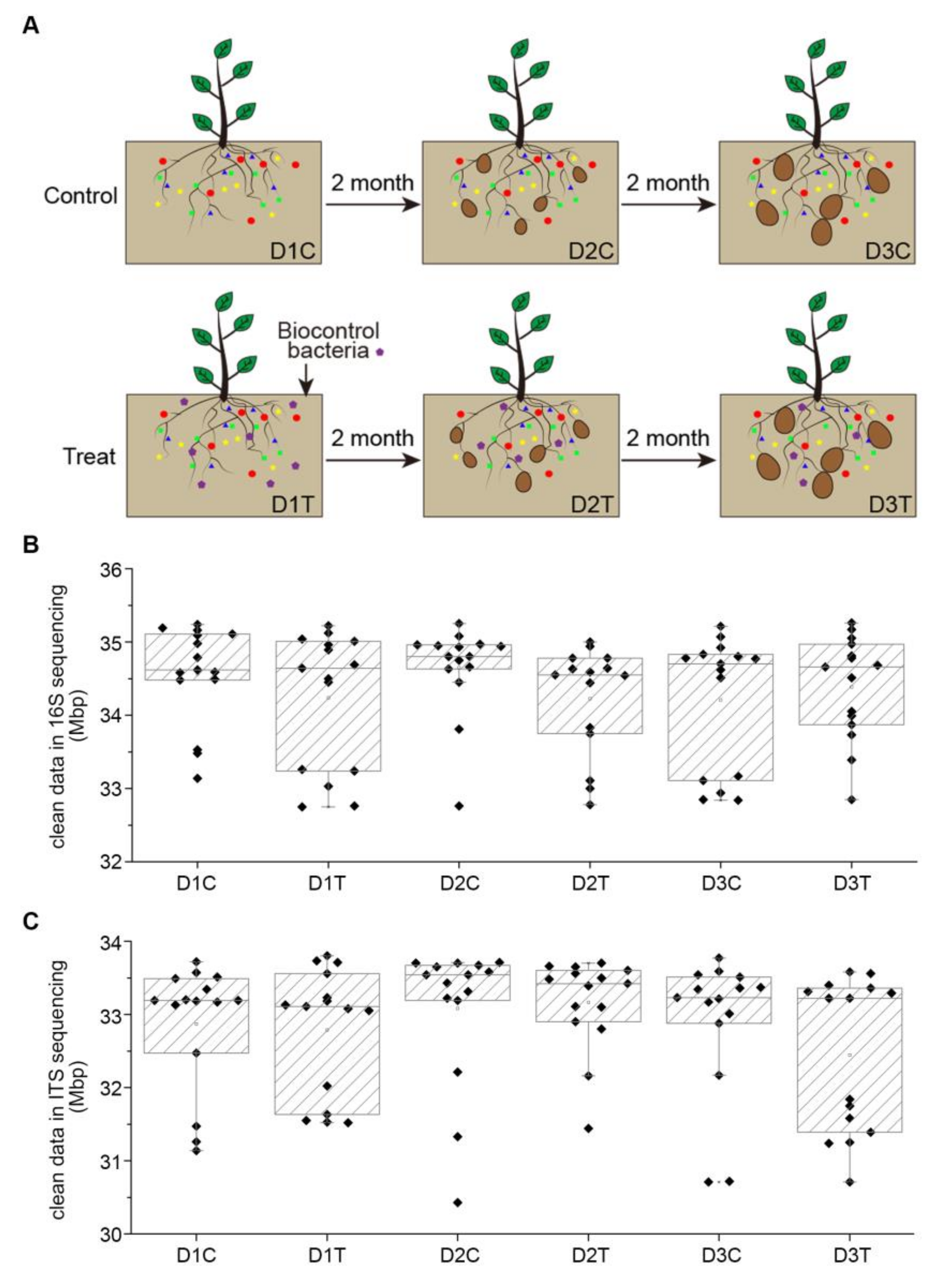
2.1. Experimental Design and Treatment Structure
2.2. Data Characteristic of Soil Samples
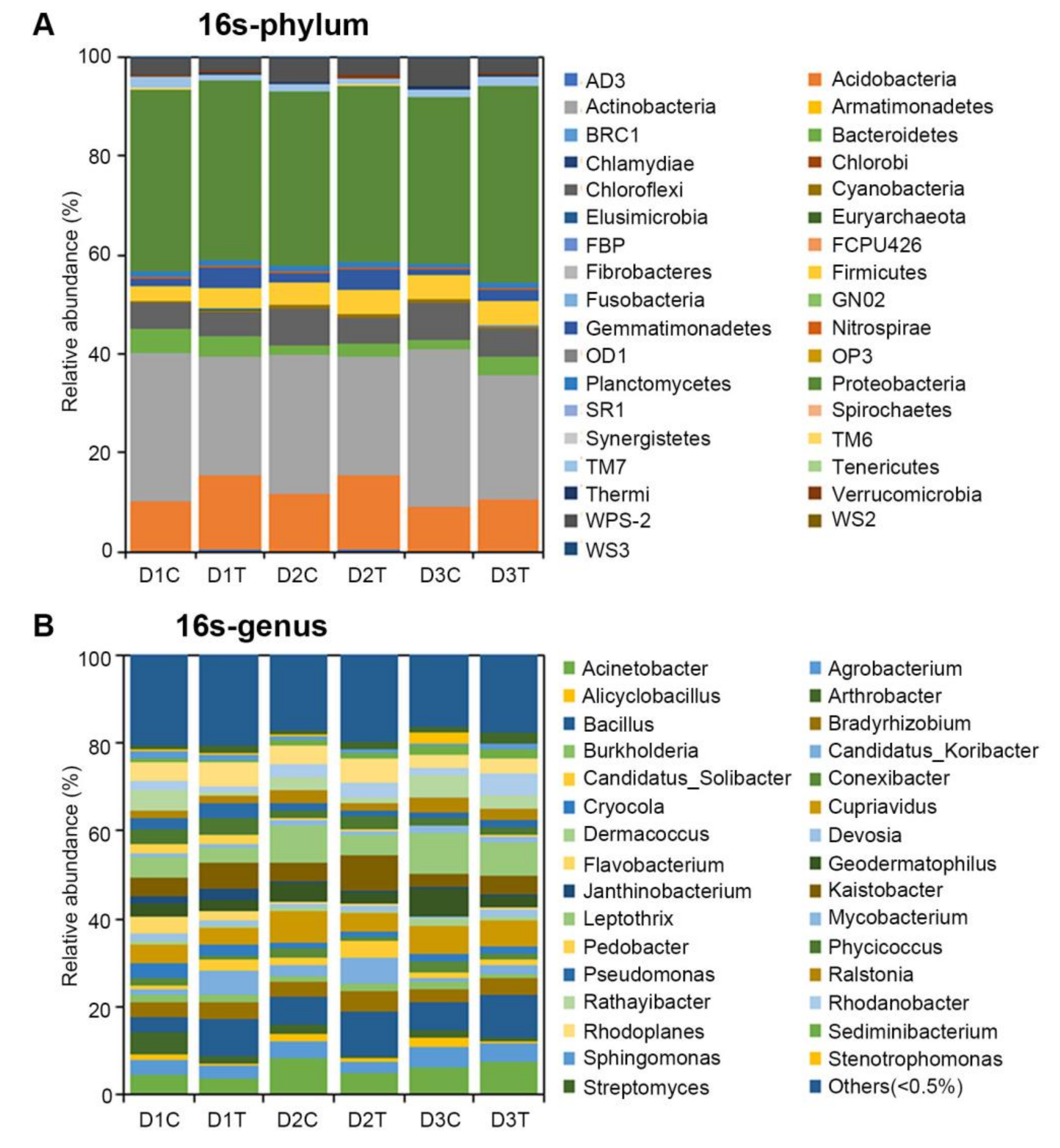
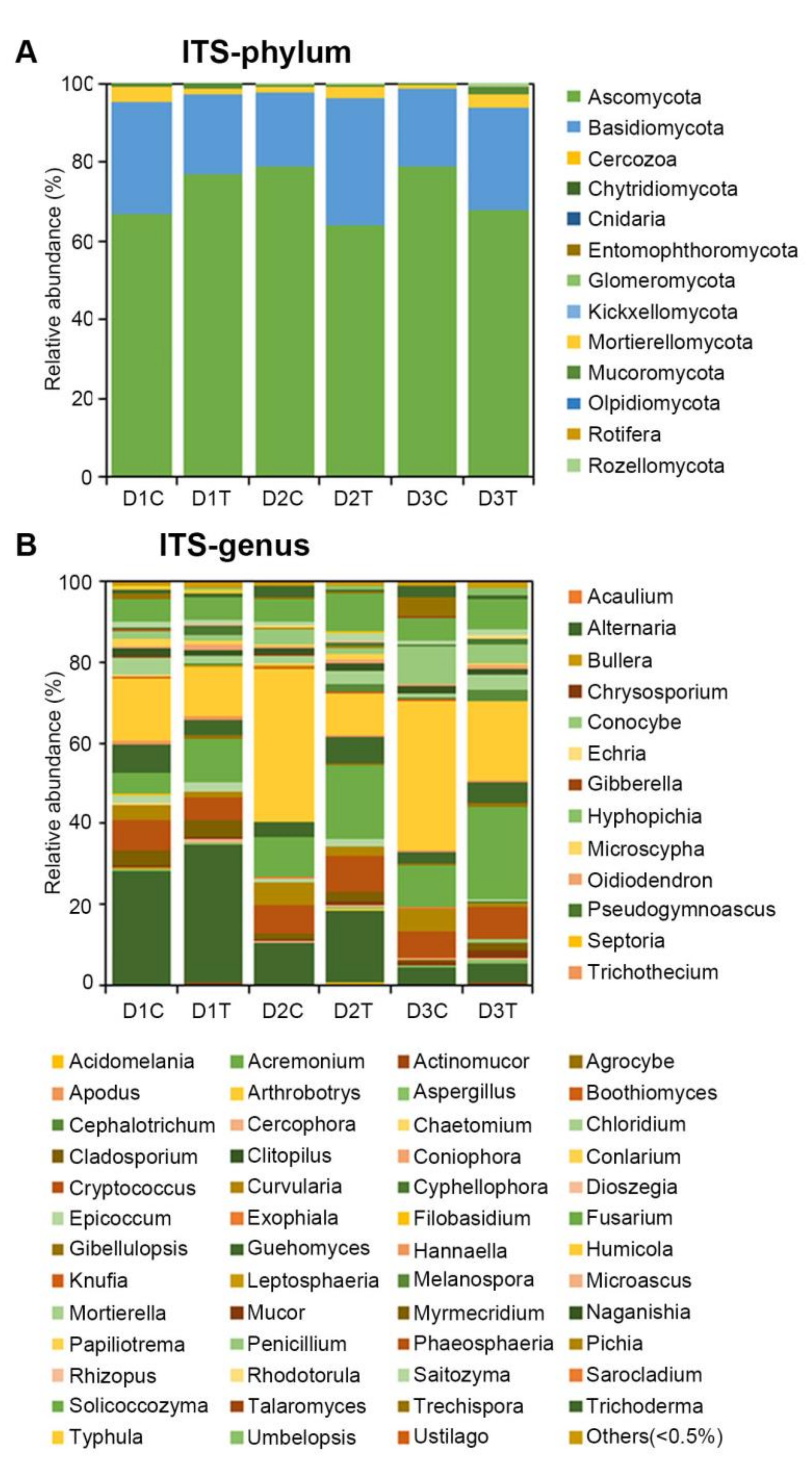
2.3. Analysis of the Microbial Community Composition between Treated and Untreated Soils
2.4. Microorganism Community Diversity in Soil Samples under Different Conditions
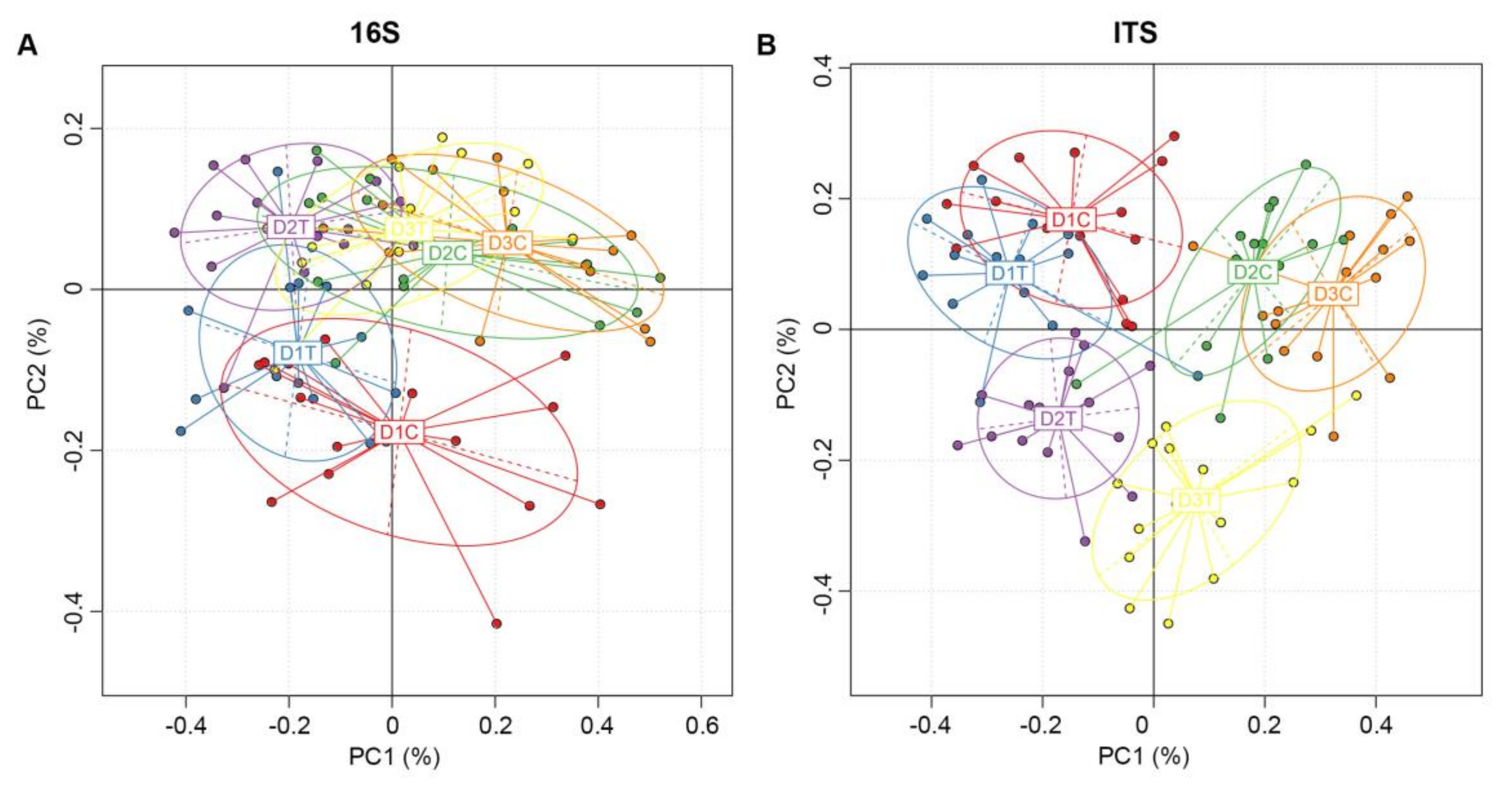
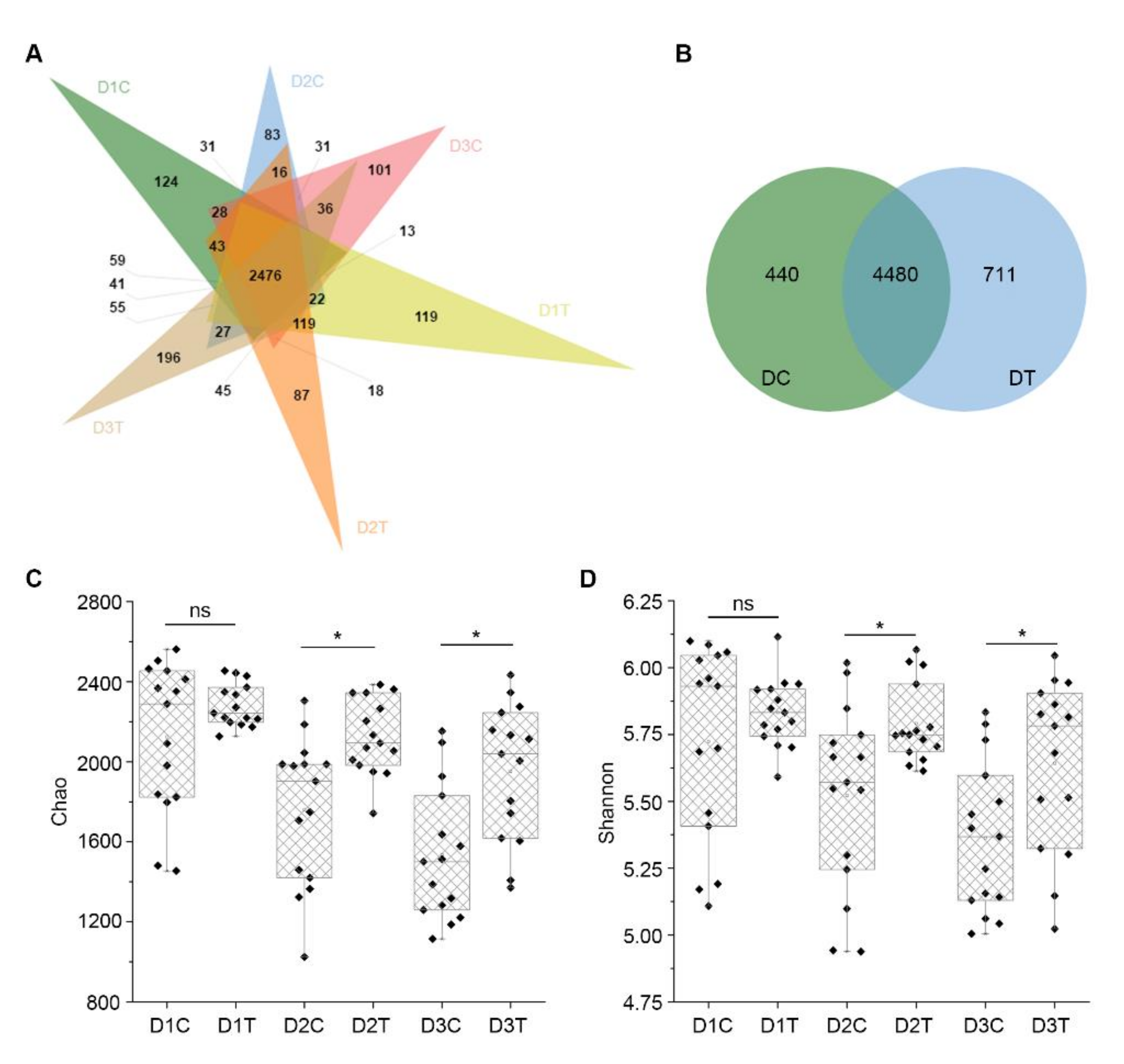
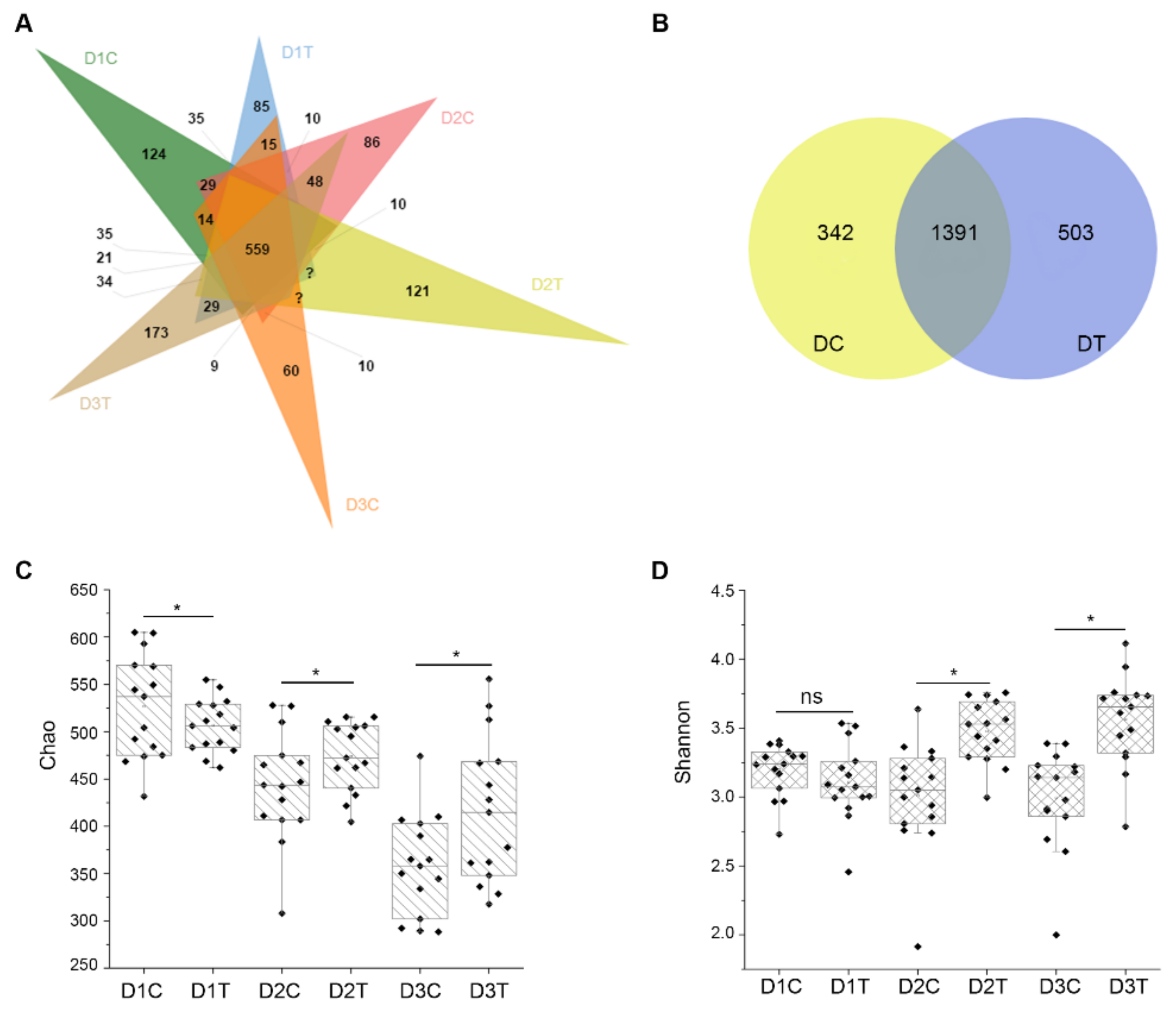
2.5. Similarities and Differences of OTUs between Six Groups of Soil Samples
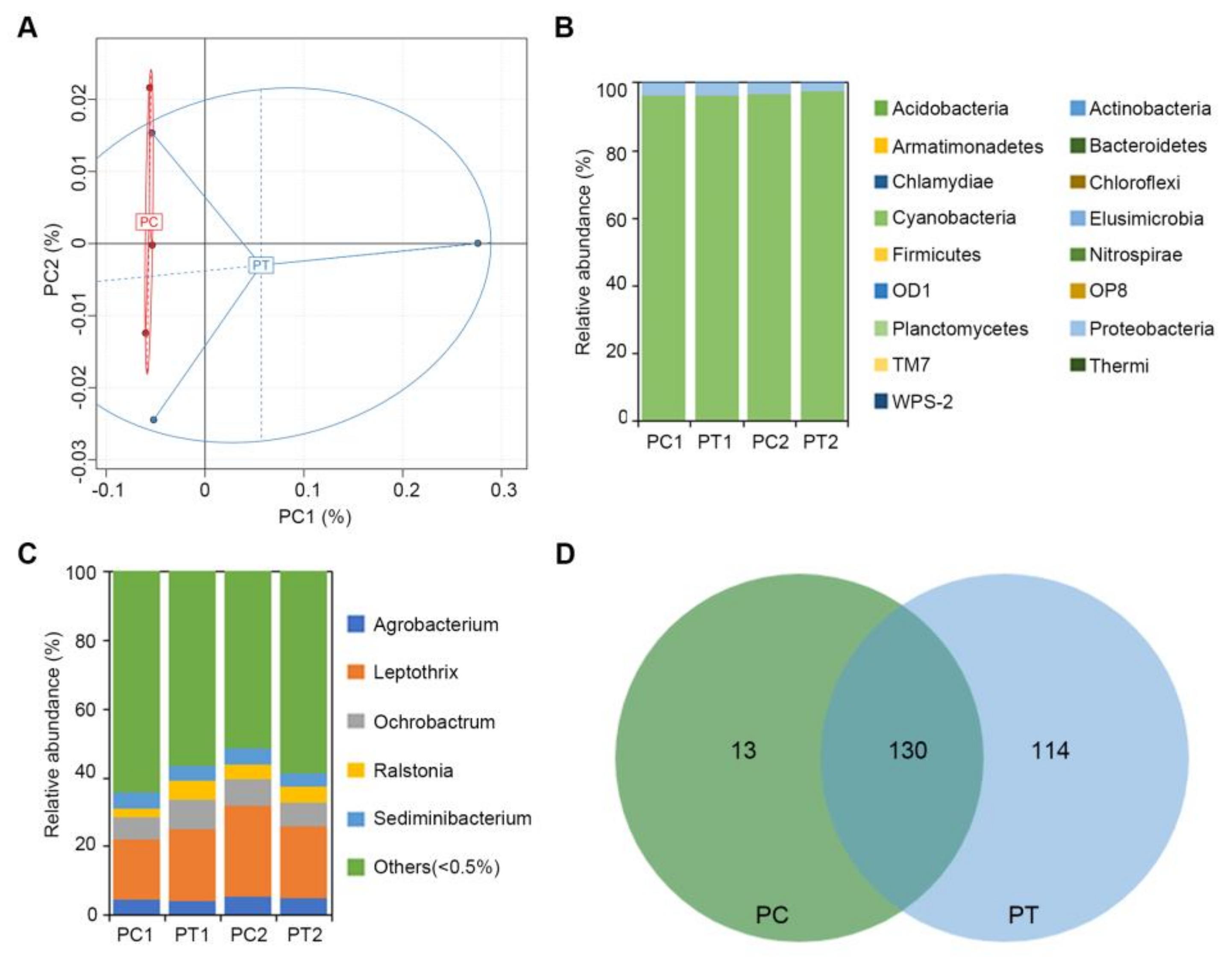
2.6. Analysis of Bacteria Diversity of Potato by 16S Sequencing
2.7. Analysis of Potato Quality Measures
3. Discussion
4. Materials and Methods
4.1. Preparation and Dosage of Bacillus subtilis Strain Bv17 in Field
4.2. Soil Treatment and Collection of Soil and Potato Sample
4.3. DNA Extraction and PCR Analysis
4.4. Sequencing Analysis
4.5. Detection of Quality of Potato Tubers
4.6. Statistical Analysis
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zarraonaindia, I.; Owens, S.M.; Weisenhorn, P.; West, K.; Marcell, J.H.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.; Taghavi, S.; et al. The soil microbiome influences grapevine-associated microbiota. mBio 2015, 6, e02527-14. [Google Scholar]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Niu, B.; Paulson, J.N.; Zheng, X.; Kolter, R. Simplified and representative bacterial community of maize roots. Proc. Natl. Acad. Sci. USA 2017, 114, 2450–2459. [Google Scholar] [CrossRef] [Green Version]
- Leach, J.E.; Triplett, L.R.; Argueso, C.T.; Trivedi, P. Communication in the Phytobiome. Cell 2017, 169, 587–596. [Google Scholar]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Cole, B.J.; Feltcher, M.E.; Waters, R.J.; Wetmore, K.M.; Mucyn, T.S.; Ryan, E.J.; Wang, G.; Hasan, S.U.; McDonald, M.; Yoshikuni, Y.; et al. Genome-wide identification of bacterial plant colonization genes. PLoS Biol. 2017, 15, e2002860. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Gonzalez, I.S.; Mittelviefhaus, M.; Clingenpeel, S.; Paredes, S.H.; Miao, J.; Wang, K.; Devescovi, G.; Stillman, K.; Monteiro, F.; et al. Genomic features of bacterial adaptation to plants. Nat. Genet. 2018, 50, 138–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [PubMed] [Green Version]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Xu, X.M.; Jeffries, P.; Pautasso, M.; Jeger, M.J. Combined use of biocontrol agents to manage plant diseases in theory and practice. Phytopathology 2011, 101, 1024–1031. [Google Scholar]
- Mazzola, M. Assessment and Management of Soil Microbial Community Structure for Disease Suppression. Annu. Rev. Phytopathol. 2004, 42, 35–59. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, R.; Luu, V.T.; Weinhold, A.; Goldberg, J.; Oh, Y.; Baldwin, I.T. Native root-associated bacteria rescue a plant from a sudden-wilt disease that emerged during continuous cropping. Proc. Natl. Acad. Sci. USA 2015, 112, 5013–5020. [Google Scholar]
- Van Bruggen, A.H.C.; Semenov, A.M.; van Diepeningen, A.D.; de Vos, O.J.; Blok, W.J. Relation between Soil Health, Wave-like Fluctuations in Microbial Populations, and Soil-borne Plant Disease Management. Eur. J. Plant Pathol. 2006, 115, 105–122. [Google Scholar]
- Zhang, Y.; Xu, J.; Riera, N.; Jin, T.; Li, J.; Wang, N. Huanglongbing impairs the rhizosphere-to-rhizoplane enrichment process of the citrus root-associated microbiome. Microbiome 2017, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Larousse, M.; Rancurel, C.; Syska, C.; Palero, F.; Etienne, C.; Industri, B.; Nesme, X.; Bardin, M.; Galiana, E. Tomato root microbiota and Phytophthora parasitica-associated disease. Microbiome 2017, 5, 56. [Google Scholar]
- Shi, W.; Li, M.; Wei, G.; Tian, R.; Li, C.; Wang, B.; Lin, R.; Shi, C.; Chi, X.; Zhou, B.; et al. The occurrence of potato common scab correlates with the community composition and function of the geocaulosphere soil microbiome. Microbiome 2019, 7, 14. [Google Scholar]
- Liu, K.; Mcinroy, J.A.; Hu, C.; Kloepper, J.W. Mixtures of Plant-Growth-Promoting Rhizobacteria Enhance Biological Control of Multiple Plant Diseases and Plant-Growth Promotion in the Presence of Pathogens. Plant Dis. 2018, 102, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Nisar, M.; Ali, H.; Hazrat, A.; Hayat, K.; Keerio, A.A.; Ihsan, M.; Laiq, M.; Ullah, S.; Fahad, S.; et al. Drought tolerance improvement in plants: An endophytic bacterial approach. Appl. Microbiol. Biotechnol. 2019, 103, 7385–7397. [Google Scholar] [CrossRef] [PubMed]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Afolabi, O.G.; Hussain, M.; Qasim, M.; Wang, L. Prospects of endophytic fungal entomopathogens as biocontrol and plant growth promoting agents: An insight on how artificial inoculation methods affect endophytic colonization of host plants. Microbiol. Res. 2018, 217, 34–50. [Google Scholar] [CrossRef]
- Fira, D.; Dimkic, I.; Beric, T.; Lozo, J.; Stankovic, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, V.P.; Araujo, N.; Schwanestrada, K.; Pasqual, M.; Doria, J. Athelia (Sclerotium) rolfsii in Allium sativum: Potential biocontrol agents and their effects on plant metabolites. An. Acad. Bras. Cienc. 2018, 90, 3949–3962. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Pang, F. Biocontrol Agents for Controlling Wheat Rust. Methods Mol. Biol. 2017, 1659, 277–288. [Google Scholar]
- Amaresan, N.; Jayakumar, V.; Kumar, K.; Thajuddin, N. Biocontrol and plant growth-promoting ability of plant-associated bacteria from tomato (Lycopersicum esculentum) under field condition. Microb. Pathog. 2019, 136, 103713. [Google Scholar]
- Iwama, K. Physiology of the Potato: New Insights into Root System and Repercussions for Crop Management. Potato Res. 2008, 51, 333–353. [Google Scholar]
- Joshi, M.; Fogelman, E.; Belausov, E.; Ginzberg, I. Potato root system development and factors that determine its architecture. J. Plant Physiol. 2016, 205, 113–123. [Google Scholar] [CrossRef]
- Weinert, N.; Piceno, Y.; Ding, G.C.; Meincke, R.; Heuer, H.; Berg, G.; Schloter, M.; Andersen, G.; Smalla, K. PhyloChip hybridization uncovered an enormous bacterial diversity in the rhizosphere of different potato cultivars: Many common and few cultivar-dependent taxa. FEMS Microbiol. Ecol. 2011, 75, 497–506. [Google Scholar] [PubMed]
- Pfeiffer, S.; Mitter, B.; Oswald, A.; Hai, B.S.; Schloter, M.; Declerck, S.; Sessitsch, A. Rhizosphere microbiomes of potato cultivated in the High Andes show stable and dynamic core microbiomes with different responses to plant development. FEMS Microbiol. Ecol. 2017, 93, 242. [Google Scholar] [CrossRef]
- Ji, X.B.; Wang, D.; Lu, Z.; Li, R.; Song, J.; Zhang, D.D.; Chen, J.Y.; Dai, X.F.; Lin, K.J. Inhibitory efficacy of BvR001 on Verticillium dahliae. Plant Prot. 2021, 47, 40–47. [Google Scholar]
- Van Dingenen, J.; Hanzalova, K.; Abd Allah Salem, M.; Abel, C.; Seibert, T.; Giavalisco, P.; Wahl, V. Limited nitrogen availability has cultivar-dependent effects on potato tuber yield and tuber quality traits. Food Chem. 2019, 288, 170–177. [Google Scholar] [CrossRef]
- Kong, H.G.; Kim, N.H.; Lee, S.Y.; Lee, S.W. Impact of a Recombinant Biocontrol Bacterium, Pseudomonas fluorescens pc78, on Microbial Community in Tomato Rhizosphere. Plant Pathol. J. 2016, 32, 136–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, A.; Sun, J.; Wang, X.; Zou, L.; Fu, B.; Chen, J. Reprogrammed endophytic microbial community in maize stalk induced by Trichoderma asperellum biocontrol agent against Fusarium diseases and mycotoxin accumulation. Fungal Biol. 2019, 123, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Kyrkou, I.; Filippi, E.; Jensen, L.E.; Hansen, L.H. Seasonal epiphytic microbial dynamics on grapevine leaves under biocontrol and copper fungicide treatments. Sci. Rep. 2020, 10, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, G.; Koberl, M.; Rybakova, D.; Muller, H.; Grosch, R.; Smalla, K. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol. Ecol. 2017, 93, fix050. [Google Scholar] [CrossRef]
- Xie, Y.; Bu, H.; Feng, Q.; Wassie, M.; Amee, M.; Jiang, Y.; Bi, Y.; Hu, L.; Chen, L. Identification of Cd-resistant microorganisms from heavy metal-contaminated soil and its potential in promoting the growth and Cd accumulation of bermudagrass. Environ. Res. 2021, 200, 111730. [Google Scholar] [PubMed]
- Leoni, C.; Piancone, E.; Sasanelli, N.; Bruno, G.L.; Manzari, C.; Pesole, G.; Ceci, L.R.; Velpicella, M. Plant Health and Rhizosphere Microbiome: Effects of the Bionematicide Aphanocladium album in Tomato Plants Infested by Meloidogyne javanica. Microorganisms 2020, 8, 1922. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Zhao, Y.; Fu, W.; Hu, C.; Chen, G.; Xiao, Z.; Chen, Y.; Wang, Z.; Cheng, H. Variation of rhizosphere microbial community in continuous mono-maize seed production. Sci. Rep. 2021, 11, 1544. [Google Scholar] [CrossRef]
- Jiang, L.; Jeong, J.C.; Lee, J.S.; Park, J.M.; Yang, J.W.; Lee, M.H.; Choi, S.H.; Kim, C.Y.; Kim, D.H.; Kim, S.W.; et al. Potential of Pantoea dispersa as an effective biocontrol agent for black rot in sweet potato. Sci. Rep. 2019, 9, 16354. [Google Scholar] [CrossRef] [Green Version]
- Raoul, D.E.Y.; Cigna, J.; Quetu Laurent, A.; Caron, A.; Munier, E.; Cirou, A.B.; Helias, A.; Faure, D. Biocontrol of the Potato Blackleg and Soft Rot Diseases Caused by Dickeya dianthicola. Appl. Environ. Microbiol. 2016, 82, 268–278. [Google Scholar]
- Sarwar, A.; Latif, Z.; Zhang, S.; Hao, J.; Bechthold, A. A Potential Biocontrol Agent Streptomyces violaceusniger AC12AB for Managing Potato Common Scab. Front. Microbiol. 2019, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Elazouni, I.; Abdel Aziz, S.; Rabea, A. Microbial efficacy as biological agents for potato enrichment as well as bio-controls against wilt disease caused by Ralstonia solanacearum. World J. Microbiol. Biotechnol. 2019, 35, 30. [Google Scholar] [PubMed]
- Gupta, R.; Singh, A.; Srivastava, M.; Shanker, K.; Pandey, R. Plant-microbe interactions endorse growth by uplifting microbial community structure of Bacopa monnieri rhizosphere under nematode stress. Microbiol. Res. 2019, 218, 87–96. [Google Scholar] [CrossRef]
- Baffoni, L.; Gaggia, F.; Dalanaj, N.; Prodi, A.; Nipoti, P.; Pisi, A.; Biavati, B.; Gioia, D. Microbial inoculants for the biocontrol of Fusarium spp. in durum wheat. BMC Microbiol. 2015, 15, 242. [Google Scholar]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microb. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.Y.; Zhang, Y.L.; He, T.J.; Niu, L.L.; Wang, Q.; Wu, Z.H.; Xiao, L.L.; Tian, S.J. Effects of potato-maize intercropping on potato growth, yield and carbohydrate. J. South. Agric. 2021, 52, 1198–1205. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Kong, Z.-Q.; Zhang, D.-D.; Chen, J.-Y.; Dai, X.-F.; Li, R. Rhizosphere Microbiomes of Potato Cultivated under Bacillus subtilis Treatment Influence the Quality of Potato Tubers. Int. J. Mol. Sci. 2021, 22, 12065. https://doi.org/10.3390/ijms222112065
Song J, Kong Z-Q, Zhang D-D, Chen J-Y, Dai X-F, Li R. Rhizosphere Microbiomes of Potato Cultivated under Bacillus subtilis Treatment Influence the Quality of Potato Tubers. International Journal of Molecular Sciences. 2021; 22(21):12065. https://doi.org/10.3390/ijms222112065
Chicago/Turabian StyleSong, Jian, Zhi-Qiang Kong, Dan-Dan Zhang, Jie-Yin Chen, Xiao-Feng Dai, and Ran Li. 2021. "Rhizosphere Microbiomes of Potato Cultivated under Bacillus subtilis Treatment Influence the Quality of Potato Tubers" International Journal of Molecular Sciences 22, no. 21: 12065. https://doi.org/10.3390/ijms222112065
APA StyleSong, J., Kong, Z.-Q., Zhang, D.-D., Chen, J.-Y., Dai, X.-F., & Li, R. (2021). Rhizosphere Microbiomes of Potato Cultivated under Bacillus subtilis Treatment Influence the Quality of Potato Tubers. International Journal of Molecular Sciences, 22(21), 12065. https://doi.org/10.3390/ijms222112065





