Decoding the Role of DVL1 in Intracranial Meningioma
Abstract
1. Introduction
2. Results
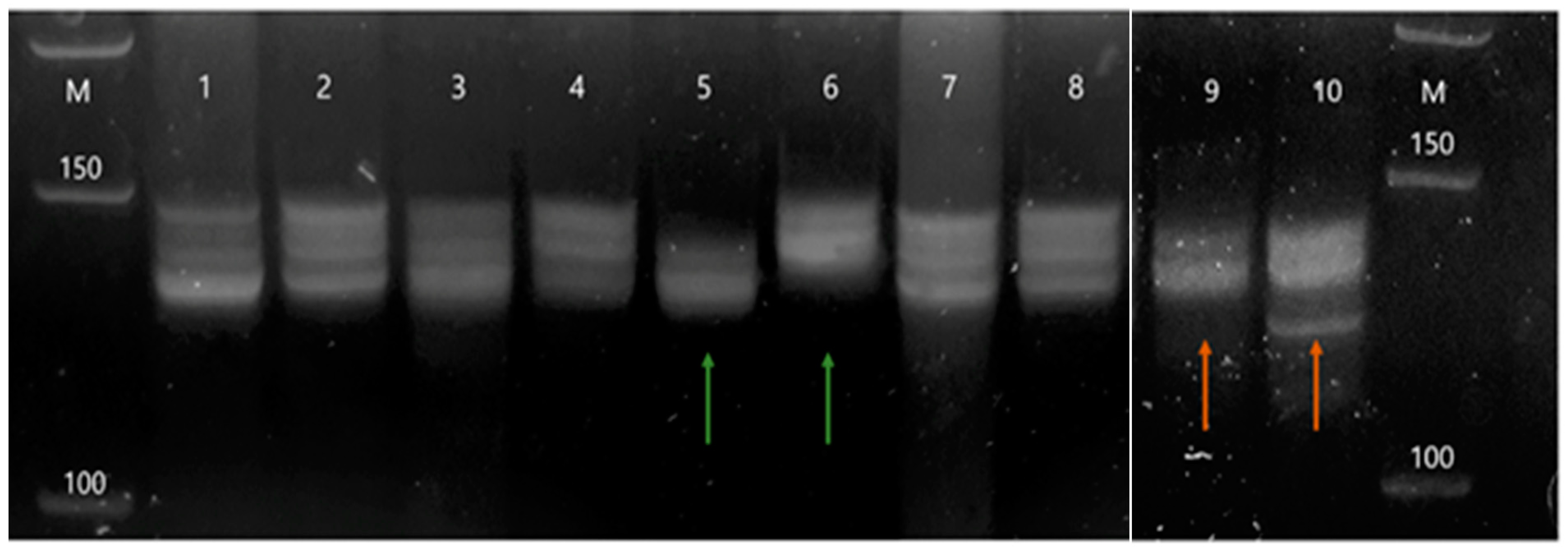
2.1. Genetic Instability of the DVL1 Gene Recorded with the D1S468 Marker
2.2. Mutations in the PDZ Region of the DVL1 Gene
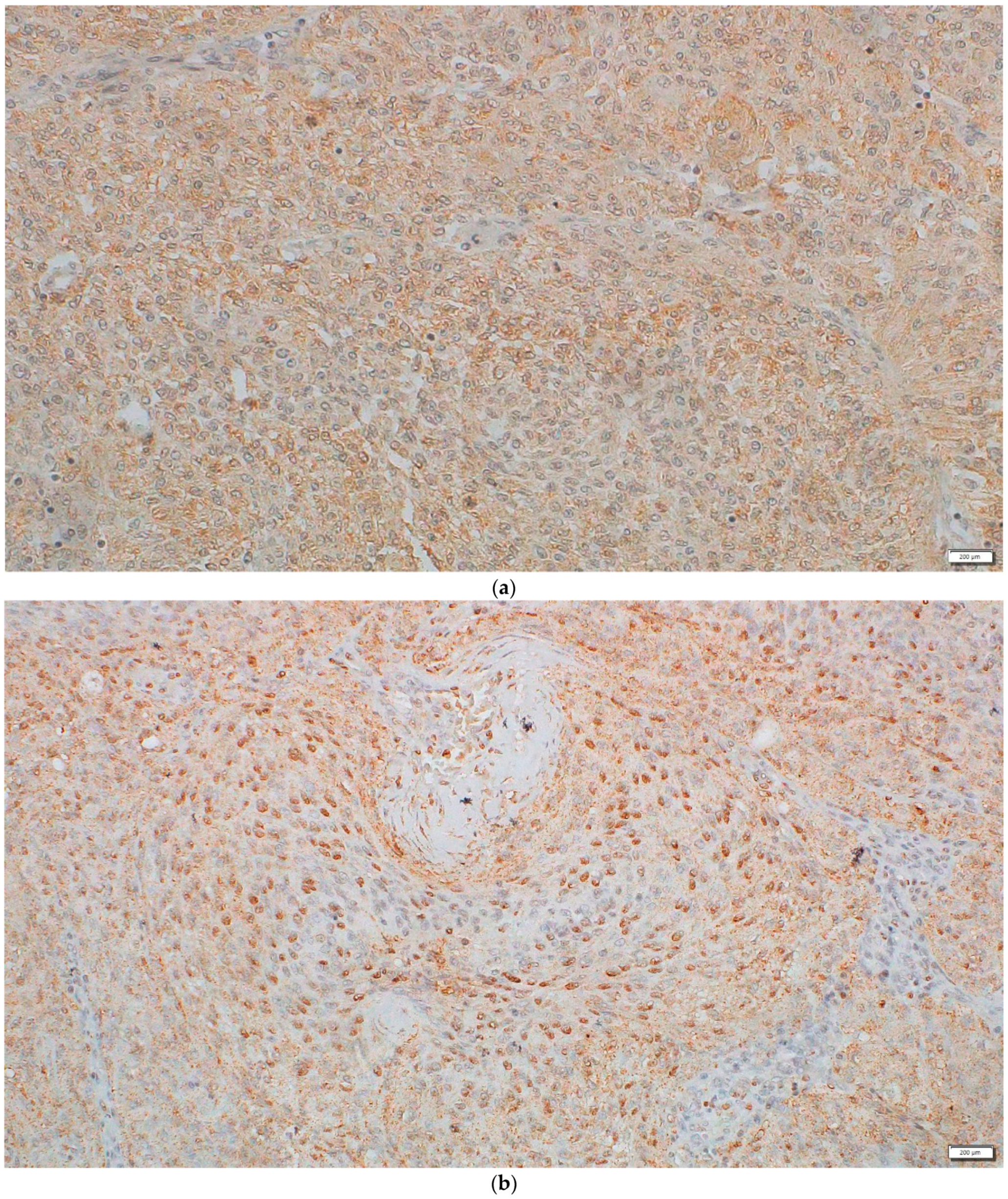
2.3. Protein Expression and Localization of the DVL1 and Active β-Catenin Form
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Central Brain Tumor Registry of the United States (CBTRUS). Available online: https://cbtrus.org/ (accessed on 30 January 2021).
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21, v1–v100. [Google Scholar] [PubMed]
- Pèrez-Magán, E.; Campos-Martín, Y.; Mur, P.; Fiaño, C.; Ribalta, T.; García, J.F.; Rey, J.A.; De Lope, A.R.; Mollejo, M.; Melendez, B. Genetic alterations associated with progression and recurrence in meningiomas. J. Neuropathol. Exp. Neurol. 2012, 71, 882–893. [Google Scholar]
- Pećina-Šlaus, N.; Kafka, A.; Lechpammer, M. Molecular genetics of intracranial meningiomas with emphasis on canonical Wnt signalling. Cancers 2016, 8, 67. [Google Scholar] [CrossRef]
- Galani, V.; Lampri, E.; Varouktsi, A.; Alexiou, G.; Mitselou, A.; Kyritsis, A.P. Genetic and epigenetic alterations in meningiomas. Clin. Neurol. Neurosurg. 2017, 158, 119–125. [Google Scholar] [CrossRef]
- Nasser, M.M.; Mehdipour, P. Exploration of involved key genes and signaling diversity in brain tumors. Cell. Mol. Neurobiol. 2017, 38, 393–419. [Google Scholar] [CrossRef]
- Preusser, M.; Brastianos, P.; Mawrin, C. Advances in meningioma genetics: Novel therapeutic opportunities. Nat. Rev. Neurol. 2018, 14, 106–115. [Google Scholar] [CrossRef]
- Wang, N.; Osswald, M. Meningiomas: Overview and new directions in therapy. Semin. Neurol. 2018, 38, 112–120. [Google Scholar]
- Pereira, B.J.A.; Oba-Shinjo, S.M.; de Almeida, A.N.; Marie, S. Molecular alterations in meningiomas: Literature review. Clin. Neurol. Neurosurg. 2019, 176, 89–96. [Google Scholar]
- Vallée, A.; LeCarpentier, Y.; Vallée, J.-N. Targeting the canonical WNT/β-catenin pathway in cancer treatment using non-steroidal anti-inflammatory drugs. Cells 2019, 8, 726. [Google Scholar]
- Kahn, M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014, 13, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Noelanders, R.; Vleminckx, K. How Wnt signaling builds the brain: Bridging development and disease. Neuroscientist 2016, 23, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Matsui, W.H. Cancer stem cell signaling pathways. Medicine 2016, 95, S8–S19. [Google Scholar] [CrossRef] [PubMed]
- Oliva, C.A.; Montecinos-Oliva, C.; Inestrosa, N.C. Wnt signaling in the central nervous system: New insights in health and disease. Prog. Mol. Biol. Transl. Sci. 2018, 153, 81–130. [Google Scholar]
- El-Sahli, S.; Xie, Y.; Wang, L.; Liu, S. Wnt signaling in cancer metabolism and immunity. Cancers 2019, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.F.; Kaur, P.; Bunnag, N.; Suresh, J.; Sung, I.C.H.; Tan, Q.H.; Gruber, J.; Tolwinski, N.S. WNT signaling in disease. Cells 2019, 8, 826. [Google Scholar]
- Pham, M.H.; Zada, G.; Mosich, G.M.; Chen, T.C.; Giannotta, S.L.; Wang, K.; Mack, W.J. Molecular genetics of meningiomas: A systematic review of the current literature and potential basis for future treatment paradigms. Neurosurg. Focus 2011, 30, E7. [Google Scholar] [CrossRef]
- Bukovac, A.; Kafka, A.; Raguž, M.; Brlek, P.; Dragičević, K.; Müller, D.; Pećina-Šlaus, N. Are we benign? What can Wnt signaling pathway and epithelial to mesenchymal transition tell us about intracranial meningioma progression. Cancers 2021, 13, 1633. [Google Scholar]
- Kafka, A.; Bašić-Kinda, S.; Pećina-Šlaus, N. The cellular story of dishevelleds. Croat. Med. J. 2014, 55, 459–46667. [Google Scholar] [CrossRef]
- Yu, J.; Virshup, D.M. Updating the Wnt pathways. Biosci. Rep. 2014, 34, e00142. [Google Scholar] [CrossRef]
- Wiese, K.; Nusse, R.; Van Amerongen, R. Wnt signalling: Conquering complexity. Development 2018, 145, 165902. [Google Scholar] [CrossRef]
- Jackstadt, R.; Hodder, M.C.; Sansom, O.J. WNT and β-catenin in cancer: Genes and therapy. Annu. Rev. Cancer Biol. 2020, 4, 177–196. [Google Scholar]
- Sharma, M.; Castro-Piedras, I.; Simmons, G.; Pruitt, K. Dishevelled: A masterful conductor of complex Wnt signals. Cell. Signal. 2018, 47, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Nagahata, T.; Shimada, T.; Harada, A.; Nagai, H.; Onda, M.; Yokoyama, S.; Shiba, T.; Jin, E.; Kawanami, O.; Emi, M. Amplification, up-regulation and over-expression of DVL-1, the human counterpart of the Drosophila disheveled gene, in primary breast cancers. Cancer Sci. 2003, 94, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Kafka, A.; Bačić, M.; Tomas, D.; Žarković, K.; Bukovac, A.; Njirić, N.; Mrak, G.; Krsnik, Z.; Pećina-Šlaus, N. Different behaviour of DVL1, DVL2, DVL3 in astrocytoma malignancy grades and their association to TCF1 and LEF1 upregulation. J. Cell. Mol. Med. 2018, 23, 641–655. [Google Scholar] [CrossRef]
- Ars, E.; Serra, E.; Garcia, J.; Kruyer, H.; Gaona, A.; Lázaro, C.; Estivill, X. Mutations affecting mRNA splicing are the most common molecular defects in patients with neurofibromatosis type 1. Hum. Mol. Genet. 2000, 9, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Buratti, E.; Baralle, F. Influence of RNA secondary structure on the pre-mRNA splicing process. Mol. Cell. Biol. 2004, 24, 10505–10514. [Google Scholar] [CrossRef]
- Rogic, S.; Montpetit, B.; Hoos, H.; Mackworth, A.; Ouellette, B.; Hieter, P. Correlation between the secondary structure of pre-mRNA introns and the efficiency of splicing in Saccharomyces cerevisiae. BMC Genom. 2008, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Rubtsov, P. Role of pre-mRNA secondary structures in the regulation of alternative splicing. Mol. Biol. 2016, 50, 823–830. [Google Scholar] [CrossRef]
- Lin, C.; Taggart, A.; Fairbrother, W.G. RNA structure in splicing: An evolutionary perspective. RNA Biol. 2016, 13, 766–771. [Google Scholar] [PubMed]
- Brennan, K.; Sancho, J.M.G.; Castelo-Soccio, L.A.; Howe, L.; Mc Brown, A. Truncated mutants of the putative Wnt receptor LRP6/Arrow can stabilize β-catenin independently of Frizzled proteins. Oncogene 2004, 23, 4873–4884. [Google Scholar] [CrossRef]
- Schneikert, J.; Behrens, J. Truncated APC is required for cell proliferation and DNA replication. Int. J. Cancer 2006, 119, 74–79. [Google Scholar] [CrossRef]
- Castro-Chavez, F. The rules of variation: Amino acid exchange according to the rotating circular genetic code. J. Theor. Biol. 2010, 264, 711–721. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, M.Y.; Yen, L.C.; Liu, H.C.; Liu, P.P.; Chung, F.Y.; Wang, T.N.; Wang, J.Y.; Lin, S.R. Significant overexpression of DVL1 in Taiwanese colorectal cancer patients with liver metastasis. Int. J. Mol. Sci. 2013, 14, 20492–20507. [Google Scholar] [CrossRef]
- Ameli, F.; Rose, I.M.; Masir, N. Expression of DDR1 and DVL1 in invasive ductal and lobular breast carcinoma does not correlate with histological type, grade and hormone receptor status. Asian Pac. J. Cancer Prev. 2015, 16, 2385–2390. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Molehin, D.; Castro-Piedras, I.; Martinez, E.G.; Pruitt, K. Acetylation of conserved DVL-1 lysines regulates its nuclear translocation and binding to gene promoters in triple-negative breast cancer. Sci. Rep. 2019, 9, 16257. [Google Scholar] [CrossRef]
- Karin-Kujundzic, V.; Kardum, V.; Sola, I.M.; Paic, F.; Skrtic, A.; Skenderi, F.; Serman, A.; Nikuseva-Martic, T.; Vranic, S.; Serman, L. Dishevelled family proteins in serous ovarian carcinomas: A clinicopathologic and molecular study. APMIS 2020, 128, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Miyamoto, S.; Nagahata, T.; Konishi, N.; Emi, M.; Onda, M. Upregulation and overexpression of DVL1, the human counterpart of the Drosophila dishevelled gene, in prostate cancer. Tumori 2005, 91, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhao, Y.; Yang, Z.Q.; Dong, Q.Z.; Dong, X.J.; Han, Y.; Zhao, C.; Wang, E.H. Dishevelled family proteins are expressed in non-small cell lung cancer and function differentially on tumor progression. Lung Cancer 2008, 62, 181–192. [Google Scholar]
- Zhang, K.; Song, H.; Yang, P.; Dai, X.; Li, Y.; Wang, L.; Du, J.; Pan, K.; Zhang, T. Silencing dishevelled-1 sensitizes paclitaxel-resistant human ovarian cancer cells via AKT/GSK-3β/β-catenin signalling. Cell Prolif. 2015, 48, 249–258. [Google Scholar]
- Kafka, A.; Tomas, D.; Beroš, V.; Pećina, H.I.; Zeljko, M.; Pećina-Šlaus, N. Brain metastases from lung cancer show increased expression of DVL1, DVL3 and beta-catenin and down-regulation of E-cadherin. Int. J. Mol. Sci. 2014, 15, 10635–10651. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Sambrook, J. Molecular Cloning—A Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2012. [Google Scholar]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information—NCBI. Available online: https://www.ncbi.nlm.nih.gov (accessed on 25 October 2020).
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar]
- National Center for Biotechnology Information—NCBI. Available online: https://www.ncbi.nlm.nih.gov/nucleotide/NG_008048?report=genbank&log$=nuclalign&blast_rank=1&RID=0 (accessed on 25 January 2021).
- Protein Variation Effect Analyzer—PROVEAN. Available online: http://provean.jcvi.org/index.php (accessed on 30 January 2021).
- Mazières, J.; Brugger, W.; Cappuzzo, F.; Middel, P.; Frosch, A.; Bara, I.; Klingelschmitt, G.; Klughammer, B. Evaluation of EGFR protein expression by immunohistochemistry using H-score and the magnification rule: Re-analysis of the SATURN study. Lung Cancer 2013, 82, 231–237. [Google Scholar] [CrossRef] [PubMed]
- RStudio. Available online: https://rstudio.com/ (accessed on 15 March 2021).
- CRAN. Available online: https://cran.r-project.org/ (accessed on 15 March 2021).
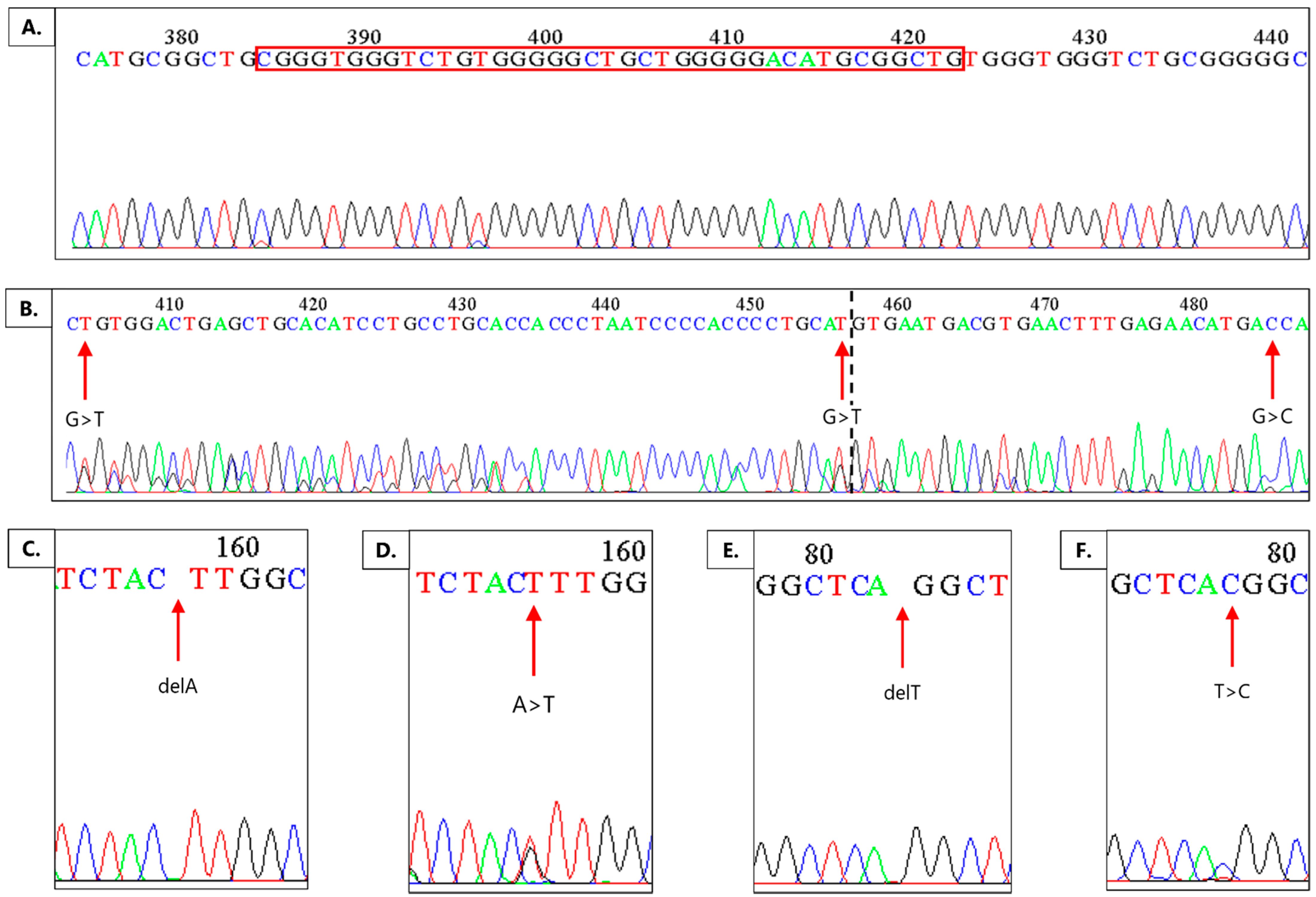
| Location of Mutation | Type of Mutation | Number of Samples with This Type of Mutation |
|---|---|---|
| Intron 7 | NG_008048.2: g.13921delT | 7 |
| Intron 7 | NG_008048.2: g.13921T>C | 4 |
| Exon 8 | NG_008048.2: g.13998delA | 1 |
| Exon 8 | NG_008048.2: g.14004delA | 9 |
| Exon 8 | NG_008048.2: g.14004A>T | 1 |
| Intron 8 | NG_008048.2: g.14228_14267dup | 25 |
| Intron 8 | NG_008048.2: g.14248G>T | 1 |
| Intron 8 | NG_008048.2: g.14300G>T | 1 |
| Exon 9 | NG_008048.2: g.14329G>C | 1 |
| (Pre) Denaturation | Denaturation | Annealing | Extending | No. of Cycles | |
|---|---|---|---|---|---|
| D1S468 | 94 °C/5 min | 94 °C/30 s | 60 °C/30 s | 72 °C/30 s | 40 |
| PDZ | 94 °C/5 min | 94 °C/35 s | 58.6 °C/35 s | 72 °C/35 s | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukovac, A.; Dragičević, K.; Kafka, A.; Orešković, D.; Cesarec-Augustinović, S.; Pećina-Šlaus, N. Decoding the Role of DVL1 in Intracranial Meningioma. Int. J. Mol. Sci. 2021, 22, 11996. https://doi.org/10.3390/ijms222111996
Bukovac A, Dragičević K, Kafka A, Orešković D, Cesarec-Augustinović S, Pećina-Šlaus N. Decoding the Role of DVL1 in Intracranial Meningioma. International Journal of Molecular Sciences. 2021; 22(21):11996. https://doi.org/10.3390/ijms222111996
Chicago/Turabian StyleBukovac, Anja, Katarina Dragičević, Anja Kafka, Darko Orešković, Sanja Cesarec-Augustinović, and Nives Pećina-Šlaus. 2021. "Decoding the Role of DVL1 in Intracranial Meningioma" International Journal of Molecular Sciences 22, no. 21: 11996. https://doi.org/10.3390/ijms222111996
APA StyleBukovac, A., Dragičević, K., Kafka, A., Orešković, D., Cesarec-Augustinović, S., & Pećina-Šlaus, N. (2021). Decoding the Role of DVL1 in Intracranial Meningioma. International Journal of Molecular Sciences, 22(21), 11996. https://doi.org/10.3390/ijms222111996







