Microcirculatory Function during Endotoxemia—A Functional Citrulline-Arginine-NO Pathway and NOS3 Complex Is Essential to Maintain the Microcirculation
Abstract
:1. Introduction
2. Results
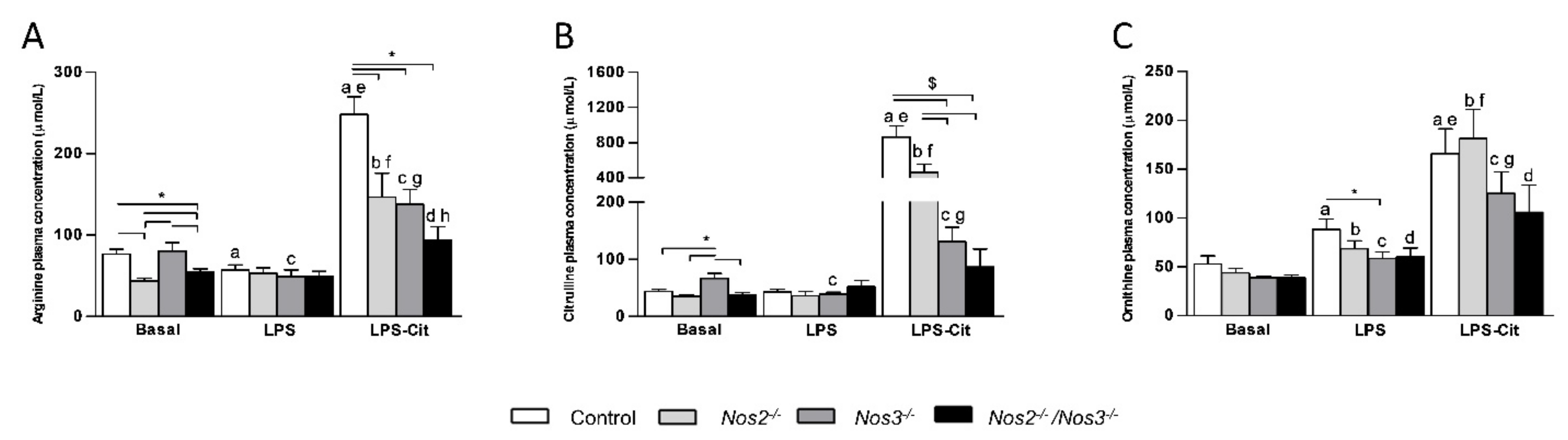
2.1. Plasma Amino Acid Concentrations in Control and Nos-Deficient Mice under Basal and Endotoxemic Conditions
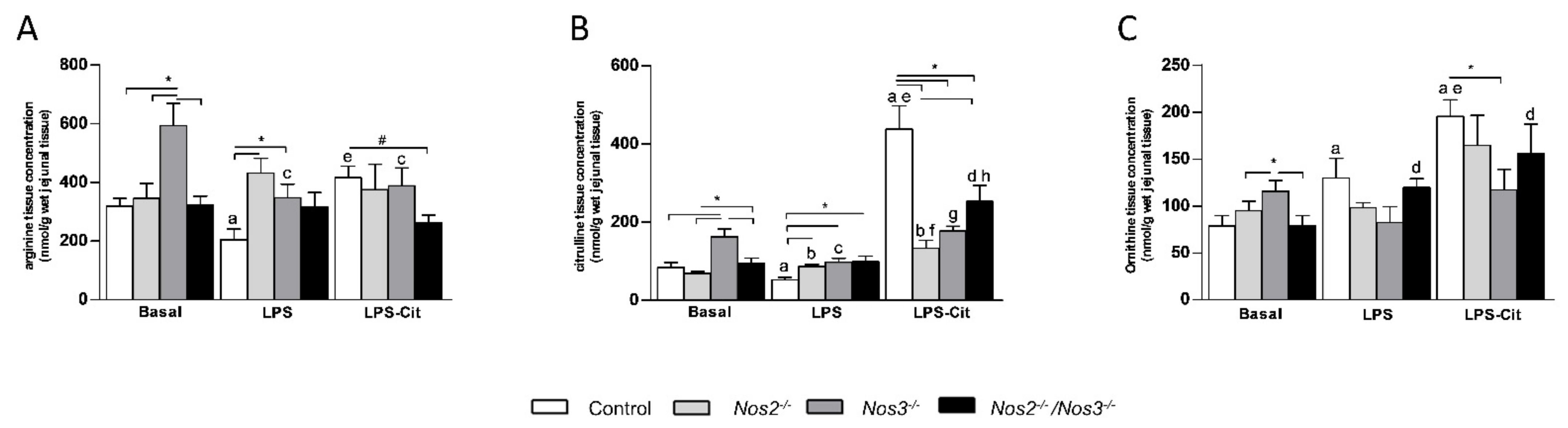
2.2. Amino Acid Concentrations in Jejunal Tissue of Control and Nos-Deficient Mice under Basal and Endotoxemic Conditions
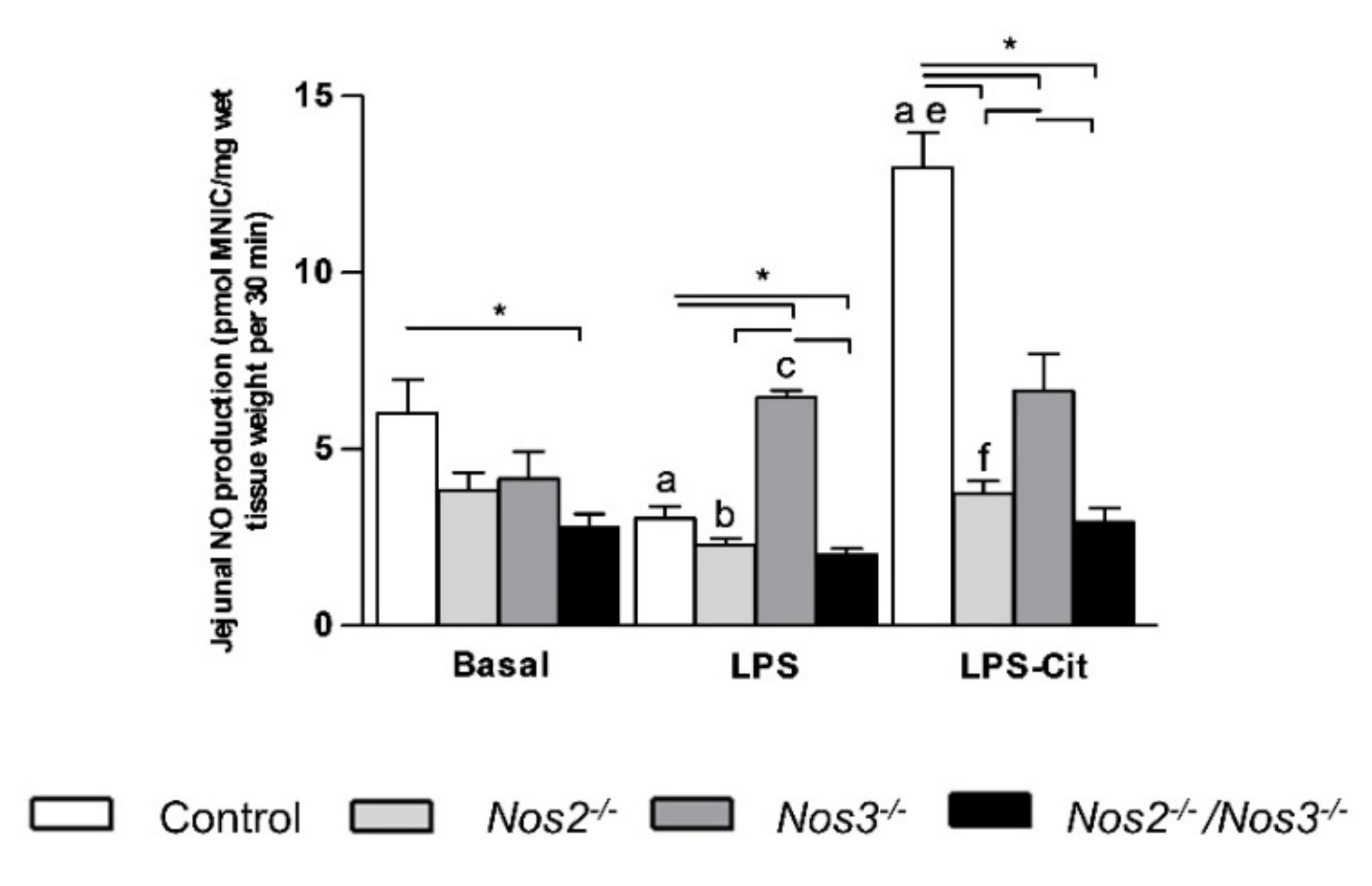
2.3. L-Citrulline Enhances the Intestinal NO Production in Control and Nos2-Deficient Mice
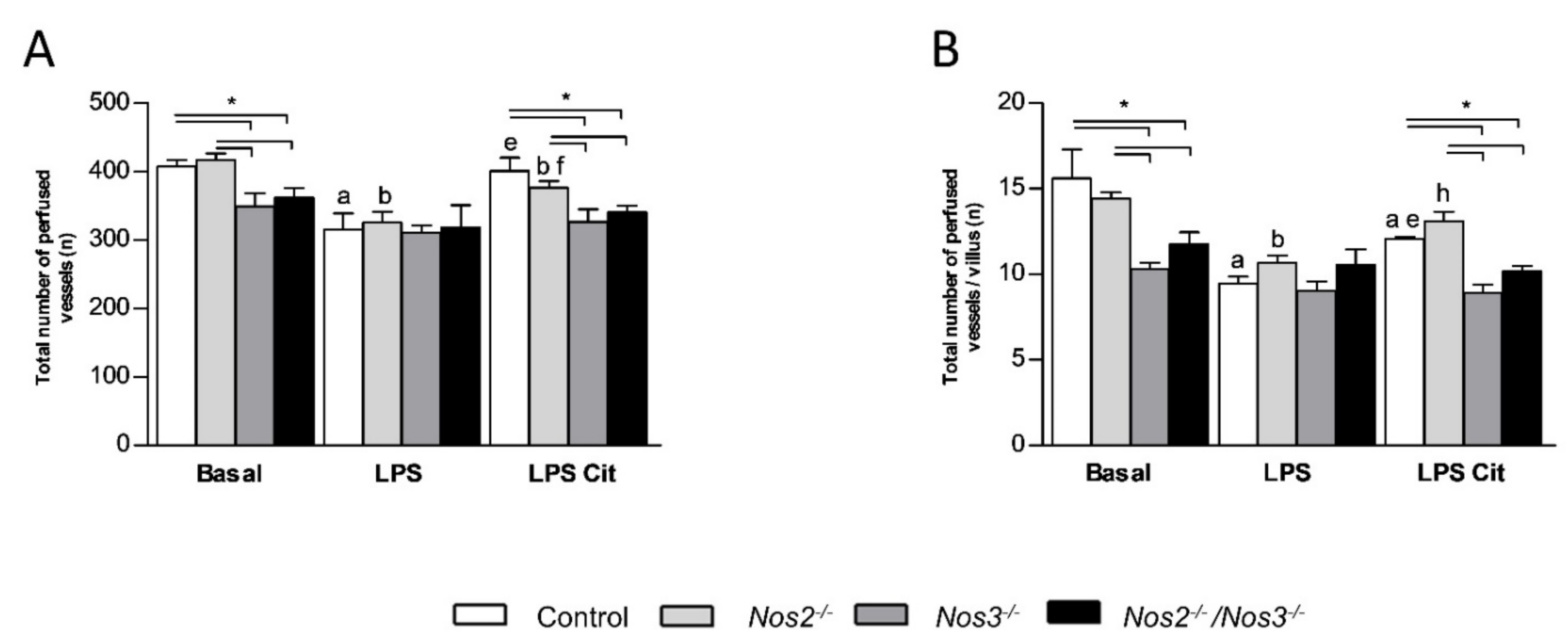
2.4. Detrimental Effects of Nos3 Deficiency on Microcirculatory Flow during Endotoxemia
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Plasma and Tissue Amino-Acid Concentration Measurements
4.4. In Vivo Tissue NO Production
4.5. Jejunal Microcirculation Measurements with SDF Imaging
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Trzeciak, S.; Cinel, I.; Phillip Dellinger, R.; Shapiro, N.I.; Arnold, R.C.; Parrillo, J.E.; Hollenberg, S.M. Resuscitating the microcirculation in sepsis: The central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Acad. Emerg. Med. 2008, 15, 399–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pool, R.; Gomez, H.; Kellum, J.A. Mechanisms of Organ Dysfunction in Sepsis. Crit. Care Clin. 2018, 34, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Sakr, Y.; Dubois, M.-J.; De Backer, D.; Creteur, J.; Vincent, J.-L. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit. Care Med. 2004, 32, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Lipinska-Gediga, M. Sepsis and septic shock-is a microcirculation a main player? Anaesthesiol. Intensive Ther. 2016, 48, 261–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joffre, J.; Hellman, J.; Ince, C.; Ait-Oufella, H. Endothelial Responses in Sepsis. Am. J. Respir. Crit. Care Med. 2020, 202, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.P.; Buchman, T.G.; Karl, I.E.; Hotchkiss, R.S. Molecular biology of multiple organ dysfunction syndrome: Injury, adaptation, and apoptosis. Surg. Infect. 2000, 1, 207–213, discussion 214-205. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, S.; Dellinger, R.P.; Parrillo, J.E.; Guglielmi, M.; Bajaj, J.; Abate, N.L.; Arnold, R.C.; Colilla, S.; Zanotti, S.; Hollenberg, S.M. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: Relationship to hemodynamics, oxygen transport, and survival. Ann. Emerg. Med. 2007, 49, 88–98.e2. [Google Scholar] [CrossRef]
- Kavdia, M.; Popel, A.S. Contribution of nNOS- and eNOS-derived NO to microvascular smooth muscle NO exposure. J. Appl. Physiol. 2004, 97, 293–301. [Google Scholar] [CrossRef]
- Wijnands, K.A.; Vink, H.; Briede, J.J.; van Faassen, E.E.; Lamers, W.H.; Buurman, W.A.; Poeze, M. Citrulline a more suitable substrate than arginine to restore NO production and the microcirculation during endotoxemia. PLoS ONE 2012, 7, e37439. [Google Scholar] [CrossRef] [Green Version]
- Bruins, M.J.; Lamers, W.H.; Meijer, A.J.; Soeters, P.B.; Deutz, N.E. In vivo measurement of nitric oxide production in porcine gut, liver and muscle during hyperdynamic endotoxaemia. Br. J. Pharmacol. 2002, 137, 1225–1236. [Google Scholar] [CrossRef] [Green Version]
- Flam, B.R.; Eichler, D.C.; Solomonson, L.P. Endothelial nitric oxide production is tightly coupled to the citrulline-NO cycle. Nitric Oxide 2007, 17, 115–121. [Google Scholar] [CrossRef]
- Kelm, M. Nitric oxide metabolism and breakdown. Biochim. Biophys. Acta 1999, 1411, 273–289. [Google Scholar] [CrossRef] [Green Version]
- Luiking, Y.C.; Engelen, M.P.; Deutz, N.E. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-A.; Wang, T.-Y.; Varadharaj, S.; Reyes, L.A.; Hemann, C.; Talukder, M.A.H.; Chen, Y.-R.; Druhan, L.J.; Zweier, J.L. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 2010, 468, 1115–1118. [Google Scholar] [CrossRef] [Green Version]
- Luiking, Y.C.; Poeze, M.; Ramsay, G.; Deutz, N.E. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am. J. Clin. Nutr. 2009, 89, 142–152. [Google Scholar] [CrossRef]
- Freund, H.; Atamian, S.; Holroyde, J.; Fischer, J.E. Plasma amino acids as predictors of the severity and outcome of sepsis. Ann. Surg. 1979, 190, 571–576. [Google Scholar] [CrossRef]
- Durante, W.; Johnson, F.K.; Johnson, R.A. Arginase: A critical regulator of nitric oxide synthesis and vascular function. Clin. Exp. Pharmacol. Physiol. 2007, 34, 906–911. [Google Scholar] [CrossRef] [Green Version]
- Ochoa, J.B.; Udekwu, A.O.; Billiar, T.R.; Curran, R.D.; Cerra, F.B.; Simmons, R.L.; Peitzman, A.B. Nitrogen oxide levels in patients after trauma and during sepsis. Ann. Surg. 1991, 214, 621–626. [Google Scholar] [CrossRef]
- Kao, C.C.; Bandi, V.; Guntupalli, K.K.; Wu, M.; Castillo, L.; Jahoor, F. Arginine, citrulline and nitric oxide metabolism in sepsis. Clin. Sci. 2009, 117, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Argaman, Z.; Young, V.R.; Noviski, N.; Castillo-Rosas, L.; Lu, X.M.; Zurakowski, D.; Cooper, M.; Davison, C.; Tharakan, J.F.; Ajami, A.; et al. Arginine and nitric oxide metabolism in critically ill septic pediatric patients. Crit. Care Med. 2003, 31, 591–597. [Google Scholar] [CrossRef]
- Crenn, P.; Neveux, N.; Chevret, S.; Jaffray, P.; Cynober, L.; Melchior, J.C.; Annane, D.; COIITSS Study Group. Plasma l-citrulline concentrations and its relationship with inflammation at the onset of septic shock: A pilot study. J. Crit. Care 2013, 29, 315.e1–315.e6. [Google Scholar] [CrossRef] [Green Version]
- Elwafi, F.; Curis, E.; Zerrouk, N.; Neveux, N.; Chaumeil, J.C.; Arnaud, P.; Cynober, L.; Moinard, C. Endotoxemia affects citrulline, arginine and glutamine bioavailability. Eur. J. Clin. Investig. 2012, 42, 282–289. [Google Scholar] [CrossRef]
- Elbers, P.W.G.; Ince, C. Bench-to-bedside review: Mechanisms of critical illness—Classifying microcirculatory flow abnormalities in distributive shock. Crit. Care 2006, 10, 221. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.J.; Beloussow, K.; Shen, W.C. Accessibility of endothelial and inducible nitric oxide synthase to the intracellular citrulline-arginine regeneration pathway. Biochem. Pharmacol. 2005, 69, 97–104. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Kashfi, K.; Ghasemi, A. Endogenous flux of nitric oxide: Citrulline is preferred to Arginine. Acta Physiol. 2021, 231, e13572. [Google Scholar] [CrossRef]
- Ince, C.; Sinaasappel, M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit. Care Med. 1999, 27, 1369–1377. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Inoue, M.; Wasa, M.; Fukuzawa, M.; Kamata, S.; Okada, A. Expression of endothelial constitutive nitric oxide synthase mRNA in gastrointestinal mucosa and its downregulation by endotoxin. Life Sci. 1997, 61, 1323–1329. [Google Scholar] [CrossRef]
- Boerma, E.C.; Mathura, K.R.; van der Voort, P.H.; Spronk, P.E.; Ince, C. Quantifying bedside-derived imaging of microcirculatory abnormalities in septic patients: A prospective validation study. Crit. Care 2005, 9, R601–R606. [Google Scholar] [CrossRef] [Green Version]
- Hollenberg, S.M.; Broussard, M.; Osman, J.; Parrillo, J.E. Increased microvascular reactivity and improved mortality in septic mice lacking inducible nitric oxide synthase. Circ. Res. 2000, 86, 774–778. [Google Scholar] [CrossRef] [Green Version]
- Kubes, P.; McCafferty, D.M. Nitric oxide and intestinal inflammation. Am. J. Med. 2000, 109, 150–158. [Google Scholar] [CrossRef]
- Cobb, J.P.; Hotchkiss, R.S.; Swanson, P.E.; Chang, K.; Qiu, Y.; Laubach, V.E.; Karl, I.E.; Buchman, T.G. Inducible nitric oxide synthase (iNOS) gene deficiency increases the mortality of sepsis in mice. Surgery 1999, 126, 438–442. [Google Scholar] [CrossRef]
- Bultinck, J.; Sips, P.; Vakaet, L.; Brouckaert, P.; Cauwels, A. Systemic NO production during (septic) shock depends on parenchymal and not on hematopoietic cells: In vivo iNOS expression pattern in (septic) shock. Faseb. J. 2006, 20, 2363–2365. [Google Scholar] [CrossRef] [PubMed]
- Cauwels, A.; Bultinck, J.; De Zwaef, R.; Vandendriessche, B.; Magez, S.; Brouckaert, P. Nitric oxide production by endotoxin preparations in TLR4-deficient mice. Nitric Oxide 2014, 36, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, I.; Abe, M.; Shibata, K.; Shimizu, N.; Sakata, N.; Katsuragi, T.; Tanaka, K. Evaluating the role of inducible nitric oxide synthase using a novel and selective inducible nitric oxide synthase inhibitor in septic lung injury produced by cecal ligation and puncture. Am. J. Respir. Crit. Care Med. 2000, 162, 716–722. [Google Scholar] [CrossRef] [Green Version]
- Al-Shabrawey, M.; El-Remessy, A.; Gu, X.; Brooks, S.S.; Hamed, M.S.; Huang, P.; Caldwell, R.B. Normal vascular development in mice deficient in endothelial NO synthase: Possible role of neuronal NO synthase. Mol. Vis. 2003, 9, 549–558. [Google Scholar]
- Wei, X.Q.; Charles, I.G.; Smith, A.; Ure, J.; Feng, G.J.; Huang, F.P.; Xu, D.; Muller, W.; Moncada, S.; Liew, F.Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 1995, 375, 408–411. [Google Scholar] [CrossRef]
- Massberg, S.; Eisenmenger, S.; Enders, G.; Krombach, F.; Messmer, K. Quantitative analysis of small intestinal microcirculation in the mouse. Res. Exp. Med. 1998, 198, 23–35. [Google Scholar] [CrossRef]
- Burnett, A.L.; Nelson, R.J.; Calvin, D.C.; Liu, J.X.; Demas, G.E.; Klein, S.L.; Kriegsfeld, L.J.; Dawson, V.L.; Dawson, T.M.; Snyder, S.H. Nitric oxide-dependent penile erection in mice lacking neuronal nitric oxide synthase. Mol. Med. 1996, 2, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.L.; Huang, Z.; Mashimo, H.; Bloch, K.D.; Moskowitz, M.A.; Bevan, J.A.; Fishman, M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 1995, 377, 239–242. [Google Scholar] [CrossRef]
- Huang, A.; Sun, D.; Shesely, E.G.; Levee, E.M.; Koller, A.; Kaley, G. Neuronal NOS-dependent dilation to flow in coronary arteries of male eNOS-KO mice. Am. J. Physiol. 2002, 282, H429–H436. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, B.L.; Solomonson, L.P.; Eichler, D.C. Argininosuccinate synthase expression is required to maintain nitric oxide production and cell viability in aortic endothelial cells. J. Biol Chem. 2004, 279, 18353–18360. [Google Scholar] [CrossRef] [Green Version]
- Elms, S.; Chen, F.; Wang, Y.; Qian, J.; Askari, B.; Yu, Y.; Pandey, D.; Iddings, J.; Caldwell, R.; Fulton, D.J.R. Insights into the arginine paradox: Evidence against the importance of subcellular location of arginase and eNOS. Am. J. Physiol. Circ. Physiol. 2013, 305, H651–H666. [Google Scholar] [CrossRef] [Green Version]
- Wijnands, K.A.; Hoeksema, M.A.; Meesters, D.M.; van den Akker, N.M.; Molin, D.G.; Briede, J.J.; Ghosh, M.; Kohler, S.E.; van Zandvoort, M.A.; de Winther, M.P.; et al. Arginase-1 deficiency regulates arginine concentrations and NOS2-mediated NO production during endotoxemia. PLoS ONE 2014, 9, e86135. [Google Scholar] [CrossRef] [Green Version]
- Van Wijck, K.; Wijnands, K.A.; Meesters, D.M.; Boonen, B.; van Loon, L.J.; Buurman, W.A.; Dejong, C.H.; Lenaerts, K.; Poeze, M. L-citrulline Improves Splanchnic Perfusion and Reduces Gut Injury during Exercise. Med. Sci. Sports Exerc. 2014. [Google Scholar] [CrossRef]
- Laubach, V.E.; Shesely, E.G.; Smithies, O.; Sherman, P.A. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc. Natl. Acad. Sci. USA 1995, 92, 10688–10692. [Google Scholar] [CrossRef] [Green Version]
- Groner, W.; Winkelman, J.W.; Harris, A.G.; Ince, C.; Bouma, G.J.; Messmer, K.; Nadeau, R.G. Orthogonal polarization spectral imaging: A new method for study of the microcirculation. Nat. Med. 1999, 5, 1209–1212. [Google Scholar] [CrossRef]
- Spronk, P.E.; Ince, C.; Gardien, M.J.; Mathura, K.R.; Oudemans-van Straaten, H.M.; Zandstra, D.F. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet 2002, 360, 1395–1396. [Google Scholar] [CrossRef]
- De Backer, D.; Hollenberg, S.; Boerma, C.; Goedhart, P.; Buchele, G.; Ospina-Tascon, G.; Dobbe, I.; Ince, C. How to evaluate the microcirculation: Report of a round table conference. Crit. Care 2007, 11, R101. [Google Scholar] [CrossRef] [Green Version]
- Verdant, C.L.; De Backer, D.; Bruhn, A.; Clausi, C.M.; Su, F.; Wang, Z.; Rodriguez, H.; Pries, A.R.; Vincent, J.L. Evaluation of sublingual and gut mucosal microcirculation in sepsis: A quantitative analysis. Crit. Care Med. 2009, 37, 2875–2881. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijnands, K.A.P.; Meesters, D.M.; Vandendriessche, B.; Briedé, J.J.; van Eijk, H.M.H.; Brouckaert, P.; Cauwels, A.; Lamers, W.H.; Poeze, M. Microcirculatory Function during Endotoxemia—A Functional Citrulline-Arginine-NO Pathway and NOS3 Complex Is Essential to Maintain the Microcirculation. Int. J. Mol. Sci. 2021, 22, 11940. https://doi.org/10.3390/ijms222111940
Wijnands KAP, Meesters DM, Vandendriessche B, Briedé JJ, van Eijk HMH, Brouckaert P, Cauwels A, Lamers WH, Poeze M. Microcirculatory Function during Endotoxemia—A Functional Citrulline-Arginine-NO Pathway and NOS3 Complex Is Essential to Maintain the Microcirculation. International Journal of Molecular Sciences. 2021; 22(21):11940. https://doi.org/10.3390/ijms222111940
Chicago/Turabian StyleWijnands, Karolina A. P., Dennis M. Meesters, Benjamin Vandendriessche, Jacob J. Briedé, Hans M. H. van Eijk, Peter Brouckaert, Anje Cauwels, Wouter H. Lamers, and Martijn Poeze. 2021. "Microcirculatory Function during Endotoxemia—A Functional Citrulline-Arginine-NO Pathway and NOS3 Complex Is Essential to Maintain the Microcirculation" International Journal of Molecular Sciences 22, no. 21: 11940. https://doi.org/10.3390/ijms222111940
APA StyleWijnands, K. A. P., Meesters, D. M., Vandendriessche, B., Briedé, J. J., van Eijk, H. M. H., Brouckaert, P., Cauwels, A., Lamers, W. H., & Poeze, M. (2021). Microcirculatory Function during Endotoxemia—A Functional Citrulline-Arginine-NO Pathway and NOS3 Complex Is Essential to Maintain the Microcirculation. International Journal of Molecular Sciences, 22(21), 11940. https://doi.org/10.3390/ijms222111940





