Hyperphosphorylation of Tau Due to the Interference of Protein Phosphatase Methylesterase-1 Overexpression by MiR-125b-5p in Melatonin Receptor Knockout Mice
Abstract
:1. Introduction
2. Results
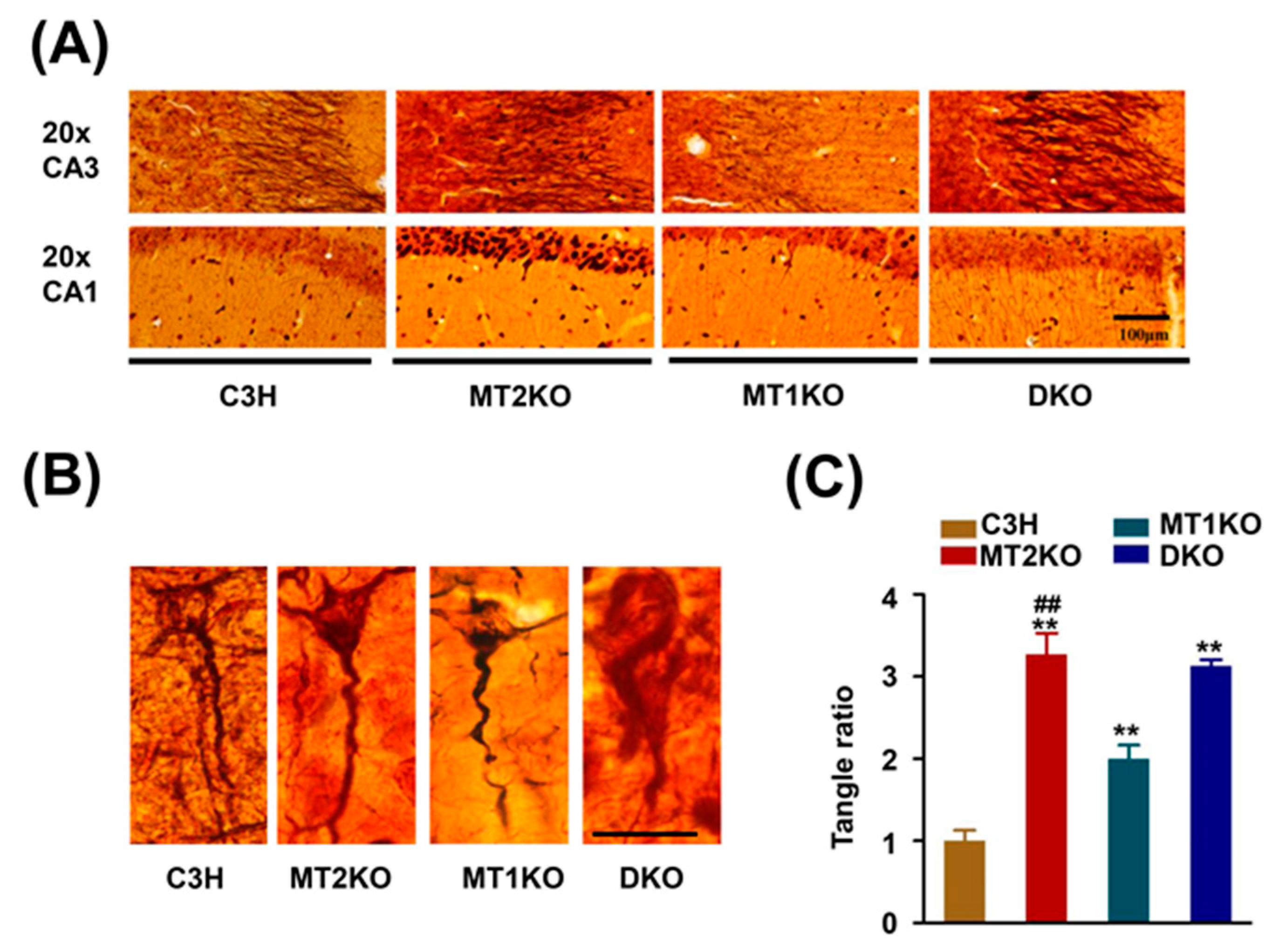
2.1. Melatonin Receptor Knockout Mice Exhibit a Strong Signal for Hypophosphorylated Tau
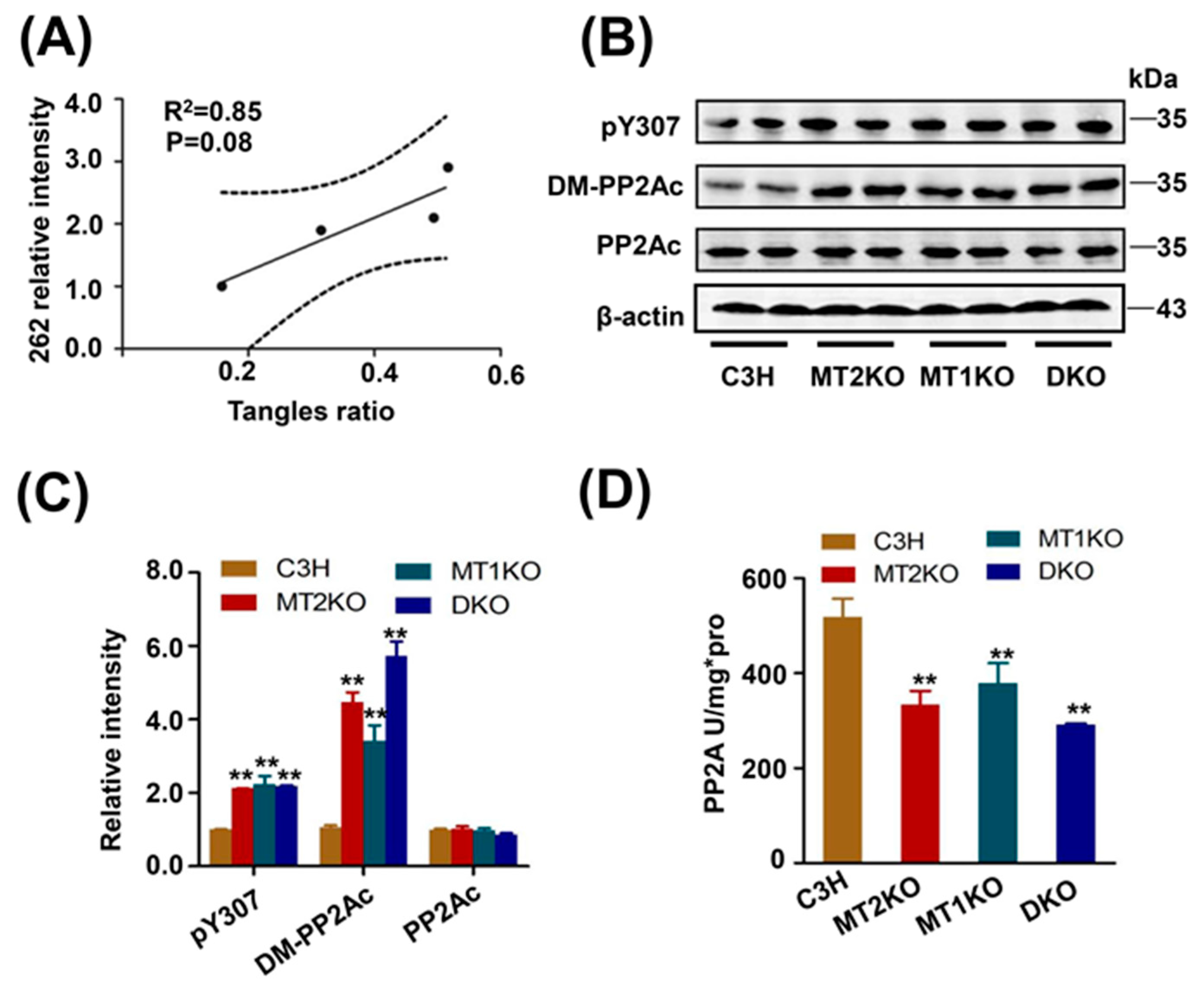
2.2. Tau Hyperphosphorylation Is Caused by the Decreased Activity of PP2A
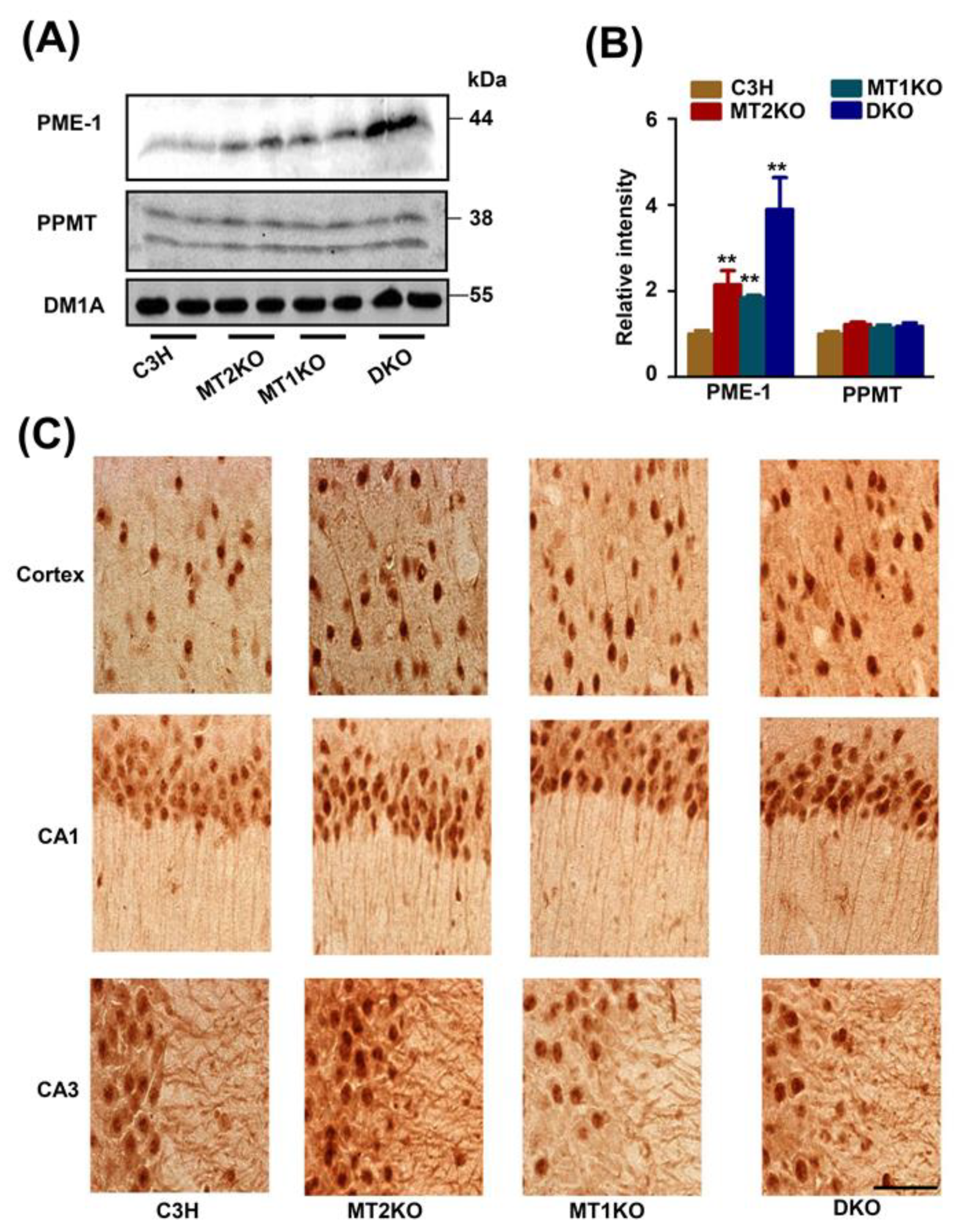
2.3. The Melatonin Receptor Regulates PP2A through PME-1
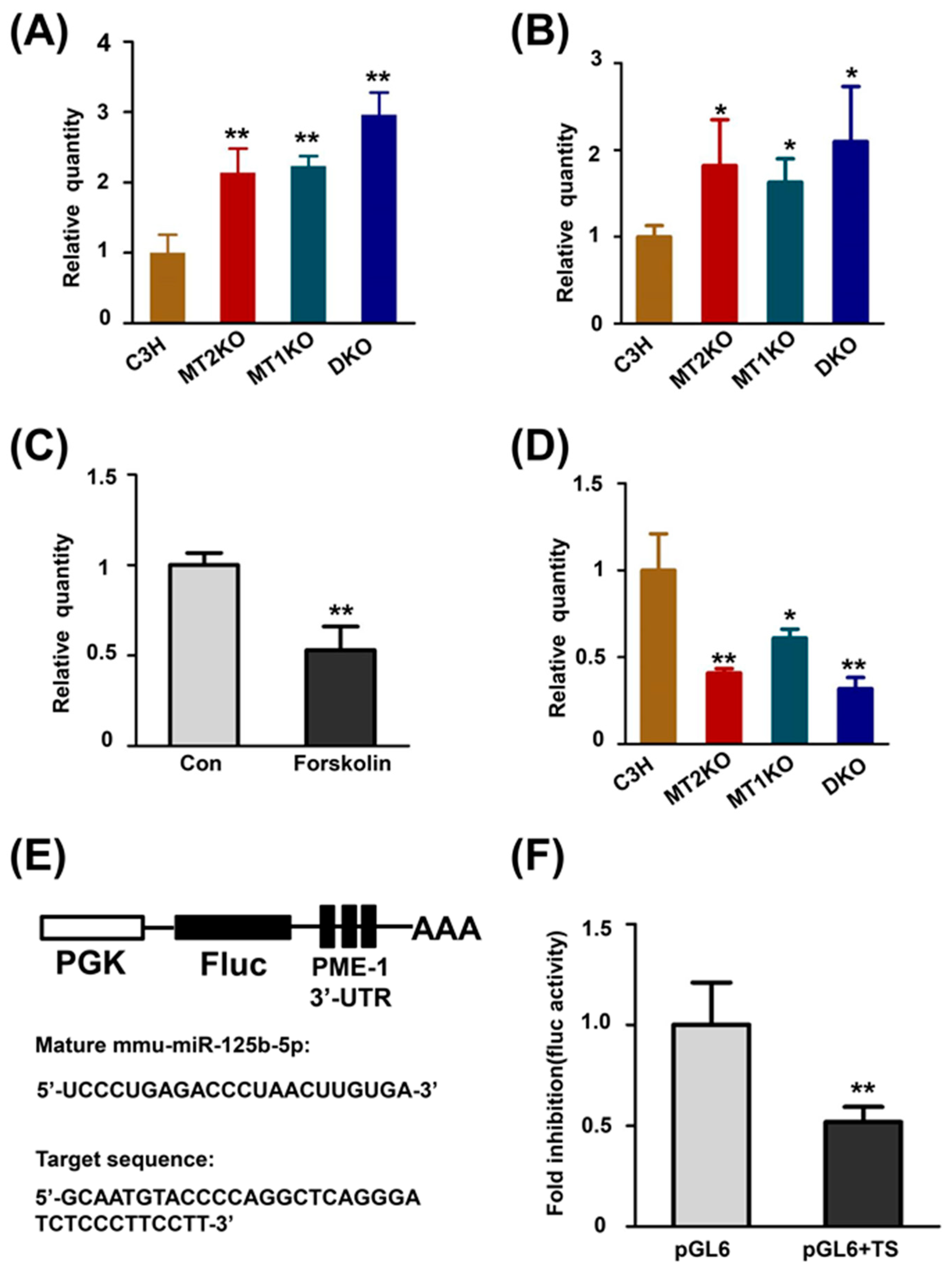
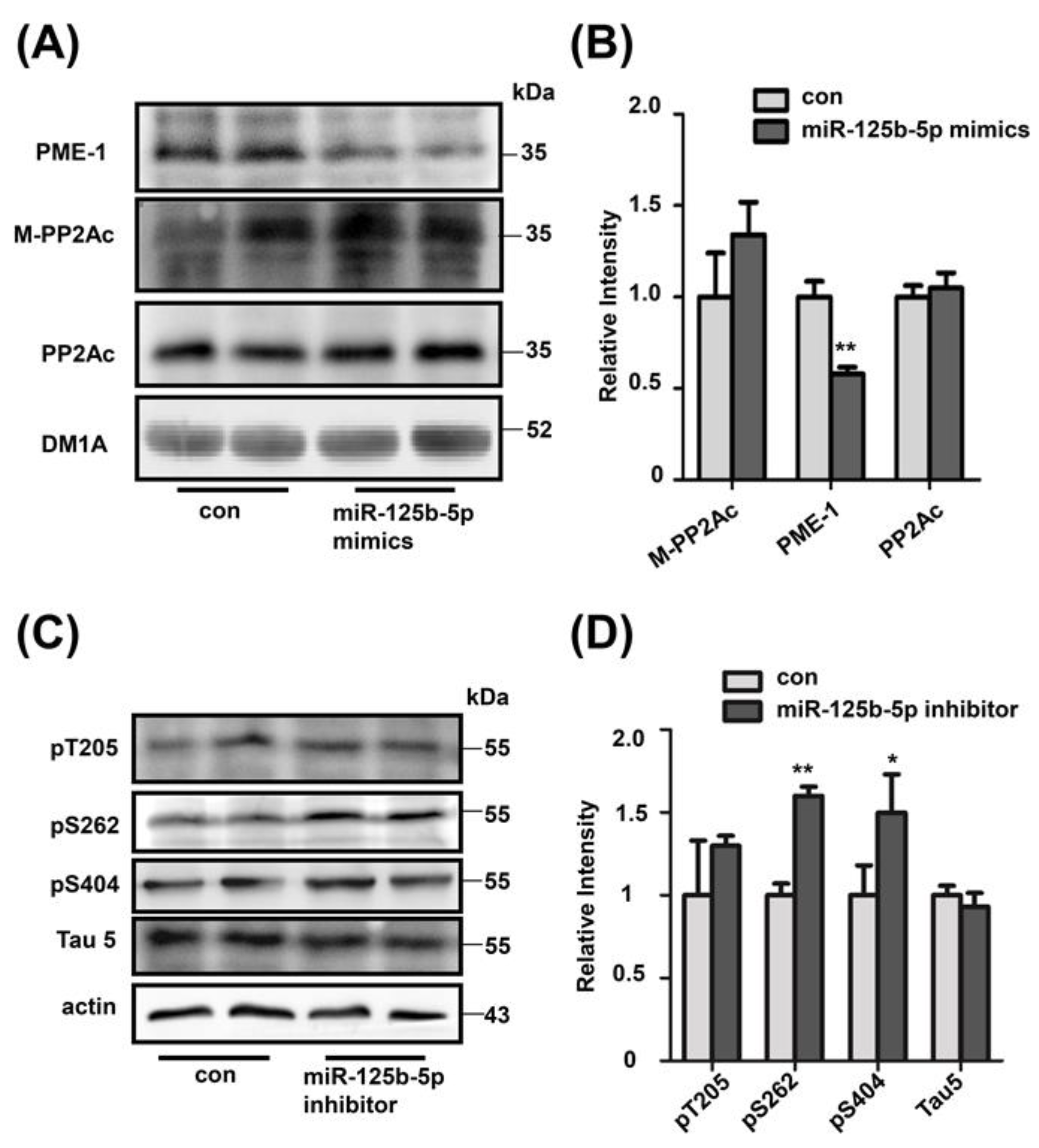
2.4. cAMP Promotes the Transcription of PME-1 by Decreasing the Production of miR-125b-5p
2.5. Decreased miR-125b-5p Induces Tau Hyperphosphorylation
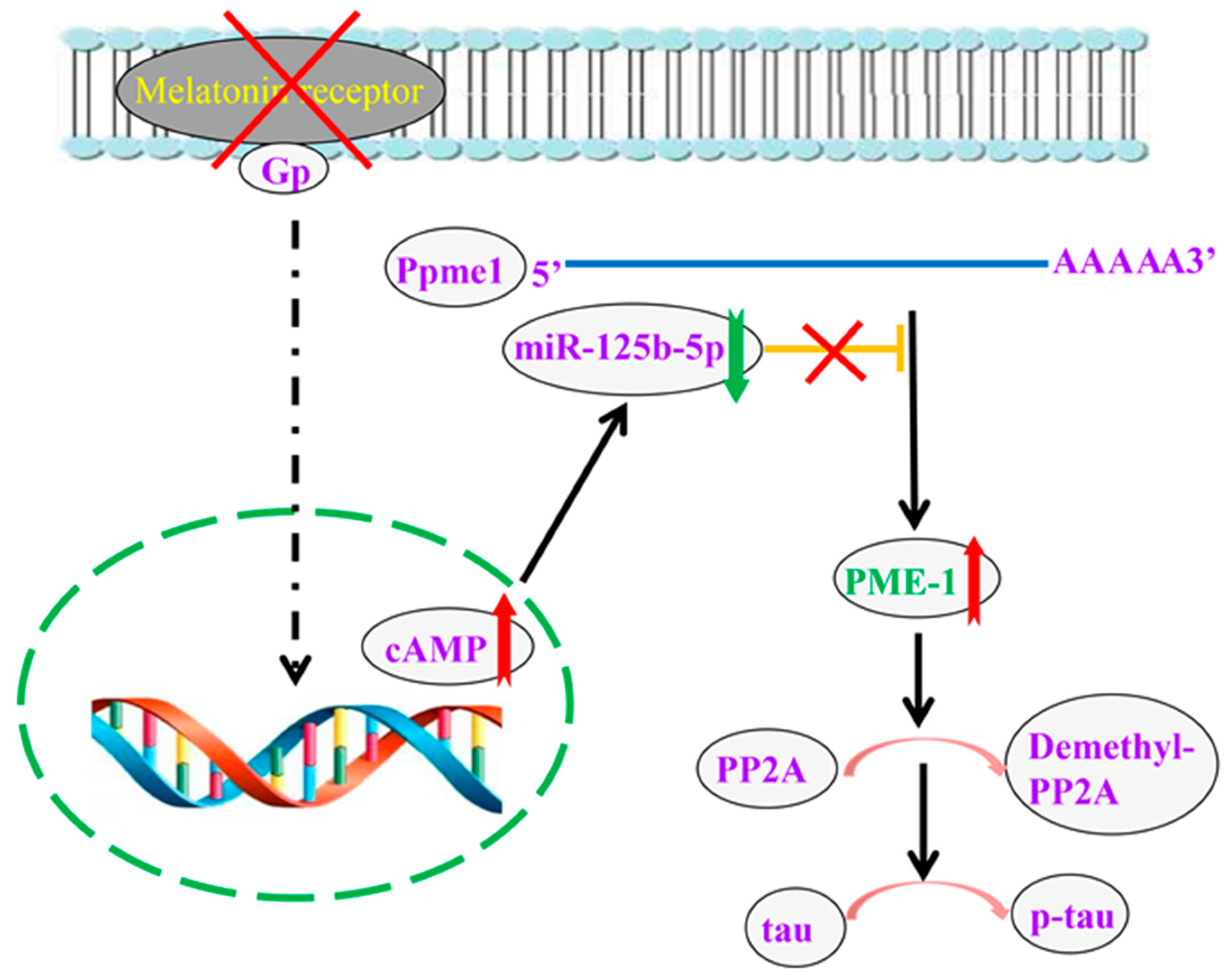
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Antibodies
4.3. Western Blot
4.4. Cell Culture and Treatments
4.5. Immunohistochemistry
4.6. PP2A Activity Assay
4.7. Bielschowsky Silver Staining and Analysis
4.8. cAMP ELISA
4.9. RNA Isolation and Real-Time PCR
4.10. Luciferase-Reporter Plasmid Construction and Transfection
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Amaral, F.G.D.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef] [Green Version]
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Medina, M.E.; Tan, D.X.; Reiter, R.J. Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: A physicochemical analysis. J. Pineal Res. 2014, 58, 107–116. [Google Scholar] [CrossRef] [PubMed]
- García, J.J.; López-Pingarrón, L.; de Souza, P.A.; Tres, A.; Escudero, P.; García-Gil, F.A.; Tan, D.-X.; Reiter, R.J.; Ramírez, J.M.; Bernal-Pérez, M. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: A review. J. Pineal Res. 2014, 56, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.M.; Kang, H.J.; McCorvy, J.D.; Glatfelter, G.; Jones, A.J.; Che, T.; Slocum, S.; Huang, X.-P.; Savych, O.; Moroz, Y.; et al. Virtual discovery of melatonin receptor ligands to modulate circadian rhythms. Nature 2020, 579, 609–614. [Google Scholar] [CrossRef]
- Mosher, A.A.; Tsoulis, M.W.; Lim, J.; Tan, C.; Agarwal, S.; Leyland, N.A.; Foster, W.G. Melatonin activity and receptor expression in endometrial tissue and endometriosis. Hum. Reprod. 2019, 34, 1215–1224. [Google Scholar] [CrossRef]
- Li, P.; Hu, F.; Cao, X.; Luo, L.; Tu, Q. Melatonin receptor protects cardiomyocyte against oxidative stress-induced apoptosis through the MAPK-ERK signaling pathway. J. Recept. Signal Transduct. 2020, 40, 117–125. [Google Scholar] [CrossRef]
- Witt-Enderby, P.A.; Li, P.-K. Melatonin receptors and ligands. Vitam. Horm. 2000, 58, 321–354. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie, R.S.; Melan, M.; Passey, D.K.; Witt-Enderby, P.A. Dual coupling of MT1 and MT2 melatonin receptors to cyclic AMP and phosphoinositide signal transduction cascades and their regulation following melatonin exposure. Biochem. Pharmacol. 2002, 63, 587–595. [Google Scholar] [CrossRef]
- Chen, L.; He, X.; Zhang, Y.; Chen, X.; Lai, X.; Shao, J.; Shi, Y.; Zhou, N. Melatonin Receptor Type 1 Signals to Extracellular Signal-Regulated Kinase 1 and 2 via Gi and Gs Dually Coupled Pathways in HEK-293 Cells. Biochemistry 2014, 53, 2827–2839. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Hoffmann, K.; Gao, C.; Petrulionis, M.; Herr, I.; Schemmer, P. Melatonin promotes sorafenib-induced apoptosis through synergistic activation of JNK/c-jun pathway in human hepatocellular carcinoma. J. Pineal Res. 2017, 62, e12398. [Google Scholar] [CrossRef]
- Kandalepas, P.C.; Mitchell, J.W.; Gillette, M.U. Melatonin Signal Transduction Pathways Require E-Box-Mediated Transcription of Per1 and Per2 to Reset the SCN Clock at Dusk. PLoS ONE 2016, 11, e0157824. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Balmik, A.A.; Chinnathambi, S. Melatonin Reduces GSK3β-Mediated Tau Phosphorylation, Enhances Nrf2 Nuclear Translocation and Anti-Inflammation. ASN Neuro 2020, 12, 1759091420981204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, F.; Chen, Z.; Su, Q.; Yan, M.; Zhang, Q.; Tan, J.; Qian, L.; Han, Y. Melatonin modulates IL-1beta-induced extracellular matrix remodeling in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration and inflammation. Aging 2019, 11, 10499. [Google Scholar] [CrossRef]
- Chitimus, D.M.; Popescu, M.R.; Voiculescu, S.E.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Zagrean, A.-M. Melatonin’s Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules 2020, 10, 1211. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef]
- Janjua, I.; Goldman, R.D. Sleep-related melatonin use in healthy children. Can. Fam. Physician Med. Fam. Can. 2016, 62, 315–316. [Google Scholar]
- Cajochen, C.; Kräuchi, K.; Wirz-Justice, A. Role of Melatonin in the Regulation of Human Circadian Rhythms and Sleep. J. Neuroendocr. 2003, 15, 432–437. [Google Scholar] [CrossRef] [Green Version]
- Iravani, S.; Eslami, P.; Moghadam, A.D.; Moazzami, B.; Mehrvar, A.; Hashemi, M.R.; Mansour-Ghanaei, F.; Mansour-Ghanaei, A.; Majidzadeh-A, K. The Role of Melatonin in Colorectal Cancer. J. Gastrointest. Cancer 2019, 51, 748–753. [Google Scholar] [CrossRef]
- Dana, P.M.; Sadoughi, F.; Mobini, M.; Shafabakhsh, R.; Chaichian, S.; Moazzami, B.; Chamani, M.; Asemi, Z. Molecular and Biological Functions of Melatonin in Endometrial Cancer. Curr. Drug Targets 2020, 21, 519–526. [Google Scholar] [CrossRef]
- Chuffa, L.G.D.A.; Carvalho, R.F.; Justulin, L.A.; Cury, S.S.; Seiva, F.R.F.; Jardim-Perassi, B.V.; Zuccari, D.A.P.D.C.; Reiter, R.J. A meta-analysis of microRNA networks regulated by melatonin in cancer: Portrait of potential candidates for breast cancer treatment. J. Pineal Res. 2020, 69, e12693. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Patel, K.K.; Dehari, D.; Agrawal, A.K.; Singh, S. Melatonin and its ubiquitous anticancer effects. Mol. Cell. Biochem. 2019, 462, 133–155. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin′s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [Green Version]
- Jenwitheesuk, A.; Nopparat, C.; Mukda, S.; Wongchitrat, P.; Govitrapong, P. Melatonin Regulates Aging and Neurodegeneration through Energy Metabolism, Epigenetics, Autophagy and Circadian Rhythm Pathways. Int. J. Mol. Sci. 2014, 15, 16848–16884. [Google Scholar] [CrossRef] [Green Version]
- Gunata, M.; Parlakpinar, H.; Acet, H. Melatonin: A review of its potential functions and effects on neurological diseases. Rev. Neurol. 2019, 176, 148–165. [Google Scholar] [CrossRef]
- Sanchez-Barcelo, E.J.; Revilla, N.; Mediavilla, M.D.; Martínez-Cué, C.; Reiter, R.J. Clinical Uses of Melatonin in Neurological Diseases and Mental and Behavioural Disorders. Curr. Med. Chem. 2017, 24, 3851–3878. [Google Scholar] [CrossRef]
- Lin, L.; Huang, Q.X.; Yang, S.S.; Chu, J.; Wang, J.Z.; Tian, Q. Melatonin in Alzheimer’s disease. Int. J. Mol. Sci. 2013, 14, 14575–14593. [Google Scholar] [CrossRef] [Green Version]
- Shukla, M.; Govitrapong, P.; Boontem, P.; Reiter, R.J.; Satayavivad, J. Mechanisms of Melatonin in Alleviating Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 1010–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamtaji, O.R.; Reiter, R.J.; Alipoor, R.; Dadgostar, E.; Kouchaki, E.; Asemi, Z. Melatonin and Parkinson Disease: Current Status and Future Perspectives for Molecular Mechanisms. Cell. Mol. Neurobiol. 2019, 40, 15–23. [Google Scholar] [CrossRef]
- Salehpour, M.Y.; Mollica, A.; Momtaz, S.; Sanadgol, N.; Farzaei, M.H. Melatonin and Multiple Sclerosis: From Plausible Neuropharmacological Mechanisms of Action to Experimental and Clinical Evidence. Clin. Drug Investig. 2019, 39, 607–624. [Google Scholar] [CrossRef]
- Taleb, H.A.A.; Alghamdi, B.S. Neuroprotective Effects of Melatonin during Demyelination and Remyelination Stages in a Mouse Model of Multiple Sclerosis. J. Mol. Neurosci. 2020, 70, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, E.A.; Jesse, C.R.; Bortolatto, C.F.; Nogueira, C.W. Correlations between behavioural and oxidative parameters in a rat quinolinic acid model of Huntington’s disease: Protective effect of melatonin. Eur. J. Pharmacol. 2013, 701, 65–72. [Google Scholar] [CrossRef]
- Jain, S.V.; Horn, P.S.; Simakajornboon, N.; Beebe, D.W.; Holland, K.; Byars, A.W.; Glauser, T.A. Melatonin improves sleep in children with epilepsy: A randomized, double-blind, crossover study. Sleep Med. 2015, 16, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Valdés-Tovar, M.; Estrada-Reyes, R.; Solís-Chagoyán, H.; Argueta, J.; Dorantes-Barrón, A.M.; Quero-Chávez, D.; Cruz-Garduño, R.; Cercós, M.G.; Trueta, C.; Oikawa-Sala, J.; et al. Circadian modulation of neuroplasticity by melatonin: A target in the treatment of depression. Br. J. Pharmacol. 2018, 175, 3200–3208. [Google Scholar] [CrossRef]
- Morera-Fumero, A.L.; Abreu-Gonzalez, P. Role of Melatonin in Schizophrenia. Int. J. Mol. Sci. 2013, 14, 9037–9050. [Google Scholar] [CrossRef] [Green Version]
- Rosales-Corral, S.A.; Acuña-Castroviejo, D.; Coto-Montes, A.; Boga, J.A.; Manchester, L.C.; Fuentes-Broto, L.; Korkmaz, A.; Ma, S.; Tan, D.X.; Reiter, R.J. Alzheimer’s disease: Pathological mechanisms and the beneficial role of melatonin. J. Pineal Res. 2011, 52, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Bahna, S.G.; Sathiyapalan, A.; Foster, J.A.; Niles, L.P. Regional upregulation of hippocampal melatonin MT2 receptors by valproic acid: Therapeutic implications for Alzheimer’s disease. Neurosci. Lett. 2014, 576, 84–87. [Google Scholar] [CrossRef]
- Ali, T.; Kim, M.O. Melatonin ameliorates amyloid beta-induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI3/Akt/GSk3beta pathway in the mouse hippocampus. J. Pineal Res. 2015, 59, 47–59. [Google Scholar] [CrossRef]
- Zheng, X.-N.; Wu, X.-F.; Guo, X.; Xie, L.-N.; Xie, Z.-Q.; Wei, X.-R.; Liu, L.; Chen, X.-L.; Yue, Z.-H. Manual Acupuncture Stimulation of Paired Acupoints Can Relieve Sleep Disorder Possibly by Upregulating Pineal Melatonin Protein and Its Receptor mRNA Levels in the Suprachiasmatic Nucleus in Insomnia Rats. Zhen Ci Yan Jiu = Acupunct. Res. 2018, 43, 360–364. [Google Scholar]
- Xie, Z.; Chen, F.; Li, W.A.; Geng, X.; Li, C.; Meng, X.; Feng, Y.; Liu, W.; Yu, F. A review of sleep disorders and melatonin. Neurol. Res. 2017, 39, 559–565. [Google Scholar] [CrossRef]
- Reynhout, S.; Jansen, S.; Haesen, D.; van Belle, S.; de Munnik, S.A.; Bongers, E.; Schieving, J.H.; Marcelis, C.; Amiel, J.; Rio, M.; et al. De Novo Mutations Affecting the Catalytic Calpha Subunit of PP2A, PPP2CA, Cause Syndromic Intellectual Disability Resembling Other PP2A-Related Neurodevelopmental Disorders. Am. J. Hum. Genet. 2019, 104, 139–156. [Google Scholar] [CrossRef] [Green Version]
- Mishima, K.; Tozawa, T.; Satoh, K.; Matsumoto, Y.; Hishikawa, Y.; Okawa, M. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep–waking. Biol. Psychiatry 1999, 45, 417–421. [Google Scholar] [CrossRef]
- Maldonado-Lasuncion, I.; Atienza, M.; Sanchez-Espinosa, M.P.; Cantero, J.L. Aging-Related Changes in Cognition and Cortical Integrity are Associated with Serum Expression of Candidate MicroRNAs for Alzheimer Disease. Cereb. Cortex 2018, 29, 4426–4437. [Google Scholar] [CrossRef]
- Asayama, K.; Yamadera, H.; Ito, T.; Suzuki, H.; Kudo, Y.; Endo, S. Double Blind Study of Melatonin Effects on the Sleep-wake Rhythm, Cognitive and Non-cognitive Functions in Alzheimer Type Dementia. J. Nippon. Med. Sch. 2003, 70, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Gehrman, P.R.; Connor, D.J.; Martin, J.L.; Shochat, T.; Corey-Bloom, J.; Ancoli-Israel, S. Melatonin Fails to Improve Sleep or Agitation in Double-Blind Randomized Placebo-Controlled Trial of Institutionalized Patients with Alzheimer Disease. Am. J. Geriatr. Psychiatry 2009, 17, 166–169. [Google Scholar] [CrossRef] [Green Version]
- Serfaty, M.; Kennell-Webb, S.; Warner, J.; Blizard, R.; Raven, P. Double blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementia. Int. J. Geriatr. Psychiatry 2002, 17, 1120–1127. [Google Scholar] [CrossRef]
- Laudon, M.; Frydman-Marom, A. Therapeutic Effects of Melatonin Receptor Agonists on Sleep and Comorbid Disorders. Int. J. Mol. Sci. 2014, 15, 15924–15950. [Google Scholar] [CrossRef] [Green Version]
- Banzhaf-Strathmann, J.; Benito, E.; May, S.; Arzberger, T.; Tahirovic, S.; Kretzschmar, H.; Fischer, A.; Edbauer, D. MicroRNA-125b induces tau hyperphosphorylation and cognitive deficits in Alzheimer’s disease. EMBO J. 2014, 33, 1667–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weil, Z.M.; Hotchkiss, A.K.; Gatien, M.L.; Pieke-Dahl, S.; Nelson, R.J. Melatonin receptor (MT1) knockout mice display depression-like behaviors and deficits in sensorimotor gating. Brain Res. Bull. 2006, 68, 425–429. [Google Scholar] [CrossRef]
- Larson, J.; Jessen, R.E.; Uz, T.; Arslan, A.D.; Kurtuncu, M.; Imbesi, M.; Manev, H. Impaired hippocampal long-term potentiation in melatonin MT2 receptor-deficient mice. Neurosci. Lett. 2006, 393, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Klosen, P.; Lapmanee, S.; Schuster, C.; Guardiola, B.; Hicks, D.; Pevet, P.; Felder-Schmittbuhl, M.P.; Sawarut, L. MT1 and MT2 melatonin receptors are expressed in nonoverlapping neuronal populations. J. Pineal Res. 2019, 67, e12575. [Google Scholar] [CrossRef] [PubMed]
- Lauckner, J.; Frey, P.; Geula, C. Comparative distribution of tau phosphorylated at Ser262 in pre-tangles and tangles. Neurobiol. Aging 2003, 24, 767–776. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Xia, Y.-Y.; Grundke-Iqbal, I.; Iqbal, K. Abnormal Hyperphosphorylation of Tau: Sites, Regulation, and Molecular Mechanism of Neurofibrillary Degeneration. J. Alzheimer’s Dis. 2012, 33, S123–S139. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wu, X.; Xu, J.; Zhou, J.; Han, X.; Guo, J. Src kinase up-regulates the ERK cascade through inactivation of protein phosphatase 2A following cerebral ischemia. BMC Neurosci. 2009, 10, 74. [Google Scholar] [CrossRef] [Green Version]
- Bryant, J.C.; Westphal, R.S.; Wadzinski, B.E. Methylated C-terminal leucine residue of PP2A catalytic subunit is important for binding of regulatory Balpha subunit. Biochem. J. 1999, 339, 241–246. [Google Scholar] [CrossRef]
- Sontag, J.M.; Nunbhakdi-Craig, V.; White, C.L., 3rd; Halpain, S.; Sontag, E. The protein phosphatase PP2A/Balpha binds to the microtubule-associated proteins Tau and MAP2 at a motif also recognized by the kinase Fyn: Implications for tauopathies. J. Biol. Chem. 2012, 287, 14984–14993. [Google Scholar] [CrossRef] [Green Version]
- Huete-Toral, F.; Crooke, A.; Martinez-Aguila, A.; Pintor, J. Melatonin Receptors Trigger cAMP Production and Inhibit Chloride Movements in Nonpigmented Ciliary Epithelial Cells. J. Pharmacol. Exp. Ther. 2014, 352, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Carlyle, B.C.; Nairn, A.C.; Wang, M.; Yang, Y.; Jin, L.E.; Simen, A.A.; Ramos, B.P.; Bordner, K.A.; Craft, G.E.; Davies, P.; et al. cAMP-PKA phosphorylation of tau confers risk for degeneration in aging association cortex. Proc. Natl. Acad. Sci. USA 2014, 111, 5036–5041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-H.; Bin, B.-H.; Kim, J.; Dong, S.E.; Park, P.J.; Choi, H.; Kim, B.J.; Yu, S.J.; Kang, H.; Kang, H.H.; et al. Novel inhibitory function of miR-125b in melanogenesis. Pigment Cell Melanoma Res. 2013, 27, 140–144. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | RT Oligonucleotide | qPCR Forward Primer | qPCR Reverse Primer |
|---|---|---|---|
| MiR-125b-5p | CACCGTTCCCCGCCGTCGGTGTCACAA | GCCTCCCTGAGACCCTA | CCGTCGGTGTCACAAGTTAG |
| SnoU6 | CACCGTTCCCCGCCGTCGGTGCTTCTC | CTCGCTTCGGCAGCA | GCCGTCGGTGCTTCTCTGT |
| Ppme1 | TCAAATGTCTTCCCAGGCTCAG | CCACCGCTCGCTATGGCTAA | |
| β-actin | GTCGTACCACAGGCATTGTGTGG | GCAATGCCTGGGTACATGGTGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Feng, L.; Zhong, W.; Zhen, H.; Chi, Q.; Wang, X. Hyperphosphorylation of Tau Due to the Interference of Protein Phosphatase Methylesterase-1 Overexpression by MiR-125b-5p in Melatonin Receptor Knockout Mice. Int. J. Mol. Sci. 2021, 22, 11850. https://doi.org/10.3390/ijms222111850
Zhao H, Feng L, Zhong W, Zhen H, Chi Q, Wang X. Hyperphosphorylation of Tau Due to the Interference of Protein Phosphatase Methylesterase-1 Overexpression by MiR-125b-5p in Melatonin Receptor Knockout Mice. International Journal of Molecular Sciences. 2021; 22(21):11850. https://doi.org/10.3390/ijms222111850
Chicago/Turabian StyleZhao, Han, Lingyan Feng, Wei Zhong, Hongyan Zhen, Qingjia Chi, and Xiang Wang. 2021. "Hyperphosphorylation of Tau Due to the Interference of Protein Phosphatase Methylesterase-1 Overexpression by MiR-125b-5p in Melatonin Receptor Knockout Mice" International Journal of Molecular Sciences 22, no. 21: 11850. https://doi.org/10.3390/ijms222111850
APA StyleZhao, H., Feng, L., Zhong, W., Zhen, H., Chi, Q., & Wang, X. (2021). Hyperphosphorylation of Tau Due to the Interference of Protein Phosphatase Methylesterase-1 Overexpression by MiR-125b-5p in Melatonin Receptor Knockout Mice. International Journal of Molecular Sciences, 22(21), 11850. https://doi.org/10.3390/ijms222111850







