Systemic Review: Ozone: A Potential New Chemotherapy
Abstract
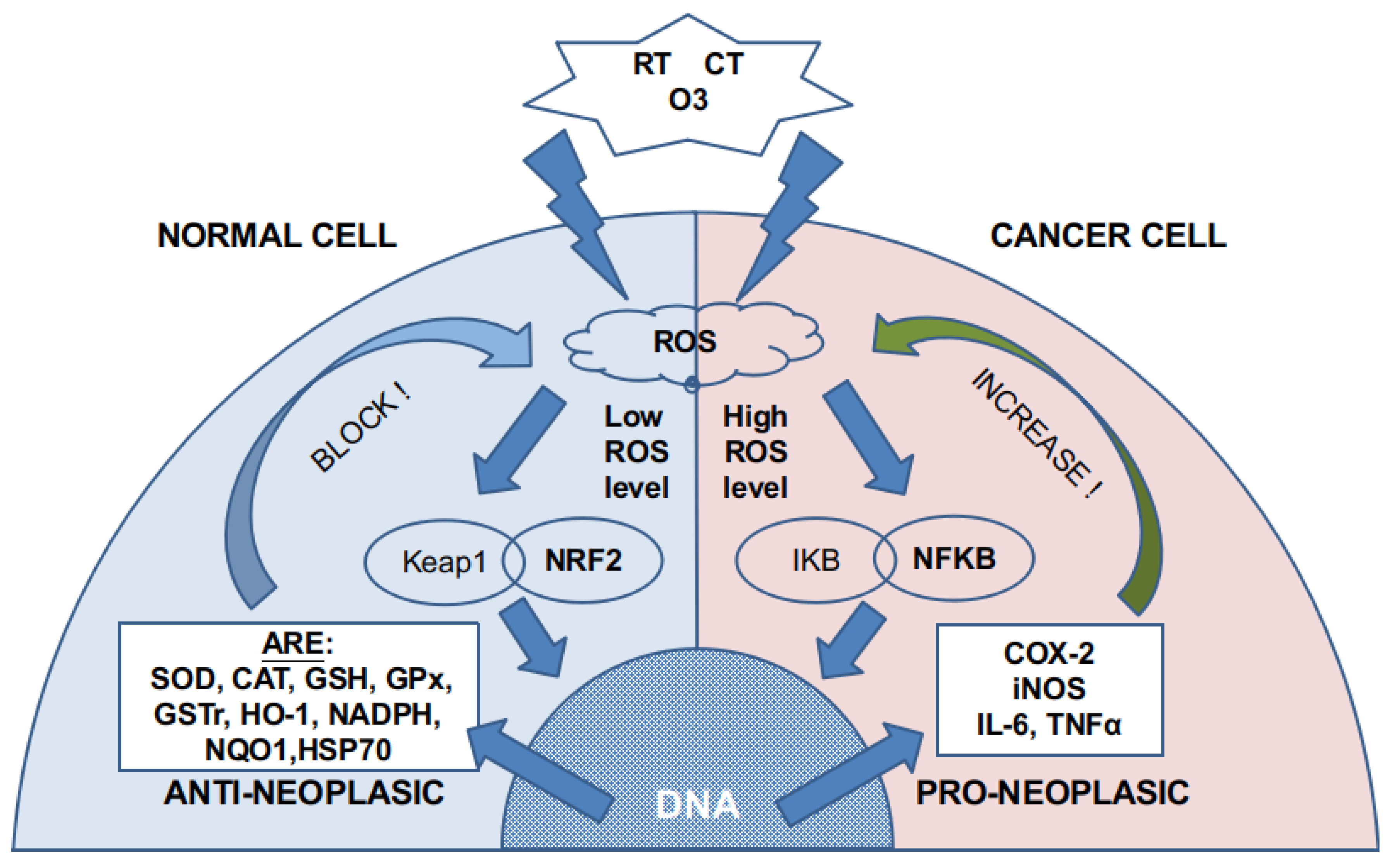
:1. Introduction
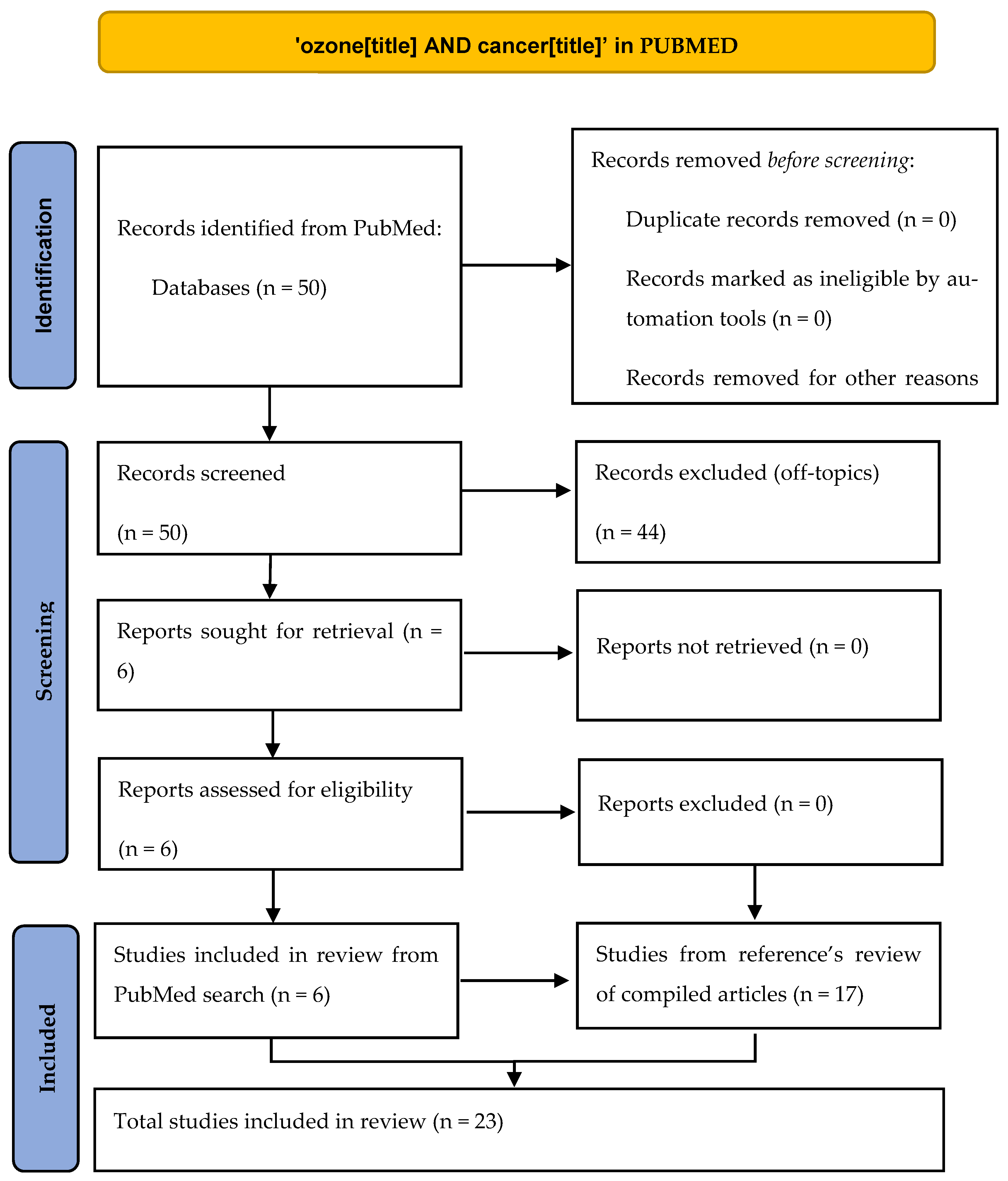
2. Material and Methods
3. Results
3.1. In Vitro Studies
3.2. In Vivo Studies
3.3. Clinical Works
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 20 September 2021).
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer. 2020. Available online: https://gco.iarc.fr/today (accessed on 20 September 2021).
- Cancer Statistics—National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/understanding/statistics (accessed on 20 September 2021).
- Clavo, B.; Santana-Rodríguez, N.; Llontop, P.; Gutiérrez, D.; Suárez, G.; López, L.; Rovira, G.; Martínez-Sánchez, G.; González, E.; Jorge, I.J.; et al. Ozone Therapy as Adjuvant for Cancer Treatment: Is Further Research Warranted? Evid. Based Complement. Altern. Med. 2018, 2018, 7931849. [Google Scholar] [CrossRef] [Green Version]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Chung, L.Y.; Tang, S.J.; Wu, Y.C.; Yang, K.C.; Huang, H.J.; Sun, G.H.; Sun, K.H. Platinum-based combination chemotherapy triggers cancer cell death through induction of BNIP3 and ROS, but not autophagy. J. Cell Mol. Med. 2020, 24, 1993–2003. [Google Scholar] [CrossRef] [Green Version]
- Glass, S.B.; Gonzalez-Fajardo, L.; Beringhs, A.O.; Lu, X. Redox potential and ROS-mediated nanomedicines for improving cancer therapy. Antioxid. Redox Signal. 2019, 30, 747–761. [Google Scholar] [CrossRef]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Chánová, E.; Syková, E.; Dejneka, A.; Kubinová, Š. Cell death induced by ozone and various non-thermal plasmas: Therapeutic perspectives and limitations. Sci. Rep. 2014, 4, 7129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baeza, J.; Cabo, J.R.; Gómez, M.; Menéndez, S.; Re, L. WFOTs Review on Evidence Based Ozone Therapy [Internet]; World Federation of Ozone Therapy: Brescia, Italy, 2015; 116p, Available online: https://www.wfoot.org/wp-content/uploads/2016/01/WFOT-OZONE-2015-ENG.pdf (accessed on 20 September 2021).
- Bocci, V. Does ozone really “cure” cancer? Int. J. Cancer 2008, 123, 1222. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Fetner, R.H. Chromosome Breakage in Vicia faba by Ozone. Nature 1958, 181, 504–505. [Google Scholar] [CrossRef]
- Fetner, R.H. Ozone-induced chromosome breakage in human cell cultures. Nature 1962, 194, 793–794. [Google Scholar] [CrossRef] [PubMed]
- Sweet, F.; Kao, M.S.; Lee, S.C.; Hagar, W.L.; Sweet, W.E. Ozone selectively inhibits growth of human cancer cells. Science 1980, 209, 931–933. [Google Scholar] [CrossRef] [Green Version]
- Karlic, H.; Kucera, H.; Metka, M.; Schönbauer, M.; Söregi, G. Zur Wirkung von Ozon und ionisierender Strahlung am In-vitro-Modell—eine Pilotstudie an vier gynäkologischen Tumoren [Effect of ozone and ionizing radiation on an in vitro model—A pilot study of 4 gynecologic tumors]. Strahlenther Onkol. 1987, 163, 37–42. [Google Scholar] [PubMed]
- Zänker, K.S.; Kroczek, R. In vitro synergistic activity of 5-fluorouracil with low-dose ozone against a chemoresistant tumor cell line and fresh human tumor cells. Chemotherapy 1990, 36, 147–154. [Google Scholar] [CrossRef] [PubMed]
- WashüTtl, J.; Viebahn, R.; Steiner, I. The Influence of Ozone on Tumor Tissue in Comparison with Healthy Tissue (in vitro). Ozone Sci. Eng. 1990, 12, 65–72. [Google Scholar] [CrossRef]
- Cannizzaro, A.; Verga Falzacappa, C.; Martinelli, M.; Misiti, S.; Brunetti, E.; Bucci, B. O(2/3) exposure inhibits cell progression affecting cyclin B1/cdk1 activity in SK-N-SH while induces apoptosis in SK-N-DZ neuroblastoma cells. J. Cell Physiol. 2007, 213, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, V.; Quagliariello, V.; Giustetto, P.; Franzini, M.; Iaffaioli, R.V. Association of Ozone with 5-Fluorouracil and Cisplatin in Regulation of Human Colon Cancer Cell Viability: In Vitro Anti-Inflammatory Properties of Ozone in Colon Cancer Cells Exposed to Lipopolysaccharides. Evid. Based Complement. Altern. Med. 2017, 2017, 7414083. [Google Scholar] [CrossRef] [Green Version]
- Mokhtari, H.; Farahmand, L.; Yaserian, K.; Jalili, N.; Majidzadeh, A.K. The antiproliferative effects of cold atmospheric plasma-activated media on different cancer cell lines, the implication of ozone as a possible underlying mechanism. J. Cell Physiol. 2019, 234, 6778–6782. [Google Scholar] [CrossRef]
- Costanzo, M.; Romeo, A.; Cisterna, B.; Calderan, L.; Bernardi, P.; Covi, V.; Tabaracci, G.; Malatesta, M. Ozone at low concentrations does not affect motility and proliferation of cancer cells in vitro. Eur. J. Histochem. 2020, 64, 3119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zeng, T.; Tang, S.; Zhong, M.; Huang, Q.; Li, X.; He, X. Medical ozone induces proliferation and migration inhibition through ROS accumulation and PI3K/AKT/NF-κB suppression in human liver cancer cells in vitro. Clin. Transl. Oncol. 2021, 23, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, S.; Cepero, J.; Borrego, L. Ozone Therapy in Cancer Treatment: State of the Art. Ozone Sci. Eng. 2008, 30, 398–404. [Google Scholar] [CrossRef]
- Schulz, S.; Häussler, U.; Mandic, R.; Heverhagen, J.T.; Neubauer, A.; Dünne, A.A.; Werner, J.A.; Weihe, E.; Bette, M. Treatment with ozone/oxygen-pneumoperitoneum results in complete remission of rabbit squamous cell carcinomas. Int. J. Cancer 2008, 122, 2360–2367. [Google Scholar] [CrossRef]
- Rossmann, A.; Mandic, R.; Heinis, J.; Höffken, H.; Küssner, O.; Kinscherf, R.; Weihe, E.; Bette, M. Intraperitoneal oxidative stress in rabbits with Papillomavirus-associated head and neck cancer induces tumoricidal immune response that Is adoptively transferable. Clin. Cancer Res. 2014, 20, 4289–4301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernuss, P.; Müller-Tyl, E.; Seitz, W. Strahlensensibilisierender Effekt von Ozon im Tierversuch [Radiosensitizing effect of ozone in animal experiment]. Strahlenther. Und Onkol. 1974, 147, 91–96. [Google Scholar]
- Grundner, H.G.; Bauer, E.; Tramer, G.; Utesch, E. Tierexperimentelle Untersuchungen über die Anwendung von Ozon auf unbestrahlte und bestrahlte Tumoren. I. Intravenöse Ozonbehandlung des Crocker-Sarkoms 180 und des Ehrlich-Karzinoms der weissen Maus [Animal experiment studies on the use of ozone in irradiated and non-irradiated tumors. I. Intravenous ozone therapy of Crocker’s sarcoma 180 and Ehrlich carcinoma in the white mouse]. Strahlenther. Und Onkol. 1976, 151, 372–381. [Google Scholar]
- Grundner, H.G.; Erler, U. Experimentation on animals for investigation of ozone treatment in tumors with and without irradiation. II. Ehrlich ascites carcinoma in vivo. Strahlenther. Und Onkol. 1976, 151, 522–529. [Google Scholar]
- Grundner, H.G. Animal experimental examinations concerning the application of ozone to non irradiated and to irradiated tumours. III. Ehrlich ascites cancer cells in vitro. Strahlenther. Und Onkol. 1976, 151, 480–486. [Google Scholar]
- Kiziltan, H.S.; Bayir, A.G.; Yucesan, G. Medical ozone and radiotherapy in a peritoneal, Erlich-ascites, tumor-cell model. Altern. Ther. Health Med. 2015, 21, 24–29. [Google Scholar] [PubMed]
- Dogan, R.; Hafız, A.M.; Kiziltan, H.S.; Yenigun, A.; Buyukpinarbaslili, N.; Eris, A.H.; Ozturan, O. Effectiveness of radiotherapy + ozone on tumoral tissue and survival in tongue cancer rat model. Auris Nasus Larynx 2018, 45, 170–174. [Google Scholar] [CrossRef]
- Re, L.; Martínez-Sánchez, G.; Bordicchia, M.; Malcangi, G.; Pocognoli, A.; Morales-Segura, M.A.; Rothchild, J.; Rojas, A. Is ozone pre-conditioning effect linked to Nrf2/EpRE activation pathway in vivo? A preliminary result. Eur. J. Pharmacol. 2014, 742, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Azuma, K.; Mori, T.; Kawamoto, K.; Murahata, Y.; Tsuka, T.; Osaki, T.; Ito, N.; Imagawa, T.; Itoh, F.; et al. The safety and anti-tumor effects of ozonated water in vivo. Int. J. Mol. Sci. 2015, 16, 25108–25120. [Google Scholar] [CrossRef] [Green Version]
- Megele, R.; Riemenschneider, M.J.; Dodoo-Schittko, F.; Feyrer, M.; Kleindienst, A. Intra-tumoral treatment with oxygen-ozone in glioblastoma: A systematic literature search and results of a case series. Oncol. Lett. 2018, 16, 5813–5822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clavo, B.; Ruiz, A.; Lloret, M.; López, L.; Suárez, G.; Macías, D.; Rodríguez, V.; Hernández, M.A.; Martín-Oliva, R.; Quintero, S.; et al. Adjuvant Ozonetherapy in Advanced Head and Neck Tumors: A Comparative Study. Evid. Based Complement. Altern. Med. 2004, 1, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E. Treatment of advanced non-small-cell lung cancer with oxygen ozone therapy and mistletoe: An integrative approach. Eur. J. Integr. Med. 2012, 4, 130. [Google Scholar]
| Author (Year) | Study Type | Disease | Route of O3 Application | Unit of Analysis | Sample Size | Findings |
|---|---|---|---|---|---|---|
| Sweet F. et al. (1980) | Basic research In vitro | Control: - Human lung diploid fibroblasts. Diseases: - Lung alveolar adenocarcinoma - Breast adenocarcinoma - Uterine carcinosarcoma - Endometrial carcinoma | Topical gas | Cell cultures | 5 groups | Control cells suffered no changes while all cancer cells had apoptosis and growth inhibition. At higher doses, control cells had growth inhibition, and cancer cells showed greater damage. |
| Simonetti V. et al. (2017) | Basic research In vitro | HT29 human cancer colon | Topical gas +/− CT (cisplatin or 5 -fluorouracil (5-FU)) | Cell cultures | 14 groups: - O3 (4 subgroups) - Cisplatin - O3 + Cisplatin (4 subgroups) - 5-FU - O3 + 5-FU (4 subgroups) | O3 damaged cancer cells and has a synergistic effect with both CT drugs. |
| Mokhtari H. et al. (2018) | Basic research In vitro | Control: - Skin fibroblasts - Mammalian gland cells Diseases: - Breast cancer (SKBR3, MCF7) - Pancreatic cancer (ASPC-1) - Lung adenocarcinoma (A-549) - Osteosarcoma (G-292) - Colon carcinoma (SW742) | Cold atmospheric plasm activated media (PAM) | Cell cultures | 8 groups: - 2 controls - 6 diseases | All cancer cell lines were affected by the exposure to PAM, directly related to the exposure time. O3 production by PAM was the reason. Colon carcinoma was the most resistant line and breast cancer and the most affected one. |
| Costanzo M. et al. (2020) | Basic research In vitro | HeLa cell line | Topical gas | Cell cultures | 2 groups (O3 different doses) | Cancer cells were not affected at these doses. |
| Li J. et al. (2021) | Basic research In vitro | Hepatocarcinoma (bel7402 and SMMC7721 cancer cell lines) | Topical gas | Cell cultures | 2 groups | Ozone restrains the proliferation and migration potential of liver cancer cells via ROS accumulation and PI3K/AKT/NF-κB suppression. |
| Dogan R. et al. (2018) | Basic research In vivo | Tongue cancer rat model (4NQO) | Rectal insufflation RT | Rats | 36: - Cancer - Cancer + RT, - Cancer + O3 + RT. - Cancer + O3 - Control | O3 increases 3 times the survival time compared with RT. RT plus O3 prolonged survival time 11 times more than RT alone. |
| Author (Year) | Study Type | Disease | Route of O3 Application | Unit of Analysis | Sample Size | Findings |
|---|---|---|---|---|---|---|
| Fetner R. (1958) | Basic research In vitro | No disease | Topical gas +/− X-ray | Vicia faba seeds | 4 groups: - O2 - O3 - X-ray - O3 + X-ray | Compared with O2, both O3 and X-ray induced chromosome breakages; more together or with higher doses |
| Fetner R. (1962) | Basic research In vitro | KB cell line (HeLa) | Topical gas +/− X-ray | Cell culture | 6 groups: - O2 - O3 - X-ray - O3 + X-ray (3 subgroups) | Compared with O2, both O3 and X-ray induced chromosome breakages; more together or with higher doses |
| Grundner HG. et al. (1976)-III | Basic research In vitro | Peritoneal carcinomatosis (Erlich ascites carcinoma) | Topical gas +/− RT | Cell culture | 3 groups: - O3 - RT - O3 + RT | O3 inhibits tumor growth and is more effective if associated with RT. |
| Karlic H. et al. (1987) | Basic research In vitro | Control: - Skin fibroblasts Diseases: - Ovarian carcinoma (OC) - 2 different lines of ovarian adenocarcinoma (OC1, OC2) - Endometrial carcinoma (EC) | Topical gas +/− RT (Ra226, Ir192, or Co60) | Cell cultures | 29 groups: - Control - Each disease: O3, Ra226, Ir192, Co60, O3 + Ra226, O3 + Ir192 O3 + Co60 (7 subgroups) | Control cells were not damaged by O3, Ra226, or both. Ir192 or Co60 alone damaged control cells. Endometrial carcinoma was resistant to O3 or Ra226, but affected by the combination. The rest of cancer cell lines were damaged by any RT or O3 +/− RT. |
| Zanker KS. et al. (1990) | Basic research In vitro | - Breast cancer - Colorectal adenocarcinoma - Glioma - Colorectal adenocarcinoma resistant to 5-FU | Topical gas +/− 5-FU | Cell cultures | 12 groups: - O3, 5-FU and both in each line. | Only glioma cells were not damaged by O3, 5-FU or both. In the other cancer cell cultures, O3 and 5-FU had a synergistic or additive effect. |
| Washuttl J. et al. (1990) | Basic research In vitro | Control: - Ovarian healthy tissue (OHT) Disease: - Ovarian carcinomatosis (OC) | Topic gas, CT (Doxorubicin or Ifosfamide) | Cell cultures | 6 groups: - Control (OHT/OC) - O3 (OHT/OC) - Doxorubicin (OC) - Ifosfamide (OC) | O3 does not harm OHT while at the same doses, it induces damage in OC, similar to the produced by the CT. There is damage in the respiratory pathway of OC caused by O3 that does not happen in OHT. |
| Cannizzaro A. et al. (2007) | Basic research In vitro | SK-N-SH and SK-N-DK neuroblastoma | Topical gas +/− CT (Cisplatin, Etoposide, or Gemcitabine) | Cell cultures | 8 groups: - Control - O3 - CT (3 subgroups) - O3 + CT (3 subgroups) | SK-N-SH cells were affected by O3, CT and both (synergistic effect). SK-N-DK cells were only affected by O3. |
| Menendez S. et al. (2008) | Basic research In vivo RCT phase III Single blind | Erlich ascites carcinoma + Sarcoma 37 Lewis lung carcinoma Prostatic adenocarcinoma (intracapsular) | Rectal insufflation Intraperitoneal insufflation before the tumor implantation RT (Co60) +/− Rectal insufflation | Mice Mice Patients | 50: - Control - 4 different O3 doses. 50: - Control - 4 different O3 doses. 70: 35 / 35 | Significant decrease in the number of metastases in the O3 group, which was directly related to the O3 dose. Significant decrease in tumor growth in pre-treated O3 groups, which was inversely related to the O3 dose. Significant decrease in side effects (radio- dermatitis, cystitis, proctitis) and PSA in O3 group. |
| Schulz S. et al. (2008) | Basic research In vivo | Head and neck squamous cell carcinoma | Intraperitoneal insufflation | Rabbits | 59 | Tumor regression that could be blocked by immunosuppressors. |
| Rossmann A. et al. (2014) | Basic research In vivo | Head and neck papillomavirus- related cancer | Intraperitoneal insufflation | Rabbits | 20 | Tumor eradication due to enhanced immunity (increase in CD3+ T-cells). |
| Hernuss P. et al. (1974) | Basic research In vivo | Walker carcinosarcoma | RT +/− Intraperitoneal insufflation | Rats | Not reported. 2 groups: - RT - RT + O3 | RT combined with O3 produced better outcomes than only RT. Tumor remission was 39% in the RT + O3 group versus 0% in RT without O3 group. |
| Grundner HG. et al. (1976)-I | Basic research In vivo | Peritoneal carcinomatosis (Erlich ascites carcinoma) | RT +/− intravenous | Mice | Not reported. 2 groups: - RT - RT + O3 | O3 does not add any inhibitory effect to RT. |
| Grundner HG. et al. (1976)-II | Basic research In vivo | Peritoneal carcinomatosis (Erlich ascites carcinoma) | RT +/− Intraperitoneal insufflation | Mice | Not reported. 2 groups: - RT - RT + O3 | O3 does not add any inhibitory effect to RT. |
| Kiziltan HS. et al. (2015) | Basic research In vivo | Peritoneal carcinomatosis (Erlich ascites carcinoma) | Intraperitoneal insufflation +/− RT | Mice | 60 3 groups: - RT - O3 - RT + O3 | O3 and RT increased the survival rates either separately or concurrently. |
| Kuroda K. et al. (2015) | Basic research In vivo | Rectal adenocarcinoma | Intratumoral injection of ozonated water | Mice | Not reported. 4 groups: - ? healthy - 5 control - 5 water - 6 O3 water | No changes in normal tissues of healthy mice with O3 water. Normal growth of cancer cells in control animals. No significant changes in water group. Significant changes in O3 in the water group. |
| Megele R. et al. (2018) | Case series | Glioblastoma: Primary (1) Recurrent (4) | Surgical re-resection + CT + intrathecal O3 | Patients | 5. 2 groups: - 1 primary - 4 recurrent | Increased survival rates compared to historical series not treated with O3. The patient treated immediately after the first surgery is alive and without recurrence. |
| Clavo B. et al. (2004) | Case-control series | Advanced head and neck tumors | RT + 5-FU RT + O3 (rectal insufflation, autohemotherapy) | Patients | 19. 2 groups: - 12 (RT + 5-FU) - 7 (RT + O3) | Same survival rates in both groups. |
| Borreli E. et al. (2012) | RCT single-blind | Lung cancer | CT CT + Viscum Alba (VA) + O3 (auto- hemotherapy) | Patients | 40. 2 groups: - 20 CT - 20 CT + O3 + VA | O3 and VA therapy was safe and seems to improve the quality of Life (QLQ30) in advanced lung cancer patients when used in association with CT. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baeza-Noci, J.; Pinto-Bonilla, R. Systemic Review: Ozone: A Potential New Chemotherapy. Int. J. Mol. Sci. 2021, 22, 11796. https://doi.org/10.3390/ijms222111796
Baeza-Noci J, Pinto-Bonilla R. Systemic Review: Ozone: A Potential New Chemotherapy. International Journal of Molecular Sciences. 2021; 22(21):11796. https://doi.org/10.3390/ijms222111796
Chicago/Turabian StyleBaeza-Noci, Jose, and Rosa Pinto-Bonilla. 2021. "Systemic Review: Ozone: A Potential New Chemotherapy" International Journal of Molecular Sciences 22, no. 21: 11796. https://doi.org/10.3390/ijms222111796
APA StyleBaeza-Noci, J., & Pinto-Bonilla, R. (2021). Systemic Review: Ozone: A Potential New Chemotherapy. International Journal of Molecular Sciences, 22(21), 11796. https://doi.org/10.3390/ijms222111796





