Modifier Role of Common RET Variants in Sporadic Medullary Thyroid Carcinoma
Abstract
:1. Introduction
2. Results
2.1. Association of Individual Variants with Sporadic MTC
2.2. Correlation of RET Variants with Clinical Data
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Genotyping
4.3. Statistics
4.4. Ethical Statement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wells, S.A.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association Guidelines for the Management of Medullary Thyroid Carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Machens, A.; Frank-Raue, K.; Lorenz, K.; Rondot, S.; Raue, F.; Dralle, H. Clinical Relevance of RET Variants G691S, L769L, S836S and S904S to Sporadic Medullary Thyroid Cancer. Clin. Endocrinol. 2012, 76, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner-Parzer, S.M.; Lang, R.; Wagner, L.; Heinze, G.; Niederle, B.; Kaserer, K.; Waldhäusl, W.; Vierhapper, H. Polymorphisms in Exon 13 and Intron 14 of the RET Protooncogene: Genetic Modifiers of Medullary Thyroid Carcinoma? J. Clin. Endocrinol. Metab. 2005, 90, 6232–6236. [Google Scholar] [CrossRef] [Green Version]
- Fugazzola, L.; Muzza, M.; Mian, C.; Cordella, D.; Barollo, S.; Alberti, L.; Cirello, V.; Dazzi, D.; Girelli, M.E.; Opocher, G.; et al. RET Genotypes in Sporadic Medullary Thyroid Cancer: Studies in a Large Italian Series. Clin. Endocrinol. 2008, 69, 418–425. [Google Scholar] [CrossRef]
- Weinhaeusel, A.; Scheuba, C.; Lauss, M.; Kriegner, A.; Kaserer, K.; Vierlinger, K.; Haas, O.A.; Niederle, B. The Influence of Gender, Age, and RET Polymorphisms on C-Cell Hyperplasia and Medullary Thyroid Carcinoma. Thyroid 2008, 18, 1269–1276. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving Access to Variant Interpretations and Supporting Evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef] [Green Version]
- Stenson, P.D.; Ball, E.V.; Mort, M.; Phillips, A.D.; Shiel, J.A.; Thomas, N.S.T.; Abeysinghe, S.; Krawczak, M.; Cooper, D.N. Human Gene Mutation Database (HGMD): 2003 Update. Hum. Mutat. 2003, 21, 577–581. [Google Scholar] [CrossRef]
- Costa, P.; Domingues, R.; Sobrinho, L.G.; Bugalho, M.J. RET Polymorphisms and Sporadic Medullary Thyroid Carcinoma in a Portuguese Population. Endocrine 2005, 27, 239–243. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Chen, X.; Huang, S.; Li, J. Quantitative Assessment of the Association between L769L and S836S Polymorphisms at RET Gene and Medullary Thyroid Carcinoma Risk. Tumour Biol. 2014, 35, 6641–6647. [Google Scholar] [CrossRef] [PubMed]
- Lantieri, F.; Caroli, F.; Ceccherini, I.; Griseri, P. The Involvement of the RET Variant G691S in Medullary Thyroid Carcinoma Enlightened by a Meta-Analysis Study. Int. J. Cancer 2013, 132, 2808–2819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceolin, L.; Siqueira, D.R.; Ferreira, C.V.; Romitti, M.; Maia, S.C.; Leiria, L.; Crispim, D.; Ashton-Prolla, P.; Prolla, P.A.; Maia, A.L. Additive Effect of RET Polymorphisms on Sporadic Medullary Thyroid Carcinoma Susceptibility and Tumor Aggressiveness. Eur. J. Endocrinol. 2012, 166, 847–854. [Google Scholar] [CrossRef] [Green Version]
- Cebrian, A.; Lesueur, F.; Martin, S.; Leyland, J.; Ahmed, S.; Luccarini, C.; Smith, P.L.; Luben, R.; Whittaker, J.; Pharoah, P.D.; et al. Polymorphisms in the Initiators of RET (Rearranged during Transfection) Signaling Pathway and Susceptibility to Sporadic Medullary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2005, 90, 6268–6274. [Google Scholar] [CrossRef] [Green Version]
- Ceolin, L.; Siqueira, D.R.; Romitti, M.; Ferreira, C.V.; Maia, A.L. Molecular Basis of Medullary Thyroid Carcinoma: The Role of RET Polymorphisms. Int. J. Mol. Sci. 2012, 13, 221–239. [Google Scholar] [CrossRef]
- Mishra, V.; Kowtal, P.; Rane, P.; Sarin, R. Modulatory Role of Single Nucleotide Polymorphisms of Distinct Genetic Pathways on Clinical Behavior of Medullary Thyroid Carcinoma. Asian Pac. J. Cancer Prev. 2020, 21, 1289–1293. [Google Scholar] [CrossRef]
- Tamanaha, R.; Camacho, C.P.; Pereira, A.C.; da Silva, A.M.Á.; Maciel, R.M.B.; Cerutti, J.M. Evaluation of RET Polymorphisms in a Six-Generation Family with G533C RET Mutation: Specific RET Variants May Modulate Age at Onset and Clinical Presentation. Clin. Endocrinol. 2009, 71, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Lebeault, M.; Pinson, S.; Guillaud-Bataille, M.; Gimenez-Roqueplo, A.-P.; Carrie, A.; Barbu, V.; Pigny, P.; Bezieau, S.; Rey, J.-M.; Delvincourt, C.; et al. Nationwide French Study of RET Variants Detected from 2003 to 2013 Suggests a Possible Influence of Polymorphisms as Modifiers. Thyroid 2017, 27, 1511–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueira, D.R.; Ceolin, L.; Ferreira, C.V.; Romitti, M.; Maia, S.C.; Maciel, L.M.Z.; Maia, A.L. Role of RET Genetic Variants in MEN2-Associated Pheochromocytoma. Eur. J. Endocrinol. 2014, 170, 821–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K. Thyroid. In AJCC Cancer Staging Manual; Amin, M.B., Edge, S., Greene, F., Byrd, D.R., Brookland, R.K., Washington, M.K., Gershenwald, J.E., Compton, C.C., Hess, K.R., Sullivan, D.C., et al., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 872–927. ISBN 978-3-319-40617-6. [Google Scholar]
- Gimm, O.; Neuberg, D.S.; Marsh, D.J.; Dahia, P.L.; Hoang-Vu, C.; Raue, F.; Hinze, R.; Dralle, H.; Eng, C. Over-Representation of a Germline RET Sequence Variant in Patients with Sporadic Medullary Thyroid Carcinoma and Somatic RET Codon 918 Mutation. Oncogene 1999, 18, 1369–1373. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, A.; Antiñolo, G.; Fernández, R.M.; Eng, C.; Marcos, I.; Borrego, S. Germline Sequence Variant S836S in the RET Proto-Oncogene Is Associated with Low Level Predisposition to Sporadic Medullary Thyroid Carcinoma in the Spanish Population. Clin. Endocrinol. 2001, 55, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-Ryś, M.; Ziemnicka, K.; Pławski, A.; Budny, B.; Michalak, M.; Hryhorowicz, S.; Hoppe-Gołębiewska, J.; Boruń, P.; Gołąb, M.; Czetwertyńska, M.; et al. Modifying Impact of RET Gene Haplotypes on Medullary Thyroid Carcinoma Clinical Course. Endocr. -Relat. Cancer 2018, 25, 421–436. [Google Scholar] [CrossRef]
- Gemignani, F.; Romei, C.; Ciampi, R.; Corrado, A.; Melaiu, O.; Figlioli, G.; Bonotti, A.; Foddis, R.; Cristaudo, A.; Pellegrini, G.; et al. Polymorphisms within the RET Proto-Oncogene and Risk of Sporadic Medullary Thyroid Carcinoma. Thyroid 2020, 30, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, D. The Human Genome Browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, V.; Kowtal, P.; Rane, P.; Sarin, R. Genetic Risk Association of CDKN1A and RET Gene SNPs with Medullary Thyroid Carcinoma: Results from the Largest MTC Cohort and Meta-Analysis. Cancer Med. 2019, 8, 6151–6161. [Google Scholar] [CrossRef] [Green Version]
- Robledo, M.; Gil, L.; Pollán, M.; Cebrián, A.; Ruíz, S.; Azañedo, M.; Benitez, J.; Menárguez, J.; Rojas, J.M. Polymorphisms G691S/S904S of RET as Genetic Modifiers of MEN 2A. Cancer Res. 2003, 63, 1814–1817. [Google Scholar]
- Borun, P.; Jerzy, S.; Ziemnicka, K.; Kubaszewski, L.; Lipinski, D.; Plawski, A. Absence of the RET+3:T Allele in the MTC Patients. Hered. Cancer Clin. Pract. 2012, 10, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and Visualization of LD and Haplotype Maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [Green Version]
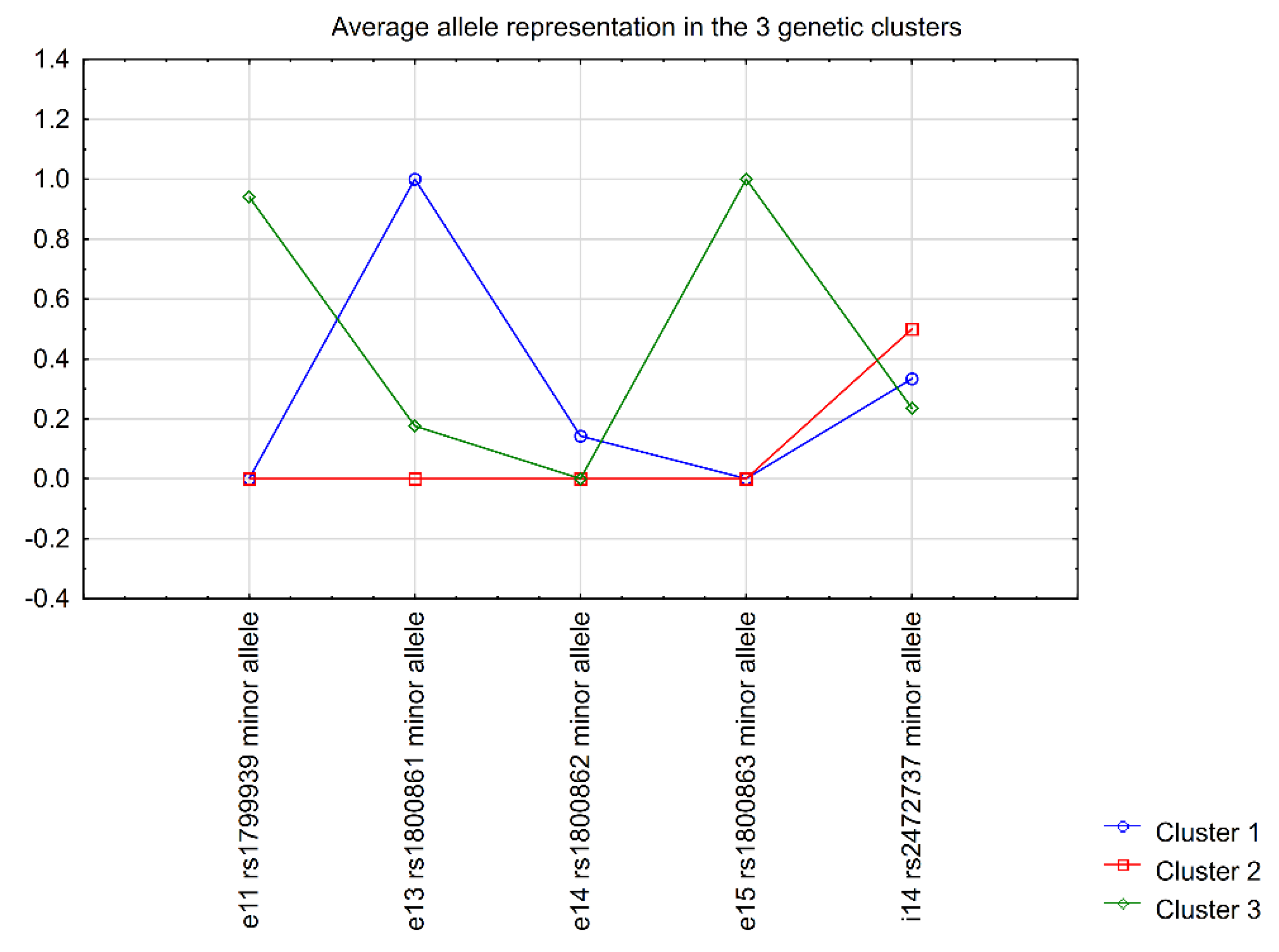

| Variant | MAF (%) Database 1 | MAF (%) MTC Group | MAF (%) Control Group | p for MTC vs. Control Group |
|---|---|---|---|---|
| e11 rs1799939 | 18.47 | 17.71 | 15.63 | 0.6990 |
| e13 rs1800861 | 23.32 | 28.13 | 34.38 | 0.3502 |
| e14 rs1800862 | 4.90 | 3.13 | 3.13 | 1.0000 |
| e15 rs1800863 | 18.58 | 18.75 | 15.63 | 0.5667 |
| i14 rs2472737 | 22.56 | 16.67 | 19.79 | 0.5756 |
| Haplotype | Control | MTC |
|---|---|---|
| GGCCG | 0.312 | 0.250 |
| GTCCG | 0.302 | 0.365 |
| GTCCA | 0.198 | 0.167 |
| ATCGG | 0.156 | 0.177 |
| GGTCG | 0.031 | 0.031 |
| GTCGG | 0.000 | 0.010 |
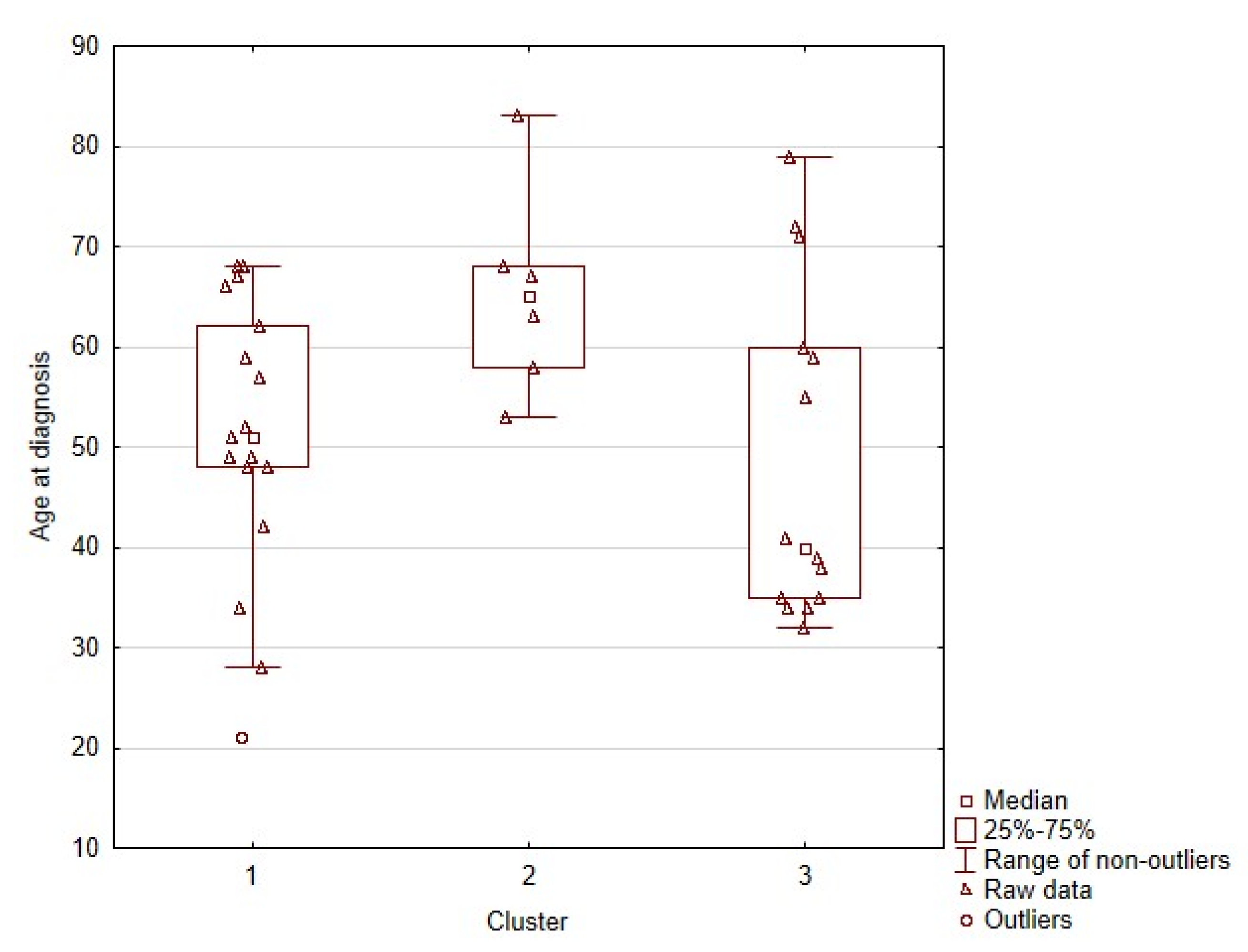
| Analysed Parameter | e11 rs1799939 * | e13 rs1800861 | e14 rs1800862 * | e15 rs1800863 * | i14 rs2472737 |
|---|---|---|---|---|---|
| Age at diagnosis | 0.0682 | 0.0162 | 0.3687 | 0.1258 | 0.0809 |
| Disease stage at diagnosis | 0.6747 | 0.8680 | 0.9261 | 0.6055 | 0.5249 |
| Lymph node metastases | 0.5804 | 0.5879 | 1.0000 | 0.5804 | 1.0000 |
| Distant metastases | 1.0000 | 0.2445 | 1.0000 | 1.0000 | 1.0000 |
| Relapses | 0.7394 | 1.0000 | 0.5716 | 0.5761 | 1.0000 |
| Remission state | 0.5594 | 0.0879 | 0.1429 | 0.5594 | 0.1429 |
| CEA concentrations | 0.6879 | 0.8208 | 0.7656 | 0.6671 | 0.8345 |
| Ct/proCt | 0.5938 | 0.9511 | 0.3711 | 0.7595 | 0.4034 |
| TSH concentrations | 0.5934 | 0.1590 | 0.0736 | 0.8541 | 0.0216 |
| Cluster 1 | Cluster 2 | Cluster 3 | p-Value | ||
|---|---|---|---|---|---|
| Other tumours in the patients | No | 11 | 6 | 11 | 0.5110 |
| Yes | 7 | 4 | 3 | ||
| Metabolic disorders in the patients | No | 17 | 6 | 10 | 0.0755 |
| Yes | 1 | 4 | 4 | ||
| Tumours (other than MTC) in first-degree relatives | No | 7 | 5 | 6 | 0.1578 |
| Yes | 6 | 0 | 5 | ||
| Metabolic disorders in first-degree relatives | No | 13 | 5 | 8 | 0.0647 |
| Yes | 0 | 0 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skalniak, A.; Trofimiuk-Müldner, M.; Przybylik-Mazurek, E.; Hubalewska-Dydejczyk, A. Modifier Role of Common RET Variants in Sporadic Medullary Thyroid Carcinoma. Int. J. Mol. Sci. 2021, 22, 11794. https://doi.org/10.3390/ijms222111794
Skalniak A, Trofimiuk-Müldner M, Przybylik-Mazurek E, Hubalewska-Dydejczyk A. Modifier Role of Common RET Variants in Sporadic Medullary Thyroid Carcinoma. International Journal of Molecular Sciences. 2021; 22(21):11794. https://doi.org/10.3390/ijms222111794
Chicago/Turabian StyleSkalniak, Anna, Małgorzata Trofimiuk-Müldner, Elwira Przybylik-Mazurek, and Alicja Hubalewska-Dydejczyk. 2021. "Modifier Role of Common RET Variants in Sporadic Medullary Thyroid Carcinoma" International Journal of Molecular Sciences 22, no. 21: 11794. https://doi.org/10.3390/ijms222111794
APA StyleSkalniak, A., Trofimiuk-Müldner, M., Przybylik-Mazurek, E., & Hubalewska-Dydejczyk, A. (2021). Modifier Role of Common RET Variants in Sporadic Medullary Thyroid Carcinoma. International Journal of Molecular Sciences, 22(21), 11794. https://doi.org/10.3390/ijms222111794








