The Entorhinal Cortex and Adult Neurogenesis in Major Depression
Abstract
:1. Introduction
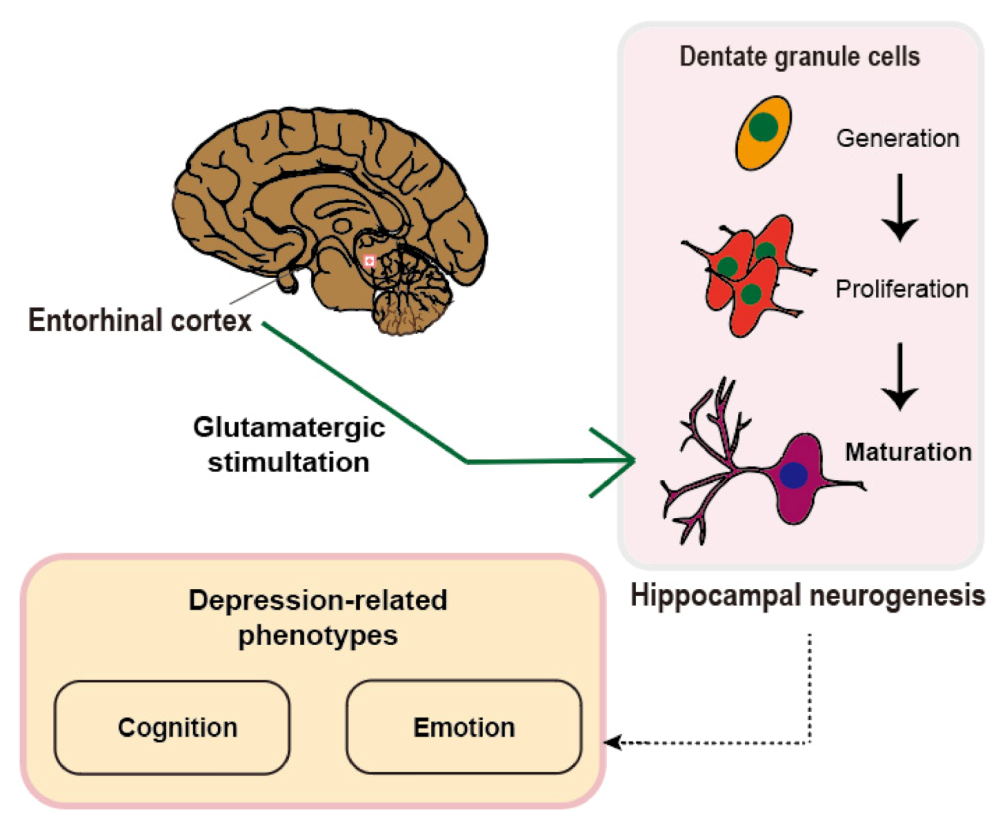
2. Entorhinal Cortex Involved in Regulation of Adult Neurogenesis
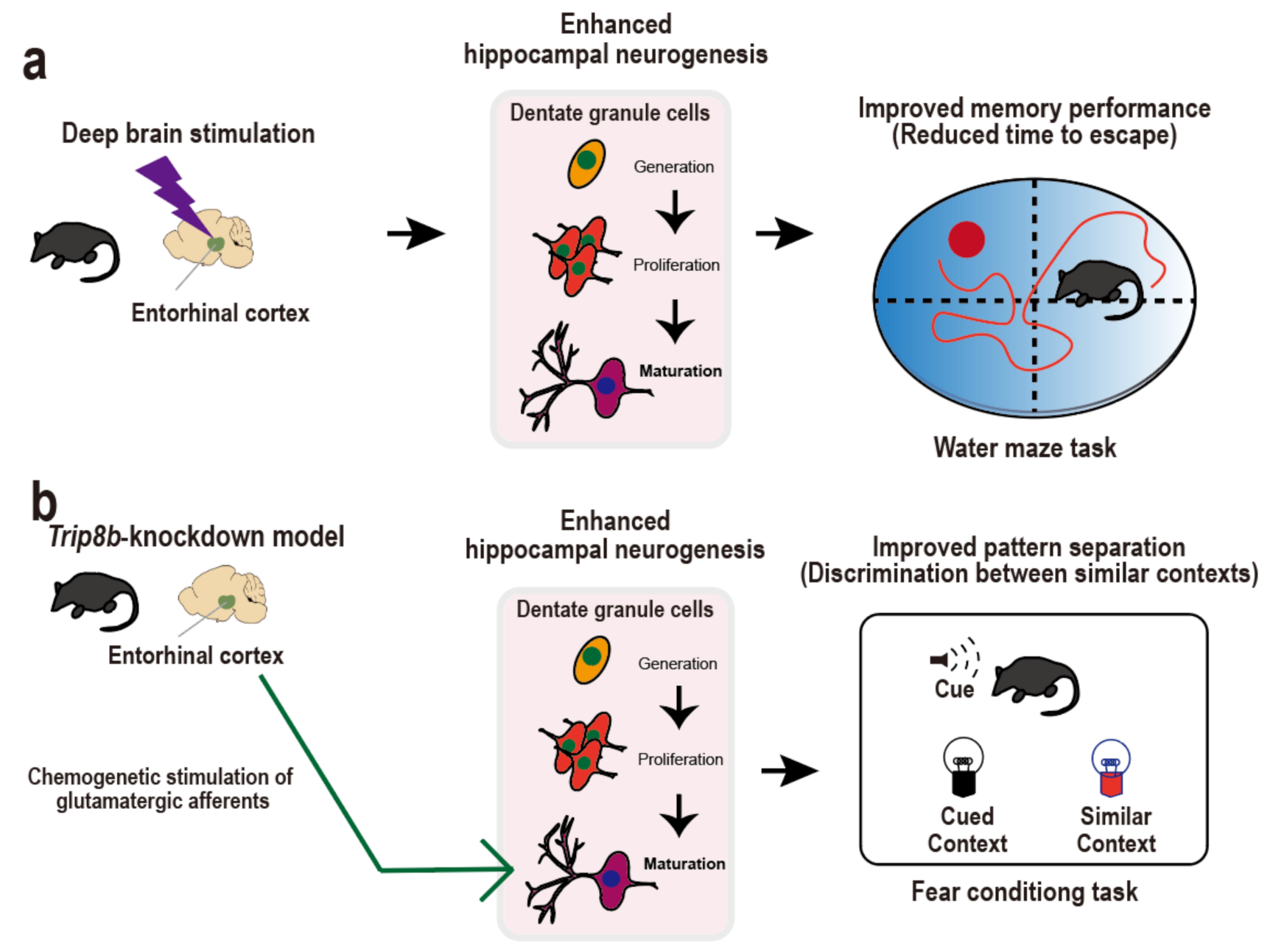
3. Entorhinal Cortex-Regulated Hippocampal Neurogenesis for Cognitive Performance
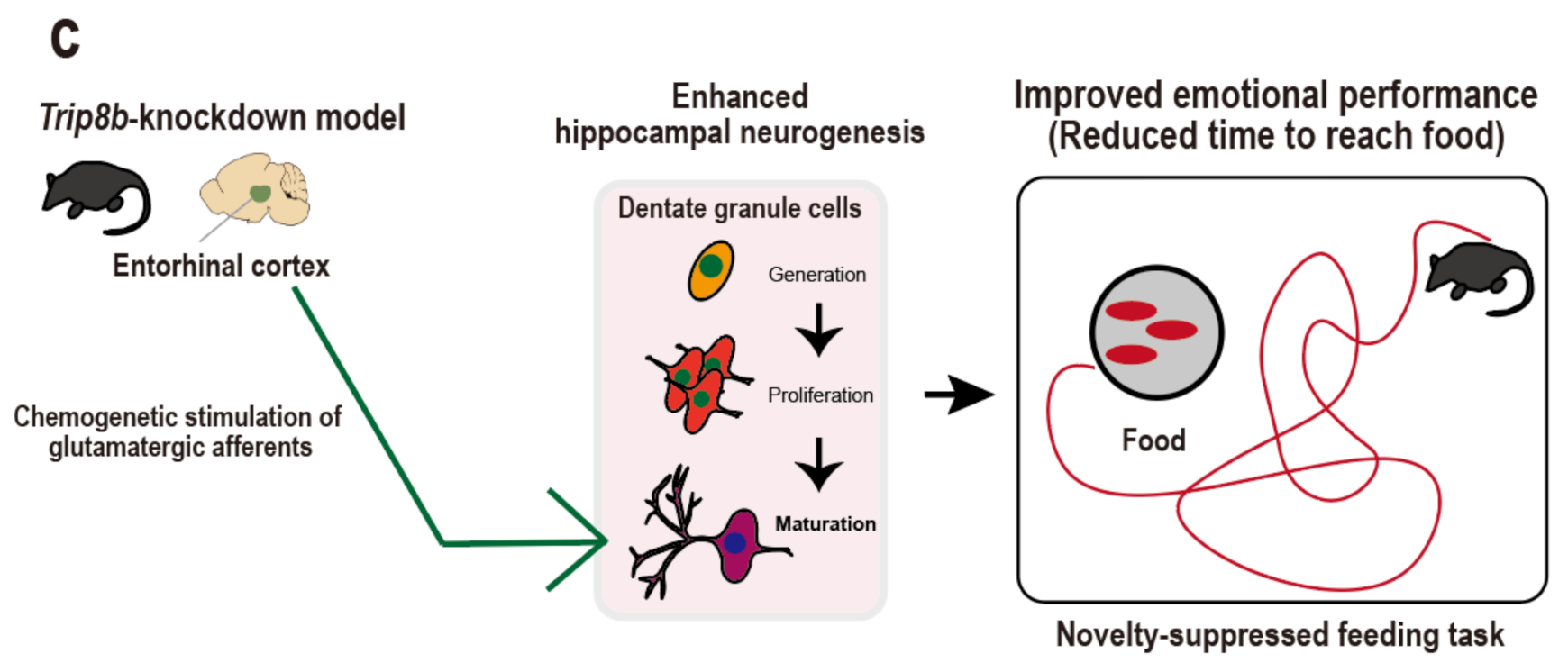
4. Entorhinal Cortex-Regulated Hippocampal Neurogenesis for Emotional Regulation
5. Suggestions for the Neural Circuitry-Neurogenesis Model of Depression
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hay, S.I.; Jayaraman, S.P.; Truelsen, T.; Sorensen, R.J.; Millear, A.; Giussani, G.; Beghi, E. GBD 2015 disease and injury incidence and prevalence collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the global burden of disease study 2015 (vol 388, pg 1545, 2016). Lancet 2017, 389, E1. [Google Scholar]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Trivedi, M.H. Modeling predictors, moderators and mediators of treatment outcome and resistance in depression. Biol. Psychiatry 2013, 74, 2–4. [Google Scholar] [CrossRef]
- Park, S.-C.; Shinfuku, N.; Maramis, M.M.; Lee, M.-S.; Park, Y.C. Adjunctive antipsychotic prescriptions for outpatients with depressive disorders in Asia: The research on Asian psychotropic prescription patterns for antidepressants (REAP-AD) study. Am. J. Psychiatry 2015, 172, 684–685. [Google Scholar] [CrossRef]
- Kupfer, D.J.; Frank, E.; Phillips, M.L. Major depressive disorder: New clinical, neurobiological, and treatment perspectives. Focus 2016, 14, 266–276. [Google Scholar] [CrossRef]
- McGrath, P.J.; Khan, A.Y.; Trivedi, M.H.; Stewart, J.W.; Morris, D.W.; Wisniewski, S.R.; Miyahara, S.; Nierenberg, A.A.; Fava, M.; Rush, A.J. Response to a selective serotonin reuptake inhibitor (citalopram) in major depressive disorder with melancholic features: A STAR* D report. J. Clin. Psychiatry 2008, 69, 1847–1855. [Google Scholar] [CrossRef]
- Insel, T.R. Next-generation treatments for mental disorders. Sci. Transl. Med. 2012, 4, 155ps19. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Park, S.-C. An alternative approach to future diagnostic standards for major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 105, 110133. [Google Scholar] [CrossRef]
- Park, S.-C.; Kim, Y.-K. Challenges and strategies for current classifications of depressive disorders: Proposal for future diagnostic standards. In Major Depressive Disorder: Rethinking and Understanding Recent Discoveries; Springer: Cham, Switzeralnd, 2021; pp. 103–116. [Google Scholar]
- Kim, I.B.; Park, S.-C. Machine learning-based definition of symptom clusters and selection of antidepressants for depressive syndrome. Diagnostics 2021, 11, 1631. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.R.; Hen, R. Adult hippocampal neurogenesis as target for the treatment of depression. CNS Neurol. Disord.-Drug Targets 2007, 6, 205–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.B.; Park, S.-C. Neural circuitry–neurogenesis coupling model of depression. Int. J. Mol. Sci. 2021, 22, 2468. [Google Scholar] [CrossRef]
- Gomes-Leal, W. Adult hippocampal neurogenesis and affective disorders: New neurons for psychic well-being. Front. Neurosci. 2021, 712, 4448. [Google Scholar] [CrossRef]
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility—linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Reynolds, R.P.; Masiulis, I.; Eisch, A.J. Re-evaluating the link between neuropsychiatric disorders and dysregulated adult neurogenesis. Nat. Med. 2016, 22, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Sahay, A.; Hen, R. Adult hippocampal neurogenesis in depression. Nat. Neurosci. 2007, 10, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Kronmüller, K.-T.; Pantel, J.; Köhler, S.; Victor, D.; Giesel, F.; Magnotta, V.A.; Mundt, C.; Essig, M.; Schröder, J. Hippocampal volume and 2-year outcome in depression. Br. J. Psychiatry 2008, 192, 472–473. [Google Scholar] [CrossRef] [Green Version]
- Kempermann, G. Regulation of adult hippocampal neurogenesis–implications for novel theories of major depression 1. Bipolar Disord. 2002, 4, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Olsen, R.H.; Sun, J.; Ming, G.-l.; Song, H. Neuronal circuitry mechanisms regulating adult mammalian neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.K.; Kim, W.R.; Ming, G.-l.; Song, H. Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann. N. Y. Acad. Sci. 2009, 1170, 664. [Google Scholar] [CrossRef] [Green Version]
- Stone, S.S.; Teixeira, C.M.; DeVito, L.M.; Zaslavsky, K.; Josselyn, S.A.; Lozano, A.M.; Frankland, P.W. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J. Neurosci. 2011, 31, 13469–13484. [Google Scholar] [CrossRef]
- Ronaghi, A.; Zibaii, M.I.; Pandamooz, S.; Nourzei, N.; Motamedi, F.; Ahmadiani, A.; Dargahi, L. Entorhinal cortex stimulation induces dentate gyrus neurogenesis through insulin receptor signaling. Brain Res. Bull. 2019, 144, 75–84. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, W.-H.; Wu, D.-L.; Zhang, K.; Zhang, J.-G. Behavioral effects of deep brain stimulation of the anterior nucleus of thalamus, entorhinal cortex and fornix in a rat model of Alzheimer’s disease. Chin. Med. J. 2015, 128, 1190. [Google Scholar] [CrossRef]
- Suthana, N.; Haneef, Z.; Stern, J.; Mukamel, R.; Behnke, E.; Knowlton, B.; Fried, I. Memory enhancement and deep-brain stimulation of the entorhinal area. N. Engl. J. Med. 2012, 366, 502–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, S.; Reynolds, R.P.; Petrof, I.; White, A.; Rivera, P.D.; Segev, A.; Gibson, A.D.; Suarez, M.; DeSalle, M.J.; Ito, N. Stimulation of entorhinal cortex–dentate gyrus circuitry is antidepressive. Nat. Med. 2018, 24, 658–666. [Google Scholar] [CrossRef]
- Claiborne, B.J.; Amaral, D.G.; Cowan, W.M. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J. Comp. Neurol. 1986, 246, 435–458. [Google Scholar] [CrossRef]
- Witter, M.P. The perforant path: Projections from the entorhinal cortex to the dentate gyrus. Prog. Brain Res. 2007, 163, 43–61. [Google Scholar] [PubMed]
- Köhler, C. Intrinsic connections of the retrohippocampal region in the rat brain. II. The medial entorhinal area. J. Comp. Neurol. 1986, 246, 149–169. [Google Scholar] [CrossRef]
- Li, Y.; Mu, Y.; Gage, F.H. Development of neural circuits in the adult hippocampus. Curr. Top. Dev. Biol. 2009, 87, 149–174. [Google Scholar]
- Kempermann, G.; Jessberger, S.; Steiner, B.; Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004, 27, 447–452. [Google Scholar] [CrossRef]
- Ge, S.; Sailor, K.A.; Ming, G.l.; Song, H. Synaptic integration and plasticity of new neurons in the adult hippocampus. J. Physiol. 2008, 586, 3759–3765. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Goh, E.L.; Sailor, K.A.; Kitabatake, Y.; Ming, G.-l.; Song, H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 2006, 439, 589–593. [Google Scholar] [CrossRef] [Green Version]
- Vivar, C.; Potter, M.C.; Choi, J.; Lee, J.-y.; Stringer, T.P.; Callaway, E.M.; Gage, F.H.; Suh, H.; Van Praag, H. Monosynaptic inputs to new neurons in the dentate gyrus. Nat. Commun. 2012, 3, 1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, S.; Yang, C.-h.; Hsu, K.-s.; Ming, G.-l.; Song, H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 2007, 54, 559–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt-Hieber, C.; Jonas, P.; Bischofberger, J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 2004, 429, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Warner-Schmidt, J.; Varela, S.; Enikolopov, G.; Greengard, P.; Flajolet, M. Norbin ablation results in defective adult hippocampal neurogenesis and depressive-like behavior in mice. Proc. Natl. Acad. Sci. USA 2015, 112, 9745–9750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; He, H.; Qiao, Y.; Zhou, T.; He, H.; Yi, S.; Zhang, L.; Mo, L.; Li, Y.; Jiang, W. Priming of microglia with IFN-γ impairs adult hippocampal neurogenesis and leads to depression-like behaviors and cognitive defects. Glia 2020, 68, 2674–2692. [Google Scholar] [CrossRef]
- Lee, M.M.; Reif, A.; Schmitt, A.G. Major depression: A role for hippocampal neurogenesis? In Behavioral Neurobiology of Depression and Its Treatment; Springer: Berlin/Heidelberg, Germany, 2012; pp. 153–179. [Google Scholar]
- Zakzanis, K.K.; Leach, L.; Kaplan, E. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol. Behav. Neurol. 1998, 11, 111–119. [Google Scholar]
- Austin, M.-P.; Mitchell, P.; Goodwin, G.M. Cognitive deficits in depression: Possible implications for functional neuropathology. Br. J. Psychiatry 2001, 178, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Bäckman, L.; Forsell, Y. Episodic memory functioning in a community-based sample of old adults with major depression: Utilization of cognitive support. J. Abnorm. Psychol. 1994, 103, 361. [Google Scholar] [CrossRef] [PubMed]
- Elderkin-Thompson, V.; Mintz, J.; Haroon, E.; Lavretsky, H.; Kumar, A. Executive dysfunction and memory in older patients with major and minor depression. Arch. Clin. Neuropsychol. 2007, 22, 261–270. [Google Scholar] [CrossRef]
- Burt, D.B.; Zembar, M.J.; Niederehe, G. Depression and memory impairment: A meta-analysis of the association, its pattern, and specificity. Psychol. Bull. 1995, 117, 285. [Google Scholar] [CrossRef]
- Söderlund, H.; Moscovitch, M.; Kumar, N.; Daskalakis, Z.J.; Flint, A.; Herrmann, N.; Levine, B. Autobiographical episodic memory in major depressive disorder. J. Abnorm. Psychol. 2014, 123, 51. [Google Scholar] [CrossRef] [Green Version]
- Murray, B.D.; Holland, A.C.; Kensinger, E.A. Episodic memory and emotion. In Handbook of Cognition and Emotion; The Guilford Press: New York, NY, USA, 2013; pp. 156–175. [Google Scholar]
- Lemogne, C.; Piolino, P.; Friszer, S.; Claret, A.; Girault, N.; Jouvent, R.; Allilaire, J.-F.; Fossati, P. Episodic autobiographical memory in depression: Specificity, autonoetic consciousness, and self-perspective. Conscious. Cognit. 2006, 15, 258–268. [Google Scholar] [CrossRef]
- Steffens, D.C.; Byrum, C.E.; McQuoid, D.R.; Greenberg, D.L.; Payne, M.E.; Blitchington, T.F.; MacFall, J.R.; Krishnan, K.R.R. Hippocampal volume in geriatric depression. Biol. Psychiatry 2000, 48, 301–309. [Google Scholar] [CrossRef]
- O’Brien, J.T.; Lloyd, A.; McKeith, I.; Gholkar, A.; Ferrier, N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am. J. Psychiatry 2004, 161, 2081–2090. [Google Scholar] [CrossRef]
- Lloyd, A.J.; Ferrier, I.N.; Barber, R.; Gholkar, A.; Young, A.H.; O’Brien, J.T. Hippocampal volume change in depression: Late-and early-onset illness compared. Br. J. Psychiatry 2004, 184, 488–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickie, I.; Naismith, S.; Ward, P.B.; Turner, K.; Scott, E.; Mitchell, P.; Wilhelm, K.; Parker, G. Reduced hippocampal volumes and memory loss in patients with early-and late-onset depression. Br. J. Psychiatry 2005, 186, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell-McGinty, S.; Butters, M.A.; Meltzer, C.C.; Greer, P.J.; Reynolds, C.F., III; Becker, J.T. Brain morphometric abnormalities in geriatric depression: Long-term neurobiological effects of illness duration. Am. J. Psychiatry 2002, 159, 1424–1427. [Google Scholar] [CrossRef]
- Déry, N.; Pilgrim, M.; Gibala, M.; Gillen, J.; Wojtowicz, J.M.; MacQueen, G.; Becker, S. Adult hippocampal neurogenesis reduces memory interference in humans: Opposing effects of aerobic exercise and depression. Front. Neurosci. 2013, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Aimone, J.B.; Gage, F.H. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010, 11, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Demic, S.; Cheng, S. The reduction of adult neurogenesis in depression impairs the retrieval of new as well as remote episodic memory. PLoS ONE 2018, 13, e0198406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, S.; Wojtowicz, J.M. A model of hippocampal neurogenesis in memory and mood disorders. Trends Cognit. Sci. 2007, 11, 70–76. [Google Scholar] [CrossRef]
- Tsao, A.; Sugar, J.; Lu, L.; Wang, C.; Knierim, J.J.; Moser, M.-B.; Moser, E.I. Integrating time from experience in the lateral entorhinal cortex. Nature 2018, 561, 57–62. [Google Scholar] [CrossRef]
- Suzuki, W.A.; Miller, E.K.; Desimone, R. Object and place memory in the macaque entorhinal cortex. J. Neurophysiol. 1997, 78, 1062–1081. [Google Scholar] [CrossRef]
- Kropff, E.; Carmichael, J.E.; Moser, M.-B.; Moser, E.I. Speed cells in the medial entorhinal cortex. Nature 2015, 523, 419–424. [Google Scholar] [CrossRef]
- Lipton, P.; Eichenbaum, H. Complementary roles of hippocampus and medial entorhinal cortex in episodic memory. Neural Plast. 2008, 2008, 92. [Google Scholar] [CrossRef] [Green Version]
- Sugar, J.; Moser, M.B. Episodic memory: Neuronal codes for what, where, and when. Hippocampus 2019, 29, 1190–1205. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Leutgeb, S.; Leutgeb, J.K. Spatial and memory circuits in the medial entorhinal cortex. Curr. Opin. Neurobiol. 2015, 32, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzsáki, G.; Moser, E.I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 2013, 16, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, J.; Miller, J.; Lee, S.A.; Coffey, T.; Watrous, A.J.; Sperling, M.R.; Sharan, A.; Worrell, G.; Berry, B.; Lega, B. Direct electrical stimulation of the human entorhinal region and hippocampus impairs memory. Neuron 2016, 92, 983–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kee, N.; Teixeira, C.M.; Wang, A.H.; Frankland, P.W. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci. 2007, 10, 355–362. [Google Scholar] [CrossRef]
- Stone, S.S.; Teixeira, C.M.; Zaslavsky, K.; Wheeler, A.L.; Martinez-Canabal, A.; Wang, A.H.; Sakaguchi, M.; Lozano, A.M.; Frankland, P.W. Functional convergence of developmentally and adult-generated granule cells in dentate gyrus circuits supporting hippocampus-dependent memory. Hippocampus 2011, 21, 1348–1362. [Google Scholar] [CrossRef]
- Robinson, N.T.; Priestley, J.B.; Rueckemann, J.W.; Garcia, A.D.; Smeglin, V.A.; Marino, F.A.; Eichenbaum, H. Medial entorhinal cortex selectively supports temporal coding by hippocampal neurons. Neuron 2017, 94, 677–688.e676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilssen, E.S.; Doan, T.P.; Nigro, M.J.; Ohara, S.; Witter, M.P. Neurons and networks in the entorhinal cortex: A reappraisal of the lateral and medial entorhinal subdivisions mediating parallel cortical pathways. Hippocampus 2019, 29, 1238–1254. [Google Scholar] [CrossRef] [Green Version]
- Eichenbaum, H.; Sauvage, M.; Fortin, N.; Komorowski, R.; Lipton, P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci. Biobehav. Rev. 2012, 36, 1597–1608. [Google Scholar] [CrossRef] [Green Version]
- Neunuebel, J.P.; Yoganarasimha, D.; Rao, G.; Knierim, J.J. Conflicts between local and global spatial frameworks dissociate neural representations of the lateral and medial entorhinal cortex. J. Neurosci. 2013, 33, 9246–9258. [Google Scholar] [CrossRef]
- Kerr, K.M.; Agster, K.L.; Furtak, S.C.; Burwell, R.D. Functional neuroanatomy of the parahippocampal region: The lateral and medial entorhinal areas. Hippocampus 2007, 17, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Fyhn, M.; Molden, S.; Witter, M.P.; Moser, E.I.; Moser, M.-B. Spatial representation in the entorhinal cortex. Science 2004, 305, 1258–1264. [Google Scholar] [CrossRef] [Green Version]
- Rolls, E. The mechanisms for pattern completion and pattern separation in the hippocampus. Front. Syst. Neurosci. 2013, 7, 74. [Google Scholar] [CrossRef] [Green Version]
- Leutgeb, J.K.; Leutgeb, S.; Moser, M.-B.; Moser, E.I. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 2007, 315, 961–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakker, A.; Kirwan, C.B.; Miller, M.; Stark, C.E. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science 2008, 319, 1640–1642. [Google Scholar] [CrossRef] [Green Version]
- Yassa, M.A.; Stark, C.E. Pattern separation in the hippocampus. Trends Neurosci. 2011, 34, 515–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clelland, C.; Choi, M.; Romberg, C.; Clemenson, G.D.; Fragniere, A.; Tyers, P.; Jessberger, S.; Saksida, L.; Barker, R.; Gage, F. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 2009, 325, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Gandy, K.; Kim, S.; Sharp, C.; Dindo, L.; Maletic-Savatic, M.; Calarge, C. Pattern separation: A potential marker of impaired hippocampal adult neurogenesis in major depressive disorder. Front. Neurosci. 2017, 11, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahay, A.; Scobie, K.N.; Hill, A.S.; O’Carroll, C.M.; Kheirbek, M.A.; Burghardt, N.S.; Fenton, A.A.; Dranovsky, A.; Hen, R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 2011, 472, 466–470. [Google Scholar] [CrossRef] [Green Version]
- Berron, D.; Schütze, H.; Maass, A.; Cardenas-Blanco, A.; Kuijf, H.J.; Kumaran, D.; Düzel, E. Strong evidence for pattern separation in human dentate gyrus. J. Neurosci. 2016, 36, 7569–7579. [Google Scholar] [CrossRef]
- Gerritsen, L.; Comijs, H.C.; van der Graaf, Y.; Knoops, A.J.; Penninx, B.W.; Geerlings, M.I. Depression, hypothalamic pituitary adrenal axis, and hippocampal and entorhinal cortex volumes—the SMART Medea study. Biol. Psychiatry 2011, 70, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.K.; Yeung, L.-K.; Noly-Gandon, A.; D’Angelo, M.C.; Kacollja, A.; Smith, V.M.; Ryan, J.D.; Barense, M.D. Human anterolateral entorhinal cortex volumes are associated with cognitive decline in aging prior to clinical diagnosis. Neurobiol. Aging 2017, 57, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, B.C.; Feczko, E.; Augustinack, J.C.; Pacheco, J.; Morris, J.C.; Fischl, B.; Buckner, R.L. Differential effects of aging and Alzheimer’s disease on medial temporal lobe cortical thickness and surface area. Neurobiol. Aging 2009, 30, 432–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reagh, Z.M.; Noche, J.A.; Tustison, N.J.; Delisle, D.; Murray, E.A.; Yassa, M.A. Functional imbalance of anterolateral entorhinal cortex and hippocampal dentate/CA3 underlies age-related object pattern separation deficits. Neuron 2018, 97, 1187–1198.e1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reagh, Z.M.; Yassa, M.A. Object and spatial mnemonic interference differentially engage lateral and medial entorhinal cortex in humans. Proc. Natl. Acad. Sci. USA 2014, 111, E4264–E4273. [Google Scholar] [CrossRef] [Green Version]
- Maurer, A.P.; Johnson, S.A.; Hernandez, A.R.; Reasor, J.; Cossio, D.M.; Fertal, K.E.; Mizell, J.M.; Lubke, K.N.; Clark, B.J.; Burke, S.N. Age-related changes in lateral entorhinal and CA3 neuron allocation predict poor performance on object discrimination. Front. Syst. Neurosci. 2017, 11, 49. [Google Scholar] [CrossRef] [Green Version]
- Leal, S.L.; Noche, J.A.; Murray, E.A.; Yassa, M.A. Disruption of amygdala–entorhinal–hippocampal network in late-life depression. Hippocampus 2017, 27, 464–476. [Google Scholar] [CrossRef] [Green Version]
- Kheirbek, M.A.; Tannenholz, L.; Hen, R. NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. J. Neurosci. 2012, 32, 8696–8702. [Google Scholar] [CrossRef]
- Wu, M.V.; Hen, R. Functional dissociation of adult-born neurons along the dorsoventral axis of the dentate gyrus. Hippocampus 2014, 24, 751–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niibori, Y.; Yu, T.-S.; Epp, J.R.; Akers, K.G.; Josselyn, S.A.; Frankland, P.W. Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region. Nat. Commun. 2012, 3, 1253. [Google Scholar] [CrossRef] [Green Version]
- Tronel, S.; Belnoue, L.; Grosjean, N.; Revest, J.M.; Piazza, P.V.; Koehl, M.; Abrous, D.N. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus 2012, 22, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Mahar, I.; Bambico, F.R.; Mechawar, N.; Nobrega, J.N. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 2014, 38, 173–192. [Google Scholar] [CrossRef]
- Paizanis, E.; Hamon, M.; Lanfumey, L. Hippocampal neurogenesis, depressive disorders, and antidepressant therapy. Neural Plast. 2007, 2007, 073754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, M.-m.; Lin, W.-j.; Pan, Y.-q.; Guan, X.-t.; Li, Y.-c. Hippocampal neurogenesis dysfunction linked to depressive-like behaviors in a neuroinflammation induced model of depression. Physiol. Behav. 2016, 161, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Sahay, A.; Drew, M.R.; Hen, R. Dentate gyrus neurogenesis and depression. Prog. Brain Res. 2007, 163, 697–822. [Google Scholar]
- Kubera, M.; Obuchowicz, E.; Goehler, L.; Brzeszcz, J.; Maes, M. In animal models, psychosocial stress-induced (neuro) inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Chesnokova, V.; Pechnick, R.N.; Wawrowsky, K. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav. Immun. 2016, 58, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, M.L.; Brachman, R.A.; Martinowich, K.; Schloesser, R.J.; Herkenham, M. Glucocorticoids orchestrate divergent effects on mood through adult neurogenesis. J. Neurosci. 2013, 33, 2961–2972. [Google Scholar] [CrossRef] [Green Version]
- Taliaz, D.; Stall, N.; Dar, D.; Zangen, A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol. Psychiatry 2010, 15, 80–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.; Son, H. Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep. 2009, 42, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-C. Neurogenesis and antidepressant action. Cell Tissue Res. 2019, 377, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Padberg, F.; George, M.S. Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. Exp. Neurol. 2009, 219, 2–13. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Rubio, B.; Pallardó, F.; Catalá, M.D. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet 1996, 348, 233–237. [Google Scholar] [CrossRef]
- Boggio, P.S.; Bermpohl, F.; Vergara, A.O.; Muniz, A.L.; Nahas, F.H.; Leme, P.B.; Rigonatti, S.P.; Fregni, F. Go-no-go task performance improvement after anodal transcranial DC stimulation of the left dorsolateral prefrontal cortex in major depression. J. Affect. Disord. 2007, 101, 91–98. [Google Scholar] [CrossRef]
- George, M.S.; Nahas, Z.; Molloy, M.; Speer, A.M.; Oliver, N.C.; Li, X.-B.; Arana, G.W.; Risch, S.C.; Ballenger, J.C. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol. Psychiatry 2000, 48, 962–970. [Google Scholar] [CrossRef]
- Li, X.; Nahas, Z.; Kozel, F.A.; Anderson, B.; Bohning, D.E.; George, M.S. Acute left prefrontal transcranial magnetic stimulation in depressed patients is associated with immediately increased activity in prefrontal cortical as well as subcortical regions. Biol. Psychiatry 2004, 55, 882–890. [Google Scholar] [CrossRef]
- Nahas, Z.; Teneback, C.C.; Kozel, A.; Speer, A.M.; DeBrux, C.; Molloy, M.; Stallings, L.; Spicer, K.M.; Arana, G.; Bohning, D.E. Brain effects of TMS delivered over prefrontal cortex in depressed adults: Role of stimulation frequency and coil–cortex distance. J. Neuropsychiatry Clin. Neurosci. 2001, 13, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Levkovitz, Y.; Harel, E.V.; Roth, Y.; Braw, Y.; Most, D.; Katz, L.N.; Sheer, A.; Gersner, R.; Zangen, A. Deep transcranial magnetic stimulation over the prefrontal cortex: Evaluation of antidepressant and cognitive effects in depressive patients. Brain Stimulat. 2009, 2, 188–200. [Google Scholar] [CrossRef]
- Hamani, C.; Diwan, M.; Macedo, C.E.; Brandão, M.L.; Shumake, J.; Gonzalez-Lima, F.; Raymond, R.; Lozano, A.M.; Fletcher, P.J.; Nobrega, J.N. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol. Psychiatry 2010, 67, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Boggio, P.S.; Nitsche, M.A.; Marcolin, M.A.; Rigonatti, S.P.; Pascual-Leone, A. Treatment of major depression with transcranial direct current stimulation. Bipolar Disord. 2006, 8, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Kito, S.; Fujita, K.; Koga, Y. Regional cerebral blood flow changes after low-frequency transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in treatment-resistant depression. Neuropsychobiology 2008, 58, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Voineskos, D.; Blumberger, D.M.; Zomorrodi, R.; Rogasch, N.C.; Farzan, F.; Foussias, G.; Rajji, T.K.; Daskalakis, Z.J. Altered transcranial magnetic stimulation–electroencephalographic markers of inhibition and excitation in the dorsolateral prefrontal cortex in major depressive disorder. Biol. Psychiatry 2019, 85, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Randver, R. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex to alleviate depression and cognitive impairment associated with Parkinson’s disease: A review and clinical implications. J. Neurol. Sci. 2018, 393, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.; McLoughlin, D.M.; O’Connell, R.; Bogue, J.; O’Connor, S.; McHugh, C.; Glennon, M. Anodal transcranial direct current stimulation of the left dorsolateral prefrontal cortex enhances emotion recognition in depressed patients and controls. J. Clin. Exp. Neuropsychol. 2017, 39, 384–395. [Google Scholar] [CrossRef]
- Philip, N.S.; Barredo, J.; van’t Wout-Frank, M.; Tyrka, A.R.; Price, L.H.; Carpenter, L.L. Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biol. Psychiatry 2018, 83, 263–272. [Google Scholar] [CrossRef]
- Baeken, C.; Marinazzo, D.; Everaert, H.; Wu, G.-R.; Van Hove, C.; Audenaert, K.; Goethals, I.; De Vos, F.; Peremans, K.; De Raedt, R. The impact of accelerated HF-rTMS on the subgenual anterior cingulate cortex in refractory unipolar major depression: Insights from 18FDG PET brain imaging. Brain Stimulat. 2015, 8, 808–815. [Google Scholar] [CrossRef]
- Boes, A.D.; Uitermarkt, B.D.; Albazron, F.M.; Lan, M.J.; Liston, C.; Pascual-Leone, A.; Dubin, M.J.; Fox, M.D. Rostral anterior cingulate cortex is a structural correlate of repetitive TMS treatment response in depression. Brain Stimulat. 2018, 11, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Berlim, M.T.; McGirr, A.; Van den Eynde, F.; Fleck, M.P.; Giacobbe, P. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: A systematic review and exploratory meta-analysis. J. Affect. Disord. 2014, 159, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Johansen-Berg, H.; Gutman, D.; Behrens, T.; Matthews, P.; Rushworth, M.; Katz, E.; Lozano, A.; Mayberg, H. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb. Cortex 2008, 18, 1374–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, A.M.; Mayberg, H.S.; Giacobbe, P.; Hamani, C.; Craddock, R.C.; Kennedy, S.H. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol. Psychiatry 2008, 64, 461–467. [Google Scholar] [CrossRef]
- Riva-Posse, P.; Choi, K.S.; Holtzheimer, P.E.; McIntyre, C.C.; Gross, R.E.; Chaturvedi, A.; Crowell, A.L.; Garlow, S.J.; Rajendra, J.K.; Mayberg, H.S. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol. Psychiatry 2014, 76, 963–969. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.S.; Riva-Posse, P.; Gross, R.E.; Mayberg, H.S. Mapping the “depression switch” during intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA Neurol. 2015, 72, 1252–1260. [Google Scholar] [CrossRef] [Green Version]
- Jing, Y.; Zhao, N.; Deng, X.P.; Feng, Z.J.; Huang, G.F.; Meng, M.; Zang, Y.F.; Wang, J. Pregenual or subgenual anterior cingulate cortex as potential effective region for brain stimulation of depression. Brain Behav. 2020, 10, e01591. [Google Scholar] [CrossRef]
- Bewernick, B.H.; Hurlemann, R.; Matusch, A.; Kayser, S.; Grubert, C.; Hadrysiewicz, B.; Axmacher, N.; Lemke, M.; Cooper-Mahkorn, D.; Cohen, M.X. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol. Psychiatry 2010, 67, 110–116. [Google Scholar] [CrossRef]
- Grubert, C.; Hurlemann, R.; Bewernick, B.H.; Kayser, S.; Hadrysiewicz, B.; Axmacher, N.; Sturm, V.; Schlaepfer, T.E. Neuropsychological safety of nucleus accumbens deep brain stimulation for major depression: Effects of 12-month stimulation. World J. Biol. Psychiatry 2011, 12, 516–527. [Google Scholar] [CrossRef]
- Bewernick, B.H.; Kayser, S.; Sturm, V.; Schlaepfer, T.E. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: Evidence for sustained efficacy. Neuropsychopharmacology 2012, 37, 1975–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauczyciel, C.; Robic, S.; Dondaine, T.; Verin, M.; Robert, G.; Drapier, D.; Naudet, F.; Millet, B. The nucleus accumbens: A target for deep brain stimulation in resistant major depressive disorder. J. Mol. Psychiatry 2013, 1, 17. [Google Scholar] [CrossRef] [Green Version]
- Falowski, S.M.; Sharan, A.; Reyes, B.A.; Sikkema, C.; Szot, P.; Van Bockstaele, E.J. An evaluation of neuroplasticity and behavior after deep brain stimulation of the nucleus accumbens in an animal model of depression. Neurosurgery 2011, 69, 1281–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tronnier, V.M.; Rasche, D.; Thorns, V.; Alvarez-Fischer, D.; Münte, T.F.; Zurowski, B. Massive weight loss following deep brain stimulation of the nucleus accumbens in a depressed woman. Neurocase 2018, 24, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, F.; Nicolini, H.; Lozano, A.M.; Piedimonte, F.; Salín, R.; Velasco, F. Electrical stimulation of the inferior thalamic peduncle in the treatment of major depression and obsessive compulsive disorders. World Neurosurg. 2013, 80, S30.e17–S30.e25. [Google Scholar] [CrossRef]
- Jiménez, F.; Velasco, F.; Salin-Pascual, R.; Hernández, J.A.; Velasco, M.; Criales, J.L.; Nicolini, H. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery 2005, 57, 585–593. [Google Scholar] [CrossRef]
- Velasco, M.; Velasco, F.; Jiménez, F.; Carrillo-Ruiz, J.D.; Velasco, A.L.; Salín-Pascual, R. Electrocortical and behavioral responses elicited by acute electrical stimulation of inferior thalamic peduncle and nucleus reticularis thalami in a patient with major depression disorder. Clin. Neurophysiol. 2006, 117, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Andy, O.J.; Jurko, F. Thalamic stimulation effects on reactive depression. Stereotact. Funct. Neurosurg. 1987, 50, 324–329. [Google Scholar] [CrossRef]
- Magdaleno-Madrigal, V.M.; Pantoja-Jiménez, C.R.; Bazaldúa, A.; Fernández-Mas, R.; Almazán-Alvarado, S.; Bolaños-Alejos, F.; Ortíz-López, L.; Ramírez-Rodriguez, G.B. Acute deep brain stimulation in the thalamic reticular nucleus protects against acute stress and modulates initial events of adult hippocampal neurogenesis. Behav. Brain Res. 2016, 314, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, D.D.; Rezai, A.R.; Carpenter, L.L.; Howland, R.H.; Bhati, M.T.; O’Reardon, J.P.; Eskandar, E.N.; Baltuch, G.H.; Machado, A.D.; Kondziolka, D. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol. Psychiatry 2015, 78, 240–248. [Google Scholar] [CrossRef]
- Malone Jr, D.A.; Dougherty, D.D.; Rezai, A.R.; Carpenter, L.L.; Friehs, G.M.; Eskandar, E.N.; Rauch, S.L.; Rasmussen, S.A.; Machado, A.G.; Kubu, C.S. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol. Psychiatry 2009, 65, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Pogarell, O.; Koch, W.; Pöpperl, G.; Tatsch, K.; Jakob, F.; Zwanzger, P.; Mulert, C.; Rupprecht, R.; Möller, H.-J.; Hegerl, U. Striatal dopamine release after prefrontal repetitive transcranial magnetic stimulation in major depression: Preliminary results of a dynamic [123I] IBZM SPECT study. J. Psychiatr. Res. 2006, 40, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kubu, C.S.; Malone, D.A.; Chelune, G.; Malloy, P.; Rezai, A.R.; Frazier, T.; Machado, A.; Rasmussen, S.; Friehs, G.; Greenberg, B.D. Neuropsychological outcome after deep brain stimulation in the ventral capsule/ventral striatum for highly refractory obsessive-compulsive disorder or major depression. Stereotact. Funct. Neurosurg. 2013, 91, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Kubu, C.S.; Brelje, T.; Butters, M.A.; Deckersbach, T.; Malloy, P.; Moberg, P.; Tröster, A.I.; Williamson, E.; Baltuch, G.H.; Bhati, M.T. Cognitive outcome after ventral capsule/ventral striatum stimulation for treatment-resistant major depression. J. Neurol. Neurosurg. Psychiatry 2017, 88, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Lozano, A.M.; Lipsman, N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron 2013, 77, 406–424. [Google Scholar] [CrossRef] [Green Version]
- Kjelstrup, K.G.; Tuvnes, F.A.; Steffenach, H.-A.; Murison, R.; Moser, E.I.; Moser, M.-B. Reduced fear expression after lesions of the ventral hippocampus. Proc. Natl. Acad. Sci. USA 2002, 99, 10825–10830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanselow, M.S.; Dong, H.-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Stone, E.A.; Lin, Y. An anti-immobility effect of exogenous corticosterone in mice. Eur. J. Pharmacol. 2008, 580, 135–142. [Google Scholar] [CrossRef]
- Murray, F.; Smith, D.W.; Hutson, P.H. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur. J. Pharmacol. 2008, 583, 115–127. [Google Scholar] [CrossRef]
- Casanova, E.; Fehsenfeld, S.; Mantamadiotis, T.; Lemberger, T.; Greiner, E.; Stewart, A.; Schütz, G. A CamKIIα iCre BAC allows brain-specific gene inactivation. Genesis 2001, 31, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Han, M.-H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; LaPlant, Q.; Graham, A.; Lutter, M.; Lagace, D.C. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Cameron, H.A.; McEwen, B.S.; Gould, E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J. Neurosci. 1995, 15, 4687–4692. [Google Scholar] [CrossRef] [PubMed]
- Toni, N.; Schinder, A.F. Maturation and functional integration of new granule cells into the adult hippocampus. Cold Spring Harb. Perspect. Biol. 2016, 8, a018903. [Google Scholar] [CrossRef] [Green Version]
- Schlett, K. Glutamate as a modulator of embryonic and adult neurogenesis. Curr. Top. Med. Chem. 2006, 6, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Melzer, S.; Michael, M.; Caputi, A.; Eliava, M.; Fuchs, E.C.; Whittington, M.A.; Monyer, H. Long-range–projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science 2012, 335, 1506–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-T.; Kao, M.-H.; Hou, W.-H.; Wei, Y.-T.; Chen, C.-L.; Lien, C.-C. Causal evidence for the role of specific GABAergic interneuron types in entorhinal recruitment of dentate granule cells. Sci. Rep. 2016, 6, 36885. [Google Scholar] [CrossRef] [PubMed]
- Colgin, L.L. Theta–gamma coupling in the entorhinal–hippocampal system. Curr. Opin. Neurobiol. 2015, 31, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna, V.M.; Anacker, C.; Burghardt, N.S.; Khandaker, H.; Andreu, V.; Millette, A.; Leary, P.; Ravenelle, R.; Jimenez, J.C.; Mastrodonato, A. Adult-born hippocampal neurons bidirectionally modulate entorhinal inputs into the dentate gyrus. Science 2019, 364, 578–583. [Google Scholar] [CrossRef]
- Acsady, L.; Kali, S. Models, structure, function: The transformation of cortical signals in the dentate gyrus. Prog. Brain Res. 2007, 163, 577–599. [Google Scholar]
- Woods, N.I.; Stefanini, F.; Apodaca-Montano, D.L.; Tan, I.M.; Biane, J.S.; Kheirbek, M.A. The dentate gyrus classifies cortical representations of learned stimuli. Neuron 2020, 107, 173–184.e176. [Google Scholar] [CrossRef]
- Liu, A.; Jain, N.; Vyas, A.; Lim, L.W. Ventromedial prefrontal cortex stimulation enhances memory and hippocampal neurogenesis in the middle-aged rats. eLife 2015, 4, e04803. [Google Scholar] [CrossRef] [PubMed]
- Kirby, E.D.; Friedman, A.R.; Covarrubias, D.; Ying, C.; Sun, W.G.; Goosens, K.A.; Sapolsky, R.M.; Kaufer, D. Basolateral amygdala regulation of adult hippocampal neurogenesis and fear-related activation of newborn neurons. Mol. Psychiatry 2012, 17, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Chamaa, F.; Sweidan, W.; Nahas, Z.; Saade, N.; Abou-Kheir, W. Thalamic stimulation in awake rats induces neurogenesis in the hippocampal formation. Brain Stimul. 2016, 9, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.; Bregman, T.; Voget, M.; Raymond, R.; Hadar, R.; Nobrega, J.N.; Hamani, C. Acute high frequency stimulation of the prefrontal cortex or nucleus accumbens does not increase hippocampal neurogenesis in rats. J. Psychiatr. Res. 2015, 68, 27–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, I.B.; Park, S.-C. The Entorhinal Cortex and Adult Neurogenesis in Major Depression. Int. J. Mol. Sci. 2021, 22, 11725. https://doi.org/10.3390/ijms222111725
Kim IB, Park S-C. The Entorhinal Cortex and Adult Neurogenesis in Major Depression. International Journal of Molecular Sciences. 2021; 22(21):11725. https://doi.org/10.3390/ijms222111725
Chicago/Turabian StyleKim, Il Bin, and Seon-Cheol Park. 2021. "The Entorhinal Cortex and Adult Neurogenesis in Major Depression" International Journal of Molecular Sciences 22, no. 21: 11725. https://doi.org/10.3390/ijms222111725
APA StyleKim, I. B., & Park, S.-C. (2021). The Entorhinal Cortex and Adult Neurogenesis in Major Depression. International Journal of Molecular Sciences, 22(21), 11725. https://doi.org/10.3390/ijms222111725






