Tetraspanins: Host Factors in Viral Infections
Abstract
1. Introduction
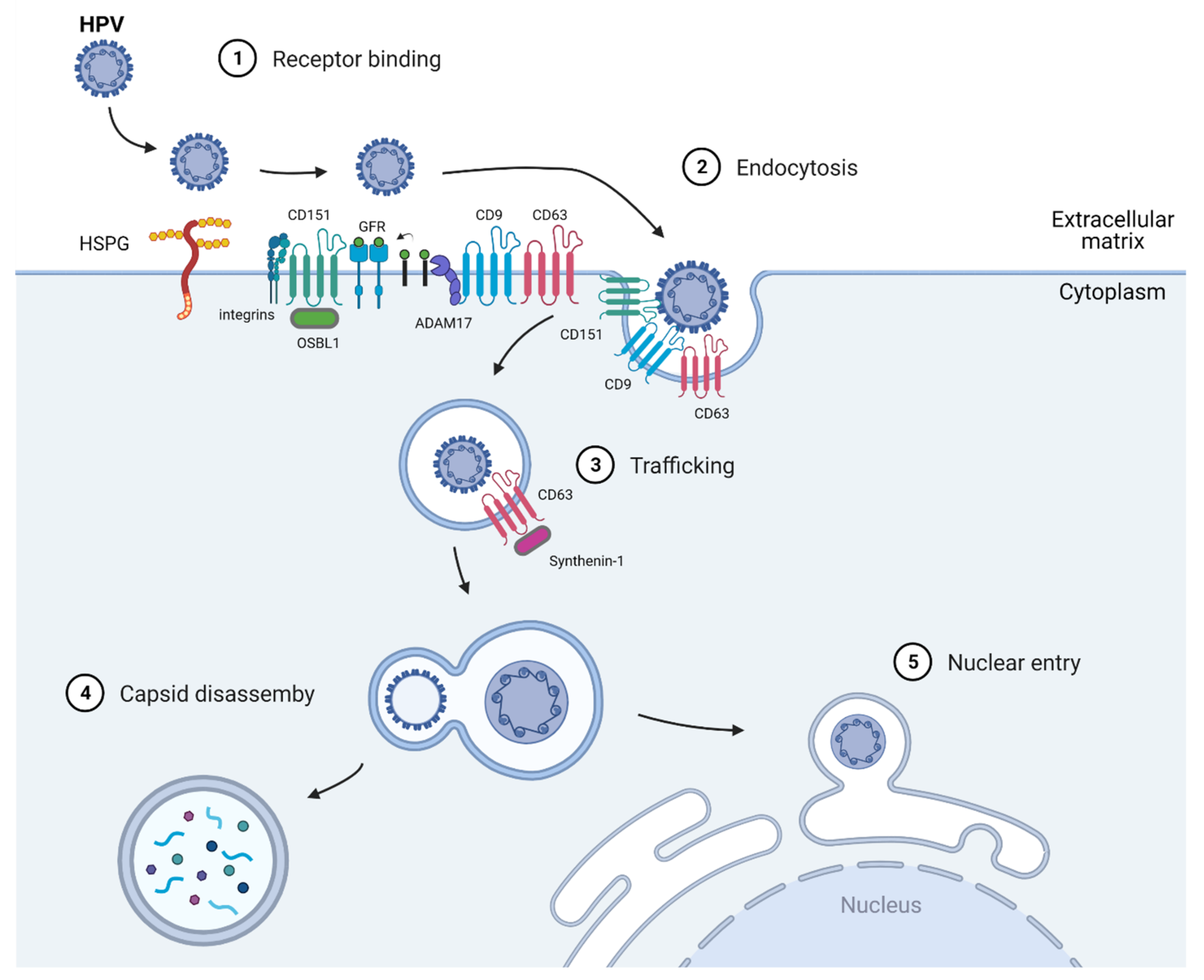
2. Human Papillomavirus (HPV)
2.1. Virus Entry
2.2. Virus Trafficking
2.3. Nuclear Entry and Replication
3. Human Immunodeficiency Virus (HIV)
3.1. Virus Entry
3.2. Transcription/Replication
3.3. Assembly and 3.4 Budding/Egress
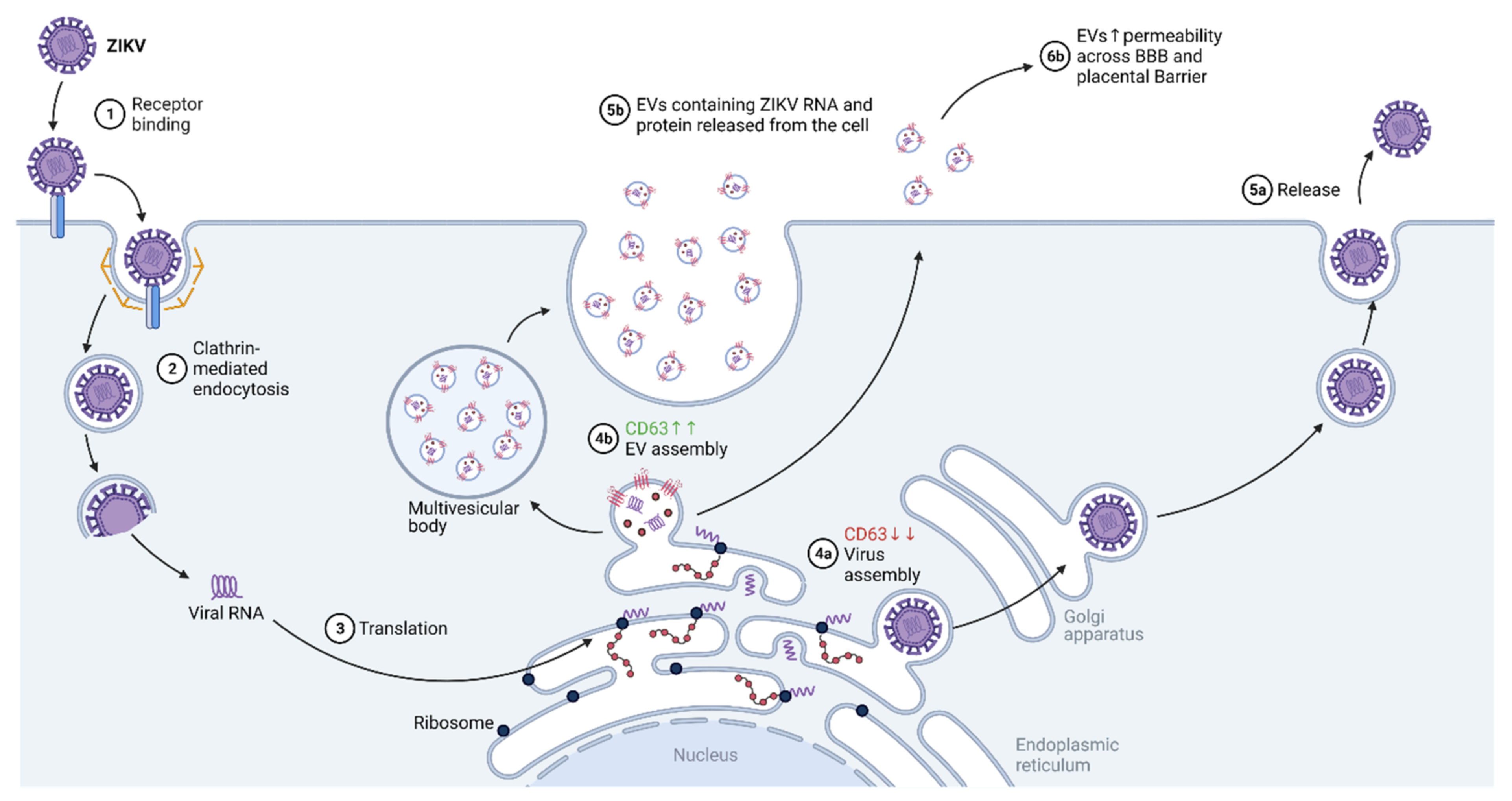
4. Zika Virus (ZIKV)
4.1. Virus Entry
4.2. Transcription/Replication
4.3. Assembly
4.4. Budding/Egress
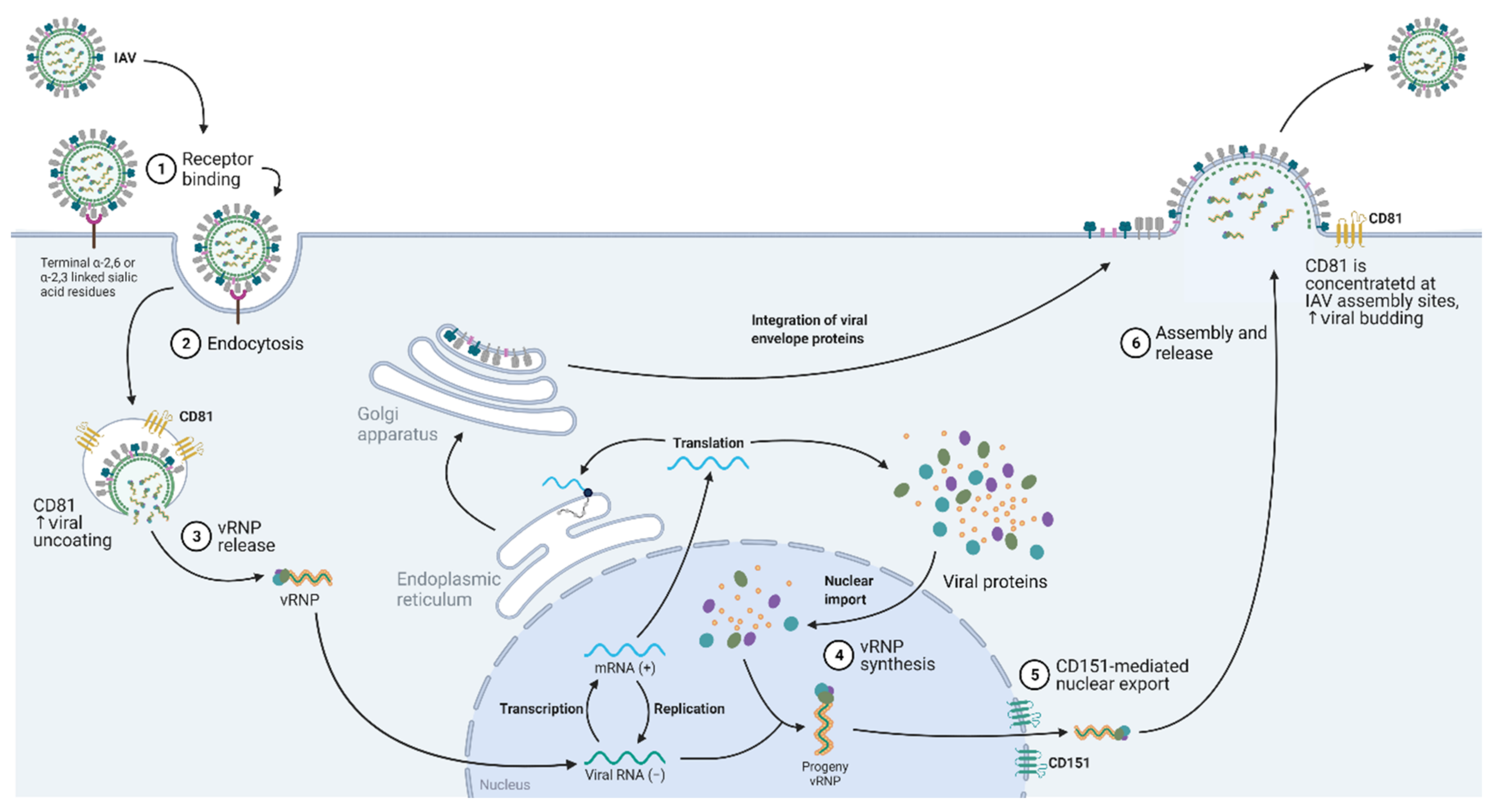
5. Influenza A Virus (IAV)
5.1. Virus Entry
5.2. Nuclear Entry and Export
5.3. Virus Budding
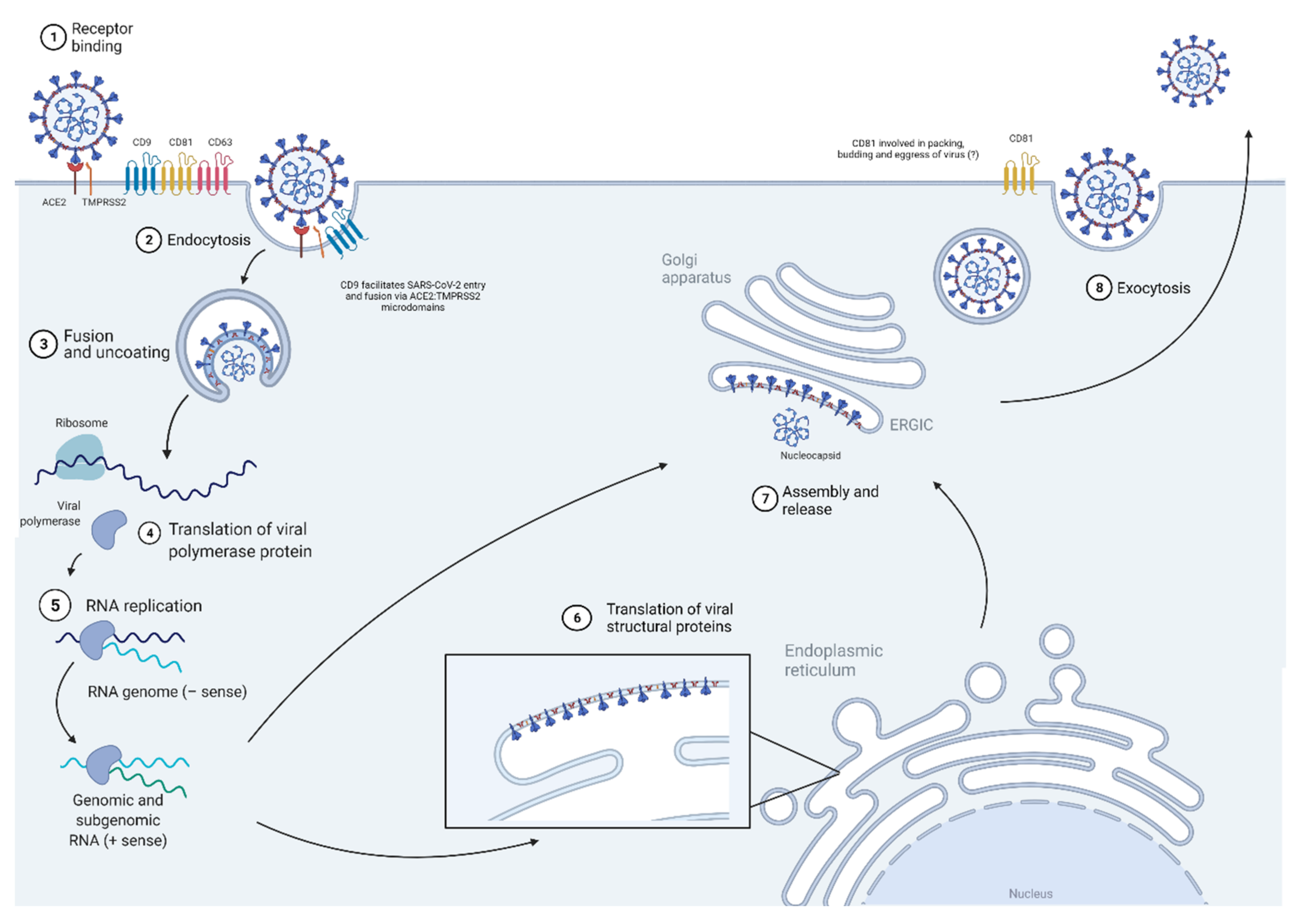
6. Coronavirus (CoV)
6.1. Virus Entry
6.2. Transcription/Replication
6.3. Assembly and 6.4 Budding/Egress
7. Utility of Tetraspanins in Viral Disease and Future Outlook as a Target for Viral Infection
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hemler, M.E. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 2005, 6, 801–811. [Google Scholar] [CrossRef]
- Robert, J.-M.H.; Amoussou, N.G.; Le Mai, H.; Logé, C.; Brouard, S. Tetraspanins: Useful multifunction proteins for the possible design and development of small-molecule therapeutic tools. Drug Discov. Today 2020, 26, 56–68. [Google Scholar] [CrossRef]
- Liu, L.; He, B.; Liu, W.M.; Zhou, D.; Cox, J.V.; Zhang, X.A. Tetraspanin CD151 Promotes Cell Migration by Regulating Integrin Trafficking. J. Biol. Chem. 2007, 282, 31631–31642. [Google Scholar] [CrossRef] [PubMed]
- Giroglou, T.; Florin, L.; Schäfer, F.; Streeck, R.E.; Sapp, M. Human Papillomavirus Infection Requires Cell Surface Heparan Sulfate. J. Virol. 2001, 75, 1565–1570. [Google Scholar] [CrossRef]
- Hemler, M.E. Tetraspanin Proteins Mediate Cellular Penetration, Invasion, and Fusion Events and Define a Novel Type of Membrane Microdomain. Annu. Rev. Cell Dev. Biol. 2003, 19, 397–422. [Google Scholar] [CrossRef]
- Stipp, C.S. Laminin-binding integrins and their tetraspanin partners as potential antimetastatic targets. Expert Rev. Mol. Med. 2010, 12, e3. [Google Scholar] [CrossRef]
- Lazo, P.A. Functional implications of tetraspanin proteins in cancer biology. Cancer Sci. 2007, 98, 1666–1677. [Google Scholar] [CrossRef]
- Tarrant, J.M.; Robb, L.; van Spriel, A.; Wright, M. Tetraspanins: Molecular organisers of the leukocyte surface. Trends Immunol. 2003, 24, 610–617. [Google Scholar] [CrossRef]
- Wright, M.; Moseley, G.; van Spriel, A. Tetraspanin microdomains in immune cell signalling and malignant disease. Tissue Antigens 2004, 64, 533–542. [Google Scholar] [CrossRef]
- Wong, A.H.; Tran, T. CD151 in Respiratory Diseases. Front. Cell Dev. Biol. 2020, 8, 64. [Google Scholar] [CrossRef]
- Zona, L.; Tawar, R.G.; Zeisel, M.B.; Xiao, F.; Schuster, C.; Lupberger, J.; Baumert, T.F. CD81-Receptor Associations—Impact for Hepatitis C Virus Entry and Antiviral Therapies. Viruses 2014, 6, 875–892. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Heal. 2019, 8, e191–e203. [Google Scholar] [CrossRef]
- Bosch, F.X.; Lorincz, A.; Munoz, N.; Meijer, C.J.L.M.; Shah, K.V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Human Papillomavirus (HPV) Vaccination: What Everyone Should Know. Available online: https://www.cdc.gov/vaccines/vpd/hpv/public/index.html (accessed on 1 September 2021).
- Zheng, Z.-M. Papillomavirus genome structure, expression, and post-transcriptional regulation. Front. Biosci. 2006, 11, 2286–2302. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J.; Gallimore, P.H. Identification of proteins encoded by the L1 and L2 open reading frames of human papillomavirus 1a. J. Virol. 1987, 61, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
- Culp, T.D.; Budgeon, L.R.; Marinkovich, M.P.; Meneguzzi, G.; Christensen, N.D. Keratinocyte-Secreted Laminin 5 Can Function as a Transient Receptor for Human Papillomaviruses by Binding Virions and Transferring Them to Adjacent Cells. J. Virol. 2006, 80, 8940–8950. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.G.; Tung, J.-S.; Przysiecki, C.T.; Cook, J.C.; Lehman, E.D.; Sands, J.A.; Jansen, K.U.; Keller, P.M. The L1 Major Capsid Protein of Human Papillomavirus Type 11 Recombinant Virus-like Particles Interacts with Heparin and Cell-surface Glycosaminoglycans on Human Keratinocytes. J. Biol. Chem. 1999, 274, 5810–5822. [Google Scholar] [CrossRef]
- Abban, C.Y.; Meneses, P.I. Usage of heparan sulfate, integrins, and FAK in HPV16 infection. Virology 2010, 403, 1–16. [Google Scholar] [CrossRef]
- Spoden, G.; Freitag, K.; Husmann, M.; Boller, K.; Sapp, M.; Lambert, C.; Florin, L. Clathrin- and Caveolin-Independent Entry of Human Papillomavirus Type 16—Involvement of Tetraspanin-Enriched Microdomains (TEMs). PLoS ONE 2008, 3, e3313. [Google Scholar] [CrossRef]
- Surviladze, Z.; Dziduszko, A.; Ozbun, M.A. Essential Roles for Soluble Virion-Associated Heparan Sulfonated Proteoglycans and Growth Factors in Human Papillomavirus Infections. PLOS Pathog. 2012, 8, e1002519. [Google Scholar] [CrossRef]
- Scheffer, K.D.; Gawlitza, A.; Spoden, G.A.; Zhang, X.A.; Lambert, C.; Berditchevski, F.; Florin, L. Tetraspanin CD151 Mediates Papillomavirus Type 16 Endocytosis. J. Virol. 2013, 87, 3435–3446. [Google Scholar] [CrossRef]
- Wüstenhagen, E.; Hampe, L.; Boukhallouk, F.; Schneider, M.A.; Spoden, G.A.; Negwer, I.; Koynov, K.; Kast, W.M.; Florin, L. The Cytoskeletal Adaptor Obscurin-Like 1 Interacts with the Human Papillomavirus 16 (HPV16) Capsid Protein L2 and Is Required for HPV16 Endocytosis. J. Virol. 2016, 90, 10629–10641. [Google Scholar] [CrossRef]
- Mikuličić, S.; Fritzen, A.; Scheffer, K.; Strunk, J.; Cabañas, C.; Sperrhacke, M.; Reiss, K.; Florin, L. Tetraspanin CD9 affects HPV16 infection by modulating ADAM17 activity and the ERK signalling pathway. Med. Microbiol. Immunol. 2020, 209, 461–471. [Google Scholar] [CrossRef]
- Gräßel, L.; Fast, L.A.; Scheffer, K.D.; Boukhallouk, F.; Spoden, G.A.; Tenzer, S.; Boller, K.; Bago, R.; Rajesh, S.; Overduin, M.; et al. The CD63-Syntenin-1 Complex Controls Post-Endocytic Trafficking of Oncogenic Human Papillomaviruses. Sci. Rep. 2016, 6, 1–18. [Google Scholar] [CrossRef]
- Siddiqa, A.; Broniarczyk, J.; Banks, L. Papillomaviruses and Endocytic Trafficking. Int. J. Mol. Sci. 2018, 19, 2619. [Google Scholar] [CrossRef]
- Day, P.M.; Lowy, D.R.; Schiller, J.T. Heparan Sulfate-Independent Cell Binding and Infection with Furin-Precleaved Papillomavirus Capsids. J. Virol. 2008, 82, 12565–12568. [Google Scholar] [CrossRef] [PubMed]
- Pyeon, D.; Pearce, S.M.; Lank, S.M.; Ahlquist, P.; Lambert, P.F. Establishment of Human Papillomavirus Infection Requires Cell Cycle Progression. PLoS Pathog. 2009, 5, e1000318. [Google Scholar] [CrossRef]
- Reinson, T.; Henno, L.; Toots, M.; Ustav, M. The Cell Cycle Timing of Human Papillomavirus DNA Replication. PLoS ONE 2015, 10, e0131675. [Google Scholar] [CrossRef] [PubMed]
- Suárez, H.; Rocha-Perugini, V.; Álvarez, S.; Yáñez-Mó, M. Tetraspanins, Another Piece in the HIV-1 Replication Puzzle. Front. Immunol. 2018, 9, 1811. [Google Scholar] [CrossRef]
- Vanangamudi, M.; Nair, P.C.; Engels, S.E.M.; Palaniappan, S.; Namasivayam, V. Structural Insights to Human Immunodeficiency Virus (HIV-1) Targets and Their Inhibition. In Antiviral Drug Discovery and Development; Liu, X., Zhan, P., Menéndez-Arias, L., Poongavanam, V., Eds.; Springer: Singapore, 2021; pp. 63–95. [Google Scholar]
- Aberg, J.A.; Kaplan, J.E.; Libman, H.; Emmanuel, P.; Anderson, J.R.; Stone, V.E.; Oleske, J.M.; Currier, J.S.; Gallant, J.E. Primary Care Guidelines for the Management of Persons Infected with Human Immunodeficiency Virus: 2009 Update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 49, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Khalid, K.; Padda, J.; Khedr, A.; Ismail, D.; Zubair, U.; Al-Ewaidat, O.A.; Padda, S.; Cooper, A.C.; Jean-Charles, G. HIV and Messenger RNA Vaccine. Cureus 2021, 13. [Google Scholar] [CrossRef]
- World Health Organization. HIV/AIDS. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 22 August 2021).
- Stein, B.S.; Gowda, S.D.; Lifson, J.D.; Penhallow, R.C.; Bensch, K.G.; Engleman, E.G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell 1987, 49, 659–668. [Google Scholar] [CrossRef]
- Gartner, S.; Markovits, P.; Markovitz, D.M.; Betts, R.F.; Popovic, M. Virus Isolation From and Identification of HTLV-III/LAV-Producing Cells in Brain Tissue From a Patient With AIDS. JAMA 1986, 256, 2365–2371. [Google Scholar] [CrossRef]
- Patterson, S.; Knight, S.C. Susceptibility of Human Peripheral Blood Dendritic Cells to Infection by Human Immunodeficiency Virus. J. Gen. Virol. 1987, 68, 1177–1181. [Google Scholar] [CrossRef]
- Shaw, G.M.; Broder, S.; Essex, M.; Gallo, R.C. Human T-cell leukemia virus: Its discovery and role in leukemogenesis and immunosuppression. Adv. Intern. Med. 1984, 30, 1–27. [Google Scholar]
- Ensoli, B.; Moretti, S.; Borsetti, A.; Maggiorella, M.T.; Buttò, S.; Picconi, O.; Tripiciano, A.; Sgadari, C.; Monini, P.; Cafaro, A. New insights into pathogenesis point to HIV-1 Tat as a key vaccine target. Arch. Virol. 2021, 166, 2955–2974. [Google Scholar] [CrossRef]
- Stewart-Jones, G.B.; Soto, C.; Lemmin, T.; Chuang, G.-Y.; Druz, A.; Kong, R.; Thomas, P.V.; Wagh, K.; Zhou, T.; Behrens, A.-J.; et al. Trimeric HIV-1-Env Structures Define Glycan Shields from Clades A, B, and G. Cell 2016, 165, 813–826. [Google Scholar] [CrossRef]
- Wagh, K.; Hahn, B.H.; Korber, B. Hitting the sweet spot. Curr. Opin. HIV AIDS 2020, 15, 267–274. [Google Scholar] [CrossRef]
- Gray, G.E.; Huang, Y.; Grunenberg, N.; Laher, F.; Roux, S.; Andersen-Nissen, E.; De Rosa, S.C.; Flach, B.; Randhawa, A.K.; Jensen, R.; et al. Immune correlates of the Thai RV144 HIV vaccine regimen in South Africa. Sci. Transl. Med. 2019, 11, eaax1880. [Google Scholar] [CrossRef]
- Robb, M.L.; Rerks-Ngarm, S.; Nitayaphan, S.; Pitisuttithum, P.; Kaewkungwal, J.; Kunasol, P.; Khamboonruang, C.; Thongcharoen, P.; Morgan, P.; Benenson, M.; et al. Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: A post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect. Dis. 2012, 12, 531–537. [Google Scholar] [CrossRef]
- Lee, J.H.; Crotty, S. HIV vaccinology: 2021 update. Semin. Immunol. 2021, 51, 101470. [Google Scholar] [CrossRef] [PubMed]
- Archin, N.M.; Sung, J.M.; Garrido, C.; Soriano-Sarabia, N.; Margolis, D.M. Eradicating HIV-1 infection: Seeking to clear a persistent pathogen. Nat. Rev. Genet. 2014, 12, 750–764. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.A.; Januário, Y.C.; Dasilva, L.L.P. HIV-1 Hijacking of Host ATPases and GTPases That Control Protein Trafficking. Front. Cell Dev. Biol. 2021, 9, 1718. [Google Scholar] [CrossRef] [PubMed]
- Gulick, R.M.; Flexner, C. Long-Acting HIV Drugs for Treatment and Prevention. Annu. Rev. Med. 2019, 70, 137–150. [Google Scholar] [CrossRef]
- Ménager, M.M. TSPAN7, effector of actin nucleation required for dendritic cell-mediated transfer of HIV-1 to T cells. Biochem. Soc. Trans. 2017, 45, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, N.C.; Masters, T.A.; Sheetz, M.P. Mechanical feedback between membrane tension and dynamics. Trends Cell Biol. 2012, 22, 527–535. [Google Scholar] [CrossRef]
- Kaur, S.; Fielding, A.B.; Gassner, G.; Carter, N.J.; Royle, S.J. An unmet actin requirement explains the mitotic inhibition of clathrin-mediated endocytosis. eLife 2014, 3, e00829. [Google Scholar] [CrossRef]
- Ménager, M.; Littman, D.R. Actin Dynamics Regulates Dendritic Cell-Mediated Transfer of HIV-1 to T Cells. Cell 2016, 164, 695–709. [Google Scholar] [CrossRef]
- Rocha-Perugini, V.; Gordon-Alonso, M.; Sánchez-Madrid, F. PIP2: Choreographer of actin-adaptor proteins in the HIV-1 dance. Trends Microbiol. 2014, 22, 379–388. [Google Scholar] [CrossRef][Green Version]
- Wiley, R.D.; Gummuluru, S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc. Natl. Acad. Sci. USA 2006, 103, 738–743. [Google Scholar] [CrossRef]
- Madison, M.N.; Okeoma, C.M. Exosomes: Implications in HIV-1 Pathogenesis. Viruses 2015, 7, 4093–4118. [Google Scholar] [CrossRef]
- Sims, B.; Farrow, A.L.; Williams, S.D.; Bansal, A.; Krendelchtchikov, A.; Gu, L.; Matthews, Q.L. Role of TIM-4 in exosome-dependent entry of HIV-1 into human immune cells. Int. J. Nanomed. 2017, 12, 4823–4833. [Google Scholar] [CrossRef] [PubMed]
- Sims, B.; Farrow, A.L.; Williams, S.D.; Bansal, A.; Krendelchtchikov, A.; Matthews, Q.L. Tetraspanin blockage reduces exosome-mediated HIV-1 entry. Arch. Virol. 2018, 163, 1683–1689. [Google Scholar] [CrossRef]
- Gordon, M.; Yáñez-Mó, M.; Barreiro, O.; Álvarez, S.; Muñoz-Fernández, M.; Valenzuela-Fernández, A.; Sánchez-Madrid, F. Tetraspanins CD9 and CD81 Modulate HIV-1-Induced Membrane Fusion. J. Immunol. 2006, 177, 5129–5137. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Yamashita, S.; Higashiyama, S.; Nakagawa, T.; Shimomura, I.; Funahashi, T.; Kameda-Takemura, K.; Kawata, S.; Taniguchi, N.; et al. Role of membrane-anchored heparin-binding epidermal growth factor-like growth factor and CD9 on macrophages. Biochem. J. 1997, 328, 923–928. [Google Scholar] [CrossRef]
- Imai, T.; Yoshie, O. C33 antigen and M38 antigen recognized by monoclonal antibodies inhibitory to syncytium formation by human T cell leukemia virus type 1 are both members of the transmembrane 4 superfamily and associate with each other and with CD4 or CD8 in T cells. J. Immunol. 1993, 151, 6470–6481. [Google Scholar] [PubMed]
- Imai, T.; Kakizaki, M.; Nishimura, M.; Yoshie, O. Molecular analyses of the association of CD4 with two members of the transmembrane 4 superfamily, CD81 and CD82. J. Immunol. 1995, 155, 1229–1239. [Google Scholar]
- Fournier, M.; Peyrou, M.; Bourgoin, L.; Maeder, C.; Tchou, I.; Foti, M. CD4 dimerization requires two cysteines in the cytoplasmic domain of the molecule and occurs in microdomains distinct from lipid rafts. Mol. Immunol. 2010, 47, 2594–2603. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kawano, Y.; Sato, K.; Ando, Y.; Aoki, J.; Miura, Y.; Komano, J.; Tanaka, Y.; Koyanagi, Y. A CD63 Mutant Inhibits T-cell Tropic Human Immunodeficiency Virus Type 1 Entry by Disrupting CXCR4 Trafficking to the Plasma Membrane. Traffic 2008, 9, 540–558. [Google Scholar] [CrossRef]
- Yoshida, T.; Ebina, H.; Koyanagi, Y. N-linked glycan-dependent interaction of CD63 with CXCR4 at the Golgi apparatus induces downregulation of CXCR4. Microbiol. Immunol. 2009, 53, 629–635. [Google Scholar] [CrossRef]
- Li, G.; Endsley, M.A.; Somasunderam, A.; Gbota, S.L.; I Mbaka, M.; Murray, J.L.; Ferguson, M.R. The dual role of tetraspanin CD63 in HIV-1 replication. Virol. J. 2014, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Piguet, V.; Sattentau, Q. Dangerous liaisons at the virological synapse. J. Clin. Investig. 2004, 114, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Jolly, C.; Sattentau, Q.J. Human Immunodeficiency Virus Type 1 Assembly, Budding, and Cell-Cell Spread in T Cells Take Place in Tetraspanin-Enriched Plasma Membrane Domains. J. Virol. 2007, 81, 7873–7884. [Google Scholar] [CrossRef]
- Ivanusic, D.; Madela, K.; Bannert, N.; Denner, J. The large extracellular loop of CD63 interacts with gp41 of HIV-1 and is essential for establishing the virological synapse. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Weng, J.; Krementsov, D.; Khurana, S.; Roy, N.H.; Thali, M. Formation of Syncytia Is Repressed by Tetraspanins in Human Immunodeficiency Virus Type 1-Producing Cells. J. Virol. 2009, 83, 7467–7474. [Google Scholar] [CrossRef]
- Ferri, K.F.; Jacotot, E.; Geuskens, M.; Kroemer, G. Apoptosis and karyogamy in syncytia induced by the HIV-1-envelope glycoprotein complex. Cell Death Differ. 2000, 7, 1137–1139. [Google Scholar] [CrossRef]
- Ferri, K.F.; Jacotot, E.; Leduc, P.; Geuskens, M.; E Ingber, D.; Kroemer, G. Apoptosis of Syncytia Induced by the HIV-1–Envelope Glycoprotein Complex: Influence of Cell Shape and Size. Exp. Cell Res. 2000, 261, 119–126. [Google Scholar] [CrossRef]
- Shin, Y.H.; Park, C.M.; Yoon, C.-H. An Overview of Human Immunodeficiency Virus-1 Antiretroviral Drugs: General Principles and Current Status. Infect. Chemother. 2021, 53, 29–45. [Google Scholar] [CrossRef]
- Rocha-Perugini, V.; Suárez, H.; Álvarez, S.; López-Martín, S.; Lenzi, G.M.; Vences-Catalán, F.; Levy, S.; Kim, B.; Muñoz-Fernández, M.A.; Sánchez-Madrid, F.; et al. CD81 association with SAMHD1 enhances HIV-1 reverse transcription by increasing dNTP levels. Nat. Microbiol. 2017, 2, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dziuba, N.; Friedrich, B.; von Lindern, J.; Murray, J.L.; Rojo, D.R.; Hodge, T.W.; O’Brien, W.A.; Ferguson, M.R. A critical role for CD63 in HIV replication and infection of macrophages and cell lines. Virology 2008, 379, 191–196. [Google Scholar] [CrossRef]
- Fu, E.; Pan, L.; Xie, Y.; Mu, D.; Liu, W.; Jin, F.; Bai, X. Tetraspanin CD63 is a regulator of HIV-1 replication. Int. J. Clin. Exp. Pathol. 2015, 8, 1184–1198. [Google Scholar]
- Li, G.; Dziuba, N.; Friedrich, B.; Murray, J.L.; Ferguson, M.R. A post-entry role for CD63 in early HIV-1 replication. Virology 2011, 412, 315–324. [Google Scholar] [CrossRef]
- Lingappa, J.; Lingappa, V.; Reed, J. Addressing Antiretroviral Drug Resistance with Host-Targeting Drugs—First Steps towards Developing a Host-Targeting HIV-1 Assembly Inhibitor. Viruses 2021, 13, 451. [Google Scholar] [CrossRef]
- Freed, E.O. HIV-1 assembly, release and maturation. Nat. Rev. Genet. 2015, 13, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Nydegger, S.; Khurana, S.; Krementsov, D.; Foti, M.; Thali, M. Mapping of tetraspanin-enriched microdomains that can function as gateways for HIV-1. J. Cell Biol. 2006, 173, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Grigorov, B.; Attuil-Audenis, V.; Perugi, F.; Nedelec, M.; Watson, S.; Pique, C.; Darlix, J.-L.; Conjeaud, H.; Muriaux, D. A role for CD81 on the late steps of HIV-1 replication in a chronically infected T cell line. Retrovirology 2009, 6, 1–16. [Google Scholar] [CrossRef]
- Ruiz-Mateos, E.; Pelchen-Matthews, A.; Deneka, M.; Marsh, M. CD63 Is Not Required for Production of Infectious Human Immunodeficiency Virus Type 1 in Human Macrophages. J. Virol. 2008, 82, 4751–4761. [Google Scholar] [CrossRef]
- Haller, C.; Müller, B.; Fritz, J.V.; Lamas-Murua, M.; Stolp, B.; Pujol, F.M.; Keppler, O.T.; Fackler, O.T. HIV-1 Nef and Vpu Are Functionally Redundant Broad-Spectrum Modulators of Cell Surface Receptors, Including Tetraspanins. J. Virol. 2014, 88, 14241–14257. [Google Scholar] [CrossRef]
- Lambelé, M.; Koppensteiner, H.; Symeonides, M.; Roy, N.H.; Chan, J.; Schindler, M.; Thali, M. Vpu Is the Main Determinant for Tetraspanin Downregulation in HIV-1-Infected Cells. J. Virol. 2015, 89, 3247–3255. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Aoki, J.; Misawa, N.; Daikoku, E.; Sano, K.; Tanaka, Y.; Koyanagi, Y. Modulation of Human Immunodeficiency Virus Type 1 Infectivity through Incorporation of Tetraspanin Proteins. J. Virol. 2008, 82, 1021–1033. [Google Scholar] [CrossRef]
- Chertova, E.; Chertov, O.; Coren, L.V.; Roser, J.D.; Trubey, C.M.; Bess, J.W.; Sowder, R.C.; Barsov, E.; Hood, B.L.; Fisher, R.J.; et al. Proteomic and Biochemical Analysis of Purified Human Immunodeficiency Virus Type 1 Produced from Infected Monocyte-Derived Macrophages. J. Virol. 2006, 80, 9039–9052. [Google Scholar] [CrossRef]
- Branham, M.T.; Bustos, M.A.; De Blas, G.A.; Rehmann, H.; Zarelli, V.E.; Trevino, C.L.; Darszon, A.; Mayorga, L.S.; Tomes, C.N. Epac Activates the Small G Proteins Rap1 and Rab3A to Achieve Exocytosis. J. Biol. Chem. 2009, 284, 24825–24839. [Google Scholar] [CrossRef]
- Kubo, Y.; Masumoto, H.; Izumida, M.; Kakoki, K.; Hayashi, H.; Matsuyama, T. Rab3a-Bound CD63 Is Degraded and Rab3a-Free CD63 Is Incorporated into HIV-1 Particles. Front. Microbiol. 2017, 8, 1653. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Mensah, G.; Al Sharif, S.; Pinto, D.; Branscome, H.; Yelamanchili, S.; Cowen, M.; Erickson, J.; Khatkar, P.; Mahieux, R.; et al. Extracellular Vesicles from Infected Cells Are Released Prior to Virion Release. Cells 2021, 10, 781. [Google Scholar] [CrossRef]
- Gould, S.J.; Booth, A.M.; Hildreth, J.E.K. The Trojan exosome hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 10592–10597. [Google Scholar] [CrossRef]
- Kulkarni, R.; Prasad, A. Exosomes Derived from HIV-1 Infected DCs Mediate Viral trans-Infection via Fibronectin and Galectin-3. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Bernatchez, J.; Tran, L.T.; Li, J.; Luan, Y.; Siqueira-Neto, J.L.; Li, R. Drugs for the Treatment of Zika Virus Infection. J. Med. Chem. 2019, 63, 470–489. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Ko, A.I.; Baud, D. Zika Virus Infection—After the Pandemic. New Engl. J. Med. 2019, 381, 1444–1457. [Google Scholar] [CrossRef]
- Shan, C.; Xie, X.; Shi, P.-Y. Zika Virus Vaccine: Progress and Challenges. Cell Host Microbe 2018, 24, 12–17. [Google Scholar] [CrossRef] [PubMed]
- White, M.K.; Wollebo, H.; Beckham, J.D.; Tyler, K.L.; Khalili, K. Zika virus: An emergent neuropathological agent. Ann. Neurol. 2016, 80, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.R.; Jamieson, D.; Powers, A.M.; Honein, M.A. Zika Virus. New Engl. J. Med. 2016, 374, 1552–1563. [Google Scholar] [CrossRef]
- Pannu, J.; Barry, M. Global health security as it pertains to Zika, Ebola, and COVID-19. Curr. Opin. Infect. Dis. 2021, 34, 401–408. [Google Scholar] [CrossRef]
- Jemielity, S.; Wang, J.J.; Chan, Y.K.; Ahmed, A.A.; Li, W.; Monahan, S.; Bu, X.; Farzan, M.; Freeman, G.J.; Umetsu, D.T.; et al. TIM-family Proteins Promote Infection of Multiple Enveloped Viruses through Virion-associated Phosphatidylserine. PLOS Pathog. 2013, 9, e1003232. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, D.S.; Ruiz-Arroyo, V.M.; Soler, N.; Usón, I.; Guarné, A.; Verdaguer, N. Supramolecular arrangement of the full-length Zika virus NS5. PLoS Pathog. 2019, 15, e1007656. [Google Scholar] [CrossRef] [PubMed]
- Calvet, G.; Aguiar, R.S.; O Melo, A.S.; A Sampaio, S.; de Filippis, I.; Fabri, A.; Araujo, E.S.M.; de Sequeira, P.C.; de Mendonça, M.C.L.; de Oliveira, L.; et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: A case study. Lancet Infect. Dis. 2016, 16, 653–660. [Google Scholar] [CrossRef]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Rus, K.R.; Vipotnik, T.V.; Vodušek, V.F.; et al. Zika Virus Associated with Microcephaly. New Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef]
- Zhang, Z.-W.; Li, Z.-L.; Yuan, S. The Role of Secretory Autophagy in Zika Virus Transfer through the Placental Barrier. Front. Cell. Infect. Microbiol. 2017, 6, 206. [Google Scholar] [CrossRef]
- York, S.B.; Sun, L.; Cone, A.S.; Duke, L.C.; Cheerathodi, M.R.; Meckes, D.G. Zika Virus Hijacks Extracellular Vesicle Tetraspanin Pathways for Cell-to-Cell Transmission. mSphere 2021, 6, e0019221. [Google Scholar] [CrossRef]
- Zhou, W.; Woodson, M.; Sherman, M.B.; Neelakanta, G.; Sultana, H. Exosomes mediate Zika virus transmission through SMPD3 neutral Sphingomyelinase in cortical neurons. Emerg. Microbes Infect. 2019, 8, 307–326. [Google Scholar] [CrossRef]
- World Health Organization. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 22 October 2021).
- De Clercq, E. Antiviral agents active against influenza A viruses. Nat. Rev. Drug Discov. 2006, 5, 1015–1025. [Google Scholar] [CrossRef]
- Hussain, M.; Galvin, H.D.; Haw, T.Y.; Nutsford, A.N.; Husain, M. Drug resistance in influenza A virus: The epidemiology and management. Infect. Drug Resist. 2017, 10, 121–134. [Google Scholar] [CrossRef]
- Prachanronarong, K.L.; Canale, A.S.; Liu, P.; Somasundaran, M.; Hou, S.; Poh, Y.-P.; Han, T.; Zhu, Q.; Renzette, N.; Zeldovich, K.B.; et al. Mutations in Influenza A Virus Neuraminidase and Hemagglutinin Confer Resistance against a Broadly Neutralizing Hemagglutinin Stem Antibody. J. Virol. 2019, 93, e01639-18. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhuang, X. Epsin 1 is a cargo-specific adaptor for the clathrin-mediated endocytosis of the influenza virus. Proc. Natl. Acad. Sci. USA 2008, 105, 11790–11795. [Google Scholar] [CrossRef]
- De Vries, E.; Tscherne, D.M.; Wienholts, M.J.; Cobos-Jiménez, V.; Scholte, F.; Garcia-Sastre, A.; Rottier, P.J.M.; de Haan, C. Dissection of the Influenza A Virus Endocytic Routes Reveals Macropinocytosis as an Alternative Entry Pathway. PLoS Pathog. 2011, 7, e1001329. [Google Scholar] [CrossRef] [PubMed]
- Sieczkarski, S.B.; Whittaker, G.R. Influenza Virus Can Enter and Infect Cells in the Absence of Clathrin-Mediated Endocytosis. J. Virol. 2002, 76, 10455–10464. [Google Scholar] [CrossRef] [PubMed]
- Wiley, D.C.; Skehel, J.J. The Structure and Function of the Hemagglutinin Membrane Glycoprotein of Influenza Virus. Annu. Rev. Biochem. 1987, 56, 365–394. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.; Steven, A.C. At Low pH, Influenza Virus Matrix Protein M1 Undergoes a Conformational Change Prior to Dissociating from the Membrane. J. Virol. 2013, 87, 5621–5628. [Google Scholar] [CrossRef]
- He, J.; Sun, E.; Bujny, M.V.; Kim, D.; Davidson, M.W.; Zhuang, X. Dual Function of CD81 in Influenza Virus Uncoating and Budding. PLoS Pathog. 2013, 9, e1003701. [Google Scholar] [CrossRef]
- Melén, K.; Fagerlund, R.; Franke, J.; Köhler, M.; Kinnunen, L.; Julkunen, I. Importin α Nuclear Localization Signal Binding Sites for STAT1, STAT2, and Influenza A Virus Nucleoprotein. J. Biol. Chem. 2003, 278, 28193–28200. [Google Scholar] [CrossRef]
- Qiao, Y.; Yan, Y.; Tan, K.S.; Tan, S.S.; Seet, J.E.; Arumugam, T.V.; Chow, V.T.; Wang, D.Y.; Tran, T. CD151, a novel host factor of nuclear export signaling in influenza virus infection. J. Allergy Clin. Immunol. 2017, 141, 1799–1817. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; De Giglio, M.A.R.; Roviello, G.N. Anti-Coronavirus Vaccines: Past Investigations on SARS-CoV-1 and MERS-CoV, the Approved Vaccines from BioNTech/Pfizer, Moderna, Oxford/AstraZeneca and others under Development Against SARS-CoV-2 Infection. Curr. Med. Chem. 2021, 28, 1. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. New Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-R.; Cao, Q.-D.; Hong, Z.-S.; Tan, Y.-Y.; Chen, S.-D.; Jin, H.-J.; Tan, K.-S.; Wang, D.-Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med Res. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Plante, J.A.; Mitchell, B.M.; Plante, K.S.; Debbink, K.; Weaver, S.C.; Menachery, V.D. The variant gambit: COVID-19’s next move. Cell Host Microbe 2021, 29, 508–515. [Google Scholar] [CrossRef]
- Boehm, E.; Kronig, I.; Neher, R.A.; Eckerle, I.; Vetter, P.; Kaiser, L. Novel SARS-CoV-2 variants: The pandemics within the pandemic. Clin. Microbiol. Infect. 2021, 27, 1109–1117. [Google Scholar] [CrossRef]
- Charrin, S.; Jouannet, S.; Boucheix, C.; Rubinstein, E. Tetraspanins at a glance. J. Cell Sci. 2014, 127, 3641–3648. [Google Scholar] [CrossRef]
- Florin, L.; Lang, T. Tetraspanin Assemblies in Virus Infection. Front. Immunol. 2018, 9, 1140. [Google Scholar] [CrossRef]
- Kim, C.-H. SARS-CoV-2 Evolutionary Adaptation toward Host Entry and Recognition of Receptor O-Acetyl Sialylation in Virus–Host Interaction. Int. J. Mol. Sci. 2020, 21, 4549. [Google Scholar] [CrossRef] [PubMed]
- Hantak, M.P.; Qing, E.; Earnest, J.T.; Gallagher, T. Tetraspanins: Architects of Viral Entry and Exit Platforms. J. Virol. 2019, 93, e01429-17. [Google Scholar] [CrossRef]
- Earnest, J.T.; Hantak, M.P.; Park, J.-E.; Gallagher, T. Coronavirus and Influenza Virus Proteolytic Priming Takes Place in Tetraspanin-Enriched Membrane Microdomains. J. Virol. 2015, 89, 6093–6104. [Google Scholar] [CrossRef] [PubMed]
- Earnest, J.T.; Hantak, M.P.; Li, K.; Jr, P.B.M.; Perlman, S.; Gallagher, T. The tetraspanin CD9 facilitates MERS-coronavirus entry by scaffolding host cell receptors and proteases. PLoS Pathog. 2017, 13, e1006546. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.B.M.M.K.; Khan, A.-A.; Ahmed, R.; Hossain, S.; Kabir, S.M.T.; Islam, S.; Siddiki, A.M.A.M.Z. Transcriptome of nasopharyngeal samples from COVID-19 patients and a comparative analysis with other SARS-CoV-2 infection models reveal disparate host responses against SARS-CoV-2. J. Transl. Med. 2021, 19, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Rappa, G.; Green, T.M.; Lorico, A. The Nuclear Pool of Tetraspanin CD9 Contributes to Mitotic Processes in Human Breast Carcinoma. Mol. Cancer Res. 2014, 12, 1840–1850. [Google Scholar] [CrossRef]
- Zismanov, V.; Drucker, L.; Attar-Schneider, O.; Matalon, S.T.; Pasmanik-Chor, M.; Lishner, M. Tetraspanins stimulate protein synthesis in myeloma cell lines. J. Cell. Biochem. 2012, 113, 2500–2510. [Google Scholar] [CrossRef]
- Berditchevski, F.; Odintsova, E. Tetraspanins as Regulators of Protein Trafficking. Traffic 2006, 8, 89–96. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Genet. 2020, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Hassuna, N.; Monk, P.N.; Moseley, G.W.; Partridge, L.J. Strategies for Targeting Tetraspanin Proteins. BioDrugs 2009, 23, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Neelakanta, G.; Sultana, H. Tetraspanins as Potential Therapeutic Candidates for Targeting Flaviviruses. Front. Immunol. 2021, 12, 1386. [Google Scholar] [CrossRef] [PubMed]
- Charrin, S.; Manié, S.; Oualid, M.; Billard, M.; Boucheix, C.; Rubinstein, E. Differential stability of tetraspanin/tetraspanin interactions: Role of palmitoylation. FEBS Lett. 2002, 516, 139–144. [Google Scholar] [CrossRef]
- Sincock, P.M.; Mayrhofer, G.; Ashman, L.K. Localization of the Transmembrane 4 Superfamily (TM4SF) Member PETA-3 (CD151) in Normal Human Tissues: Comparison with CD9, CD63, and α5β1 Integrin. J. Histochem. Cytochem. 1997, 45, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Crew, V.K.; Burton, N.; Kagan, A.; Green, C.A.; Levene, C.; Flinter, F.; Brady, R.L.; Daniels, G.; Anstee, D.J. CD151, the first member of the tetraspanin (TM4) superfamily detected on erythrocytes, is essential for the correct assembly of human basement membranes in kidney and skin. Blood 2004, 104, 2217–2223. [Google Scholar] [CrossRef]
- Sachs, N.; Kreft, M.; Weerman, M.A.V.D.B.; Beynon, A.J.; Peters, T.; Weening, J.J.; Sonnenberg, A. Kidney failure in mice lacking the tetraspanin CD151. J. Cell Biol. 2006, 175, 33–39. [Google Scholar] [CrossRef]
- Seu, L.; Tidwell, C.; Timares, L.; Duverger, A.; Wagner, F.H.; Goepfert, P.A.; Westfall, A.O.; Sabbaj, S.; Kutsch, O. CD151 Expression Is Associated with a Hyperproliferative T Cell Phenotype. J. Immunol. 2017, 199, 3336–3347. [Google Scholar] [CrossRef]
- Reyes, R.; Cardeñes, B.; Machado-Pineda, Y.; Cabañas, C. Tetraspanin CD9: A Key Regulator of Cell Adhesion in the Immune System. Front. Immunol. 2018, 9, 863. [Google Scholar] [CrossRef]
- Zou, F.; Wang, X.; Han, X.; Rothschild, G.; Zheng, S.G.; Basu, U.; Sun, J. Expression and Function of Tetraspanins and Their Interacting Partners in B Cells. Front. Immunol. 2018, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- Benites, E.C.; Cabrini, D.P.; Silva, A.C.; Silva, J.C.; Catalan, D.T.; Berezin, E.N.; Cardoso, M.R.; Passos, S.D. Acute respiratory viral infections in pediatric cancer patients undergoing chemotherapy. J. Pediatr. 2014, 90, 370–376. [Google Scholar] [CrossRef][Green Version]
- Gosain, R.; Abdou, Y.; Singh, A.; Rana, N.; Puzanov, I.; Ernstoff, M.S. COVID-19 and Cancer: A Comprehensive Review. Curr. Oncol. Rep. 2020, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hijano, D.; Maron, G.; Hayden, R.T. Respiratory Viral Infections in Patients With Cancer or Undergoing Hematopoietic Cell Transplant. Front. Microbiol. 2018, 9, 3097. [Google Scholar] [CrossRef] [PubMed]
- Robilotti, E.V.; Babady, N.E.; Mead, P.A.; Rolling, T.; Perez-Johnston, R.; Bernardes, M.; Bogler, Y.; Caldararo, M.; Figueroa, C.J.; Glickman, M.S.; et al. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 2020, 26, 1218–1223. [Google Scholar] [CrossRef]
- Kischel, P.; Bellahcene, A.; Deux, B.; Lamour, V.; Dobson, R.; De Pauw, E.; Clézardin, P.; Castronovo, V. Overexpression of CD9 in human breast cancer cells promotes the development of bone metastases. Anticancer. Res. 2012, 32, 5211–5220. [Google Scholar] [PubMed]
- Lewitowicz, P.; Matykiewicz, J.; Koziel, D.; Chrapek, M.; Horecka-Lewitowicz, A.; Gluszek, S. CD63 and GLUT-1 Overexpression Could Predict a Poor Clinical Outcome in GIST: A Study of 54 Cases with Follow-Up. Gastroenterol. Res. Pr. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zuo, L.; Zheng, H.; Li, G.; Hu, X. Increased Expression of CD81 in Breast Cancer Tissue is Associated with Reduced Patient Prognosis and Increased Cell Migration and Proliferation in MDA-MB-231 and MDA-MB-435S Human Breast Cancer Cell Lines In Vitro. Med. Sci. Monit. 2018, 24, 5739–5747. [Google Scholar] [CrossRef] [PubMed]

| Tetraspanin | Virus | Role of Tetraspanins In Virus’s Life Cycles | ||
|---|---|---|---|---|
| Viral Entry | Replication | Viral Exit | ||
| CD151 | HPV | [21,22] | - | - |
| IAV | - | [114] | - | |
| CD9 | HPV | [23] | - | - |
| HIV | [55,56,57,65] | - | [79,86] | |
| CoV | [121,122,123,124,125,126] | - | - | |
| CD63 | HPV | - | [19,24] | - |
| HIV | [61,62,63,65,66] | [63,72,73,74] | [79,82,85] | |
| Zika | - | - | [100] | |
| CoV | [121,123,124] | - | - | |
| CD81 | HIV | [55,56,58,59,60,65] | [71] | [65,78,79,81,86] |
| IAV | [112] | - | [112] | |
| CoV | [121,122,123,124] | - | - | |
| TSPAN7 | HIV | [50] | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
New, C.; Lee, Z.-Y.; Tan, K.S.; Wong, A.H.-P.; Wang, D.Y.; Tran, T. Tetraspanins: Host Factors in Viral Infections. Int. J. Mol. Sci. 2021, 22, 11609. https://doi.org/10.3390/ijms222111609
New C, Lee Z-Y, Tan KS, Wong AH-P, Wang DY, Tran T. Tetraspanins: Host Factors in Viral Infections. International Journal of Molecular Sciences. 2021; 22(21):11609. https://doi.org/10.3390/ijms222111609
Chicago/Turabian StyleNew, ChihSheng, Zhao-Yong Lee, Kai Sen Tan, Amanda Huee-Ping Wong, De Yun Wang, and Thai Tran. 2021. "Tetraspanins: Host Factors in Viral Infections" International Journal of Molecular Sciences 22, no. 21: 11609. https://doi.org/10.3390/ijms222111609
APA StyleNew, C., Lee, Z.-Y., Tan, K. S., Wong, A. H.-P., Wang, D. Y., & Tran, T. (2021). Tetraspanins: Host Factors in Viral Infections. International Journal of Molecular Sciences, 22(21), 11609. https://doi.org/10.3390/ijms222111609






