Antiviral Gene Expression in Young and Aged Murine Lung during H1N1 and H3N2
Abstract
1. Introduction
2. Results
2.1. Impact of Chronological Aging on Host Responses to Mouse-Adapted H1N1 and H3N2 Strains of Influenza
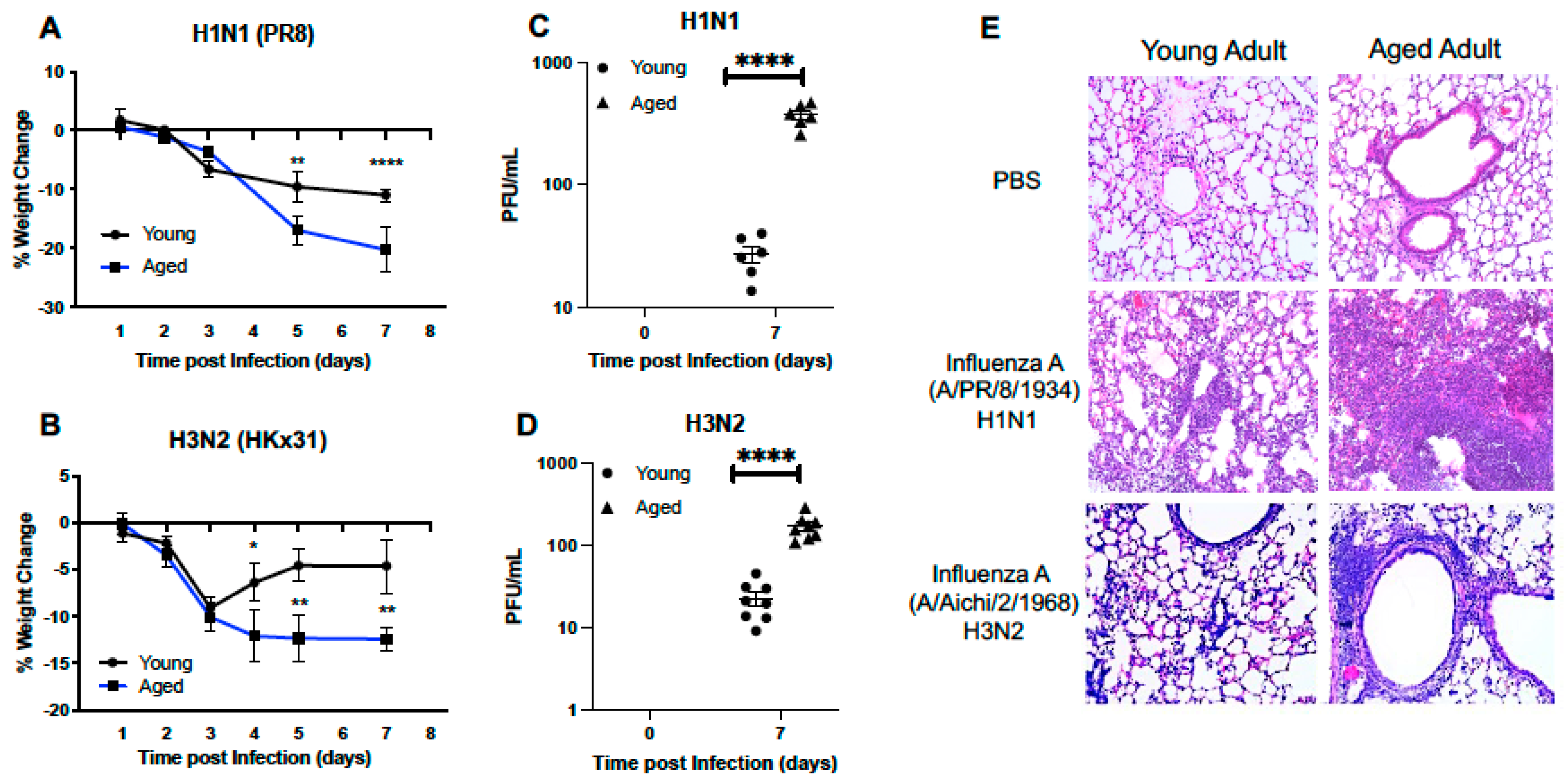
2.1.1. Morbidity and Histological Changes in the Young and Aged Murine Adult Lung
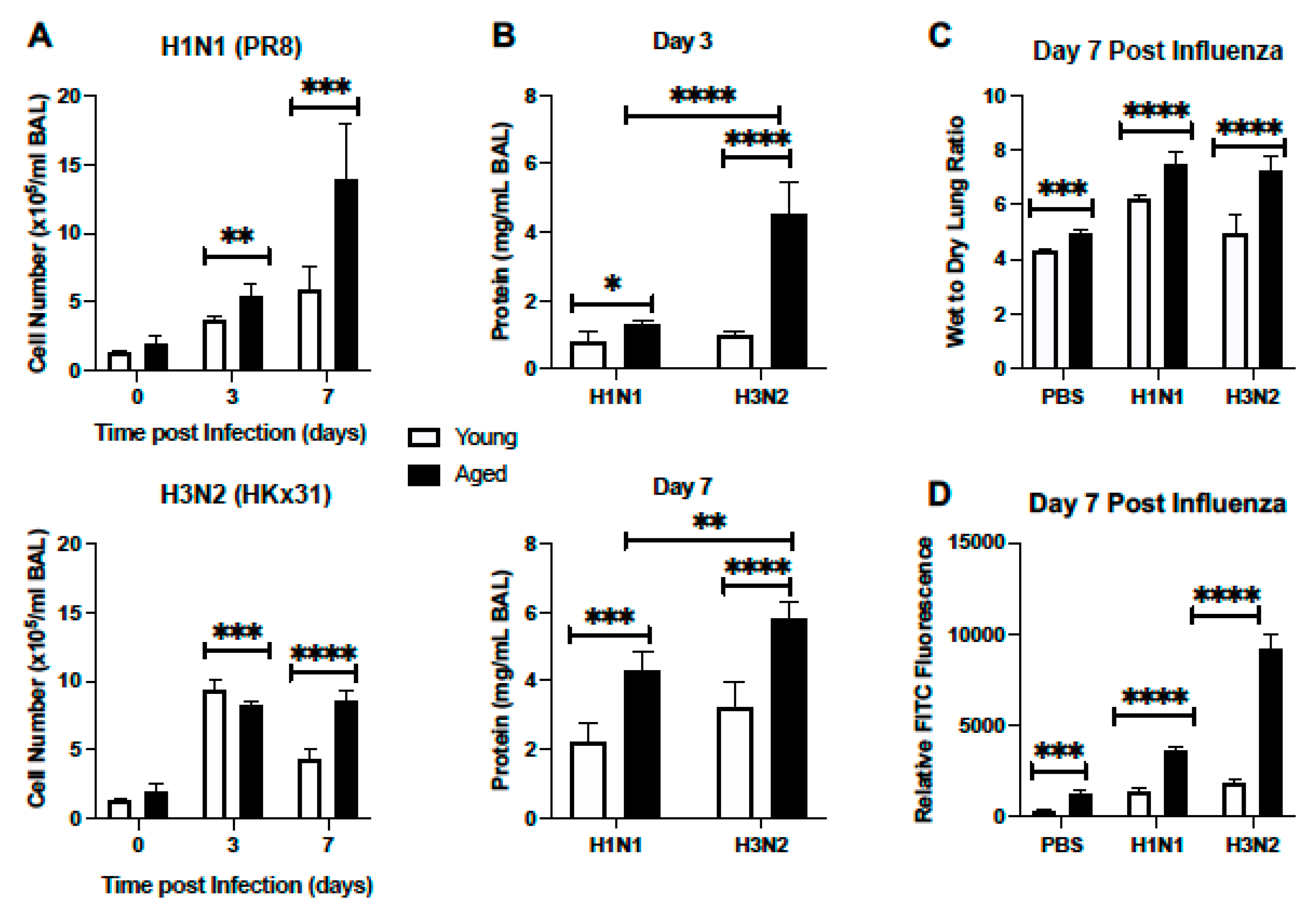
2.1.2. Cellular Infiltration and Lung Injury Is Increased in Aged Murine Lung in Response to Influenza
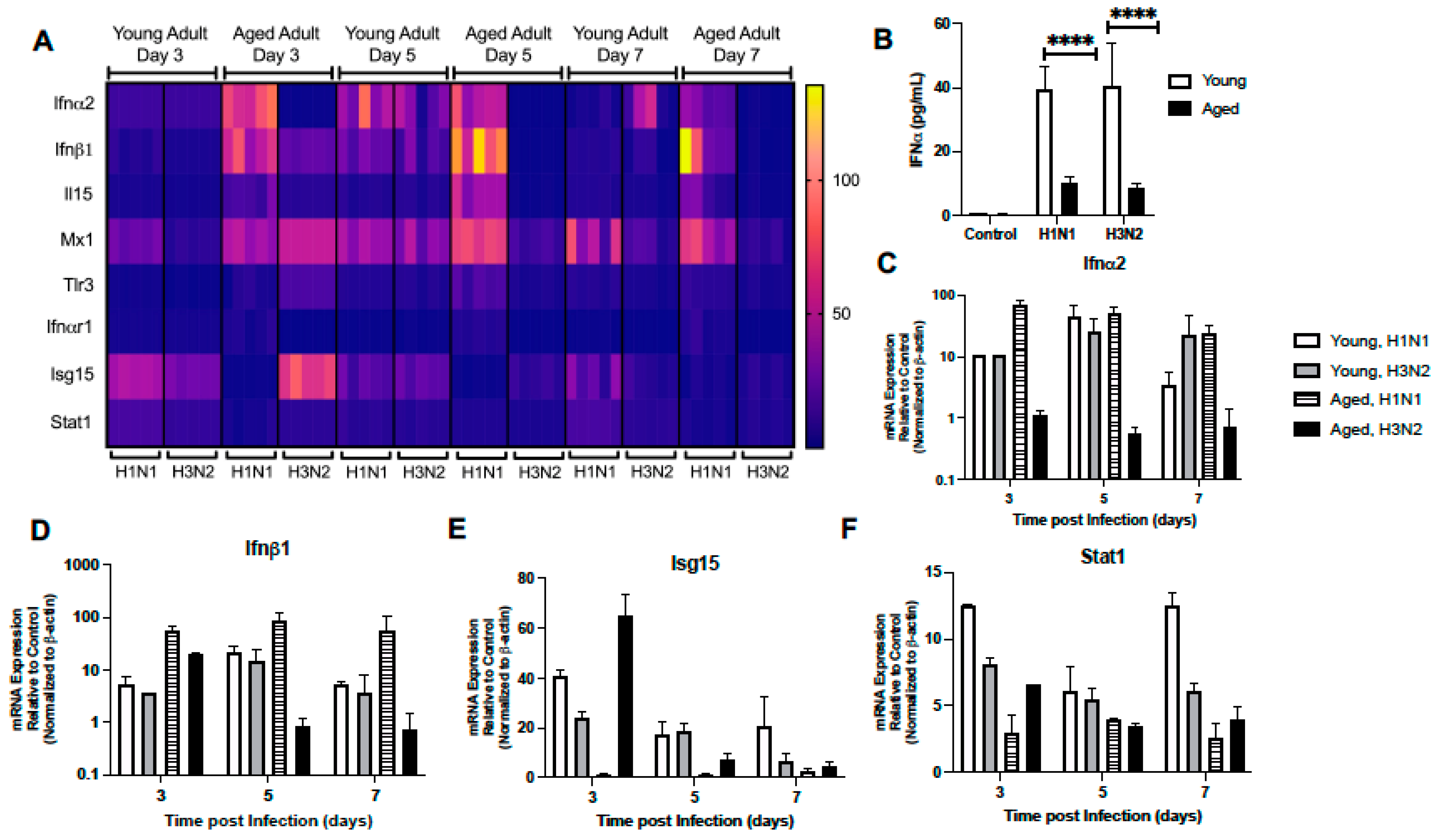
2.1.3. Dysregulated Type I IFN Signaling in Aged Lung
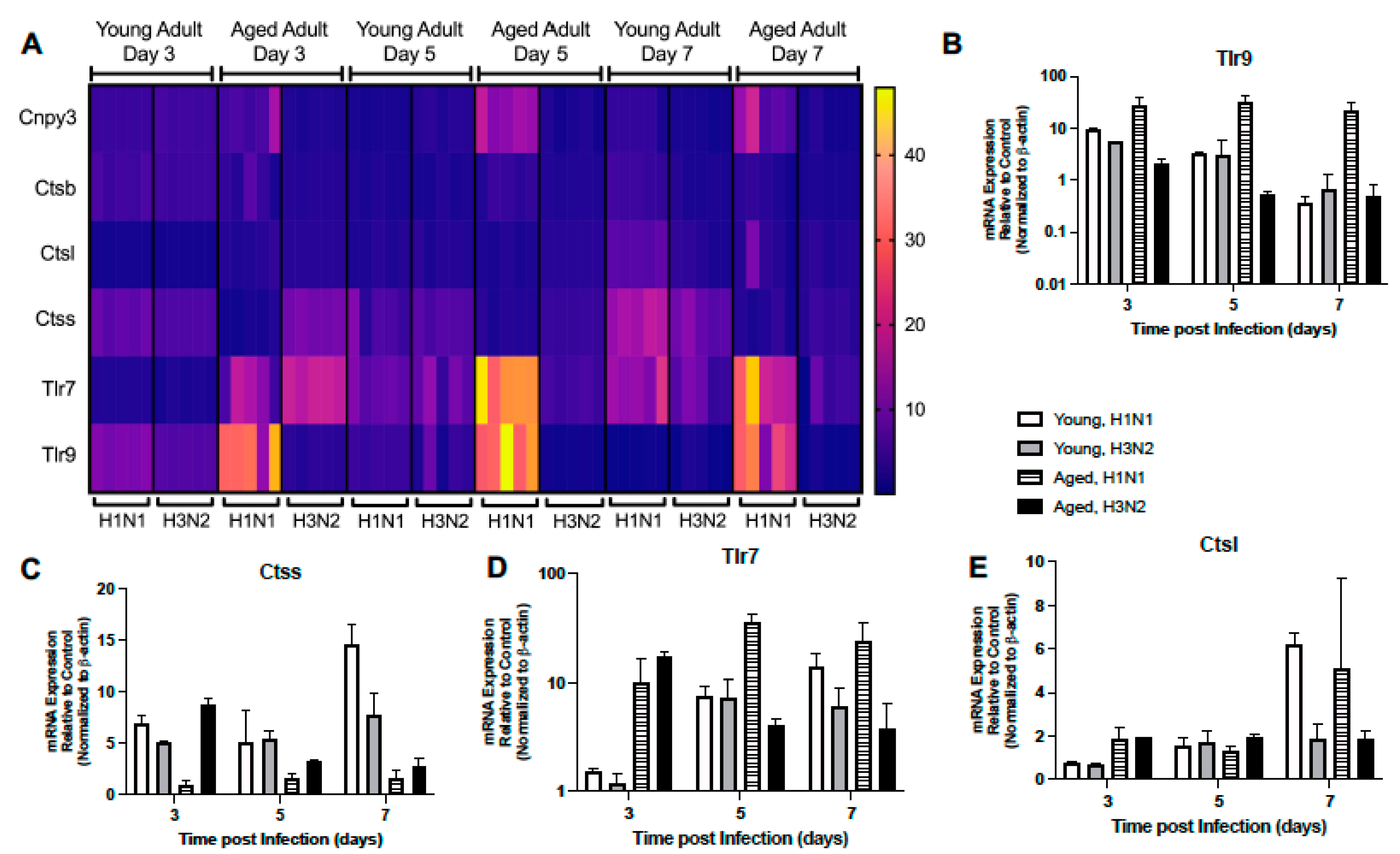
2.1.4. Altered Expression of TLR Signaling Responsive Genes
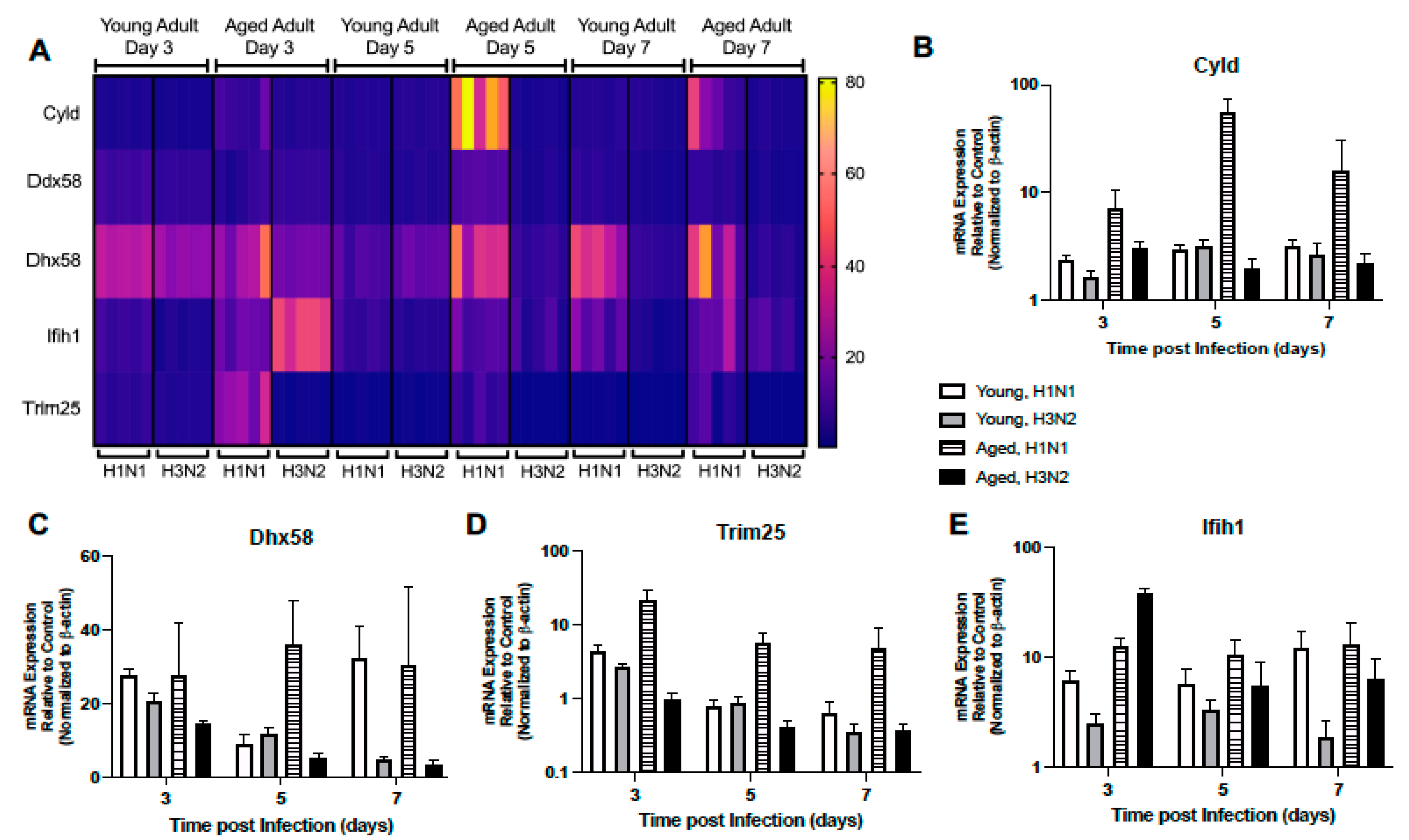
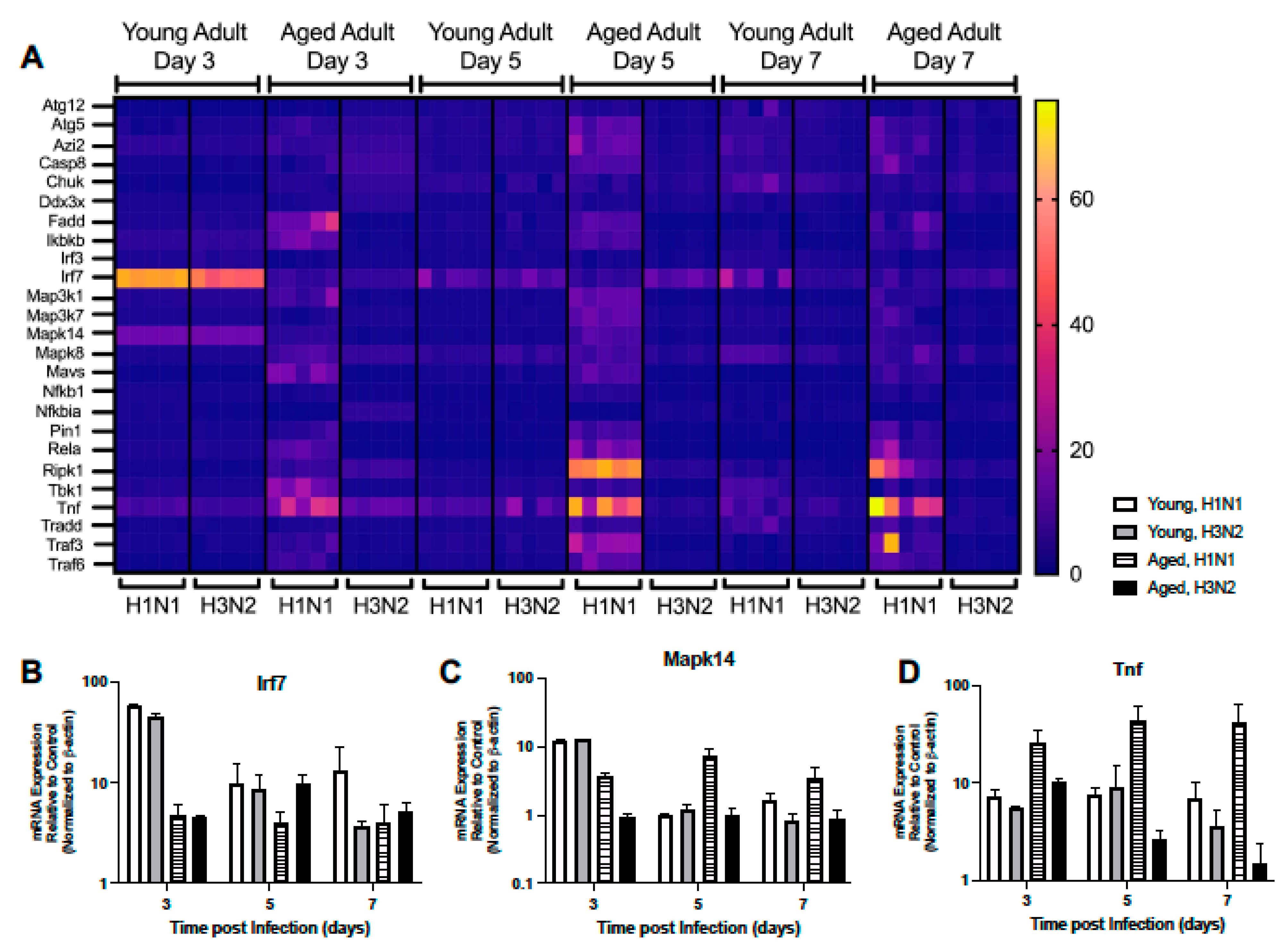
2.1.5. Altered Expression of RIG-I-like Receptor Signaling
2.1.6. Dysregulated Expression of TLR Receptors and Chaperones
3. Discussion
4. Materials and Methods
- i.
- Influenza: Viral stocks: H1N1 (strain: A/Puerto Rico/8/1934, PR8, material #: 10100374, batch #: 4XP170531, EID50 per ml: 1010.3) and H3N2 (strain: A/Aichi/2/1968, HKx31, material #:10100375, batch #: 4XX171019, EID50 per ml: 1010.5) were purchased from Charles River (Norwich, CT).
- ii.
- In Vivo Procedures and Tissue Collection: Influenza infection: All mice were anesthetized with isoflurane (5% for induction and 2% for maintenance) prior to intranasal instillation with 12.5 PFU of influenza (50 μL volume in PBS). Bronchoalveolar lavage (BAL): BAL was collected using previously published methods [49]. Briefly, 0.8-mL of PBS was slowly injected and aspirated 4 times prior to saving the recovered lavage fluid on ice. Lavage was clarified at 7000 rpm for 10 min at 4 °C. Viral titer assay of BAL: TCID50 in was calculated using the Viral ToxGlo Assay (Promega, Madison WI). Briefly, 3.16-fold serial dilutions of virus were plated for 24–48 h on >80% confluent MDCK cells. Upon visualization of cytopathic effect, ATP detection reagent was added, and luminescence was measured. Values were calculated by plotting net relative luminescence units (RLU) values after subtracting average blank wells against viral dilution. The TCID50 value is the reciprocal of the dilution that produced a 50% decline in ATP levels compared to untreated controls. Validated regression analysis was performed using GraphPad Prism. Protein quantification in BAL: Protein levels in clarified lavage were calculated using the BioRad protein assay (BioRad) per manufacturer’s instructions. IFNα ELISA: IFNα2 and 4 levels in clarified BAL were assessed by ELISA (ThermoFisher Scientific, Catalog # BMS6027) per manufacturer’s instructions. Lung tissue collection: At select time points of infection lung tissue was collected from control and influenza infected young and aged adult mice. Tissue was snap frozen or placed into Allprotect (Qiagen) for future analysis. FITC-Dextran Lung Permeability Assay: Young and aged adult mice were intranasally instilled with 50-μL of FITC-Dextran (3 mg/kg). After 1 h, blood was collected from euthanized mice, and plasma was isolated after centrifugation (7000 rpm, 10 min). Fluorescence was assessed (excitation 485, emission 528). Lung Wet to Dry Ratio: Lung tissue was collected from control and influenza infected young and aged adult mice. Lung tissue weight was assessed at harvest (wet weight) and after being placed in a 60 °C drying over for 48 h (dry weight). Histology: Mice were euthanized, and right lung tissue was collected for downstream analysis. To maintain architecture, left lung was distended with 1% low melting agarose and placed into cold formalin [50]. Tissue samples were processed, and H&E stained by the Translational Research Program at WCM Pathology and Laboratory of Medicine. Images were scanned using the EVOS FL Auto Imaging System (ThermoFisher Scientific). For all animal experiments, we used 5–10 mice per group and experiments were repeated at least three times.
- iii.
- RNA Purification and Real Time PCR: RNA samples were extracted using the automated Maxwell RNA extraction protocol (Madison, WI). Samples were quantified and A260/280 ratios were recorded. Samples were reverse transcribed using the First Stand Synthesis Kit and quantified RT2 ProfilerTM Assays (RT2 Profiler PCR Array, Mouse Antiviral Response, PAMM-122Z). Results were quantified using analytical software provided by Qiagen Gene Globe.
- iv.
- Statistical Analysis: Survival analysis between groups was calculated using the Mantel Cox test. Comparison of groups was performed using a two-tailed t-test and comparisons between groups were verified by one-way ANOVA. For two component comparisons (time post infection and age), two-way ANOVA was used to calculate statistical significance. All samples were independent and contained the same sample size for analysis. All data were analyzed using GraphPad Prism software (San Diego, CA). Statistical significance was considered by a * p < 0.05, **p < 0.01, *** p < 0.001, and **** p < 0.0001.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Global Seasonal Influenza-associated Mortality Collaborator, N. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Zhou, F.; Li, H.; Gu, L.; Liu, M.; Xue, C.X.; Cao, B.; Wang, C. National Influenza Apdm09 Clinical Investigation Group of, C., Risk factors for nosocomial infection among hospitalised severe influenza A(H1N1)pdm09 patients. Respir. Med. 2018, 134, 86–91. [Google Scholar] [CrossRef]
- Matsumoto, M.; Seya, T. TLR3: Interferon induction by double-stranded RNA including poly(I:C). Adv. Drug Deliv. Rev. 2008, 60, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Sanjo, H.; Takeuchi, O.; Sugiyama, M.; Okabe, M.; Takeda, K.; et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 2003, 301, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Oshiumi, H.; Matsumoto, M.; Funami, K.; Akazawa, T.; Seya, T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat. Immunol. 2003, 4, 161–167. [Google Scholar] [CrossRef]
- Sato, A.; Iwasaki, A. Induction of antiviral immunity requires Toll-like receptor signaling in both stromal and dendritic cell compartments. Proc. Natl. Acad Sci. USA 2004, 101, 16274–16279. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef]
- Lund, J.M.; Alexopoulou, L.; Sato, A.; Karow, M.; Adams, N.C.; Gale, N.W.; Iwasaki, A.; Flavell, R.A. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad Sci. USA 2004, 101, 5598–5603. [Google Scholar] [CrossRef]
- Graef, K.M.; Vreede, F.T.; Lau, Y.F.; McCall, A.W.; Carr, S.M.; Subbarao, K.; Fodor, E. The PB2 subunit of the influenza virus RNA polymerase affects virulence by interacting with the mitochondrial antiviral signaling protein and inhibiting expression of beta interferon. J. Virol. 2010, 84, 8433–8445. [Google Scholar] [CrossRef]
- Gack, M.U.; Albrecht, R.A.; Urano, T.; Inn, K.S.; Huang, I.C.; Carnero, E.; Farzan, M.; Inoue, S.; Jung, J.U.; Garcia-Sastre, A. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 2009, 5, 439–449. [Google Scholar] [CrossRef]
- Koliopoulos, M.G.; Lethier, M.; van der Veen, A.G.; Haubrich, K.; Hennig, J.; Kowalinski, E.; Stevens, R.V.; Martin, S.R.; Reis e Sousa, C.; Cusack, S.; et al. Molecular mechanism of influenza A NS1-mediated TRIM25 recognition and inhibition. Nat. Commun. 2018, 9, 1820. [Google Scholar] [CrossRef] [PubMed]
- Mibayashi, M.; Martinez-Sobrido, L.; Loo, Y.M.; Cardenas, W.B.; Gale, M., Jr.; Garcia-Sastre, A. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J. Virol. 2007, 81, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, I.; Ye, F.; McNally, B.; Willette, M.; Flano, E. Toll-like receptor expression and induction of type I and type III interferons in primary airway epithelial cells. J. Virol. 2013, 87, 3261–3270. [Google Scholar] [CrossRef]
- Schulz, K.S.; Mossman, K.L. Viral Evasion Strategies in Type I IFN Signaling—A Summary of Recent Developments. Front. Immunol. 2016, 7, 498. [Google Scholar] [CrossRef] [PubMed]
- Schindler, C.; Levy, D.E.; Decker, T. JAK-STAT signaling: From interferons to cytokines. J. Biol. Chem. 2007, 282, 20059–20063. [Google Scholar] [CrossRef]
- Hernandez-Vargas, E.A.; Wilk, E.; Canini, L.; Toapanta, F.R.; Binder, S.C.; Uvarovskii, A.; Ross, T.M.; Guzman, C.A.; Perelson, A.S.; Meyer-Hermann, M. Effects of aging on influenza virus infection dynamics. J. Virol. 2014, 88, 4123–4131. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Qian, F.; Mohanty, S.; van Duin, D.; Newman, F.K.; Zhang, L.; Chen, S.; Towle, V.; Belshe, R.B.; Fikrig, E.; et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J. Immunol. 2010, 184, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Haq, K.; McElhaney, J.E. Immunosenescence: Influenza vaccination and the elderly. Curr. Opin. Immunol. 2014, 29, 38–42. [Google Scholar] [CrossRef]
- Mbawuike, I.N.; Acuna, C.L.; Walz, K.C.; Atmar, R.L.; Greenberg, S.B.; Couch, R.B. Cytokines and impaired CD8+ CTL activity among elderly persons and the enhancing effect of IL-12. Mech. Ageing Dev. 1997, 94, 25–39. [Google Scholar] [CrossRef]
- Lee, N.; Chan, P.K.; Hui, D.S.; Rainer, T.H.; Wong, E.; Choi, K.W.; Lui, G.C.; Wong, B.C.; Wong, R.Y.; Lam, W.Y.; et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J. Infect. Dis. 2009, 200, 492–500. [Google Scholar] [CrossRef]
- Kang, K.S.; Lee, N.; Shin, M.S.; Kim, S.D.; Yu, Y.; Mohanty, S.; Belshe, R.B.; Montgomery, R.R.; Shaw, A.C.; Kang, I. An altered relationship of influenza vaccine-specific IgG responses with T cell immunity occurs with aging in humans. Clin. Immunol. 2013, 147, 79–88. [Google Scholar] [CrossRef][Green Version]
- Van Duin, D.; Allore, H.G.; Mohanty, S.; Ginter, S.; Newman, F.K.; Belshe, R.B.; Medzhitov, R.; Shaw, A.C. Prevaccine determination of the expression of costimulatory B7 molecules in activated monocytes predicts influenza vaccine responses in young and older adults. J. Infect. Dis. 2007, 195, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Blomberg, B.B.; Garcia, D.; Keilich, S.R.; Haynes, L. Age-related factors that affect B cell responses to vaccination in mice and humans. Immunol. Rev. 2020, 296, 142–154. [Google Scholar] [CrossRef]
- Bufan, B.; Arsenovic-Ranin, N.; Petrovic, R.; Zivkovic, I.; Stoiljkovic, V.; Leposavic, G. Strain specificities in influence of ageing on germinal centre reaction to inactivated influenza virus antigens in mice: Sex-based differences. Exp. Gerontol. 2020, 133, 110857. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Seong, R.K.; Shin, O.S. Enhanced Viral Replication by Cellular Replicative Senescence. Immune Netw. 2016, 16, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Van der Geest, K.S.; Abdulahad, W.H.; Tete, S.M.; Lorencetti, P.G.; Horst, G.; Bos, N.A.; Kroesen, B.J.; Brouwer, E.; Boots, A.M. Aging disturbs the balance between effector and regulatory CD4+ T cells. Exp. Gerontol. 2014, 60, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Molony, R.D.; Nguyen, J.T.; Kong, Y.; Montgomery, R.R.; Shaw, A.C.; Iwasaki, A. Aging impairs both primary and secondary RIG-I signaling for interferon induction in human monocytes. Sci. Signal. 2017, 10, 509. [Google Scholar] [CrossRef] [PubMed]
- Toapanta, F.R.; Ross, T.M. Impaired immune responses in the lungs of aged mice following influenza infection. Respir. Res. 2009, 10, 112. [Google Scholar] [CrossRef]
- Keef, E.; Zhang, L.A.; Swigon, D.; Urbano, A.; Ermentrout, G.B.; Matuszewski, M.; Toapanta, F.R.; Ross, T.M.; Parker, R.S.; Clermont, G. Discrete Dynamical Modeling of Influenza Virus Infection Suggests Age-Dependent Differences in Immunity. J. Virol. 2017, 91, e00395-17. [Google Scholar] [CrossRef]
- Boianelli, A.; Nguyen, V.K.; Ebensen, T.; Schulze, K.; Wilk, E.; Sharma, N.; Stegemann-Koniszewski, S.; Bruder, D.; Toapanta, F.R.; Guzman, C.A.; et al. Modeling Influenza Virus Infection: A Roadmap for Influenza Research. Viruses 2015, 7, 5274–5304. [Google Scholar] [CrossRef]
- McElhaney, J.E.; Upshaw, C.M.; Hooton, J.W.; Lechelt, K.E.; Meneilly, G.S. Responses to influenza vaccination in different T-cell subsets: A comparison of healthy young and older adults. Vaccine 1998, 16, 1742–1747. [Google Scholar] [CrossRef]
- Sridharan, A.; Esposo, M.; Kaushal, K.; Tay, J.; Osann, K.; Agrawal, S.; Gupta, S.; Agrawal, A. Age-associated impaired plasmacytoid dendritic cell functions lead to decreased CD4 and CD8 T cell immunity. Age 2011, 33, 363–376. [Google Scholar] [CrossRef]
- Kang, I.; Hong, M.S.; Nolasco, H.; Park, S.H.; Dan, J.M.; Choi, J.Y.; Craft, J. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J. Immunol. 2004, 173, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.S.; Dan, J.M.; Choi, J.Y.; Kang, I. Age-associated changes in the frequency of naive, memory and effector CD8+ T cells. Mech. Ageing Dev. 2004, 125, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Beli, E.; Clinthorne, J.F.; Duriancik, D.M.; Hwang, I.; Kim, S.; Gardner, E.M. Natural killer cell function is altered during the primary response of aged mice to influenza infection. Mech. Ageing Dev. 2011, 132, 503–510. [Google Scholar] [CrossRef]
- Nogusa, S.; Ritz, B.W.; Kassim, S.H.; Jennings, S.R.; Gardner, E.M. Characterization of age-related changes in natural killer cells during primary influenza infection in mice. Mech. Ageing Dev. 2008, 129, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: A mini-review. Gerontology 2013, 59, 421–426. [Google Scholar] [CrossRef]
- Prakash, S.; Agrawal, S.; Cao, J.N.; Gupta, S.; Agrawal, A. Impaired secretion of interferons by dendritic cells from aged subjects to influenza: Role of histone modifications. Age 2013, 35, 1785–1797. [Google Scholar] [CrossRef]
- Plowden, J.; Renshaw-Hoelscher, M.; Gangappa, S.; Engleman, C.; Katz, J.M.; Sambhara, S. Impaired antigen-induced CD8+ T cell clonal expansion in aging is due to defects in antigen presenting cell function. Cell. Immunol. 2004, 229, 86–92. [Google Scholar] [CrossRef]
- Lu, J.; Duan, X.; Zhao, W.; Wang, J.; Wang, H.; Zhou, K.; Fang, M. Aged Mice are More Resistant to Influenza Virus Infection due to Reduced Inflammation and Lung Pathology. Aging Dis. 2018, 9, 358–373. [Google Scholar] [CrossRef]
- Walker, M.A.; Volpi, S.; Sims, K.B.; Walter, J.E.; Traggiai, E. Powering the immune system: Mitochondria in immune function and deficiency. J. Immunol. Res. 2014, 2014, 164309. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, S.E.; Sena, L.A.; Chandel, N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity 2015, 42, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Kelly, B.; O’Neill, L.A.J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017, 18, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Moore, C.B.; Liesman, R.M.; O’Connor, B.P.; Bergstralh, D.T.; Chen, Z.J.; Pickles, R.J.; Ting, J.P. MAVS-mediated apoptosis and its inhibition by viral proteins. PLoS ONE 2009, 4, e5466. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, X.; Nie, X.; Sun, L.; Tang, T.S.; Chen, D.; Sun, Q. COX5B regulates MAVS-mediated antiviral signaling through interaction with ATG5 and repressing ROS production. PLoS Pathog. 2012, 8, e1003086. [Google Scholar] [CrossRef]
- Borgeling, Y.; Schmolke, M.; Viemann, D.; Nordhoff, C.; Roth, J.; Ludwig, S. Inhibition of p38 mitogen-activated protein kinase impairs influenza virus-induced primary and secondary host gene responses and protects mice from lethal H5N1 infection. J. Biol. Chem. 2014, 289, 13–27. [Google Scholar] [CrossRef]
- Marchant, D.; Singhera, G.K.; Utokaparch, S.; Hackett, T.L.; Boyd, J.H.; Luo, Z.; Si, X.; Dorscheid, D.R.; McManus, B.M.; Hegele, R.G. Toll-like receptor 4-mediated activation of p38 mitogen-activated protein kinase is a determinant of respiratory virus entry and tropism. J. Virol. 2010, 84, 11359–11373. [Google Scholar] [CrossRef]
- Luig, C.; Kother, K.; Dudek, S.E.; Gaestel, M.; Hiscott, J.; Wixler, V.; Ludwig, S. MAP kinase-activated protein kinases 2 and 3 are required for influenza A virus propagation and act via inhibition of PKR. FASEB J. 2010, 24, 4068–4077. [Google Scholar] [CrossRef]
- Sun, F.; Xiao, G.; Qu, Z. Murine Bronchoalveolar Lavage. Bio-Protoc. 2017, 7, e2287. [Google Scholar] [CrossRef]
- Halbower, A.C.; Mason, R.J.; Abman, S.H.; Tuder, R.M. Agarose infiltration improves morphology of cryostat sections of lung. Lab. Investig. 1994, 71, 149–153. [Google Scholar]

| Symbol | Gene Name |
|---|---|
| Atg12 | Autophagy-related 12 |
| Atg5 | Autophagy-related 5 |
| Azi2 | 5-azacytidine induced gene 2 |
| Casp8 | Caspase 8 |
| Ccl3 | Chemokine (C-C motif) ligand 3 |
| Ccl4 | Chemokine (C-C motif) ligand 4 |
| Ccl5 | Chemokine (C-C motif) ligand 5 |
| Cd40 | CD40 antigen |
| Cd80 | CD80 antigen |
| Cd86 | CD86 antigen |
| Chuk | Conserved helix-loop-helix ubiquitous kinase |
| Cnpy3 | Canopy 3 homolog |
| Ctsb | Cathepsin B |
| Ctsl | Cathepsin L |
| Ctss | Cathepsin S |
| Cxcl10 | Chemokine (C-X-C motif) ligand 10 |
| Cxcl9 | Chemokine (C-X-C motif) ligand 9 |
| Cyld | Cylindromatosis |
| Ddx3x | DEAD/H (Asp-Glu-Ala-Asp/His) box polypeptide 3 |
| Ddx58 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 58 |
| Dhx58 | DEXH (Asp-Glu-X-His) box polypeptide 58 |
| Fadd | Fas (TNFRSF6)-associated via death domain |
| Ifih1 | Interferon induced with helicase C domain 1 |
| Ifnα2 | Interferon alpha 2 |
| Ifnαr1 | Interferon (alpha and beta) receptor 1 |
| Ifnβ1 | Interferon beta 1 |
| Ikbkb | Inhibitor of kappaB kinase beta |
| Il12a | Interleukin 12A |
| Il12b | Interleukin 12b |
| Il15 | Interleukin 15 |
| Il6 | Interleukin 6 |
| Irf3 | Interferon regulatory factor 3 |
| Irf7 | Interferon regulatory factor 7 |
| Isg15 | ISG15 ubiquitin-like modifier |
| Map3k1 | Mitogen-activated protein 3 kinase 1 |
| Map3k7 | Mitogen-activated protein 3 kinase 7 |
| Mapk14 | Mitogen-activated protein kinase 14 |
| Mapk8 | Mitogen-activated protein kinase 8 |
| Mavs | Mitochondrial antiviral signaling protein |
| Mx1 | Myxovirus (Influenza virus) resistance 1 |
| Nfκb1 | Nuclear factor of kappa light polypeptide gene enhancer in B- cells 1, p105 |
| Nfκbia | Nuclear factor of kappa light polypeptide gene enhancer in B- cells inhibitor, alpha |
| Pin1 | Protein (peptidyl-proyl cis/trans isomerase) NIMA-interacting 1 |
| Rela | V-rel reticuloendotheliosis viral oncogene homolog A |
| Ripk1 | Receptor (TNFRSF)-interacting serine-threonine kinase 1 |
| Stat1 | Signal transducer and activator of transcription 1 |
| Tbk1 | TANK-binding kinase 1 |
| Tlr3 | Toll-like receptor 3 |
| Tlr9 | Toll-like receptor 9 |
| Tnf | Tumor necrosis factor |
| Tradd | TNFRSF1A-associated via death domain |
| Traf3 | Tnf receptor-associated factor 3 |
| Traf6 | Tnf receptor-associated factor 6 |
| Trim25 | Tripartite motif-containing 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, R.; Yang, J.; Pagan, K.; Cho, S.J.; Stout-Delgado, H. Antiviral Gene Expression in Young and Aged Murine Lung during H1N1 and H3N2. Int. J. Mol. Sci. 2021, 22, 12097. https://doi.org/10.3390/ijms222212097
Harris R, Yang J, Pagan K, Cho SJ, Stout-Delgado H. Antiviral Gene Expression in Young and Aged Murine Lung during H1N1 and H3N2. International Journal of Molecular Sciences. 2021; 22(22):12097. https://doi.org/10.3390/ijms222212097
Chicago/Turabian StyleHarris, Rebecca, Jianjun Yang, Kassandra Pagan, Soo Jung Cho, and Heather Stout-Delgado. 2021. "Antiviral Gene Expression in Young and Aged Murine Lung during H1N1 and H3N2" International Journal of Molecular Sciences 22, no. 22: 12097. https://doi.org/10.3390/ijms222212097
APA StyleHarris, R., Yang, J., Pagan, K., Cho, S. J., & Stout-Delgado, H. (2021). Antiviral Gene Expression in Young and Aged Murine Lung during H1N1 and H3N2. International Journal of Molecular Sciences, 22(22), 12097. https://doi.org/10.3390/ijms222212097






