ncRNAs in Therapeutics: Challenges and Limitations in Nucleic Acid-Based Drug Delivery
Abstract
1. Introduction
2. Strategies for Fine-Tuning Therapeutic ncRNA Levels by Using Specific Inhibitors or Activators
3. Physiological Barriers Hindering the Clinical Use of Oligonucleotide Therapies
4. Improving the Performance of Nucleic Acid-Based Therapies
4.1. Nucleic Acid Stability Enhancement
4.2. Nanoparticles as Delivery Vehicles: Advantages and Limitations
4.3. Nanoparticles as Delivery Vehicles: Effects of Structure and Composition
5. Future Trends in the Clinical Use of Nucleic Acids for ncRNA Therapy
5.1. Avoiding Immune-Related Adverse Reactions: Nucleic Acid–TLR Interactions
5.2. Improving the Specificity of Targeting Nucleic Acids
5.3. Improving the Delivery of Nucleic Acid-Nanoparticle Systems
5.4. Selecting Appropriate Patients
5.5. Integrating Complex Regulatory Systems
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kowara, M.; Borodzicz-Jazdzyk, S.; Rybak, K.; Kubik, M.; Cudnoch-Jedrzejewska, A. Therapies Targeted at Non-Coding RNAs in Prevention and Limitation of Myocardial Infarction and Subsequent Cardiac Remodeling—Current Experience and Perspectives. Int. J. Mol. Sci. 2021, 22, 5718. [Google Scholar] [CrossRef] [PubMed]
- Aurilia, C.; Donati, S.; Palmini, G.; Miglietta, F.; Iantomasi, T.; Brandi, M. The Involvement of Long Non-Coding RNAs in Bone. Int. J. Mol. Sci. 2021, 22, 3909. [Google Scholar] [CrossRef] [PubMed]
- De Benedittis, S.; Fortunato, F.; Cava, C.; Gallivanone, F.; Iaccino, E.; Caligiuri, M.E.; Castiglioni, I.; Bertoli, G.; Manna, I.; Labate, A.; et al. irculating microRNA: The Potential Novel Diagnostic Biomarkers to Predict Drug Resistance in Temporal Lobe Epilepsy, a Pilot Study. Int. J. Mol. Sci. 2021, 22, 702. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, R.; Suleiman, S.; Pentimalli, F.; O’Toole, S.; O’Leary, J.; Ward, M.; Conlon, N.; Sabol, M.; Ozretić, P.; Erson-Bensan, A.; et al. Could MicroRNAs Be Useful Tools to Improve the Diagnosis and Treatment of Rare Gynecological Cancers? A Brief Overview. Int. J. Mol. Sci. 2021, 22, 3822. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016, 44, 6518–6548. [Google Scholar] [CrossRef]
- Navarro, E.; Mallén, A.; Cruzado, J.M.; Torras, J.; Hueso, M. Unveiling ncRNA regulatory axes in atherosclerosis progression. Clin. Transl. Med. 2020, 9, e5. [Google Scholar] [CrossRef]
- Bajan, S.; Hutvagner, G. RNA-Based Therapeutics: From Antisense Oligonucleotides to miRNAs. Cells 2020, 9, 137. [Google Scholar] [CrossRef]
- Ideue, T.; Hino, K.; Kitao, S.; Yokoi, T.; Hirose, T. Efficient oligonucleotide-mediated degradation of nuclear noncoding RNAs in mammalian cultured cells. RNA 2009, 15, 1578–1587. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Endo, H.; Shiroki, T.; Nakagawa, T.; Yokoyama, M.; Tamai, K.; Yamanami, H.; Fujiya, T.; Sato, I.; Yamaguchi, K.; Tanaka, N.; et al. Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS ONE 2013, 8, e77070. [Google Scholar] [CrossRef] [PubMed]
- Koch, L. The driving force of cancer evolution. Nat. Rev. Genet. 2017, 18, 703. [Google Scholar] [CrossRef]
- Liu, S.J.; Horlbeck, M.A.; Cho, S.W.; Birk, H.S.; Malatesta, M.; He, D.; Attenello, F.J.; Villalta, J.E.; Cho, M.Y.; Chen, Y.; et al. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science 2017, 355, 6320. [Google Scholar] [CrossRef] [PubMed]
- Arun, G.; Diermeier, S.; Akerman, M.; Chang, K.-C.; Wilkinson, J.E.; A Hearn, S.; Kim, Y.; MacLeod, A.R.; Krainer, A.; Norton, L.; et al. Differentiation of mammary tumors and reduction in metastasis uponMalat1lncRNA loss. Genes Dev. 2016, 30, 34–51. [Google Scholar] [CrossRef]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Bader, A.G.; Brown, D.; Winkler, M. The Promise of MicroRNA Replacement Therapy. Cancer Res. 2010, 70, 7027–7030. [Google Scholar] [CrossRef]
- Juliano, R.L.; Carver, K. Cellular uptake and intracellular trafficking of oligonucleotides. Adv. Drug Deliv. Rev. 2015, 87, 35–45. [Google Scholar] [CrossRef]
- Kole, R.; Krainer, A.R.; Altman, S. RNA therapeutics: Beyond RNA interference and antisense oligonucleotides. Nat. Rev. Drug Discov. 2012, 11, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Houseley, J.; Tollervey, D. The Many Pathways of RNA Degradation. Cell 2009, 136, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tiruppathi, C.; Cho, J.; Minshall, R.D.; Malik, A.B. Delivery of nanoparticle: Complexed drugs across the vascular endothelial barrier via caveolae. IUBMB Life 2011, 63, 659–667. [Google Scholar] [CrossRef]
- Allen, T.M.; Hansen, C.B.; Guo, L.S. Subcutaneous administration of liposomes: A comparison with the intravenous and intraperitoneal routes of injection. Biochim. Biophys. Acta (BBA) Biomembr. 1993, 1150, 9–16. [Google Scholar] [CrossRef]
- Wagenaar, T.R.; Tolstykh, T.; Shi, C.; Jiang, L.; Zhang, J.; Li, Z.; Yu, Q.; Qu, H.; Sun, F.; Cao, H.; et al. Identification of the endosomal sorting complex required for transport-I (ESCRT-I) as an important modulator of anti-miR uptake by cancer cells. Nucleic Acids Res. 2015, 43, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.I.; Moazzam, M.; Kato, S.; Cho, K.Y.; Tiwari, R.K. Overcoming Barriers for siRNA Therapeutics: From Bench to Bedside. Pharmaceuticals 2020, 13, 294. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Sheng, Y.; Shi, J.; Yu, B.; Yu, Z.; Liao, G. Long Circulating Polymeric Nanoparticles for Gene/Drug Delivery. Curr. Drug Metab. 2018, 19, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Piella, J.; Bastús, N.G.; Puntes, V. Size-Dependent Protein–Nanoparticle Interactions in Citrate-Stabilized Gold Nanoparticles: The Emergence of the Protein Corona. Bioconj. Chem. 2017, 28, 88–97. [Google Scholar] [CrossRef]
- Gheibihayat, S.M.; Jaafari, M.R.; Hatamipour, M.; Sahebkar, A. Improvement of the pharmacokinetic characteristics of liposomal doxorubicin using CD47 biomimickry. J. Pharm. Pharmacol. 2021, 73, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Okude, H.; Ori, D.; Kawai, T. Signaling Through Nucleic Acid Sensors and Their Roles in Inflammatory Diseases. Front. Immunol. 2021, 11, 625833. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, S.; Milam, V.T. Modified Nucleic Acids: Expanding the Capabilities of Functional Oligonucleotides. Molecules 2020, 25, 4659. [Google Scholar] [CrossRef]
- Das, S.K.; Menezes, M.E.; Bhatia, S.; Wang, X.-Y.; Emdad, L.; Sarkar, D.; Fisher, P.B. Gene Therapies for Cancer: Strategies, Challenges and Successes. J. Cell. Physiol. 2015, 230, 259–271. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Dane, K.Y.; Nembrini, C.; Tomei, A.A.; Eby, J.K.; O’Neil, C.P.; Velluto, D.; Swartz, M.A.; Inverardi, L.; Hubbell, J.A. Nano-sized drug-loaded micelles deliver payload to lymph node immune cells and prolong allograft survival. J. Control. Release 2011, 156, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Foged, C.; Brodin, B.; Frokjaer, S.; Sundblad, A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int. J. Pharm. 2005, 298, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.D.; Scholzen, A.; Minigo, G.; David, C.; Apostolopoulos, V.; Mottram, P.L.; Plebanski, M. Pathogen recognition and development of particulate vaccines: Does size matter? Methods 2006, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold Nanoparticles as a Vaccine Platform: Influence of Size and Shape on Immunological Responses in Vitro and in Vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Galdiero, S. Peptide chemistry encounters nanomedicine: Recent applications and upcoming scenarios in cancer. Future Med. Chem. 2018, 10, 1877–1880. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Montis, C.; Paccosi, S.; Silvano, A.; Michelucci, E.; Berti, D.; Bosi, A.; Parenti, A.; Romagnoli, P. Inorganic nanoparticles as potential regulators of immune response in dendritic cells. Nanomedicine 2017, 12, 1647–1660. [Google Scholar] [CrossRef]
- Vlamidis, Y.; Voliani, V. Bringing Again Noble Metal Nanoparticles to the Forefront of Cancer Therapy. Front. Bioeng. Biotechnol. 2018, 6, 143. [Google Scholar] [CrossRef]
- Habibullah, G.; Viktorova, J.; Ruml, T. Current Strategies for Noble Metal Nanoparticle Synthesis. Nanoscale Res. Lett. 2021, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Qiao, S.-Z. A mesoporous organosilica nano-bowl with high DNA loading capacity—A potential gene delivery carrier. Nanoscale 2016, 8, 17446–17450. [Google Scholar] [CrossRef] [PubMed]
- Yildirimer, L.; Thanh, N.T.; Loizidou, M.; Seifalian, A. Toxicology and clinical potential of nanoparticles. Nano Today 2011, 6, 585–607. [Google Scholar] [CrossRef]
- Svoboda, O.; Fohlerova, Z.; Baiazitova, L.; Mlynek, P.; Samouylov, K.; Provaznik, I.; Hubalek, J. Transfection by Polyethyleneimine-Coated Magnetic Nanoparticles: Fine-Tuning the Condition for Electrophysiological Experiments. J. Biomed. Nanotechnol. 2018, 14, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Cortajarena, A.L.; Ortega, D.; Ocampo, S.M.; Gonzalez-García, A.; Couleaud, P.; Miranda, R.; Belda-Iniesta, C.; Ayuso-Sacido, A. Engineering Iron Oxide Nanoparticles for Clinical Settings. Nanobiomedicine 2014, 1, 2. [Google Scholar] [CrossRef]
- Singh, D.P.; Herrera, C.E.; Singh, B.; Singh, S.; Singh, R.K.; Kumar, R. Graphene oxide: An efficient material and recent approach for biotechnological and biomedical applications. Mater. Sci. Eng. C 2018, 86, 173–197. [Google Scholar] [CrossRef] [PubMed]
- Lage, T.; Rodrigues, R.O.; Catarino, S.; Gallo, J.; Bañobre-López, M.; Minas, G. Graphene-Based Magnetic Nanoparticles for Theranostics: An Overview for Their Potential in Clinical Application. Nanomaterials 2021, 11, 1073. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, Q.; Zhang, Z.; Gong, T.; Sun, X. Cationic Bovine Serum Albumin Based Self-Assembled Nanoparticles as siRNA Delivery Vector for Treating Lung Metastatic Cancer. Small 2014, 10, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Le, V.-M.; Lang, M.-D.; Shi, W.-B.; Liu, J.-W. A collagen-based multicellular tumor spheroid model for evaluation of the efficiency of nanoparticle drug delivery. Artif. Cells Nanomed. Biotechnol. 2014, 44, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.; Fauzi, M.B. Current Update of Collagen Nanomaterials—Fabrication, Characterisation and Its Applications: A Review. Pharmaceutics 2021, 13, 316. [Google Scholar] [CrossRef] [PubMed]
- Vaghasiya, K.; Ray, E.; Singh, R.; Jadhav, K.; Sharma, A.; Khan, R.; Katare, O.P.; Verma, R.K. Efficient, enzyme responsive and tumor receptor targeting gelatin nanoparticles decorated with concanavalin-A for site-specific and controlled drug delivery for cancer therapy. Mater. Sci. Eng. C 2021, 123, 112027. [Google Scholar] [CrossRef] [PubMed]
- Gessner, I.; Neundorf, I. Nanoparticles Modified with Cell-Penetrating Peptides: Conjugation Mechanisms, Physicochemical Properties, and Application in Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2020, 21, 2536. [Google Scholar] [CrossRef]
- Quader, S.; Kataoka, K. Nanomaterial-Enabled Cancer Therapy. Mol. Ther. 2017, 25, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Spadari, C.D.C.; Bastiani, F.W.M.D.S.D.; Lopes, L.B.; Ishida, K. Alginate nanoparticles as non-toxic delivery system for miltefosine in the treatment of candidiasis and cryptococcosis. Int. J. Nanomed. 2019, 14, 5187–5199. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Wei, J.; Yu, C.; Han, X.; Qin, X.; Zhang, C.; Liao, W.; Li, L.; Huang, W. Non-viral nanocarriers for intracellular delivery of microRNA therapeutics. J. Mater. Chem. B 2019, 7, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rana, T.M. Therapeutic targeting of microRNAs: Current status and future challenges. Nat. Rev. Drug Discov. 2014, 13, 622–638. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, D.K.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef]
- dos Reis, L.G.; Lee, W.-H.; Svolos, M.; Moir, L.M.; Jaber, R.; Windhab, N.; Young, P.M.; Traini, D. Nanotoxicologic Effects of PLGA Nanoparticles Formulated with a Cell-Penetrating Peptide: Searching for a Safe pDNA Delivery System for the Lungs. Pharmaceutics 2019, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Chen, L.; Zhao, B.; Yuan, W. Rationale and Application of PEGylated Lipid-Based System for Advanced Target Delivery of siRNA. Front. Pharmacol. 2021, 11, 598175. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Qiao, M.; Wang, Q.; Ye, Y.; Ba, S.; Ma, J.; Hu, H.; Zhao, X.; Chen, D. Dual-responsive polyplexes with enhanced disassembly and endosomal escape for efficient delivery of siRNA. Biomaterials 2018, 162, 47–59. [Google Scholar] [CrossRef]
- Bakan, B.; Gülcemal, S.; Akgöl, S.; Hoet, P.; Yavaşoğlu, N. Ülkü K. Synthesis, characterization and toxicity assessment of a new polymeric nanoparticle, l-glutamic acid-g-p(HEMA). Chem. Biol. Interactions 2020, 315, 108870. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Bao, Y.; Xu, C.; Chen, L.; Tang, Z. Poly(L-Glutamic Acid)-Drug Conjugates for Chemo- and Photodynamic Combination Therapy. Macromol. Biosci. 2021, 21, e2000192. [Google Scholar] [CrossRef]
- Dzmitruk, V.; Apartsin, E.; Ihnatsyeu-Kachan, A.; Abashkin, V.; Shcharbin, D.; Bryszewska, M. Dendrimers Show Promise for siRNA and microRNA Therapeutics. Pharmaceutics 2018, 10, 126. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, C.J.; Vargas, J.R.; Blake, T.R.; Hardy, J.W.; Kanada, M.; Contag, C.H.; Wender, P.A.; Waymouth, R.M. Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proc. Natl. Acad. Sci. USA 2017, 114, E448–E456. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Oskuee, R.K.; Nassirli, H.; Malaekeh-Nikouei, B. Progress in the development of lipopolyplexes as efficient non-viral gene delivery systems. J. Control. Release 2016, 236, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Guo, P.; Wen, W.-C.; Wong, H. Lipid-Based Nanocarriers for RNA Delivery. Curr. Pharm. Des. 2015, 21, 3140–3147. [Google Scholar] [CrossRef]
- Park, K.S.; Sun, X.; Aikins, M.E.; Moon, J.J. Non-viral COVID-19 vaccine delivery systems. Adv. Drug Deliv. Rev. 2021, 169, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Artegiani, B.; Clevers, H. Use and application of 3D-organoid technology. Hum. Mol. Genet. 2018, 27, R99–R107. [Google Scholar] [CrossRef]
- Aparicio, S.; Hidalgo, M.; Kung, A.L. Examining the utility of patient-derived xenograft mouse models. Nat. Rev. Cancer 2015, 15, 311–316. [Google Scholar] [CrossRef]
- Lee, J.; Lilly, G.D.; Doty, R.C.; Podsiadlo, P.; Kotov, N.A. In vitro Toxicity Testing of Nanoparticles in 3D Cell Culture. Small 2009, 5, 1213–1221. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. Online 2020, 19, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics—Challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.N.; Wolken, N.; Brown, T.; Dauer, W.T.; Ehrlich, M.E.; Gonzalez-Alegre, P. Lethal toxicity caused by expression of shRNA in the mouse striatum: Implications for therapeutic design. Gene Ther. 2011, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Bander, N.H. Antibody–Drug Conjugate Target Selection: Critical Factors. Methods Mol. Biol. 2013, 1045, 29–40. [Google Scholar] [CrossRef]
- Orellana, E.A.; Tenneti, S.; Rangasamy, L.; Lyle, L.T.; Low, P.S.; Kasinski, A.L. FolamiRs: Ligand-targeted, vehicle-free delivery of microRNAs for the treatment of cancer. Sci. Transl. Med. 2017, 9, eaam9327. [Google Scholar] [CrossRef]
- Viney, N.J.; van Capelleveen, J.C.; Geary, R.S.; Xia, S.; Tami, J.A.; Yu, R.Z.; Marcovina, S.M.; Hughes, S.G.; Graham, M.J.; Crooke, R.M.; et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): Two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 2016, 388, 2239–2253. [Google Scholar] [CrossRef]
- Landmesser, U.; Lüscher, T.F. Advancing RNA-targeted therapy for personalised prevention of coronary disease: Focus on ANGPLT3. Eur. Heart J. 2020, 41, 3946–3948. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kim, M. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef]
- Syn, N.L.; Wang, L.; Chow, E.K.; Lim, C.T.; Goh, B.-C. Exosomes in Cancer Nanomedicine and Immunotherapy: Prospects and Challenges. Trends Biotechnol. 2017, 35, 665–676. [Google Scholar] [CrossRef]
- Whitford, W.; Guterstam, P. Exosome manufacturing status. Futur. Med. Chem. 2019, 11, 1225–1236. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, H. Notch-1 siRNA and Methotrexate towards a Multifunctional Approach in Rhematoid Arthritis Management: A Nanomedicine Approach. Pharm. Res. 2018, 35, 123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Y.; Kilchrist, K.V.; Li, J.; Duvall, C.L.; Oupický, D. Endosomolytic and Tumor-Penetrating Mesoporous Silica Nanoparticles for siRNA/miRNA Combination Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 4308–4322. [Google Scholar] [CrossRef]
- Petrek, H.; Batra, N.; Ho, P.Y.; Tu, M.-J.; Yu, A.-M. Bioengineering of a single long noncoding RNA molecule that carries multiple small RNAs. Appl. Microbiol. Biotechnol. 2019, 103, 6107–6117. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.S.; Bose, R.J.; McCarthy, J.R.; Azmi, I.D.M.; Madheswaran, T. Biomimetic bacterial and viral-based nanovesicles for drug delivery, theranostics, and vaccine applications. Drug Discov. Today 2021, 26, 902–915. [Google Scholar] [CrossRef]
- Chen, B.; Mei, L.; Wang, Y.; Guo, G. Advances in intelligent DNA nanomachines for targeted cancer therapy. Drug Discov. Today 2021, 26, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Parab, S.; Dabholkar, N.; Agrawal, M.; Singhvi, G.; Alexander, A.; Bapat, R.A.; Kesharwani, P. Oral peptide delivery: Challenges and the way ahead. Drug Discov. Today 2021, 26, 931–950. [Google Scholar] [CrossRef] [PubMed]

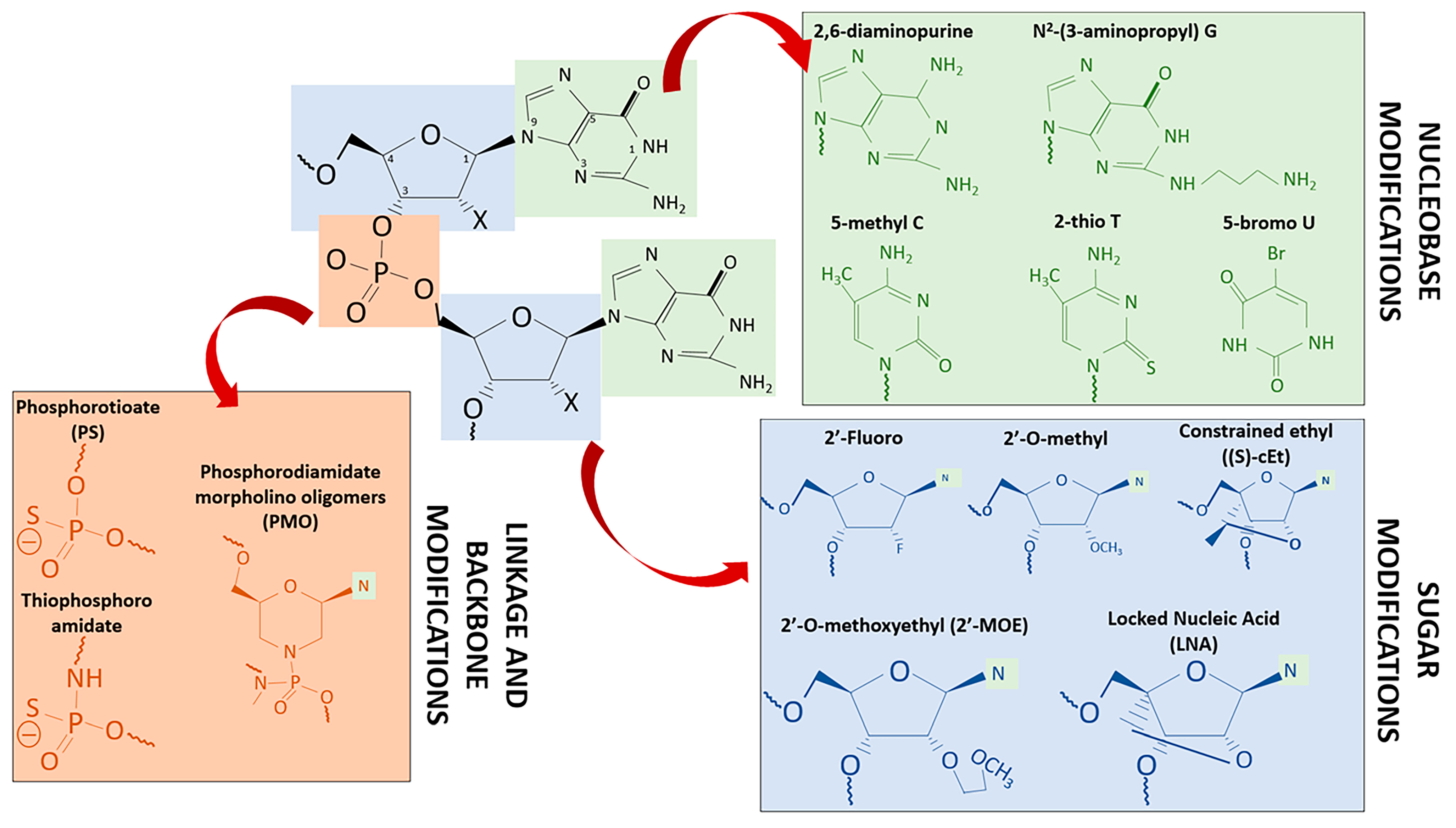
| Chemical Modification | ASOs Improvement | Commercialized or Phase 3 ASOs Drug |
|---|---|---|
| Nucleobase Modifications | ||
| 2,6-diaminopurine | Enhance electrostatic interactions with phosphate backbone Enhance target binding affinity and specificity Enhance duplex thermal stability | |
| N2-(3-aminopropyl) G | ||
| 5-methyl C | ||
| 2-thio T | ||
| 5-bromo U | ||
| Sugar Modifications | ||
| 2′-Fluoro | Shows duplex stabilizing properties and binding to dsDNA Enhance binding affinity for target RNA sequences Reduce susceptibility toward nuclease degradation | |
| 2′-MO | ||
| (S)-cEt | ||
| LNA | Miravirsen | |
| 2′-MOE | Mipomersen, Nusinersen, Volanesorsen | |
| 2′-H | Fomivirsen, Mongersen | |
| Phosphodiester Linkage & Backbone Modifications | ||
| Phosphorotioate (PS) | Improvement of resistance to nuclease cleavage Enhance binding to albumin and heparin proteins Improvement in cellular uptake | |
| Thiophosphoroamidate | ||
| Phosphorodiamidate morpholino oligomers (PMO) | Eteplirsen, Golodirsen | |
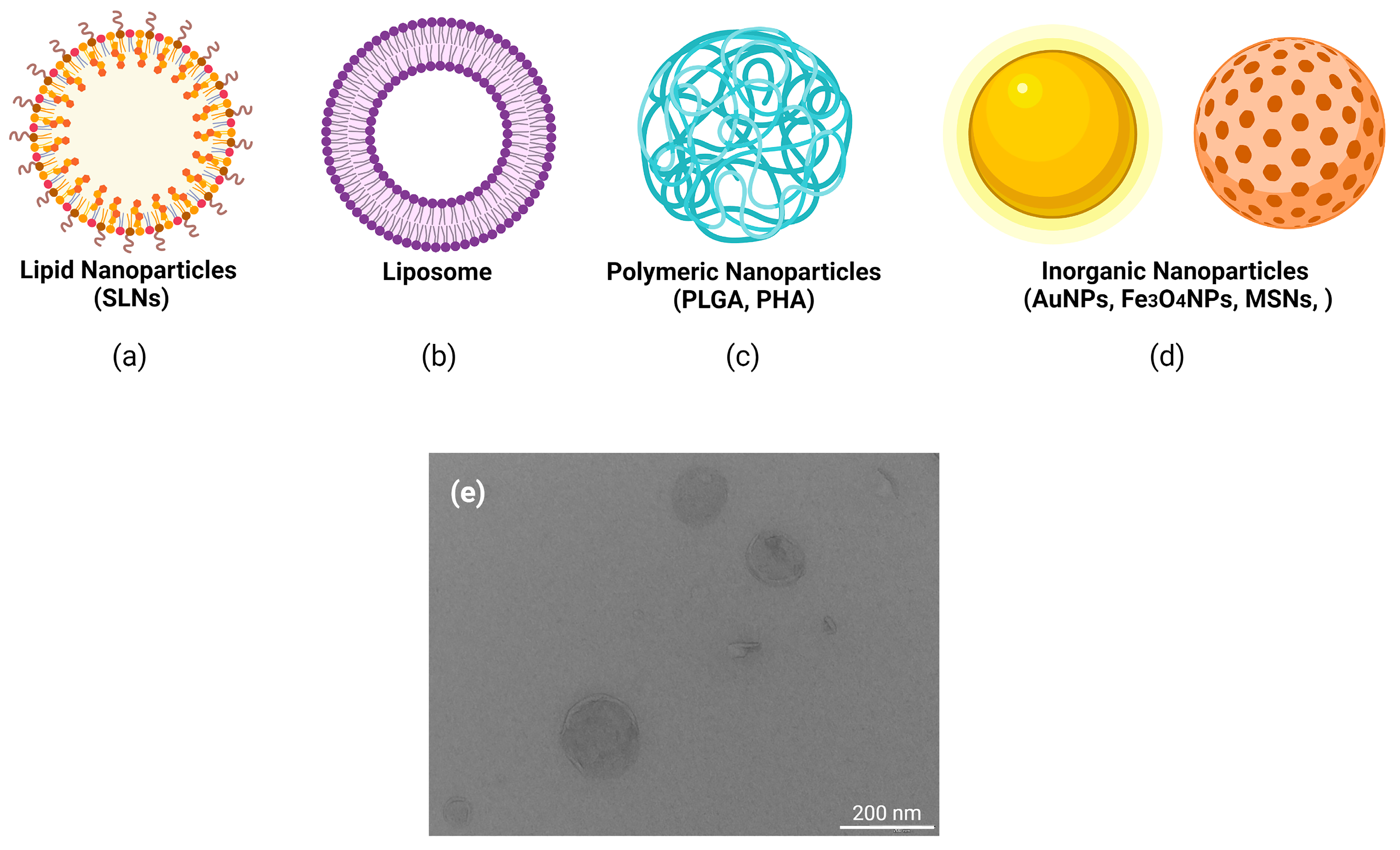
| SMaterials | Properties | Current 2021 Clinical Trials | Toxical Profiling | References |
|---|---|---|---|---|
| Inorganic | ||||
| Noble metal (Au, Ag, Pt) NPs | Biocompatible, surfaces with multiple cargo, | SP1–SP4 | Cytotoxicity, inflammation, apoptosis | [36,37,38] |
| Silica | inmunotherapy | SP5 | Cytotoxicity dose dependent. Oxidative stress, inflammation | [39,40] |
| Iron oxide (IONPs and SPIONs), Ferritine | Biocomptability, wide range of sizes and shapes | SP6–SP10 | Cytotoxicity | [41,42] |
| Carbon nontubes | ||||
| Graphene base nanomaterials | Large surface area, high charge carrier mobility and high stability | SP11–SP14 | Cytotoxicity dose dependent, particle aggregation | [43,44] |
| Organic polymers | ||||
| Proteins-stabilized NPs | ||||
| Albumin | Biocomptability, facilitate endocytosis, great loading efficiency | SP15–SP51 | Low | [45] |
| Collagen | Biocompatible, control drug releasing | Non | Low | [46,47] |
| Gelatin | Biocompatible, biodegradable | Non | Low | [48] |
| CPPs (Cell Penetrating peptides) | Translocate across biological membranes | Non | Low | [49] |
| Polysaccharides | ||||
| Chitosan | Biocompatible, biodegradable, sustain drug release, low immunogeneity | SP52 | Low | [50] |
| Alginate | Biocompatible, low immunogeneity | Non | Low | [51] |
| Lipid-based nanoparticles (LNPs) | Enhance internalization and endosomal scape | SP53–SP62 | Disruption of cell membranes and protein aggregation | [52,53,54] |
| Covalent complexation with polymers | ||||
| PLGA (poly-D,L-lactic-co-glycolic acid) | Biocompatible, biodegradable. | Non | Low | [55] |
| PEG (polyethylen glycol) | Increase circulation time and efficiency | Non | Immune-mediated side effects | [56] |
| PEI (polyethylenimine) | “Proton sponge” and facilitate endosomal scape. | Non | Oxidative stress and DNA damage. | [57] |
| Poly-L-glutamate | Biocompatible | Non | Low | [58,59] |
| Dendrimers | Well physical characterized | Non | Oxidative stress and DNA damage. | [60,61] |
| Charge-altering releasable transporters (CARTs) | Endosomal scape | Non | No tested | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hueso, M.; Mallén, A.; Suñé-Pou, M.; Aran, J.M.; Suñé-Negre, J.M.; Navarro, E. ncRNAs in Therapeutics: Challenges and Limitations in Nucleic Acid-Based Drug Delivery. Int. J. Mol. Sci. 2021, 22, 11596. https://doi.org/10.3390/ijms222111596
Hueso M, Mallén A, Suñé-Pou M, Aran JM, Suñé-Negre JM, Navarro E. ncRNAs in Therapeutics: Challenges and Limitations in Nucleic Acid-Based Drug Delivery. International Journal of Molecular Sciences. 2021; 22(21):11596. https://doi.org/10.3390/ijms222111596
Chicago/Turabian StyleHueso, Miguel, Adrián Mallén, Marc Suñé-Pou, Josep M. Aran, Josep M. Suñé-Negre, and Estanislao Navarro. 2021. "ncRNAs in Therapeutics: Challenges and Limitations in Nucleic Acid-Based Drug Delivery" International Journal of Molecular Sciences 22, no. 21: 11596. https://doi.org/10.3390/ijms222111596
APA StyleHueso, M., Mallén, A., Suñé-Pou, M., Aran, J. M., Suñé-Negre, J. M., & Navarro, E. (2021). ncRNAs in Therapeutics: Challenges and Limitations in Nucleic Acid-Based Drug Delivery. International Journal of Molecular Sciences, 22(21), 11596. https://doi.org/10.3390/ijms222111596









