Phytoestrogens (Resveratrol and Equol) for Estrogen-Deficient Skin—Controversies/Misinformation versus Anti-Aging In Vitro and Clinical Evidence via Nutraceutical-Cosmetics
Abstract
1. Introduction
2. Characteristics of Estrogens
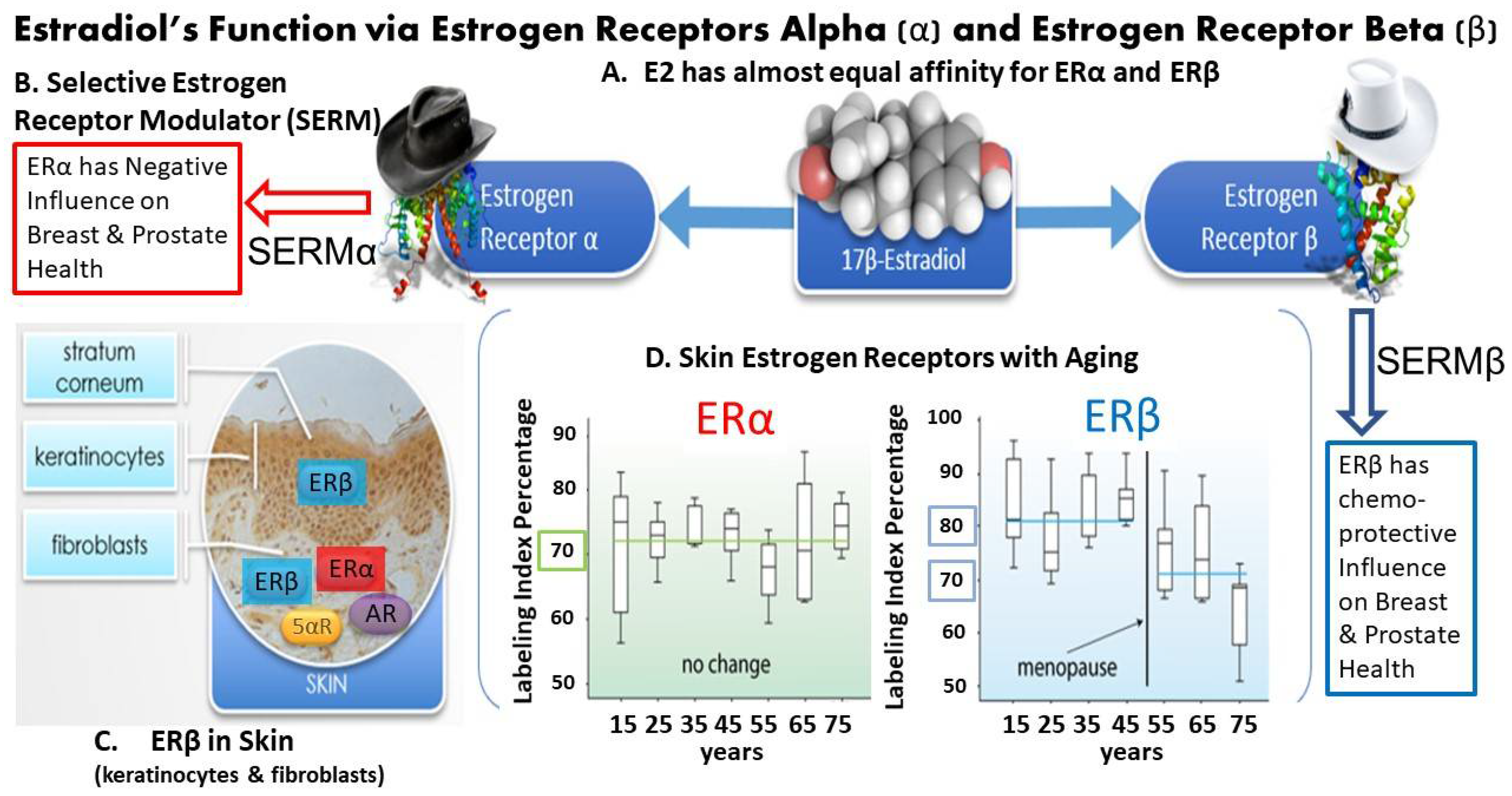
2.1. Characteristics of Estrogen Receptors
2.2. The Endocrine System, Skin Health and Hormonal Imbalance Associated with Menopause
2.3. Characteristics of Estrogen-Deficient Skin
3. How Do Things Get off Track? How Can Perspective Be Influenced Away from What Is True?
4. How Do We Know What Is True? Four Basic Elements
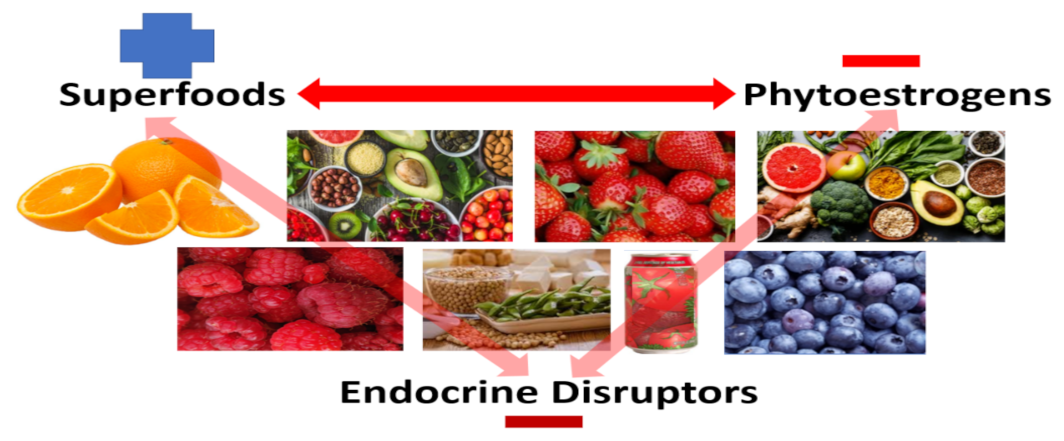
5. To Complicate Matters, “Natural,” “Pure,” “Clean,” “Organic,” and “Whole” When Applied to Food Sources May or May Not Be Entirely What One Perceives
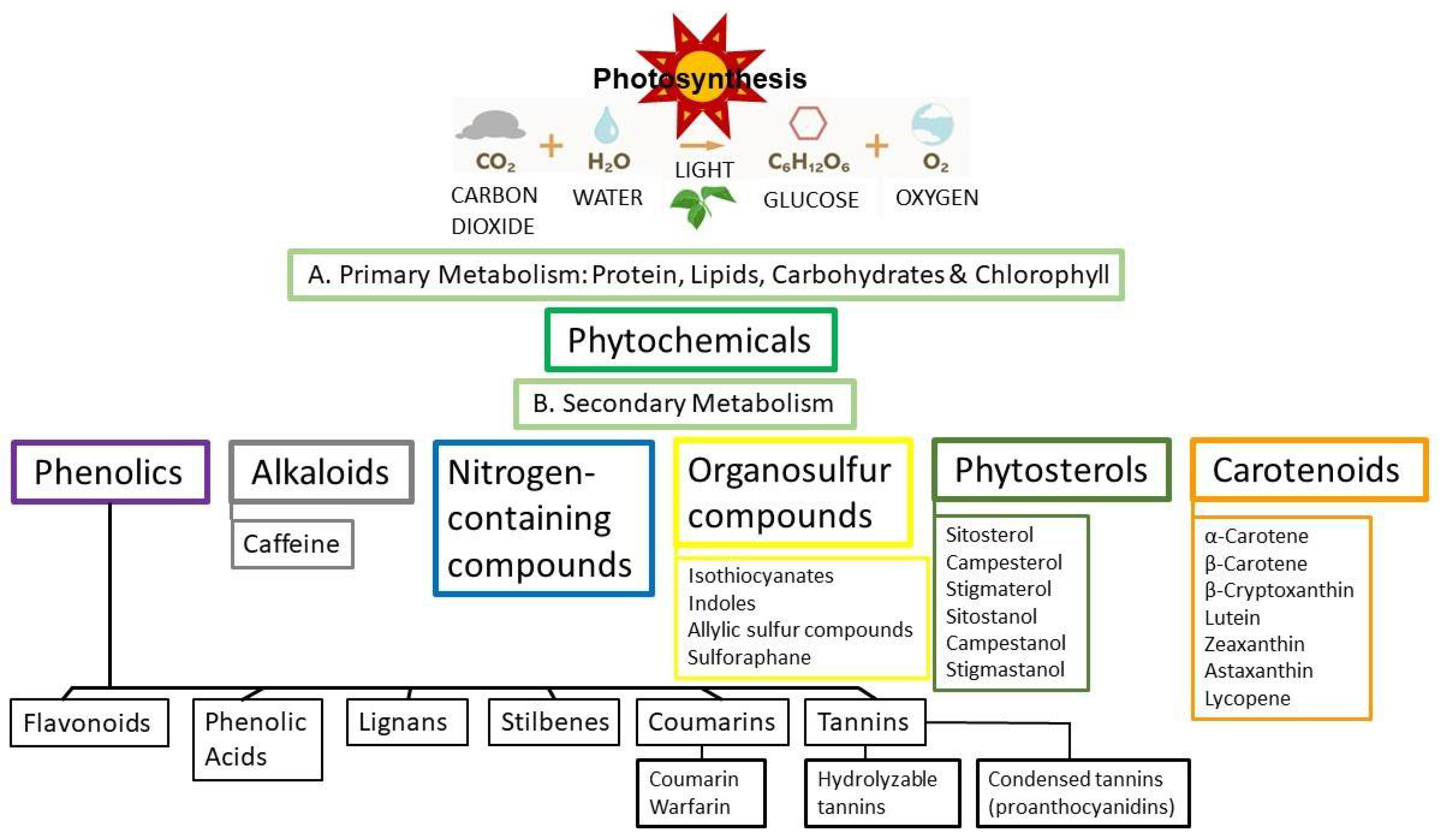
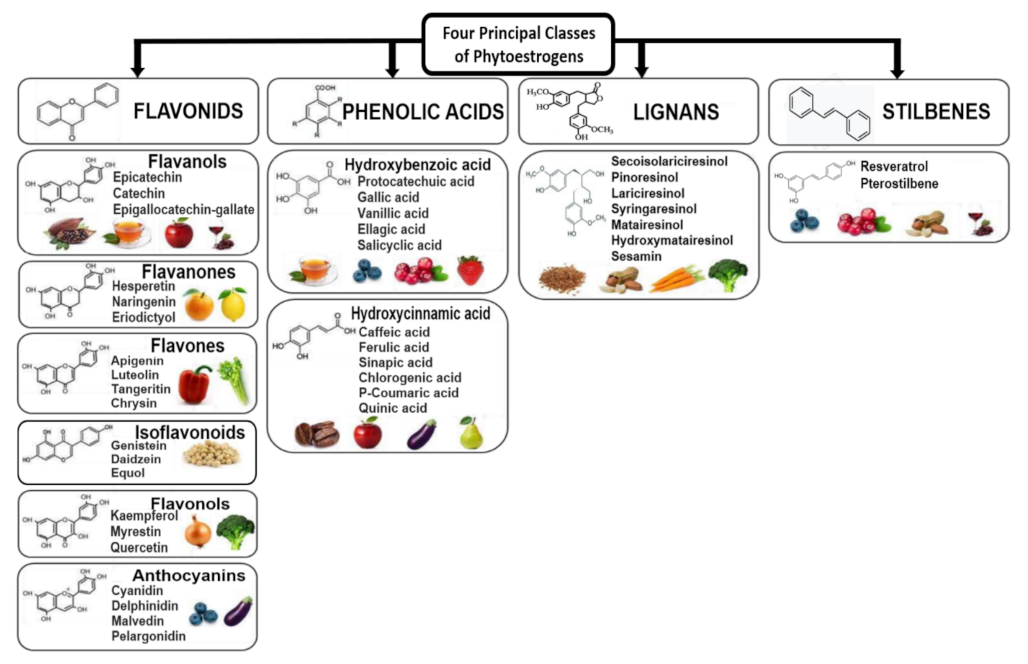
6. Phytochemicals, Polyphenols and Phytoestrogens
6.1. Soy/Phytoestrogen Controversies
6.1.1. Soy/Phytoestrogens Increase the Risk of Cancer
6.1.2. Soy/Phytoestrogens Negatively Impact Thyroid Function
6.1.3. Soy/Phytoestrogens Have Feminizing Effects on Males (Neonates, Infants, or Adults)
6.1.4. Soy/Phytoestrogens Alter Reproductive Function in Males or Females (Puberty or Biomarkers in Adults)
6.1.5. Summary and Conclusions on Controversies Associated with Phytoestrogens
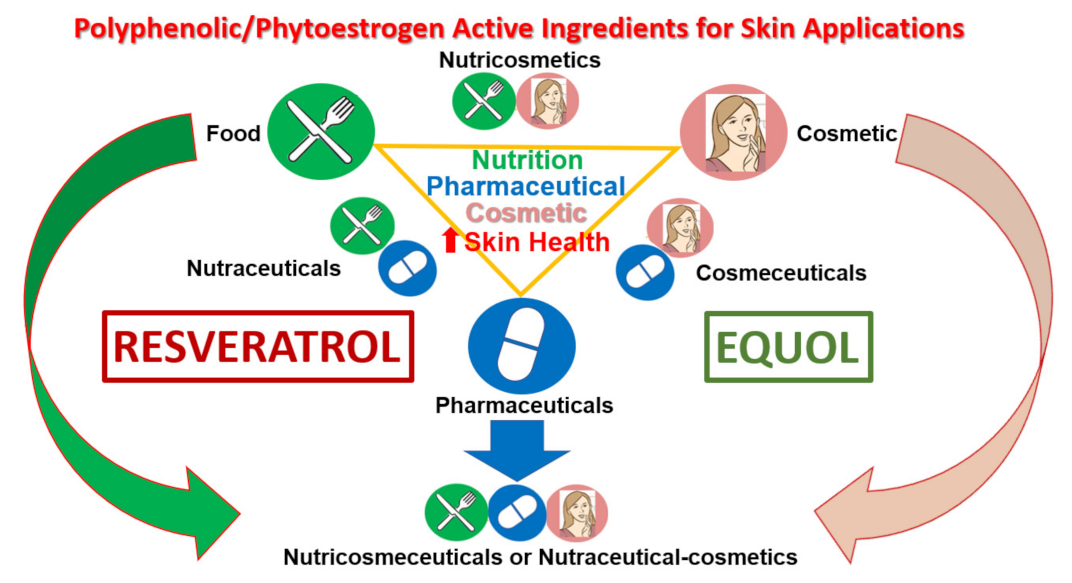
7. Polyphenols/Phytoestrogens as Nutraceutical-Cosmetics for Skin Health
7.1. Short History: From Ancient Cosmetics to Nutraceutical-Cosmetics Today
7.2. Plants and Cosmetic Innovations
8. Brief Historical Background of Resveratrol and Equol
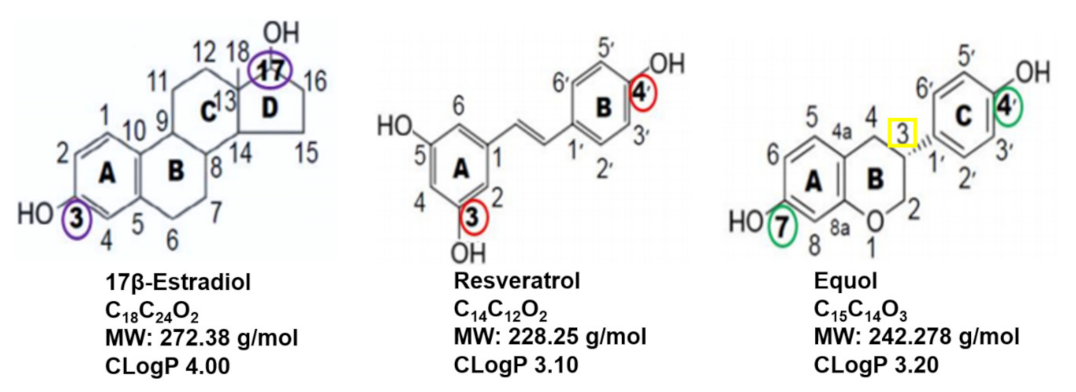
8.1. Scientific Literature Comparison of Resveratrol and Equol
8.2. Comparisons of 17β-Estradiol, Resveratrol and Equol
8.3. ER Binding Characteristics of Resveratrol, Equol and Topical/Oral Dosing
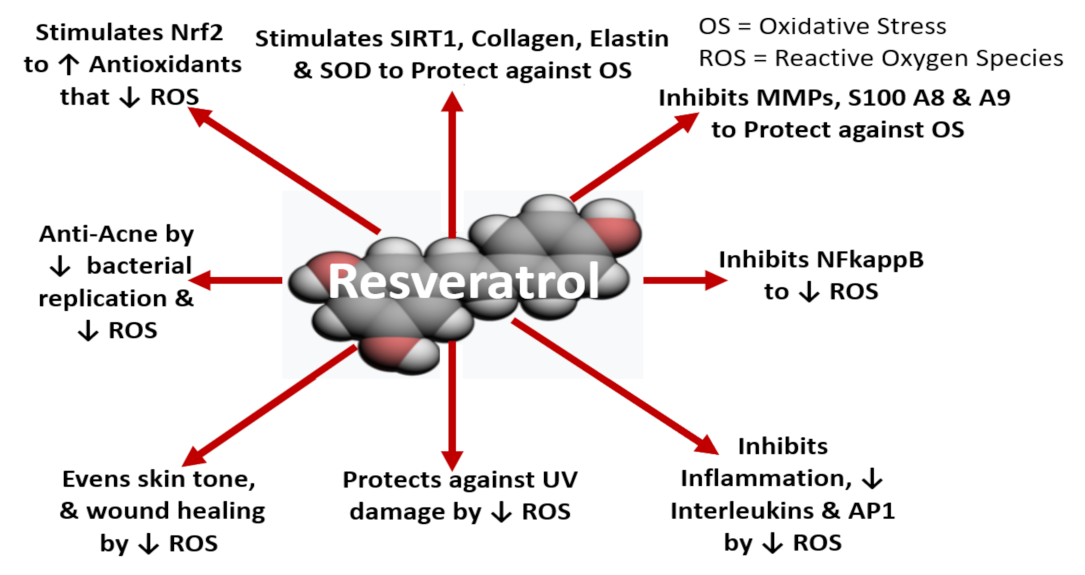
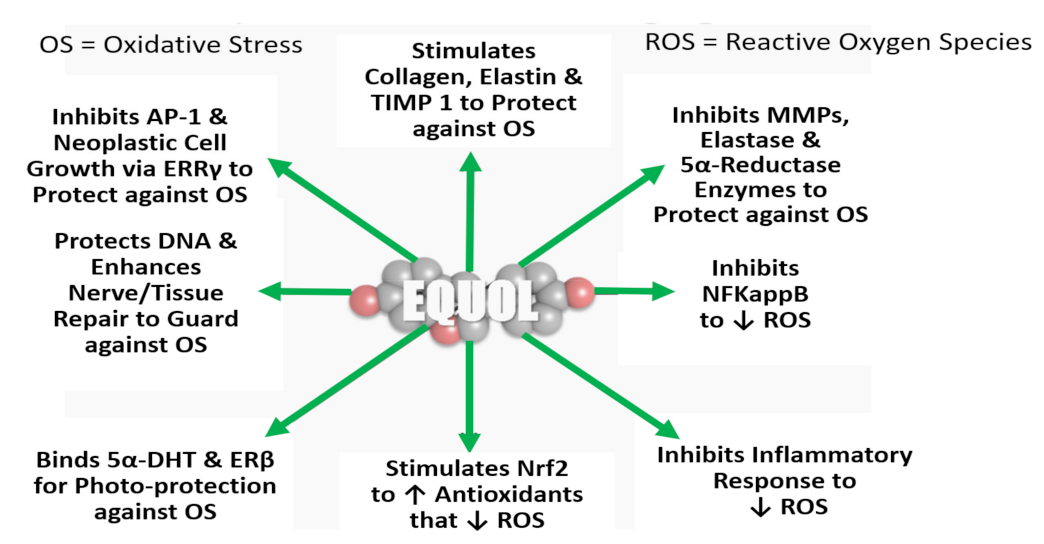
8.4. In Vitro/In Situ Evidence of Resveratrol and Equol for Nutraceutical-Cosmetics
8.4.1. Human Gene Expression of Skin Biomarkers
8.4.2. Human Skin Cell Culture Studies and Molecular Mechanism of Action
8.4.3. Clinical Skin Studies Examining Resveratrol and Equol: Oral and Topical
9. Future Directions
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Arshani, S. Before and After Photos Show How Stay-at-Home Orders Helped Los Angeles Significantly Reduce Its Notorious Smog. Business Insider. 8 April 2020. Available online: https://www.businessinsider.in/international/news/before-and-after-photos-show-how-stay-at-home-orders-helped-los-angeles-significantly-reduce-its-notorious-smog/articleshow/75045024.cms (accessed on 26 August 2021).
- Popa, D.-S.; Rusu, M.E. Isoflavones: Vegetable Sources, Biological Activity, and Analytical Methods for the Assessment. In Superfoods and Functional Food—The Development of Superfoods and Their Roles as Medicine; InTechOpen Limited: London, UK, 2017; Chapter 7; pp. 133–155. [Google Scholar] [CrossRef]
- van den Dressche, J.J.; Plat, J.; Mensink, R.P. Effects of superfoods on risk factor of metabolic syndrome: A systematic review of human intervention trials. Food Funct. 2018, 9, 1944–1966. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.A.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–233. [Google Scholar] [CrossRef]
- Resende, S.I.S.P.; Ferreira, M.; Magalhaes, C.; Sousa Lobo, J.M.; Sousa, E.; Almedia, I.F. Trends in the use of marine ingredients in anti-aging cosmetics. Algal Res. 2021, 55, 102273. [Google Scholar] [CrossRef]
- Teas, J.; Hurley, T.G.; Hebert, J.R.; Franke, A.A.; Sepkovic, D.W.; Kurzer, M.S. Dietary seaweed modifies estrogen and phytoestrogen metabolism in healthy postmenopausal women. J. Nutr. 2009, 139, 939–944. [Google Scholar] [CrossRef]
- Sychrova, E.; Stpankova, T.; Novakova, K.; Blaha, L.; Giesy, J.P.; Hilscherova, K. Estrogenic activity in extracts and exudates of cyanobacteria and green algae. Environ. Int. 2012, 39, 134–140. [Google Scholar] [CrossRef]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef]
- Lephart, E.D.; Naftolin, F. Menopause and the skin: Old favorites and new innovations in cosmeceuticals for estrogen-deficient skin. Dermatol. Ther. 2021, 11, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. A role for estrogen in skin aging and dermal biomechanics. Mech. Ageing Dev. 2021, 197, 111513. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Miki, Y.; Abe, K.; Haton, M.; Hosaka, M.; Kariya, Y.; Kakuo, S.; Fujimura, T.; Hachiya, A.; Aiba, S.; et al. The role of estrogen-metabolizing enzymes and estrogen receptors in human epidermis. Mol. Cell. Endocrinol. 2011, 344, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.-A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Blair, R.M.; Fang, H.; Branham, W.S.; Hass, B.S.; Dial, S.L.; Moland, C.L.; Tong, W.; Shi, L.; Perkins, R.; Sheehan, D.M. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: Structural diversity of ligands. Toxicol. Sci. 2000, 54, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C.J.; Tschugguel, W.; Schneeberger, C.; Huber, J.C. Production and actions of estrogens. N. Engl. J. Med. 2002, 346, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Strom, A.; Treuter, E.; Warner, M.; et al. Estrogen receptors: How do they signal and what are their targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef] [PubMed]
- Estrogen Receptor. Wikipedia. 2021. Available online: https://en.wikipedia.org/wiki/Estrogen_receptor (accessed on 31 August 2021).
- Farazneh, S.; Zarghi, A. Estrogen receptor ligands: A review (2013–2015). Sci. Pharm. 2016, 84, 409–427. [Google Scholar] [CrossRef]
- Slominski, A.; Zbytek, B.; Nikolakis, G.; Manna, P.R.; Skobowiat, C.; Zmijewski, M.; Li, W.; Janjetovic, Z.; Postlethwaite, A.; Zouboulis, C.C.; et al. Steroidogenesis in the skin: Implications for local immune function. J. Steroid Biochem. Mol. Biol. 2013, 137, 107–123. [Google Scholar] [CrossRef]
- Antonini, D.; Siblo, A.; Dentice, M.; Missero, C. An intimate relationship between thyroid hormone and skin: Regulation of gene expression. Front. Endocrinol. 2013, 4, 104. [Google Scholar] [CrossRef]
- Safer, J.D. Thyroid hormone action on skin. Dermato-Endocrinology 2011, 3, 211–215. [Google Scholar] [CrossRef]
- Chen, Y.; Luga, J. Brain-skin connection: Stress, inflammation and skin health. Inflamm. Allergy Drug Targets 2014, 13, 177–190. [Google Scholar] [CrossRef]
- Choe, S.J.; Kim, D.; Kim, E.J.; Ahn, J.-S.; Choi, E.-J.; Son, E.D.; Lee, T.R.; Choi, E.H. Psychological stress deteriorates skin barrier function by activating 11β-hydroxy steroid dehydrogenase 1 and the HPA axis. Sci. Rep. 2018, 8, 6334. [Google Scholar] [CrossRef]
- Thorton, M.J. Estrogens and skin aging. Dermato-Endocrinology 2013, 5, 264–270. [Google Scholar] [CrossRef]
- Archer, D.F. Postmenopausal skin and estrogen. Gynecol. Endocrinol. 2012, 28, 2–6. [Google Scholar] [CrossRef]
- Reus, T.L.; Brohem, C.A.; Schuck, D.C.; Lorencini, M. Revisiting the effects of menopause on the skin: Functional changes, clinical studies in vitro models and therapeutic alternatives. Mech. Ageing Dev. 2020, 185, 111193. [Google Scholar] [CrossRef]
- Reed, B.G.; Carr, B.R. The Normal Menstrual Cycle and the Control of Ovulation. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Darmouth, MA, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279054/ (accessed on 27 September 2021).
- Labrie, F.; Martel, C.; Belanger, A.; Pelletier, G. Androgens in women are essentially made from DHEA in each peripheral tissue according to intracrinology. J. Steroid Biochem. Mol. Biol. 2017, 168, 9–18. [Google Scholar] [CrossRef]
- Brzozowska, M.; Lewinski, A. Changes in androgen levels in menopausal women. Menopause Rev. 2020, 19, 151–154. [Google Scholar] [CrossRef]
- Stevenson, S.; Thornton, J. Effects of estrogens on skin aging and the potential role of SERMs. Clin. Interv. Aging. 2007, 2, 283–297. [Google Scholar]
- Lephart, E.D. Brain androgen and progesterone metabolizing enzymes: Biosynthesis, distribution, and function. Brain Res. Rev. 2001, 37, 25–37. [Google Scholar] [CrossRef]
- Gopaul, R.; Knaggs, H.; Lephart, E.D. Biochemical investigation and gene analysis of equol: A plant and soy-derived isoflavonoid with antiaging and antioxidant properties with potential human skin applications. BioFactors 2012, 38, 44–52. [Google Scholar] [CrossRef]
- Lephart, E.D. A review of the role of estrogen in dermal aging and facial attractiveness in women. J. Cosmet. Dermatol. 2018, 17, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Menczer, F.; Hills, T. Information Overload Helps Fake News Spread, and Social Media Knows It. Sci. Am. 2020, 323, 54–61. Available online: https://www.scientificamerican.com/article/information-overload-helps-fake-news-spread-and-social-media-knows-it/ (accessed on 28 August 2021).
- Armstrong, R.L. New Survey Suggests 10% of Americans Believe the Moon Landing Was Fake. 2019. Available online: https://www.satelliteinternet.com/resources/moom-landing-real-survey/ (accessed on 28 August 2021).
- McRobbie, D.W.; Moore, E.A.; Graves, M.J.; Prince, M.R. What’s the attraction? In MRI from Picture to Proton, 2nd ed.; Cambridge University Press: Cambridge, UK, 2007; pp. 1–8. [Google Scholar]
- Gazzaniga, M.S. Are there “left brain” and “right brain” types of people. In Psychological Science, 6nd ed.; W.W. Norton: New York, NY, USA, 2018; pp. 96–97. [Google Scholar]
- Nielsen, J.A.; Zielinski, B.A.; Ferguson, M.A.; Lainhart, J.E.; Anderson, J.S. An evaluation of the left-brain vs. right-brain hypothesis with resting state functional connectivity magnetic resonance imaging. PLoS ONE 2013, 8, e71275. [Google Scholar] [CrossRef]
- USA Today. 2011. Available online: https://www.indystar.com/story/news/education/2021/08/11/dan-stock-indiana-doctors-viral-mt-vernon-school-board-testimony-full-misinformation/5551476001/ (accessed on 1 September 2021).
- Science. 2021. Available online: https://www.lexico.com/en/definition/science (accessed on 2 September 2021).
- Food and Drug Administration (FDA) USA Report. Defect levels handbook: The Food Defect Action Levels of Natural or Unavoidable Defects in Foods That Present No Health Hazards for Humans. 2018. Available online: https://www.fda.gov/food/ingredients-additives-gras-packaging-guidance-documents-regulatory-information/food-defect-levels-handbook (accessed on 1 September 2021).
- Dolan, L.C.; Matulka, R.A.; Burdock, G.A. Naturally occurring food toxins. Toxins 2010, 2, 2289–2332. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.M.; Cortes, H.N.; Jenks, B.H.; Maki, K.C. Naturally occurring hormones in foods and potential health effects. Toxic. Res. Appl. 2020, 4, 1–12. [Google Scholar] [CrossRef]
- Buckingham, J. Dictionary of Natural Products on DVD; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, F.F.; de Paulo, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Banan-Mwine-Daliri, E.; Elahi, F.; Chelliah, R.; Lee, B.-H.; Oh, D.-H. New insights on the use of polyphenols as natural preservatives and their emerging safety concerns. Front. Sustain. Food Syst. 2020, 4, 52581. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmaceutical activity. BioMed Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef]
- Hassanpour, S.; Maheri-Sis, N.; Eshratkhah, B.; Mehmandar, F.B. Plants and secondary metabolites (tannins): A review. Int. J. For. Soil Erosion. 2011, 1, 47–53. [Google Scholar]
- Xavier, V.; Monti, F.-P.; Vercauteven, J.; Deffieux, G.; Merillon, J.-M. Direct liquid chromatography analysis of resveratrol derivatives and flavanonols in wines with absorbance with fluorescence detection. Anal. Chim. Acta 2002, 458, 103–110. [Google Scholar]
- Maur, W.; Aldercreutz, H. Natural and anthropogenic environmental oestrogens: The scientific basis of risk assessment. Pure Appl. Chem. 1998, 70, 1759–1776. [Google Scholar]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Woodward, K.A.; Draijer, R.; Thijssen, D.H.; Low, D.A. Polyphenols and microvascular function in humans: A systemic review. Curr. Pharm. Design 2018, 24, 203–226. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Zhao, Z.; Moghadasian, N.H. Bioavailability of hydroxycinnamates: A brief review of the in vivo and in vitro studies. Phytochem. Rev. 2010, 9, 133–145. [Google Scholar] [CrossRef]
- Ayres, D.C.; Loike, J.D. Lignans: Chemical, Biological and Clinical Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Yeung, A.W.K.; Tzvetko, N.T.; Balacheva, A.A.; Georgieva, M.G.; Gan, R.-Y.; Jozwik, A.; Pyzel, B.; Horbanczuk, J.O.; Novellino, E.; Durazzo, A.; et al. Lignans: Quantitative analysis of the research literature. Front. Pharmacol. 2020, 11, 37. [Google Scholar] [CrossRef]
- Goncalves, A.C.; Campos, G.; Alves, G.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Physical and phytochemical composition of 23 Portuguese sweet cherries as conditioned by variety (or genotype). Food Chem. 2021, 355, 127637. [Google Scholar] [CrossRef]
- Pezzuto, J. Resveratrol: Twenty years of growth, development and controversy. Biomol. Ther. 2019, 27, 1–14. [Google Scholar] [CrossRef]
- Mccormack, D.; McFadden, D. A Review of Pterostilbene Antioxidant Activity and Disease Modification. Oxid. Med. Cell. Longev. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Gorzkiewicz, J.; Bartosz, G.; Sadowska-Bartosz, I. The potential effects of phytoestrogens: The role in neuroprotection. Molecules 2021, 26, 2954. [Google Scholar] [CrossRef] [PubMed]
- Degen, G.H.; Blaszkewicz, M.; Shi, L.; Buyken, A.E.; Remer, T. Urinary isoflavone phytoestrogens in German children and adolescents- A longitudinal examination in the DONALD cohort. Mol. Nutr. Food Res. 2011, 55, 359–367. [Google Scholar] [CrossRef]
- Valentin-Blasini, L.; Sadowski, M.A.; Walden, D.; Caltabiano, L.; Needham, L.L.; Barr, D.B. Urinary phytoestrogens concentrations in the U.S. population (1999–2000). J. Expo. Anal. Environ. Epidemiol. 2005, 15, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. Soy and health update: Evaluation of the clinical and epidemiologic literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R. Assessing risks and benefit of genistein and soy. Environ. Health Perspect. 2006, 114, A332–A333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Messina, M. Soy foods, isoflavones, and the health of postmenopausal women. Am. J. Clin. Nutr. 2014, 100 (Suppl. S1), 423S–430S. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L. Soy, soy foods and their role in Vegetarian diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef]
- Messina, M.; Rogero, M.M.; Fisberg, M.; Waitzberz, D. Health impact of childhood and adolescent soy consumption. Nutr. Rev. 2017, 75, 500–515. [Google Scholar] [CrossRef]
- Reed, K.E.; Camargo, J.; Hamiltion-Reeves, J.; Kurzer, S.; Messina, M. Neither soy nor isoflavone intake affects male reproductive hormones: An expanded and updated meta-analysis of clinical studies. Reprod. Toxicol. 2021, 100, 60–67. [Google Scholar] [CrossRef]
- Messina, M. Soybean isoflavone exposure does not have feminizing effects on men: A critical examination of the clinical evidence. Fertil. Steril. 2010, 93, 2095–2104. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Mazur, W. Photo-oestrogens and Western disease. Ann. Med. 1997, 29, 95–120. [Google Scholar] [CrossRef]
- Akaza, H.; Miyanaga, N.; Takashima, N.; Naito, S.; Hirao, Y.; Tsukamoto, T.; Fujoka, T.; Mori, M.; Kim, W.-J.; Song, J.M.; et al. Comparisons of percent equol producers between prostate cancer patients and controls: Case-controlled studies of isoflvones in Japanese, Korean and American residents. Jpn. J. Clin. Oncol. 2004, 34, 86–89. [Google Scholar] [CrossRef]
- Setchell, K.D.; Clerici, C. Equol: Pharmacokinetics and biological actions. J. Nutr. 2010, 140, 1363S–1368S. [Google Scholar] [CrossRef]
- Douglas, C.C.; Johnson, S.A.; Arjmandi, B.H. Soy and its isoflavones: The truth behind the science in breast cancer. Anti-Cancer Agents Med. Chem. 2013, 13, 1178–1187. [Google Scholar] [CrossRef]
- Minatoya, M.; Kutomi, G.; Asakura, S.; Otokozawa, S.; Sugiyama, Y.; Nagata, Y.; Mori, M.; Hirata, K. Equol, adiponectin, insulin levels and risk of breast cancer. Asian Pac. J. Cancer Prev. 2013, 14, 2191–2199. [Google Scholar] [CrossRef][Green Version]
- Fitz, H.; Seely, D.; Flower, G.; Skidmore, B.; Fernandes, R.; Vadeboncoeur, S.; Kennedy, D.; Cooley, K.; Wong, R.; Sagars, S.; et al. Soy, red clover, and isoflavones and breast cancer: A systematic review. PLoS ONE 2013, 8, e81968. [Google Scholar] [CrossRef]
- Lephart, E.D. Review: Anti-oxidant and anti-aging properties of equol in prostate health (BPH). Open J. Endocrinol. Metab. Dis. 2014, 2014, 42404. [Google Scholar] [CrossRef]
- Simon, S. Soy and Cancer Risk: Our Expert’s Advice. 29 April 2019. Available online: https//www.cancer.org/latest-news/soy-and-cancer-risk-our-experts-advice.html (accessed on 6 September 2021).
- Horn-Ross, P.L.; Higgatt, K.L.; Lee, M.M. Phytoestrogens and thyroid cancer risk: The San Francisco Bay area thyroid cancer study. Cancer Epidemiol. Biomark. Prevent. 2002, 11, 43–49. [Google Scholar]
- Hampl, R.; Ostantnikova, D.; Celec, P.; Putz, Z.; Lapcik, O.; Matucha, P. Short-term effect of soy consumption on thyroid hormone levels and correlation with phytoestrogen levels in healthy subjects. Endocr. Regul. 2008, 42, 53–61. [Google Scholar]
- Messina, M.; Redmond, G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: A review of the relevant literature. Thyroid 2006, 16, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.; Polito, F.; Adamo, E.B.; Bitto, A.; Squadrito, R.; Denvenga, S. Update on genistein and thyroid: An overall message of safety. Front. Endocrinol. 2012, 3, 94. [Google Scholar] [CrossRef] [PubMed]
- EFSA ANS PANEL (EFSA Panel on Food Additives and Nutrient Sources added to Food). Scientific opinion on the risk assessment for per- and post-menopausal women taking food supplements containing isolated isoflavones. EFSA J. 2015, 4246, 342. [Google Scholar]
- Sathyapalan, T.; Dawson, A.J.; Rigby, A.S.; Thatcher, N.J.; Kilpatrick, E.S.; Atkin, S.L. The effect of phytoestrogen on thyroid in subclinical hypothyroidism: Randomized, double blind, crossover study. Front. Endocrinol. 2018, 9, 531. [Google Scholar] [CrossRef]
- Otun, J.; Sahebkar, A.; Ostlundh, L.; Atkin, L.; Sathyapalan, T. Systematic review and meta-analysis on the effect of soy on thyroid function. Sci. Rep. 2019, 9, 3964. [Google Scholar] [CrossRef]
- Nippoldt, T.B. Soy: Does It Worsen Hypothyroidism? Mayo Clinic E-Newsletter. 10 October 2019. Available online: https://www.mayoclinic.org/disease-conditions/hypothyroidism/expert-answers/hypothyroidism/faq-20055188 (accessed on 27 September 2021).
- Jing, H.; Gilchrist, J.M.; Badger, T.M.; Pivik, R.T. A longitudinal study of differences in electroencephalographic activity among breastfed, milk-formula fed, and soy-formula-fed infants during the first year of life. Early Hum. Dev. 2010, 86, 119–125. [Google Scholar] [CrossRef]
- Gilchrist, J.M.; Moore, M.B.; Andres, A.; Estroff, J.A.; Badger, T.M. Ultrasonographic patterns of reproductive organs in infants fed soy formula: Comparison to infants fed breast milk and milk formula. J. Pediatr. 2010, 156, 215–220. [Google Scholar] [CrossRef]
- Andres, A.; Moore, M.B.; Linam, L.E.; Casey, P.H.; Cleves, M.S.; Badger, T.M. Compared with feeding infants breast milk or cow-milk formula, soy formula feeding does not affect subsequent reproductive organ size at 5 years of age. J. Nutr. 2015, 145, 871–875. [Google Scholar] [CrossRef]
- Giampietro, P.G.; Bruno, G.; Furcolo, G.; Casati, A.; Brunetti, E.; Spadoni, G.L.; Galli, E. Soy protein formulas in children: No hormonal effects in long-term feeding. J. Pediatr. Endocrinol. Metab. 2004, 17, 191–196. [Google Scholar] [CrossRef]
- Hamilton-Reeves, J.M.; Vazquez, G.; Duval, S.J.; Phipps, W.R.; Kurzer, M.S.; Messina, M.J. Clinical studies show no effects of soy protein or isoflavones on reproductive hormones in men: Results of a meta-analysis. Fertil. Steril. 2010, 94, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Sathyapalan, T.; Rigby, A.S.; Bhasin, S.; Thatcher, N.J.; Kilpatrick, E.S.; Atkin, S.L. Effect of soy in men with type 2 diabetes mellitus and subclinical hypogonadism: A randomized controlled study. J. Clin. Endocrinol. Metab. 2017, 102, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Fara, G.M.; Del Covo, G.; Bermuzzi, S.; Bigatello, A.; Di Pietro, C.; Scaglioni, S.; Chiumello, G. Epidemic of breast enlargement in an Italian school. Lancet 1979, 314, 295–297. [Google Scholar] [CrossRef]
- Sanez de Rodriguez, C.A.; Bongiovanni, A.M.; Condo de Begrego, L. An epidemic of precocious development in Puerto Rican children. J. Pediatr. 1985, 107, 393–396. [Google Scholar] [CrossRef]
- Safe, S.H. Endocrine disruptors and human health-Is there a problem? An update. Environ. Health Perspect. 2000, 108, 487–493. [Google Scholar] [PubMed]
- Schwarcz, J. Silent Spring; McGill University, Office of Science and Society: Montreal, QC, Canada, 2017; Available online: https://www.mcgill/ca.oss/article/health-nutrition-history-quackery/silent-spring (accessed on 8 September 2021).
- Parish, L.C.; Crissey, J.T. Cosmetics: A historical review. Clin. Dermatol. 1988, 6, 1–4. [Google Scholar] [CrossRef]
- Kurke, J. A History of Cosmetics from Ancient Times. 2021. Available online: https://cosmeticsinfo.org/Ancient-history-cosmetics (accessed on 8 September 2021).
- Chaudhri, S.K.; Jain, N.K. History of cosmetics. Asian J. Pharm. 2009, 3, 164–167. [Google Scholar]
- Draelos, Z.D. Cosmetics and skin care products. A historical perspective. Dermatol. Clin. 2000, 18, 557–559. [Google Scholar] [CrossRef]
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalherio, M.; Ribeiro, H.M.; Simoes, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Feetham, H.J.; Jeong, H.S.; McKesey, J.; Wickless, H.; Jacobe, H. Skin care and cosmeceuticals: Attitudes and trends among trainees and educators. J. Cosmet. Dermatol. 2018, 17, 220–226. [Google Scholar] [CrossRef]
- Hernandez, D.F.; Cervantes, E.L.; Luna-Vital, D.A.; Mojica, L. Food-derived bioactive compounds with anti-aging potential for nutricosmetic and cosmeceutical products. Crit. Rev. Food Sci. Nutr. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Faccio, G. Plant complexity and cosmetic innovation. iScience 2020, 23, 101358. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Allshouse, A.; Pavlovic, J.; Santoro, N. Menstrual cycle hormone changes associated with reproductive aging and how they relate to symptoms. Obstet. Gynecol. Clin. N. Am. 2018, 45, 613–628. [Google Scholar] [CrossRef]
- Grieger, J.A.; Norman, R.J. Menstrual cycle length and patterns of global cohort of women using a mobile phone app: Retrospective cohort study. J. Med. Internet Res. 2020, 22, e17109. [Google Scholar] [CrossRef]
- Cavinato, M. Cosmetics and Cosmeceuticals. Encycl. Biomed. Gerontol. 2020, 1, 446–460. [Google Scholar] [CrossRef]
- Lephart, E.D. Resveratrol, 4′ acetoxy resveratrol, R-equol, racemic equol or S-equol as cosmeceuticals to improve dermal health. Int. J. Mol. Sci. 2017, 18, 1193. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Milanovic, M.; Natasa, N.; Aiello, V.; De Lorenzo, A.; Abenavoli, L.A. A systematic review of natural antioxidant properties of resveratrol. Nat. Prod. Commun. 2018, 13, 1195–1203. [Google Scholar] [CrossRef]
- Takaoka, M. Resveratrol, a new phenolic compound form Veratrum Grandiflorum. J. Chem. Jpn. 1939, 60, 1090–1100. [Google Scholar]
- Burns, J.; Yokota, T.; Asihara, H.; Lean, M.E.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhou, J.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health benefits and molecular mechanisms of resveratrol: A narrative review. Foods 2020, 9, 340. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005, 81, 1072–1079. [Google Scholar] [CrossRef]
- Bannwart, T.M.; Adlercretuz, H.; Fotsis, T.; Wahala, K.; Hare, T.; Barrow, G. Identification of isoflavonic phytoestrogens and lignans in urine of human and in cow milk by GC/MS. Adv. Mass. Spec. 1986, 10, 622–661. [Google Scholar]
- Lephart, E.D. Determination of S- and R-equol in plant-based food products and efficacy of topical or oral 4′,7-isoflavandiol (R/S/ equol) to improve skin health in adult men, a placebo-controlled pilot study. J. Funct. Foods 2021, 83, 104563. [Google Scholar] [CrossRef]
- Fatima, A.; Khan, M.S.; Ahmad, M.W. Therapeutic potential of equol: A comprehensive review. Curr. Pharm. Des. 2020, 26, 5837–5843. [Google Scholar] [CrossRef]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptor α and β. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef]
- Mueller, S.O.; Simon, S.; Chae, K.; Metzler, M.; Korach, K.S. Phytoestrogens and their human metabolites show distinct agonistic and antagonist properties on estrogen receptor α (ERα) and ERβ in human cells. Toxicol. Sci. 2004, 80, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D.; Acerson, M.J.; Andrus, M.B. Synthesis and skin gene analysis of 4′acetoxy-resveratrol (4AR), therapeutic potential for dermal applications. Bioorg. Med. Chem. Lett. 2016, 26, 3258–3262. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D.; Andrus, M.B. Human skin gene expression: Natural (trans) resveratrol versus five resveratrol analogs for dermal applications. Exp. Biol. Med. 2017, 242, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.A.; Sanguansri, L.; Lockett, R. Nano- and micro-encapsulated systems for enhancing the delivery of resveratrol. Ann. N. Y. Acad. Sci. 2013, 1, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.; Vaz-da-Silva, M.; Falcao, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.F.; Nunes, T.; Wright, L.; et al. Pharmacokinetics and safety profile of trans-resveratrol in a rising multiple-dose study in health voluneeters. Mol. Nutr. Food Res. 2009, 52 (Suppl. S1), S7–S15. [Google Scholar] [CrossRef]
- Cottant, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Bwaudeux, J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Farris, P.; Krutmann, J.; Li, Y.-H.; McDaniel, D.; Krol, Y. Resveratrol: A unique antioxidant offering a multi-mechanistic approach for treating aging skin. J. Drugs Dermatol. 2013, 12, 1389–1394. [Google Scholar]
- Qasem, R.J. The estrogenic activity of resveratrol: A comprehensive review of in vitro and in vivo evidence and the potential for endocrine disruption. Crit. Rev. Toxicol. 2020, 5, 439–462. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Zhao, X.; Jha, P.; Heubi, J.E.; Brown, N.M. The pharmacokinetic behavior of the soy isoflavone metabolite s-equol and its diastereoisomer r-equol in healthy adults determined by using stable-isotope-labeled tracers. Am. J. Clin. Nutr. 2009, 90, 1029–1037. [Google Scholar] [CrossRef]
- Lephart, E.D. Equol’s efficacy is greater than astaxanthin for antioxidants, extracellular matrix integrity & breakdown, growth factors and inflammatory biomarkers via human skin gene expression analysis. J. Funct. Foods 2019, 59, 380–393. [Google Scholar]
- Davinelli, S.; Nadia, S.; Visentin, M.; Zella, D.; Scapagnini, G. Enhancement of mitochondrial biogenesis with polyphenols: Combined effects of resveratrol and equol in human endothelial cells. Immun. Aging 2013, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D.; Sommerfeldt, J.M.; Andrus, M.B. Resveratrol: Influences on gene expression in human skin. J. Funct. Foods. 2014, 10, 377–384. [Google Scholar] [CrossRef]
- Chedea, V.S.; Vicas, S.I.; Sticozzi, C.; Pessina, F.; Frosini, M.; Maioli, E.; Valacchi, G. Resveratrol: From diet to topical usage. Food Funct. 2017, 8, 3879–3892. [Google Scholar] [CrossRef]
- Ratz-Lyko, A.; Arct, J. Resveratrol as an active ingredient for cosmetic and dermatological applications: A review. J. Cosmet. Laser Ther. 2019, 21, 84–90. [Google Scholar] [CrossRef]
- Gugleva, V.; Zasheva, S.; Hristova, M.; Andonova, V. Topical use of resveratrol: Technological aspects. Pharmacia 2020, 67, 89–94. [Google Scholar] [CrossRef]
- Lephart, E.D. 4′,7-Isoflavandiol (Equol) enhances human dermal fibroblast renewal and has effects similar to 17β-estradiol in stimulating collagen and elastin expression. Cell cycle and RT-PCR analysis without Phenol Red. Cosmetics 2021, 8, 5. [Google Scholar] [CrossRef]
- Kang, J.A.; Yoon, Y.D.; Han, M.H.; Han, S.B.; Lee, K.; Kang, M.R.; Moon, E.Y.; Jeon, Y.J.; Park, S.K.; Kim, H.M. Estrogen receptor-independent inhibition of tumor necrosis factor-alpha gene expression by phytoestrogen equol is mediated by blocking nuclear factor-kappa β activation in mouse macrophages. Biochem. Pharm. 2005, 71, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.J.; Lee, K.W.; Rogozin, E.A.; Cho, Y.-Y.; Heo, Y.-S.; Bode, A.M.; Lee, H.J.; Dong, Z. Equol inhibits neoplastic cell transformation by targetin the MEK/ERK/p90RSK/activator protein—1 pathway. J. Biol. Chem. 2007, 45, 32856–32866. [Google Scholar] [CrossRef]
- Hirvonen, J.; Rajalin, A.M.; Wohlfart, G.; Adlercreutz, H.; Wahala, K.; Aarnisalo, P. Transcriptional activation of estrogen-receptor-related receptor gamma (ERRgamma) is stimulated by the phytoestrogen equol. J. Steroid. Biochem. Mol. Biol. 2011, 123, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liang, X.Y.; Shi, L.Y.; Wang, L.; Chen, J.L.; Kang, C.; Zhu, J.D.; Mi, M.T. Estrogen receptor and PI3K/Akt signaling pathway involvement in S-equol-induced activation of Nrf2/ARE in endothelia cells. PLoS ONE 2013, 8, e79075. [Google Scholar] [CrossRef]
- Widyarini, S.; Domanski., D.; Painter, N.; Reeve, V.E. Estrogen receptor signaling protects against immune suppression by UV radiation exposure. Proc. Natl. Acad. Sci. USA 2006, 103, 12837–12842. [Google Scholar] [CrossRef] [PubMed]
- Pomari, E.; Valle, L.D.; Pertile, P.; Colombo, L.; Thorton, M.J. Intracrine sex steroid synthesis and signaling in human epidermal keratinocytes and dermal fibroblasts. FASEB J. 2015, 29, 508–524. [Google Scholar] [CrossRef]
- Widyarini, S.; Domanski, D.; Painter, N.; Reeve, V.E. Photoimmune protective effect of the phytoestrogenic isoflavonoid equol is partially due to its antioxidant activities. Photochem. Photobiol. Sci. 2012, 11, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Widyarini, C.; Allanson, M.; Gallagher, N.L.; Pedley, J.; Boyle, G.M.; Parsons, P.G.; Whiteman, D.C.; Walker, C.; Reeve, V.E. Isoflavonoid photoprotection in mouse and human skin is dependent on metallothionein. J. Investig. Dermatol. 2006, 126, 198–204. [Google Scholar]
- Oyama, A.; Ueno, T.; Uchiyama., S.; Aihara, T.; Miyake, A.; Kondo, S.; Matsunage, K. The effects of natural S-equol supplementation on skin aging in postmenopausal women: A polit randomized placebo-controlled trail. Menopause 2012, 19, 202–210. [Google Scholar] [CrossRef]
- Magnet, U.; Urbanek, C.; Gaisberger, D.; Tomeva, E.; Dum, E.; Pointner, A.; Haslberger, A.G. Topical equol preparation improves structural and molecular skin parameters. Int. J. Cosmet. Sci. 2017, 39, 535–542. [Google Scholar] [CrossRef]
- Woodby, B.; Penta, K.; Perorelli, A.; Lila, M.A.; Valacchi, G. Skin health from the inside out. Annu. Rev. Food Sci. Technol. 2020, 11, 235–254. [Google Scholar] [CrossRef]
- Lin, M.-H.; Hung, C.-F.; Sung, H.-C.; Yang, S.-C.; Yu, H.-P.; Fang, J.-Y. The bioactivities of resveratrol and its naturally occurring derivatives on skin. J. Food Drug Analysis. 2021, 29, 15–38. [Google Scholar] [CrossRef]
- Liu, T.; Li, N.; Yan, Y.-Q.; Liu, Y.; Xiong, K.; Liu, Y.; Xia, Q.-M.; Zhang, H.; Liu, Z.-D. Recent advances in the anti-aging effects of phytoestrogens on collagen, water content, and oxidative stress. Phytother. Res. 2020, 34, 435–447. [Google Scholar] [CrossRef]
- Hausenblas, H. Effects of Resveratrol and Collagen Supplementation on Facial Aging. Nat. Med. J. 2013, 5, 1–8. Available online: https://www.naturalmedicinejournal.com/journal/2013/effects–resveratrol–and–collagen–supplementation–facial–aging (accessed on 28 September 2021).
- Davinelli, S.; Scapagnini, G.; Marzatico, F.; Nobile, V.; Ferrara, N.; Corbi, G. Influence of equol and resveratrol supplementation on health-related quality of life in menopausal women: A randomized, placebo-controlled study. Maturitas 2017, 96, 77–83. [Google Scholar] [CrossRef]
- Farris, P.; Yatskayer, M.; Chen, N.; Krol, Y.; Oresajo, C. Evaluation of efficacy and tolerance of a nighttime topical antioxidant containing resveratrol, baicalin, and vitamin E for treatment of mild to moderate photodamaged skin. J. Drugs Dermatol. 2014, 13, 1467–1472. [Google Scholar] [PubMed]
- Brinke, A.S.; Janssens-Bocker, C.; Kerscher, M. Skin anti-aging benefits of a 2% resveratrol emulsion. J. Cosmet. Dermatol. Sci. Appl. 2021, 11, 155–168. [Google Scholar]
- Lephart, E.D. Protective effects of equol and their polyphenolic isomers against dermal aging: Microarray/protein evidence with clinical implications and unique delivery into human skin. Pharm. Biol. 2014, 51, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Poluzzi, E.; Piccinni, C.; Raschi, E.; Rampa, A.; Recanatini, M.; De Ponti, F. Phytoestrogens in postmenopause: The state of the art from a chemical, pharmacological, and regulatory perspective. Curr. Med. Chem. 2014, 21, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Desmawati, D.; Sulastri, D. Phytoestrogens and their health effects. Maced. J. Med. Sci. 2019, 7, 495–499. [Google Scholar] [CrossRef]
- Pabich, M.; Materska, M. Biological effect of soy isoflavones in the prevention of civilization diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef]
- Kim, I.-S. Current perspectives on the biological effects of soybean isoflavones and their metabolites for humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef]
- Hernando, C.; Ortega-Morillo, B.; Tapia, M.; Moragon, S.; Matrinez, M.T.; Eroles, P.; Garrido-Cano, I.; Adam-Artigues, A.; Lluch, A.; Bermejo, B.; et al. Oral selective estrogen receptor degraders (SERDs) as a novel breast cancer therapy: Present and future form a clinical prespective. Int. J. Mol. Sci. 2021, 22, 7812. [Google Scholar] [CrossRef]
- Boo, Y.C. Human skin lightening efficacy of resveratrol and its analogs: From in vitro studies to cosmetic applications. Antioxidants 2019, 8, 332. [Google Scholar] [CrossRef]
- Lephart, E.D. The nutricosmeceutical: Topical 4′,7-isoflavandiol (equol) plus other active ingredients improves skin parameters in adult and post-menopausal women greater than equol treatment alone. Cutis 2021, in press. [Google Scholar]
- Yarkent, C.; Gurlek, C.; Oncel, S.S. Potential of microalgal compounds in trending natural cosmetics: A review. Sustain. Chem. Pharm. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Vollmer, D.L.; West, V.A.; Lephart, E.D. Enhancing skin health: By oral administration of natural compounds and minerals with implications to the dermal microbiome. Int. J. Mol. Sci. 2018, 19, 3059. [Google Scholar] [CrossRef] [PubMed]
- Nobile, V.; Schiano, I.; Peral, A.; Girdina, S.; Sparta, E.; Caturla, N. Antioxidant and reduced skin-ageing effects of a polyphenol-enriched dietary supplement in response to air pollution: A randomized, double-blind, placebo-controlled study. Food Nutr. Res. 2021, 65, 5619. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. Equol’s anti-aging effects protect against environmental assaults by increasing skin antioxidant defense and ECM proteins while decreasing oxidative stress and inflammation. Cosmetics 2018, 5, 16. [Google Scholar] [CrossRef]
- Boo, Y.C. Can plant phenolic compounds protect the skin from airborne particulate matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef]

| Gene | Resveratrol | 4′ Acetoxy-Resveratrol | R-Equol | Racemic Equol | S-Equol |
|---|---|---|---|---|---|
| Anti-Aging ▲ and Aging Factors ▼ | |||||
| SIRT1 ▲ | +180 | +335 | NA | +190;↑ with Resveratrol • | NA |
| PCNA ▲ | +780 | +540 | +235 | +285 to +300 | +325 |
| NGF ▲ | +800 | +672 | +3350 | +2860 | +1620 |
| 5α-Reductase ▼ | NSA | NSA | NA | −180 | NA |
| S100 A8 ▼ | −340 | −270 | −2050 | −1000 to −2230 | −580 |
| S100 A9 ▼ | −290 | −160 | −1850 | −1180 to −2250 | −525 |
| Extracellular Matrix Proteins: (that enhance collagen & elastin) | |||||
| COLI alpha 1 | +225 | NSA | +210 | +235 | +185 |
| COL III alpha 1 | +230 | +220 | NSA | NSA | NSA |
| COL IV alpha 1 | +160 | +170 | NSA | +210 | NSA |
| Elastin | +180 | +280 | NSA | +175 to +270 | +1 |
| TIMP 1 | +215 | +250 | +2 | +200 to +540 | +150 |
| LOX | +180 | +190 | NA | NA | NA |
| Degrading Collagen/Elastin Enzymes | |||||
| MMP 1 | −180 | NA | −890 | −540 | −325 |
| MMP 3 | NSA | NA | −885 | −800 | −330 |
| MMP 9 | −485 | NA | −1375 | −1010 to −1080 | −710 |
| Antioxidants ■ plus Heavy Metal Binder/Anti-inflammatory Mediators ◊ | |||||
| CAT ■ | +180 | +160 | NA | NA | NA |
| SOD 1 ■ | +160 | +160 | NA | +200 | NA |
| SOD 2 ■ | +160 | +170 | NA | +130 to +200 | NA |
| TXNRD1 ■ ◊ | NSA | NSA | NA | +215 to +250 | NA |
| MTH 1 ■ ◊ | +4100 | +6400 | +2100 | +1800 to +2310 | +3840 |
| MTH 2 ■ ◊ | +200 | +340 | NA | +510 | NA |
| Inflammatory Factors | |||||
| IL-1A | −2200 | −1010 | −1385 | +1700 | −990 |
| IL-1R2 | −590 | −190 | −1730 | +2200 | −1675 |
| IL-6 | −3200 | −3520 | −550 | −455 | −375 |
| IL-8 | −790 | −380 | −295 | −345 | −445 |
| TNFRSF1A | −160 | −140 | −310 | −250 to −665 | −205 |
| COX 1 | NSA | NSA | −360 | −265 | −200 |
| COX 2 | NSA | −170 | NSA | +155 | NSA |
| Week 12 | Equol Plus Natural Ingredients (ENI) | Equol Alone | Increase Over (EA) |
|---|---|---|---|
| 1. Skin Firmness | 91% * | 73% | 18% |
| 2. Smoothness | 98% * | 63% | 35% |
| 3. Even Skin Tone | 98% * | 57% | 41% |
| 4. Frown Lines/Wrinkles | 89% * | 65% | 24% |
| 5. Radiance/Brightness | 98% * | 63% | 35% |
| 6. Pore Size | 93% * | 20% | 73% |
| 7. Spots/Discoloration | 84% * | 31% | 53% |
| 8. Hydration | 95% * | 61% | 34% |
| Number of Subjects | 42 | 49 | |
| Mean Age (years + SEM) | 57.3 + 7.3 | 56.7 + 8.78 | |
| Age Range (years) | 40–70 | 40–70 | |
| Caucasian (number subjects) | 23 | 30 | |
| Chinese (number subjects) | 4 | 8 | |
| Japanese (number subjects) | 15 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lephart, E.D. Phytoestrogens (Resveratrol and Equol) for Estrogen-Deficient Skin—Controversies/Misinformation versus Anti-Aging In Vitro and Clinical Evidence via Nutraceutical-Cosmetics. Int. J. Mol. Sci. 2021, 22, 11218. https://doi.org/10.3390/ijms222011218
Lephart ED. Phytoestrogens (Resveratrol and Equol) for Estrogen-Deficient Skin—Controversies/Misinformation versus Anti-Aging In Vitro and Clinical Evidence via Nutraceutical-Cosmetics. International Journal of Molecular Sciences. 2021; 22(20):11218. https://doi.org/10.3390/ijms222011218
Chicago/Turabian StyleLephart, Edwin D. 2021. "Phytoestrogens (Resveratrol and Equol) for Estrogen-Deficient Skin—Controversies/Misinformation versus Anti-Aging In Vitro and Clinical Evidence via Nutraceutical-Cosmetics" International Journal of Molecular Sciences 22, no. 20: 11218. https://doi.org/10.3390/ijms222011218
APA StyleLephart, E. D. (2021). Phytoestrogens (Resveratrol and Equol) for Estrogen-Deficient Skin—Controversies/Misinformation versus Anti-Aging In Vitro and Clinical Evidence via Nutraceutical-Cosmetics. International Journal of Molecular Sciences, 22(20), 11218. https://doi.org/10.3390/ijms222011218





