Extracellular Environment-Controlled Angiogenesis, and Potential Application for Peripheral Nerve Regeneration
Abstract
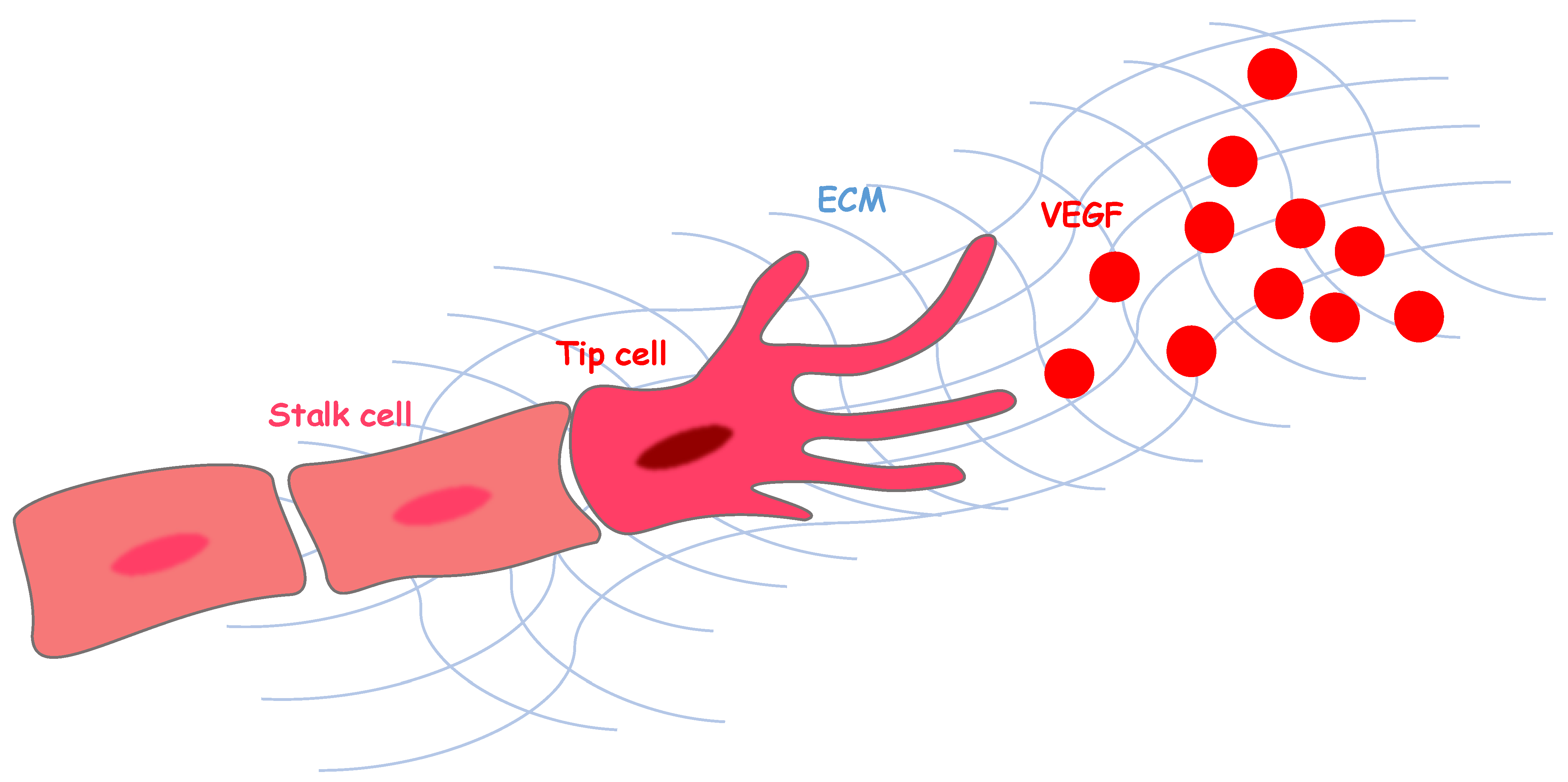
1. Angiogenesis and Vascular Endothelial Growth Factor Signaling
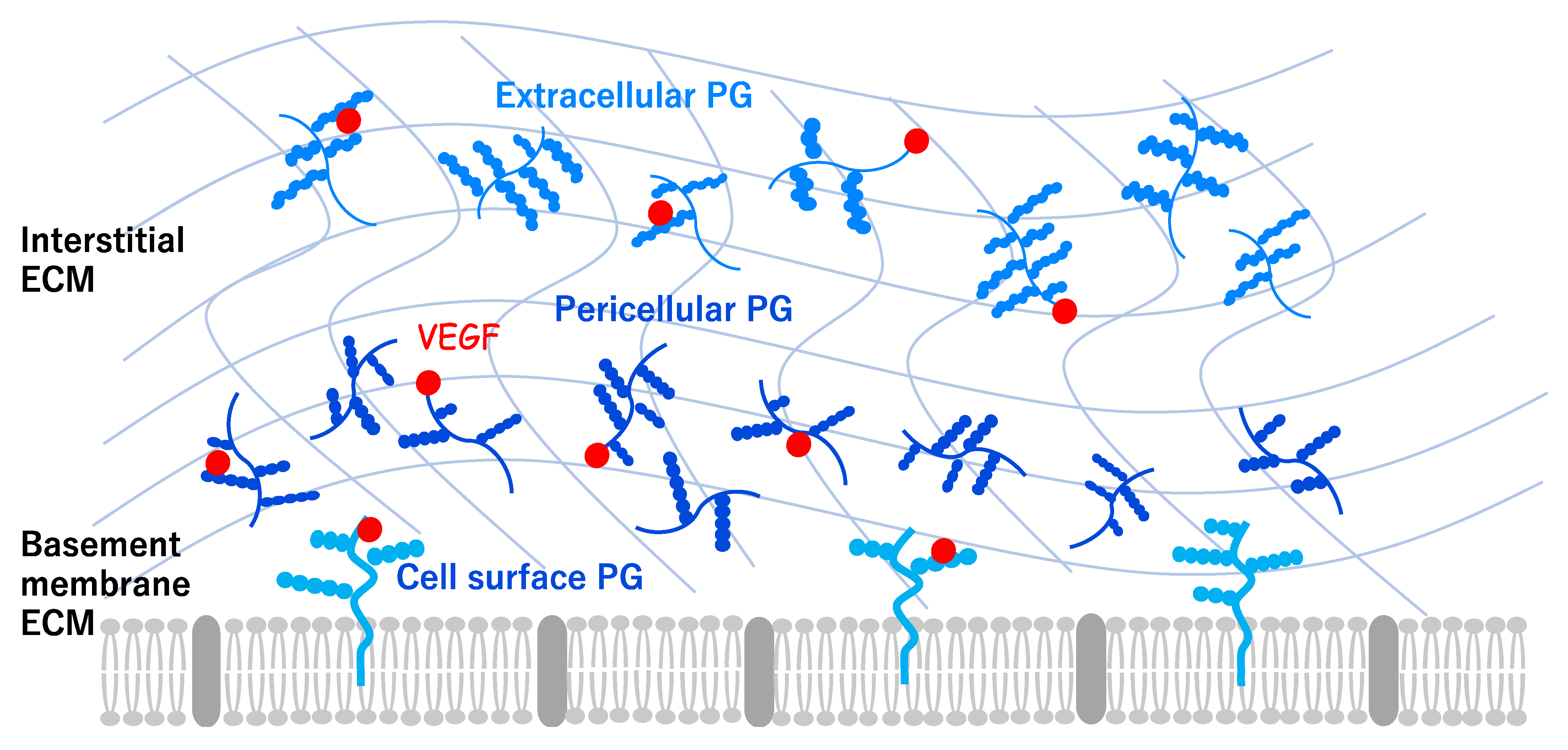
2. Angiogenesis and Extracellular Environments
3. Angiogenesis in Peripheral Axon Regeneration
4. Vascularization Strategies for Peripheral Nerve Regeneration
5. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watanabe, C.; Imaizumi, T.; Kawai, H.; Suda, K.; Honma, Y.; Ichihashi, M.; Ema, M.; Mizutani, K.-I. Aging of the vascular system and neural diseases. Front. Aging Neurosci. 2020, 12, 309. [Google Scholar] [CrossRef]
- Risau, W.; Flamme, I. Vasculogenesis. Annu. Rev. Cell. Dev. Bio. 1995, 11, 73–91. [Google Scholar] [CrossRef]
- Martino, M.M.; Hubbell, J.A. The 12th–14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 2010, 24, 4711–4721. [Google Scholar]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Ferrara, N.; Adamis, A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Simons, M.; Gordon, E.; Claesson-Welsh, E.G.L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. EGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, L.; Franco, C.; Bentley, K.; Collins, R.T.; Ponsioen, B.; Aspalter, I.M.; Rosewell, I.; Busse, M.; Thurston, G.; Medvinsky, A.B.; et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 2010, 12, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Arima, S.; Nishiyama, K.; Ko, T.; Arima, Y.; Hakozaki, Y.; Sugihara, K.; Koseki, H.; Uchijima, Y.; Kurihara, Y.; Kurihara, H. Angiogenic morphogenesis driven by dynamic and heterogeneous collective endothelial cell movement. Development 2011, 138, 4763–4776. [Google Scholar] [CrossRef] [PubMed]
- Komabayashi-Suzuki, M.; Yamanishi, E.; Watanabe, C.; Okamura, M.; Tabata, H.; Iwai, R.; Ajioka, I.; Matsushita, J.; Kidoya, H.; Takakura, N.; et al. Spatiotemporally dependent vascularization is differently utilized among neural progenitor subtypes during neocortical development. Cell Rep. 2019, 29, 1113–1129. [Google Scholar] [CrossRef]
- Rhodes, J.M.; Simons, M. The extracellular matrix and blood vessel formation: Not just a scaffold. J. Cell. Mol. Med. 2007, 11, 176–205. [Google Scholar] [CrossRef] [PubMed]
- Avraamides, C.J.; Garmy-Susini, B.; Varner, J.A. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 2008, 8, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Stratman, A.N.; Davis, G.E. Endothelial cell-pericyte interactions stimulate basement membrane matrix assembly: Influence on vascular tube remodeling, maturation, and stabilization. Microsc. Microanal. 2011, 18, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Marchand, M.; Monnota, C.; Mullera, L.; Germaina, S. Extracellular matrix scaffolding in angiogenesis and capillary homeostasis. Semin. Cell Dev. Biol. 2019, 89, 147–156. [Google Scholar] [CrossRef]
- Jakobsson, L.; Kreuger, J.; Holmborn, K.; Lundin, L.; Eriksson, I.; Kjellén, L.; Claesson-Welsh, L. Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis. Dev. Cell 2006, 10, 625–634. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Dyck, S.M.; Karimi-Abdolrezaee, S. Chondroitin sulfate proteoglycans: Key modulators in the developing and pathologic central nervous system. Exp. Neurol. 2015, 269, 169–187. [Google Scholar] [CrossRef]
- Melrose, J.; Hayes, A.; Bix, G. The CNS/PNS extracellular matrix provides instructive guidance cues to neural cells and neuroregulatory proteins in neural development and repair. Int. J. Mol. Sci. 2021, 22, 5583. [Google Scholar] [CrossRef]
- Masutani, T.; Yamada, S.; Hara, A.; Takahashi, T.; Green, P.G.; Niwa, M. Exogenous application of proteoglycan to the cell surface microenvironment facilitates to chondrogenic differentiation and maintenance. Int. J. Mol. Sci. 2020, 21, 7744. [Google Scholar] [CrossRef]
- Koch, C.D.; Lee, C.M.; Apte, S.S. Aggrecan in cardiovascular development and disease. J. Histochem. Cytochem. 2020, 68, 777–795. [Google Scholar] [CrossRef]
- Chikach, F.S.; Koch, D.C.; Mead, J.D.; Galatioto, J.; Willard, B.B.; Emerton, B.K.; Eagleton, J.M.; Blackstone, H.E.; Ramirez, F.; Roselli, E.E.; et al. Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection. JCI Insight 2018, 3, e97167. [Google Scholar] [CrossRef] [PubMed]
- Bode-Lesniewska, B.; Dours-Zimmermann, M.T.; Odermatt, B.F.; Briner, J.; Heitz, P.U.; Zimmermann, D.R. Distribution of the large aggregating proteoglycan versican in adult human tissues. J. Histochem. Cytochem. 1996, 44, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Chuang, C.Y.-N.; Melrose, J.; Davies, M.; Iozzo, R.; Whitelock, J. The role of vascular-derived perlecan in modulating cell adhesion, proliferation and growth factor signaling. Matrix Biol. 2014, 35, 112–122. [Google Scholar] [CrossRef]
- Zoeller, J.J.; McQuillan, A.; Whitelock, J.; Ho, S.-Y.; Iozzo, R.V. A central function for perlecan in skeletal muscle and cardiovascular development. J. Cell Biol. 2008, 181, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, J.J.; Whitelock, J.; Iozzo, R.V. Perlecan regulates developmental angiogenesis by modulating the VEGF-VEGFR2 axis. Matrix Biol. 2009, 28, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Sasse, P.; Malan, D.; Fleischmann, M.; Roell, W.; Gustafsson, E.; Bostani, T.; Fan, Y.; Kolbe, T.; Breitbach, M.; Addicks, K.; et al. Perlecan is critical for heart stability. Cardiovasc. Res. 2008, 80, 435–444. [Google Scholar] [CrossRef]
- Lee, B.; Clarke, D.; Alahmad, A.; Kahle, M.; Parham, C.; Auckland, L.; Shaw, C.; Fidanboylu, M.; Orr, A.; Ogunshola, O.; et al. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J. Clin. Investig. 2011, 121, 3005–3023. [Google Scholar] [CrossRef]
- Clarke, D.N.; Alahmad, A.; Lee, B.; Parham, C.; Auckland, L.; Fertala, A.; Kahle, M.; Shaw, C.S.; Roberts, J.; Bix, G.J. Perlecan domain V induces VEGf secretion in brain endothelial cells through integrin α5β1 and ERK-dependent signaling pathways. PLoS ONE 2012, 7, e45257. [Google Scholar] [CrossRef]
- Nakamura, K.; Ikeuchi, T.; Nara, K.; Rhodes, C.S.; Zhang, P.; Chiba, Y.; Kazuno, S.; Miura, Y.; Ago, T.; Arikawa-Hirasawa, E.; et al. Perlecan regulates pericyte dynamics in the maintenance and repair of the blood-brain barrier. J. Cell Biol. 2019, 218, 3506–3525. [Google Scholar] [CrossRef]
- Steiner, E.; Enzmann, G.U.; Lyck, R.; Lin, S.; Rüegg, M.A.; Kroger, S.; Engelhardt, B. The heparan sulfate proteoglycan agrin contributes to barrier properties of mouse brain endothelial cells by stabilizing adherens junctions. Cell Tissue Res. 2014, 358, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Njah, K.; Chakraborty, S.; Qiu, B.; Arumugam, S.; Raju, A.; Pobbati, A.V.; Lakshmanan, M.; Tergaonkar, V.; Thibault, G.; Wang, X.; et al. A role of agrin in maintaining the stability of vascular endothelial growth factor receptor-2 during tumor angiogenesis. Cell Rep. 2019, 28, 949–965. [Google Scholar] [CrossRef]
- Adams, R.H.; Eichmann, A. Axon guidance molecules in vascular patterning. Cold Spring Harb. Perspect. Biol. 2010, 2, a001875. [Google Scholar] [CrossRef] [PubMed]
- Peguera, B.; Segarra, M.; Acker-Palmer, A. Neurovascular crosstalk coordinates the central nervous system development. Curr. Opin. Neurobiol. 2021, 69, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Wälchli, T.; Wacker, A.; Frei, K.; Regli, L.; Schwab, M.E.; Hoerstrup, S.P.; Gerhardt, H.; Engelhardt, B. Wiring the vascular network with neural cues: A CNS perspective. Neuron 2015, 87, 271–296. [Google Scholar] [CrossRef] [PubMed]
- Takashima, S.; Watanabe, C.; Ema, M.; Mizutani, K.-I. Interaction of the nervous system and vascular system is required for the proper assembly of the neocortex. Neurochem. Int. 2019, 129, 104481. [Google Scholar] [CrossRef] [PubMed]
- Keilhoff, G.; Schild, L.; Fansa, H. Minocycline protects schwann cells from ischemia-like injury and promotes axonal outgrowth in bioartificial nerve grafts lacking wallerian degeneration. Exp. Neurol. 2008, 212, 189–200. [Google Scholar] [CrossRef]
- Cattin, A.-L.; Burden, J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.; Calavia, N.G.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-induced blood vessels guide schwann cell-mediated regeneration of peripheral nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Napoli, I.; Noon, L.; Ribeiro, S.; Kerai, A.P.; Parrinello, S.; Rosenberg, L.H.; Collins, M.; Harrisingh, M.C.; White, I.J.; Woodhoo, A.; et al. A central role for the ERK-signaling pathway in controlling schwann cell plasticity and peripheral nerve regeneration In Vivo. Neuron 2012, 73, 729–742. [Google Scholar] [CrossRef]
- Nukada, H. Post-traumatic endoneurial neovascularization and nerve regeneration: A morphometric study. Brain Res. 1988, 449, 89–96. [Google Scholar] [CrossRef]
- Vane, J.R.; Botting, R.M. Secretory functions of the vascular endothelium. J. Physiol. Pharmacol. 1992, 43, 195–207. [Google Scholar]
- Rafii, S.; Butler, J.M.; Ding, B.-S. Angiocrine functions of organ-specific endothelial cells. Nature 2016, 529, 316–325. [Google Scholar] [CrossRef]
- Toma, J.S.; Karamboulas, K.; Carr, M.; Kolaj, A.; Yuzwa, S.A.; Mahmud, N.; Storer, M.; Kaplan, D.R.; Miller, F.D. Peripheral nerve single-cell analysis identifies mesenchymal ligands that promote axonal growth. eNeuro 2020, 7, 0066. [Google Scholar] [CrossRef] [PubMed]
- Zochodne, D.W.; Nguyen, C. Angiogenesis at the site of neuroma formation in transected peripheral nerve. J. Anat. 1997, 191, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Dun, X.-P.; Parkinson, D.B. Visualizing peripheral nerve regeneration by whole mount staining. PLoS ONE 2015, 10, e0119168. [Google Scholar] [CrossRef] [PubMed]
- Saffari, T.M.; Mathot, F.; Friedrich, P.F.; Bishop, A.T.; Shin, A.Y. Revascularization patterns of nerve allografts in a rat sciatic nerve defect model. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 460–468. [Google Scholar] [CrossRef]
- Iijima, Y.; Ajiki, T.; Murayama, A.; Takeshita, K. Effect of artificial nerve conduit vascularization on peripheral nerve in a necrotic bed. Plast. Reconstr. Surg. Glob. Open 2016, 4, e665. [Google Scholar] [CrossRef] [PubMed]
- Muangsanit, P.; Roberton, V.; Costa, E.; Phillips, J.B. Engineered aligned endothelial cell structures in tethered collagen hydrogels promote peripheral nerve regeneration. Acta Biomater. 2021, 126, 224–237. [Google Scholar] [CrossRef]
- Pan, D.; Acevedo-Cintrón, J.A.; Sayanagi, J.; Snyder-Warwick, A.K.; Mackinnon, S.E.; Wood, M.D. The CCL2/CCR2 axis is critical to recruiting macrophages into acellular nerve allograft bridging a nerve gap to promote angiogenesis and regeneration. Exp. Neurol. 2020, 331, 113363. [Google Scholar] [CrossRef]
- Daines, J.M.; Schellhardt, L.; Wood, M.D. The role of the IL-4 signaling pathway in traumatic nerve injuries. Neurorehabilit. Neural Repair 2021, 35, 431–443. [Google Scholar] [CrossRef]
- Sondell, M.; Lundborg, G.; Kanje, M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and schwann cell proliferation in the peripheral nervous system. J. Neurosci. 1999, 19, 5731–5740. [Google Scholar] [CrossRef]
- Lopes, F.R.P.; Lisboa, B.C.G.; Frattini, F.; Almeida, F.M.; Tomaz, M.A.; Matsumoto, P.K.; Langone, F.; Lora, S.; Melo, P.A.; Borojevic, R.; et al. Enhancement of sciatic nerve regeneration after vascular endothelial growth factor (VEGF) gene therapy. Neuropathol. Appl. Neurobiol. 2011, 37, 600–612. [Google Scholar] [CrossRef]
- Hobson, M.I.; Green, C.J.; Terenghi, G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J. Anat. 2000, 197, 591–605. [Google Scholar] [CrossRef]
- Moimas, S.; Novati, F.; Ronchi, G.; Zacchigna, S.; Fregnan, F.; Zentilin, L.; Papa, G.; Giacca, M.; Geuna, S.; Perroteau, I.; et al. Effect of vascular endothelial growth factor gene therapy on post-traumatic peripheral nerve regeneration and denervation-related muscle atrophy. Gene Ther. 2013, 20, 1014–1021. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, P.; Tong, Z.; Luo, L.; Zhao, Y.; Chen, F.; Li, Y.; Huselstein, C.; Ye, Q.; Ye, Q.; Chen, Y. Comprehensive strategy of conduit guidance combined with VEGF producing Schwann cells accelerates peripheral nerve repair. Bioact. Mater. 2021, 6, 3515–3527. [Google Scholar] [CrossRef]
- Geuna, S.; Tos, P.; Battiston, B.; Giacobini-Robecchi, M.G. Bridging peripheral nerve defects with muscle-vein combined guides. Neurol. Res. 2004, 26, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Hsia, K.; Ma, H.; Lee, H.; Lu, J.-H. In Vivo performance of decellularized vascular grafts: A review article. Int. J. Mol. Sci. 2018, 19, 2101. [Google Scholar] [CrossRef] [PubMed]
- D’Arpa, S.; Claes, K.E.Y.; Stillaert, F.; Colebunders, B.; Monstrey, S.; Blondeel, P. Vascularized nerve “grafts”: Just a graft or a worthwhile procedure? Plast. Aesthetic Res. 2015, 2, 183–194. [Google Scholar] [CrossRef]
- Matsuo, I.; Kimura-Yoshida, C. Extracellular distribution of diffusible growth factors controlled by heparan sulfate proteoglycans during mammalian embryogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130545. [Google Scholar] [CrossRef]
- Kim, S.-H.; Turnbull, J.; Guimond, S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Urbanczyk, M.; Layland, S.L.; Schenke-Layland, K. The role of extracellular matrix in biomechanics and its impact on bioengineering of cells and 3D tissues. Matrix Biol. 2020, 85–86, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.L.; Wang, S.; Zhang, X.; Li, Y.; Huang, G.; Qi, H.; Pingguan-Murphy, B.; Li, Y.; Lu, T.J.; Xu, F. Engineering physical microenvironment for stem cell based regenerative medicine. Drug Discov. Today 2014, 19, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, E.; Sunderkötter, C.; Iozzo, R.V.; Schaefer, L. Decorin, a novel player in the insulin-like growth factor system. J. Biol. Chem. 2005, 280, 15767–15772. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saio, S.; Konishi, K.; Hohjoh, H.; Tamura, Y.; Masutani, T.; Iddamalgoda, A.; Ichihashi, M.; Hasegawa, H.; Mizutani, K.-i. Extracellular Environment-Controlled Angiogenesis, and Potential Application for Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2021, 22, 11169. https://doi.org/10.3390/ijms222011169
Saio S, Konishi K, Hohjoh H, Tamura Y, Masutani T, Iddamalgoda A, Ichihashi M, Hasegawa H, Mizutani K-i. Extracellular Environment-Controlled Angiogenesis, and Potential Application for Peripheral Nerve Regeneration. International Journal of Molecular Sciences. 2021; 22(20):11169. https://doi.org/10.3390/ijms222011169
Chicago/Turabian StyleSaio, Shingo, Kanna Konishi, Hirofumi Hohjoh, Yuki Tamura, Teruaki Masutani, Arunasiri Iddamalgoda, Masamitsu Ichihashi, Hiroshi Hasegawa, and Ken-ichi Mizutani. 2021. "Extracellular Environment-Controlled Angiogenesis, and Potential Application for Peripheral Nerve Regeneration" International Journal of Molecular Sciences 22, no. 20: 11169. https://doi.org/10.3390/ijms222011169
APA StyleSaio, S., Konishi, K., Hohjoh, H., Tamura, Y., Masutani, T., Iddamalgoda, A., Ichihashi, M., Hasegawa, H., & Mizutani, K.-i. (2021). Extracellular Environment-Controlled Angiogenesis, and Potential Application for Peripheral Nerve Regeneration. International Journal of Molecular Sciences, 22(20), 11169. https://doi.org/10.3390/ijms222011169






