An Insight into Anti-Inflammatory Activities and Inflammation Related Diseases of Anthocyanins: A Review of Both In Vivo and In Vitro Investigations
Abstract
:1. Introduction
2. Therapeutic Effect of Anthocyanins on Inflammation Related Diseases
2.1. Therapeutic Effect of Anthocyanins on Obesity
2.1.1. In Vivo Study
2.1.2. In Vitro Study
2.2. Therapeutic Effect of Anthocyanins on Diabetes and Cardiovascular Disease
2.2.1. In Vivo Study
2.2.2. In Vitro Study
2.3. Therapeutic Effect of Anthocyanins on Cancer
2.3.1. In Vivo Study
2.3.2. In Vitro Study
3. Anti-Inflammatory Mechanism of Anthocyanins
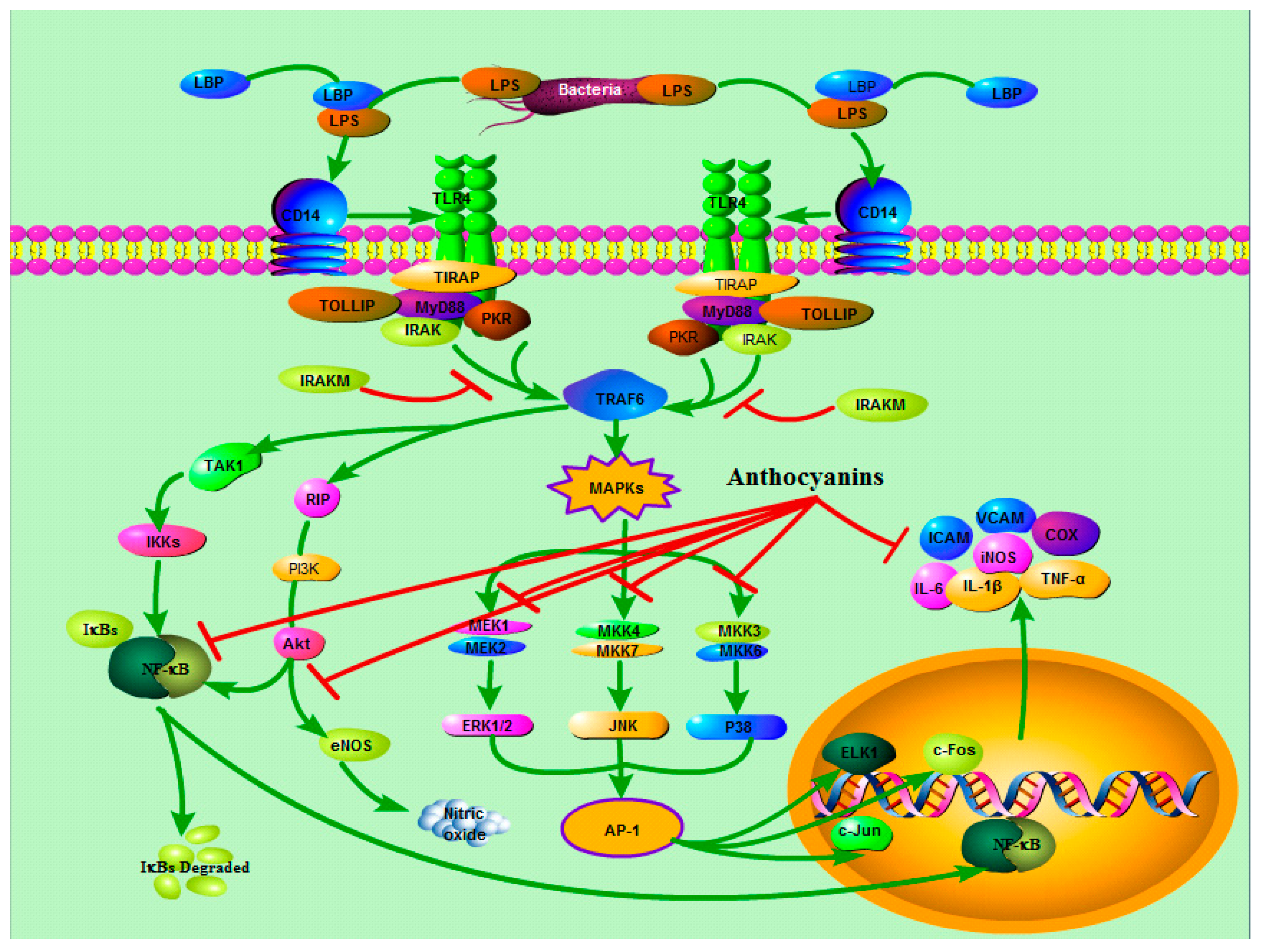
3.1. Nuclear Factor-κB Pathway (NF-κB)
3.2. TLRs and MAPKs
3.3. Nitric Oxide (NO)
3.4. Reactive Oxygen (ROS)
3.5. Prostaglandin E2 (PGE2)
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Nuclear factor-kappa B | NF-κB |
| Tumor necrosis factor-α | TNF-α |
| Interleukin-6 | IL-6 |
| Interleukin-1β | IL-1β |
| Monocyte chemoattractant protein-1 | MCP-1 |
| soluble vascular cell adhesion molecule-1 | sICAM-1 |
| C-reactive protein | CRP |
| Pattern recognition receptors | PRRS |
| Toll like receptors | TLRs |
| Mitogen activated protein kinases | MAPK |
| c-Jun N-terminal kinases | JNK |
| Reactive oxygen species | ROS |
| Cyclooxygenase-2 | COX-2 |
| Vascular endothelial growth factor | VEGF |
| Inducible nitric oxide synthase | iNOS |
| Hydrogen peroxide | H2O2 |
| Hydroxyl radical | OH− |
| Nicotinamide adenine dinucleotide phosphate | NADPH |
| Alzheimer’s disease | AD |
| Lipopolysaccharide binding protein | LBP |
| Lipopolysaccharide | LPS |
| Myeloid differentiation factor 88 | MyD88 |
| IL-1 receptor associated Kinase | IRAK |
| TNF-receptor association factor 6 | TRAF-6 |
| TGF-activated kinase 1 | TAK1 |
| Toll-interacting protein | TOLLIP |
| TIR | Toll/IL-1 receptor |
| RNA-activated protein kinase | PKR |
| Tissue-type plasminogen activator | tP A |
| Prostacyclin | PGI2 |
| Human retinal capillary endothelial cells | HRCECs |
| Catalase | CAT |
| Superoxide dismutase | SOD |
| Glutathione peroxidase | GPX |
| C-X-C motif ligand 9 | CXCL9 |
| Arginase | ARG1 |
| Chitinase-like 3 | CHIL3 |
| Lipoxygenase | LOX |
| Sulfated glycosaminoglycan | s-GAG |
| Hyaluronic acid | HA |
| Matrix metalloproteinases | MMP |
| Dipeptidyl peptidase-4 | DPP-IV |
| Proliferator-activated receptor γ | PPARγ |
References
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Berries: Anti-inflammatory Effects in Humans. J. Agric. Food Chem. 2014, 62, 3886–3903. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Klimis-Zacas, D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor- B and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015, 73, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, A.; Beart, P.M. Inflammation: Maladies, models, mechanisms and molecules. Br. J. Pharmacol. 2016, 173, 631–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellulu, M.S. Obesity, cardiovascular disease, and role of vitamin C on inflammation: A review of facts and underlying mechanisms. Inflammopharmacology 2017, 25, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Liuzzo, G.; Pedicino, D.; Flego, D.; Crea, F. Inflammation and Atherothrombosis. Clin. Immunol. 2018, 935–946.e1. [Google Scholar] [CrossRef]
- Nandkeolyar, S.; Naqvi, A.; Fan, W.; Sharma, A.; Rana, J.S.; Rozanski, A.; Shaw, L.; Friedman, J.D.; Hayes, S.; Dey, D.; et al. Utility of novel serum biomarkers to predict subclinical atherosclerosis: A sub-analysis of the EISNER study. Atherosclerosis 2019, 282, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Aboonabi, A.; Meyer, R.R.; Gaiz, A.; Singh, I. Anthocyanins in berries exhibited anti-atherogenicity and antiplatelet activities in a metabolic syndrome population. Nutr. Res. 2020, 76, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-J.; Frei, B. α-Lipoic acid inhibits TNF-a-induced NF-κB activation and adhesion molecule expression in human aortic endothelial cells. FASEB J. 2001, 15, 2423–2432. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Tsoy, I.; Park, J.M.; Chung, J.I.; Shin, S.C.; Chang, K.C. Anthocyanins from soybean seed coat inhibit the expression of TNF-α-induced genes associated with ischemia/reperfusion in endothelial cell by NF-κB-dependent pathway and reduce rat myocardial damages incurred by ischemia and reperfusion in vivo. FEBS Lett. 2006, 580, 1391–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-Alpha: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Singh, G.; Jeandet, P.; Pandey, A.; Giri, L.; Ramola, S.; Bhatt, I.D.; Venskutonis, P.R.; Georgiev, M.; Clément, C.; et al. Anthocyanins, multi-functional natural products of industrial relevance: Recent biotechnological advances. Biotechnol. Adv. 2020, 43, 107600. [Google Scholar] [CrossRef]
- Sendri, N.; Devidas, S.B.; Katoch, S.; Patial, V.; Bhandari, P. Copigmentation and UPLC-ESI-MS/MS of anthocyanin in Ipomoea nil as potential source of food colorant. Nat. Prod. Res. 2020, 1–6. [Google Scholar] [CrossRef]
- Martinsen, B.K.; Aaby, K.; Skrede, G. Effect of temperature on stability of anthocyanins, ascorbic acid and color in strawberry and raspberry jams. Food Chem. 2020, 316, 126297. [Google Scholar] [CrossRef] [PubMed]
- Moraes, L.F.D.S.; Sun, X.; Peluzio, M.D.C.G.; Zhu, M.-J. Anthocyanins/anthocyanidins and colorectal cancer: What is behind the scenes? Crit. Rev. Food Sci. Nutr. 2017, 59, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Blando, F.; Calabriso, N.; Berland, H.; Maiorano, G.; Gerardi, C.; Carluccio, M.A.; Andersen, M. Radical Scavenging and Anti-Inflammatory Activities of Representative Anthocyanin Groupings from Pigment-Rich Fruits and Vegetables. Int. J. Mol. Sci. 2018, 19, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wu, J.; Liu, F.; Tong, L.; Chen, Z.; Chen, J.; He, H.; Xu, R.; Ma, Y.; Huang, C. Neuroprotective effects of anthocyanins and its major component cyanidin-3-O-glucoside (C3G) in the central nervous system: An outlined review. Eur. J. Pharmacol. 2019, 858, 172500. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Monica, G.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifford, M.N. Anthocyanins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1118–1125. [Google Scholar] [CrossRef]
- Saigo, T.; Wang, T.; Watanabe, M.; Tohge, T. Diversity of anthocyanin and proanthocyanin biosynthesis in land plants. Curr. Opin. Plant Biol. 2020, 55, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.; Preston, T.; Kroon, P.; Botting, N.P.; Kay, C. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Giusti, M.M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Slimani, N.; Romieu, I.; Touillaud, M.; Kaaks, R.; Teucher, B.; Mattiello, A.; Grioni, S.; et al. Estimation of the intake of anthocyanidins and their food sources in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2011, 106, 1090–1099. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High Anthocyanin Intake Is Associated With a Reduced Risk of Myocardial Infarction in Young and Middle-Aged Women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Tarone, A.G.; Cazarin, C.B.B.; Junior, M.R.M. Anthocyanins: New techniques and challenges in microencapsulation. Food Res. Int. 2020, 133, 109092. [Google Scholar] [CrossRef]
- Ersus, S.; Yurdagel, U. Microencapsulation of anthocyanin pigments of black carrot (Daucus carota L.) by spray drier. J. Food Eng. 2007, 80, 805–812. [Google Scholar] [CrossRef]
- Riaz, M.; Zia-Ul-Haq, M.; Saad, B. Introduction to Anthocyanins. In Techniques for Nanoencapsulation of Food Ingredients; Springer: New York, NY, USA, 2016; pp. 21–33. [Google Scholar]
- Turturică, M.; Rapeanu, A.M.; Bahrim, G. Anthocyanins: Naturally occuring fruit pigments with functional properties. Ann. Univ. Dunarea de Jos Galati. Fascicle VI-Food Technol. 2015, 39, 9–24. [Google Scholar]
- Zhang, K.; Liu, Z.; Guan, L.; Zheng, T.; Jiu, S.; Zhu, X.; Jia, H.; Fang, J. Changes of Anthocyanin Component Biosynthesis in ‘Summer Black’ Grape Berries after the Red Flesh Mutation Occurred. J. Agric. Food Chem. 2018, 66, 9209–9218. [Google Scholar] [CrossRef]
- Pirzadeh, M.; Caporaso, N.; Rauf, A.; Shariati, M.A.; Yessimbekov, Z.; Khan, M.U.; Imran, M.; Mubarak, M.S. Pomegranate as a source of bioactive constituents: A review on their characterization, properties and applications. Crit. Rev. Food Sci. Nutr. 2020, 61, 982–999. [Google Scholar] [CrossRef]
- Strauch, R.C.; Mengist, M.F.; Pan, K.; Yousef, G.G.; Iorizzo, M.; Brown, A.F.; Lila, M.A. Variation in anthocyanin profiles of 27 genotypes of red cabbage over two growing seasons. Food Chem. 2019, 301, 125289. [Google Scholar] [CrossRef]
- Yoon, B.I.; Bae, W.J.; Choi, Y.S.; Kim, S.J.; Ha, U.S.; Hong, S.-H.; Sohn, D.W.; Kim, S.W. Anti-inflammatory and Antimicrobial Effects of Anthocyanin Extracted from Black Soybean on Chronic Bacterial Prostatitis Rat Model. Chin. J. Integr. Med. 2013, 24, 621–626. [Google Scholar] [CrossRef]
- Frond, A.D.; Iuhas, C.I.; Stirbu, I.; Leopold, L.; Socaci, S.; Andreea, S.; Ayvaz, H.; Andreea, S.; Mihai, S.; Diaconeasa, Z.; et al. Phytochemical Characterization of Five Edible Purple-Reddish Vegetables: Anthocyanins, Flavonoids, and Phenolic Acid Derivatives. Molecules 2019, 24, 1536. [Google Scholar] [CrossRef] [Green Version]
- Luna-Vital, D.A.; Luzardo-Ocampo, I.; Cuellar-Nuñez, M.L.; Loarca-Piña, G.; de Mejia, E.G. Maize extract rich in ferulic acid and anthocyanins prevents high-fat-induced obesity in mice by modulating SIRT1, AMPK and IL-6 associated metabolic and inflammatory pathways. J. Nutr. Biochem. 2020, 79, 108343. [Google Scholar] [CrossRef]
- DeFuria, J.; Bennett, G.; Strissel, K.J.; Perfield, J.W.; Milbury, P.E.; Greenberg, A.S.; Obin, M.S. Dietary Blueberry Attenuates Whole-Body Insulin Resistance in High Fat-Fed Mice by Reducing Adipocyte Death and Its Inflammatory Sequelae. J. Nutr. 2009, 139, 1510–1516. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Gu, L.; Hager, T.J.; Hager, A.; Howard, L.R. Whole Berries versus Berry Anthocyanins: Interactions with Dietary Fat Levels in the C57BL/6J Mouse Model of Obesity. J. Agric. Food Chem. 2008, 56, 647–653. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Shin, T.S.; Kim, M.Y.; Cho, N.J.; Kim, J.D. Anthocyanins from Cornus kousa ethanolic extract attenuate obesity in association with anti-angiogenic activities in 3T3-L1 cells by down-regulating adipogeneses and lipogenesis. PLoS ONE 2018, 13, e0208556. [Google Scholar] [CrossRef]
- Vilhena, R.; Figueiredo, I.; Baviera, A.; Silva, D.; Marson, B.; Oliveira, J.; Peccinini, R.; Borges, I.; Pontarolo, R. Antidiabetic activity of Musa x paradisiaca extracts in streptozotocin-induced diabetic rats and chemical characterization by HPLC-DAD-MS. J. Ethnopharmacol. 2020, 254, 112666. [Google Scholar] [CrossRef]
- Fang, J.; Huang, J. Accumulation of plasma levels of anthocyanins following multiple saskatoon berry supplements. Xenobiotica 2019, 50, 454–457. [Google Scholar] [CrossRef]
- Skates, E.; Overall, J.; DeZego, K.; Wilson, M.; Esposito, D.; Lila, M.A.; Komarnytsky, S. Berries containing anthocyanins with enhanced methylation profiles are more effective at ameliorating high fat diet-induced metabolic damage. Food Chem. Toxicol. 2018, 111, 445–453. [Google Scholar] [CrossRef]
- Aboonabi, A.; Aboonabi, A. Anthocyanins reduce inflammation and improve glucose and lipid metabolism associated with inhibiting nuclear factor-kappaB activation and increasing PPAR-γ gene expression in metabolic syndrome subjects. Free Radic. Biol. Med. 2020, 150, 30–39. [Google Scholar] [CrossRef]
- Holvoet, P. Relations between metabolic syndrome, oxidative stress and inflammation and cardiovascular disease. Verh.-K. Acad. Geneeskd. Belg. 2008, 70, 193–219. [Google Scholar]
- Bakuradze, T.; Tausend, A.; Galan, J.; Groh, I.A.M.; Berry, D.; Tur, J.A.; Marko, D.; Richling, E. Antioxidative activity and health benefits of anthocyanin-rich fruit juice in healthy volunteers. Free Radic. Res. 2019, 53, 1045–1055. [Google Scholar] [CrossRef]
- Curtis, P.J.; Van Der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome—results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef] [Green Version]
- Feng, R.; Ni, H.-M.; Wang, S.Y.; Tourkova, I.L.; Shurin, M.; Harada, H.; Yin, X.-M. Cyanidin-3-rutinoside, a Natural Polyphenol Antioxidant, Selectively Kills Leukemic Cells by Induction of Oxidative Stress. J. Biol. Chem. 2007, 282, 13468–13476. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, L.; Jing, N.; Jiang, G.; Liu, Z. Biostimulating Gut Microbiome with Bilberry Anthocyanin Combo to Enhance Anti-PD-L1 Efficiency against Murine Colon Cancer. Microorganisms 2020, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Losso, J.N.; Finley, J.W.; Karki, N.; Liu, A.G.; Prudente, A.; Tipton, R.; Yu, Y.; Greenway, F.L. Pilot Study of the Tart Cherry Juice for the Treatment of Insomnia and Investigation of Mechanisms. Am. J. Ther. 2018, 25, e194–e201. [Google Scholar] [CrossRef]
- Sangsefidi, Z.S.; Hosseinzadeh, M.; Ranjbar, A.M.; Akhondi-Meybodi, M.; Fallahzadeh, H.; Mozaffari-Khosravi, H. The effect of total anthocyanin-base standardized (Cornus mas L.) fruit extract on liver function, tumor necrosis factor α, malondealdehyde, and adiponectin in patients with non-alcoholic fatty liver: A study protocol for a double-blind randomized clinical trial. Nutr. J. 2019, 18, 1–7. [Google Scholar] [CrossRef]
- Hollands, W.J.; Armah, C.N.; Doleman, J.F.; Perez-Moral, N.; Winterbone, M.S.; Kroon, P.A. 4-Week consumption of anthocyanin-rich blood orange juice does not affect LDL-cholesterol or other biomarkers of CVD risk and glycaemia compared with standard orange juice: A randomised controlled trial. Br. J. Nutr. 2018, 119, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Ohguro, H.; Ohguro, I.; Katai, M.; Tanaka, S. Two-Year Randomized, Placebo-Controlled Study of Black Currant Anthocyanins on Visual Field in Glaucoma. Ophthalmologica 2012, 228, 26–35. [Google Scholar] [CrossRef]
- Fan, D.; Alamri, Y.; Liu, K.; Macaskill, M.; Harris, P.; Brimble, M.; Dalrymple-Alford, J.; Prickett, T.; Menzies, O.; Laurenson, A.; et al. Supplementation of Blackcurrant Anthocyanins Increased Cyclic Glycine-Proline in the Cerebrospinal Fluid of Parkinson Patients: Potential Treatment to Improve Insulin-Like Growth Factor-1 Function. Nutrients 2018, 10, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.-W.; Chen, F.-X.; Li, D.; Ling, W.-H.; Guo, H.-H. A CONSORT-Compliant, Randomized, Double-Blind, Placebo-Controlled Pilot Trial of Purified Anthocyanin in Patients With Nonalcoholic Fatty Liver Disease. Medicine 2015, 94, e758. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Sorn, S.R.; Park, Y.; Park, H.-K. Anthocyanin Rich-Black Soybean Testa Improved Visceral Fat and Plasma Lipid Profiles in Overweight/Obese Korean Adults: A Randomized Controlled Trial. J. Med. Food 2016, 19, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Chen, X.-L.; Yang, H.-W.; Ma, Y.-R. Effects of nuclear factor κB expression on retinal neovascularization and apoptosis in a diabetic retinopathy rat model. Int. J. Ophthalmol. 2015, 8, 448–452. [Google Scholar] [CrossRef]
- Tilborghs, S.; Corthouts, J.; Verhoeven, Y.; Arias, D.; Rolfo, C.; Trinh, X.B.; Van Dam, P.A. The role of Nuclear Factor-kappa B signaling in human cervical cancer. Crit. Rev. Oncol. 2017, 120, 141–150. [Google Scholar] [CrossRef]
- Duarte, L.J.; Chaves, V.C.; Nascimento, M.V.P.D.S.; Calvete, E.; Li, M.; Ciraolo, E.; Ghigo, A.; Hirsch, E.; Simões, C.M.O.; Reginatto, F.H.; et al. Molecular mechanism of action of Pelargonidin-3- O -glucoside, the main anthocyanin responsible for the anti-inflammatory effect of strawberry fruits. Food Chem. 2017, 247, 56–65. [Google Scholar] [CrossRef]
- Lee, H.-H.; Lee, S.-G.; Shin, J.-S.; Lee, H.-Y.; Yoon, K.; Ji, Y.W.; Jang, D.S.; Lee, K.-T. p-Coumaroyl Anthocyanin Mixture Isolated from Tuber Epidermis of Solanum tuberosum Attenuates Reactive Oxygen Species and Pro-inflammatory Mediators by Suppressing NF-κB and STAT1/3 Signaling in LPS-Induced RAW264.7 Macrophages. Biol. Pharm. Bull. 2017, 40, 1894–1902. [Google Scholar] [CrossRef] [Green Version]
- Le Phuong Nguyen, T.; Fenyvesi, F.; Remenyik, J.; Homoki, J.R.; Gogolák, P.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Vasvári, G.; Vecsernyés, M.; et al. Protective Effect of Pure Sour Cherry Anthocyanin Extract on Cytokine-Induced Inflammatory Caco-2 Monolayers. Nutrients 2018, 10, 861. [Google Scholar] [CrossRef] [Green Version]
- Roth, S.; Spalinger, M.R.; Gottier, C.; Biedermann, L.; Zeitz, J.; Lang, S.; Weber, A.; Rogler, G.; Scharl, M. Bilberry-Derived Anthocyanins Modulate Cytokine Expression in the Intestine of Patients with Ulcerative Colitis. PLoS ONE 2016, 11, e0154817. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Mirza, A.Z.; AlThagafi, I.I.; Shamshad, H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur. J. Med. Chem. 2019, 166, 502–513. [Google Scholar] [CrossRef]
- Lanki, M.E.; Seppänen, H.; Mustonen, H.K.; Böckelman, C.; Juuti, A.T.; Hagström, J.K.; Haglund, C.H. Toll-like receptor 2 and Toll-like receptor 4 predict favorable prognosis in local pancreatic cancer. Tumor Biol. 2018, 40. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.K.; Lim, T.-G.; Seo, S.G.; Lee, H.J.; Hwang, Y.-S.; Choung, M.-G.; Lee, K.W. Cyanidin-3-O-(2″-xylosyl)-glucoside, an anthocyanin from Siberian ginseng (Acanthopanax senticosus) fruits, inhibits UVB-induced COX-2 expression and AP-1 transactivation. Food Sci. Biotechnol. 2013, 22, 507–513. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Wu, Z.; Yao, L.; Wu, Y.; Huang, L.; Liu, K.; Zhou, X.; Gou, D. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci. Rep. 2014, 4, srep06234. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.-X.; Chen, J.-H.; Li, J.-W.; Cheng, F.-R.; Yuan, K. Protection of Anthocyanin from Myrica rubra against Cerebral Ischemia-Reperfusion Injury via Modulation of the TLR4/NF-κB and NLRP3 Pathways. Molecules 2018, 23, 1788. [Google Scholar] [CrossRef] [Green Version]
- Thummayot, S.; Tocharus, C.; Jumnongprakhon, P.; Suksamrarn, A.; Tocharus, J. Cyanidin attenuates Aβ25-35-induced neuroinflammation by suppressing NF-κB activity downstream of TLR4/NOX4 in human neuroblastoma cells. Acta Pharmacol. Sin. 2018, 39, 1439–1452. [Google Scholar] [CrossRef]
- Karunarathne, W.A.H.M.; Lee, K.T.; Choi, Y.H.; Jin, C.-Y.; Kim, G.-Y. Anthocyanins isolated from Hibiscus syriacus L. attenuate lipopolysaccharide-induced inflammation and endotoxic shock by inhibiting the TLR4/MD2-mediated NF-κB signaling pathway. Phytomedicine 2020, 76, 153237. [Google Scholar] [CrossRef]
- Mansell, A.; Jenkins, B.J. Dangerous liaisons between interleukin-6 cytokine and toll-like receptor families: A potent combination in inflammation and cancer. Cytokine Growth Factor Rev. 2013, 24, 249–256. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, Q.; Yin, J.; Zhang, Z.; Bao, H.; Wang, X. Anthocyanins attenuate neuroinflammation through the suppression of MLK3 activation in a mouse model of perioperative neurocognitive disorders. Brain Res. 2019, 1726, 146504. [Google Scholar] [CrossRef]
- Li, K.; Zhang, M.; Chen, H.; Peng, J.; Jiang, F.; Shi, X.; Bai, Y.; Jian, M.; Jia, Y. Anthocyanins from black peanut skin protect against UV-B induced keratinocyte cell and skin oxidative damage through activating Nrf 2 signaling. Food Funct. 2019, 10, 6815–6828. [Google Scholar] [CrossRef]
- Wongwichai, T.; Teeyakasem, P.; Pruksakorn, D.; Kongtawelert, P.; Pothacharoen, P. Anthocyanins and metabolites from purple rice inhibit IL-1β-induced matrix metalloproteinases expression in human articular chondrocytes through the NF-κB and ERK/MAPK pathway. Biomed. Pharmacother. 2019, 112, 108610. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Elsenduny, F.; Momtaz, S.; Parvizi, F.; Iranpanah, A.; Tewari, D.; Naseri, R.; Abdolghaffari, A.H.; Rezaei, N. An update on dietary consideration in inflammatory bowel disease: Anthocyanins and more. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 1007–1024. [Google Scholar] [CrossRef]
- Loscalzo, J. The Identification of Nitric Oxide as Endothelium-Derived Relaxing Factor. Circ. Res. 2013, 113, 100–103. [Google Scholar] [CrossRef] [Green Version]
- Baudouin, E. The language of nitric oxide signalling. Plant. Biol. 2010, 13, 233–242. [Google Scholar] [CrossRef]
- Eqian, J.; Efulton, D. Post-translational regulation of endothelial nitric oxide synthase in vascular endothelium. Front. Physiol. 2013, 4, 347. [Google Scholar] [CrossRef] [Green Version]
- Gardlik, R.; Fusekova, I. Pharmacologic Therapy for Diabetic Retinopathy. Semin. Ophthalmol. 2013, 30, 252–263. [Google Scholar] [CrossRef]
- Gupta, K.; Sirohi, V.K.; Kumari, S.; Shukla, V.; Manohar, M.; Popli, P.; Dwivedi, A. Sorcin is involved during embryo implantation via activating VEGF/PI3K/Akt pathway in mice. J. Mol. Endocrinol. 2018, 60, 119–132. [Google Scholar] [CrossRef]
- Huang, W.; Yan, Z.; Li, D.; Ma, Y.; Zhou, J.; Sui, Z. Antioxidant and Anti-Inflammatory Effects of Blueberry Anthocyanins on High Glucose-Induced Human Retinal Capillary Endothelial Cells. Oxid. Med. Cell. Longev. 2018, 2018, 1862462. [Google Scholar] [CrossRef]
- Nizamutdinova, I.T.; Kim, Y.M.; Chung, J.I.; Shin, S.C.; Jeong, Y.-K.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. Anthocyanins from black soybean seed coats stimulate wound healing in fibroblasts and keratinocytes and prevent inflammation in endothelial cells. Food Chem. Toxicol. 2009, 47, 2806–2812. [Google Scholar] [CrossRef]
- Winter, A.N.; Brenner, M.C.; Punessen, N.; Snodgrass, M.; Byars, C.; Arora, Y.; Linseman, D.A. Comparison of the Neuro-protective and Anti-Inflammatory Effects of the Anthocyanin Metabolites, Protocatechuic Acid and 4-Hydroxybenzoic Acid. Oxid. Med. Cell. Longev. 2017, 2017, 6297080. [Google Scholar] [CrossRef] [Green Version]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [Green Version]
- Palungwachira, P.; Tancharoen, S.; Phruksaniyom, C.; Klungsaeng, S.; Srichan, R.; Kikuchi, K.; Nararatwanchai, T. Antioxidant and Anti-Inflammatory Properties of Anthocyanins Extracted from Oryza sativa L. in Primary Dermal Fibroblasts. Oxid. Med. Cell. Longev. 2019, 2019, 2089817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sánchez, K.; Ariza-Salamanca, D.; Mora-Muñoz, L. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front. Mol. Neurosci. 2017, 10, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Johnson, S.L.; Liu, W.; DaSilva, N.A.; Meschwitz, S.; Dain, J.A.; Seeram, N.P. Evaluation of Polyphenol Anthocyanin-Enriched Extracts of Blackberry, Black Raspberry, Blueberry, Cranberry, Red Raspberry, and Strawberry for Free Radical Scavenging, Reactive Carbonyl Species Trapping, Anti-Glycation, Anti-β-Amyloid Aggregation, and Microglial Neuroprotective Effects. Int. J. Mol. Sci. 2018, 19, 461. [Google Scholar] [CrossRef] [Green Version]
- Furuuchi, R.; Shimizu, I.; Yoshida, Y.; Hayashi, Y.; Ikegami, R.; Suda, M.; Katsuumi, G.; Wakasugi, T.; Nakao, M.; Minamino, T. Boysenberry polyphenol inhibits endothelial dysfunction and improves vascular health. PLoS ONE 2018, 13, e0202051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodward, D.F.; Jones, R.L.; Narumiya, S. International Union of Basic and Clinical Pharmacology. LXXXIII: Classification of Prostanoid Receptors, Updating 15 Years of Progress. Pharmacol. Rev. 2011, 63, 471–538. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2014, 1851, 414–421. [Google Scholar] [CrossRef]
- He, Y.; Hu, Y.; Jiang, X.; Chen, T.; Ma, Y.; Wu, S.; Sun, J.; Jiao, R.; Li, X.; Deng, L.; et al. Cyanidin-3-O-glucoside inhibits the UVB-induced ROS/COX-2 pathway in HaCaT cells. J. Photochem. Photobiol. B Biol. 2017, 177, 24–31. [Google Scholar] [CrossRef]
- Park, S.-J.; Shin, W.-H.; Seo, J.-W.; Kim, E.-J. Anthocyanins inhibit airway inflammation and hyperresponsiveness in a murine asthma model. Food Chem. Toxicol. 2007, 45, 1459–1467. [Google Scholar] [CrossRef]
- Van De Velde, F.; Esposito, D.; Grace, M.; Pirovani, M.E.; Lila, M.A. Anti-inflammatory and wound healing properties of polyphenolic extracts from strawberry and blackberry fruits. Food Res. Int. 2018, 121, 453–462. [Google Scholar] [CrossRef]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2017, 55, 6076–6093. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, P.; He, W.; Du, X.; Chen, C.; Suo, L.; Liang, M.; Zhang, N.; Na, A.; Zhang, Y. The Prevention and Inhibition Effect of Anthocyanins on Colorectal Cancer. Curr. Pharm. Des. 2020, 25, 4919–4927. [Google Scholar] [CrossRef] [PubMed]
- Biro, A.; Markovich, A.; Homoki, J.R.; Szőllősi, E.; Hegedűs, C.; Tarapcsák, S.; Lukács, J.; Stündl, L.; Remenyik, J. Anthocyanin-Rich Sour Cherry Extract Attenuates the Lipopolysaccharide-Induced Endothelial Inflammatory Response. Molecules 2019, 24, 3427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, S.R.; Pereira, R.; Figueiredo, I.V.; Freitas, V.; Dinis, T.C.P.; Almeida, L.M. Comparison of anti-inflammatory activities of an anthocyanin-rich fraction from Portuguese blueberries (Vaccinium corymbosum L.) and 5-aminosalicylic acid in a TNBS-induced colitis rat model. PLoS ONE 2017, 12, e0174116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Lee, J.-Y. Blackcurrant (Ribes nigrum) Extract Exerts an Anti-Inflammatory Action by Modulating Macrophage Phenotypes. Nutrients 2019, 11, 975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymanowska, U.; Baraniak, B.; Bogucka-Kocka, A. Antioxidant, Anti-Inflammatory, and Postulated Cytotoxic Activity of Phenolic and Anthocyanin-Rich Fractions from Polana Raspberry (Rubus idaeus L.) Fruit and Juice—In Vitro Study. Molecules 2018, 23, 1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; de Mejia, E.G.; Luna-Vital, D.A.; Tao, T.; Chandrasekaran, S.; Chatham, L.; Juvik, J.; Singh, V.; Kumar, D. Relationship of phenolic composition of selected purple maize (Zea mays L.) genotypes with their anti-inflammatory, anti-adipogenic and anti-diabetic potential. Food Chem. 2019, 289, 739–750. [Google Scholar] [CrossRef] [PubMed]
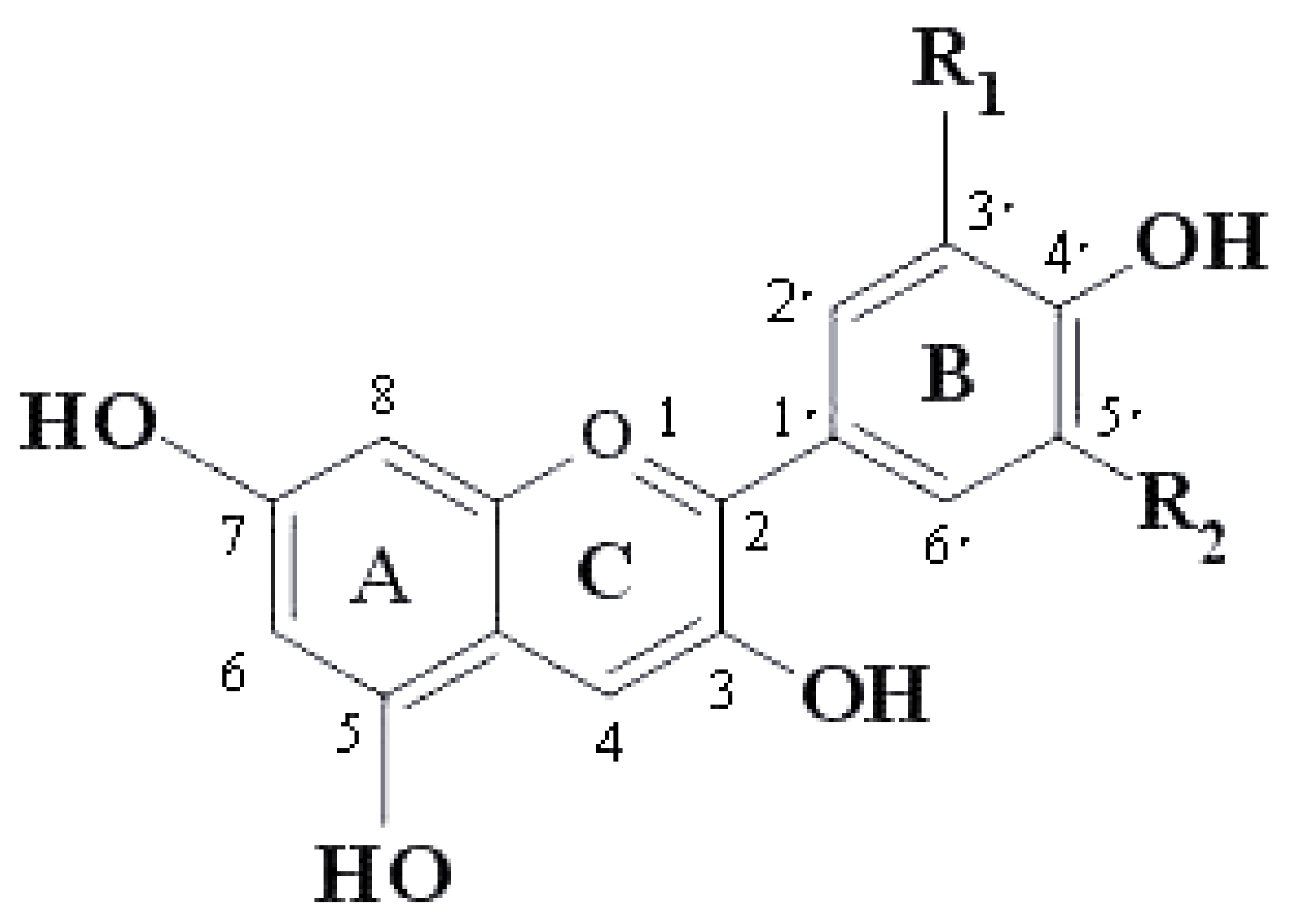

| Anthocyanins | R1 | R2 |
|---|---|---|
| Pelargonidin | H | H |
| Cyanidin | OH | H |
| Delphinidin | OH | OH |
| Peonodin | OCH3 | H |
| Petunidin | OCH3 | OH |
| Malvidin | OCH3 | OCH3 |
| Scheme | Dose and Duration of the Intervention | Participants | Study Design | Health Effects | References |
|---|---|---|---|---|---|
| Wild Norwegian bilberries and blackcurrant | Two capsules twice a day 4 weeks | 35 male and female subjects (MetS + healthy) age = 25–75 | Randomized, control design Intervention group (n = 20)-two capsule twice a day Control group (n = 15)-two capsule twice a day | Lowering inflammation and improving glucose and lipid metabolism | [42] |
| Fruit juice (Apples, strawberries, blueberries, grapes) | 750 mL fruit juice taken in three equal portions 55 days | 62 healthy male volunteers age = 20–50 | Randomized, control design Intervention group (n = 30)-750 mL fruit juice is taken in three equal portions Control group (n = 27)–750 mL placebo is taken in three equal portions | Improve DNA integrity and might influence lipid metabolism in humans | [46] |
| Blueberries | 150 g or 75 g fresh blueberries per day 21 days | 115 male and female subjects (MetS) age = 50–75 | A double-blind, placebo-controlled, parallel study | Improved endothelial Function, Improving metabolic syndrome | [47] |
| Tart cherry juice | 240 mL of tart cherry juice twice a day 2 weeks | 11 healthy male or female subjects with chronic insomnia age ≥ 50 | A randomized, double-blind, placebo controlled clinical trial | improving insomnia | [48] |
| Fresh ripe berries of cornelian cherry | total anthocyanin 320 mg/d 12 weeks | 80 patients with NAFLD age = 25–65 | A double-blind randomized clinical trial | Improving NAFLD | [49] |
| Blood orange juice | 50 mg anthocyanins/d and 500 mL blonde orange juice 4 weeks | 41 participants (aged 25–84) with a waist circumference > 94 cm (men) and > 80 cm (women) | A randomized controlled trial | Lowering cholesterol | [50] |
| Black currant | Black currant anthocyanins 50 mg/d 2 years | 38 patients with OAG | A randomized, placebo-controlled, double-masked trial | Increase eye blood flow and improve glaucoma | [51] |
| Black currant | Black currant capsules 300 mg | 11 male patients with Parkinson’s disease | Plasma and cerebrospinal fluid were collected from 11 male patients before and after 28 day supplementation of black currant capsules. | Treat neurological conditions with IGF-1 deficiency. | [52] |
| Bilberry and black currant | Purified anthocyanin 320 mg/d 12 weeks | 21 patients with NAFLD | A randomized, double-blind, placebo-controlled pilot trial | Improving NAFLD | [53] |
| Black soybeans | anthocyanin-rich black soybean testa extracts 2.5 g/d 8 weeks | 63 participants defined as overweight or obese by their body mass index (BMI > 23) or waist circumference (WC > 90 cm for males, >85 cm for females) | A randomized, double-blinded, and placebo-controlled clinical trial | Improve blood lipid status, Prevention of abdominal obesity caused by high fiber and low cholesterol diet | [54] |
| Source of Anthocyanins | Major Anthocyanins and Dose | Model | Biological Effects | References |
|---|---|---|---|---|
| Strawberry | Pelargonidin-3-O-glucoside Dose: 100–400 mg/kg | Mouse model of pleurisy | Decreased: ADA and MPO Inhibited: IkB-α, JNKMAPK | [57] |
| Sour cherry | cyanidin-3-rutinoside, cyanidin-3-O-glucoside, and cyanidin-3-O-glucosyl-rutinoside Dose: 50 μg/mL | HUVECs were treated with 100 ng/mL LPS | Decreased: ROS, TNF-α, IL-6, tPA, PGI2, COX-2 | [95] |
| Mahaleb Cherry | Cyanidin 3-(6-(rhamnosyl)glucoside), Cyanidin 3-glucoside, Cyanidin 3-(6-(rhamnosyl)-2-(xylosyl)glucoside), Cyanidin 3-(2-(xylosyl)glucoside) Dose: 60 µg/mL, 50 μg/mL | TEAC, ORAC and model of vascular inflammation | Decreased: ROS, VCAM-1 and ICAM-1 | [17] |
| Black currant | Delphinidin 3-(6-(rhamnosyl)glucoside), Cyanidin 3-(6-(rhamnosyl)glucoside) Dose: 60 µg/mL, 50 μg/mL | TEAC, ORAC and model of vascular inflammation | Decreased: ROS, VCAM-1 and ICAM-1 | |
| Black Carrot | Cyanidin 3-(6-(6-(feruloyl)glucosyl)-2-(xylosyl)galactoside), Cyanidin 3-(6-(6-(sinapoyl)glucosyl)-2-(xylosyl)galactoside) | TEAC, ORAC and model of vascular inflammation | Decreased: ROS, VCAM-1 and ICAM-1 | |
| “Sun Black” T omato | Petunidin 3-(6-(4-(E-p-coumaroyl)rhamnosyl)glucoside)-5-glucoside (petanin), Malvidin 3-(6-(4-(E-p-coumaroyl)rhamnosyl)glucoside)-5-glucoside Dose: 60 µg/mL, 50 μg/mL | TEAC, ORAC and model of vascular inflammation | Decreased: ROS, VCAM-1 and ICAM-1 | |
| Blueberries | malvidin, malvidin-3-glucoside, malvidin-3-galactoside Dose: 10 μg/mL | HRCECs | Decreased: ROS, VEGF, ICAM-1 Inhibited: Akt, NF-κB Increased: CAT, SOD | [80] |
| Portuguese blueberries | malvidin-3-galactoside, petunidin-3-arabinoside Dose: 100 mg/kg | TNBS induced colitis in rats | Decreased: iNOS, COX2, MPO, GPX | [96] |
| Black currant | delphinidin-3-rutinoside, cyanidin-3-rutinoside, delphinidin-3-glucoside Dose: 50 μg/mL | RAW 264.7 macrophages and human THP-1 monocytes | Decreased: IL-1β, iNOS, CXCL9, TNFα Increased: ARG1, CHIL3 | [97] |
| Raspberries | Cyanidin-3-O-sophoroside, Cyanidin-3-O-glucosylrutinoside, Cyanidin-3-O-glucoside, Cyanidin-3-O-rutinoside Dose: 125 μg/mL | HL-60-Human Caucasian promyelocytic leukemia, J45.01-Human acute T cell leukemia | Decreased: LOX, COX-2 | [98] |
| Black rice | cyanidin-3-O-glucoside, peonidin-3-O-glucoside Dose: 25 μg/mL | Rat primary dermal fibroblasts | Decreased: NF-κB p50 and p65 mRNA Increased: Induce Collagen, Type I Alpha 2 mRNA | [84] |
| Purple rice | Cyanidin-3-O-glucoside, peonidin-3-O-glucoside Dose: 50 μg/mL | Porcine cartilage explant | Decreased: s-GAG, HA, MMP-1, 3 and 13, Inhibited: NF-κB, ERK | [73] |
| Purple maize | Cyanidin-3-O-glucoside, pelargonidin-3-O-glucoside, peonidin-3-O-glucoside | RAW264.7 macrophages, 3T3-L1 adipocytes | Decreased: PGE2, NO, MCP, iNOS, COX-2, ROS Inhibited: PPARγ, DPP-IV | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Du, B.; Li, J.; Yang, Y.; Zhu, F. An Insight into Anti-Inflammatory Activities and Inflammation Related Diseases of Anthocyanins: A Review of Both In Vivo and In Vitro Investigations. Int. J. Mol. Sci. 2021, 22, 11076. https://doi.org/10.3390/ijms222011076
Ma Z, Du B, Li J, Yang Y, Zhu F. An Insight into Anti-Inflammatory Activities and Inflammation Related Diseases of Anthocyanins: A Review of Both In Vivo and In Vitro Investigations. International Journal of Molecular Sciences. 2021; 22(20):11076. https://doi.org/10.3390/ijms222011076
Chicago/Turabian StyleMa, Zilong, Bin Du, Jun Li, Yuedong Yang, and Fengmei Zhu. 2021. "An Insight into Anti-Inflammatory Activities and Inflammation Related Diseases of Anthocyanins: A Review of Both In Vivo and In Vitro Investigations" International Journal of Molecular Sciences 22, no. 20: 11076. https://doi.org/10.3390/ijms222011076
APA StyleMa, Z., Du, B., Li, J., Yang, Y., & Zhu, F. (2021). An Insight into Anti-Inflammatory Activities and Inflammation Related Diseases of Anthocyanins: A Review of Both In Vivo and In Vitro Investigations. International Journal of Molecular Sciences, 22(20), 11076. https://doi.org/10.3390/ijms222011076






