Epigenetic Regulation and Post-Translational Modifications of SNAI1 in Cancer Metastasis
Abstract
1. Introduction
2. Regulation of SNAI1
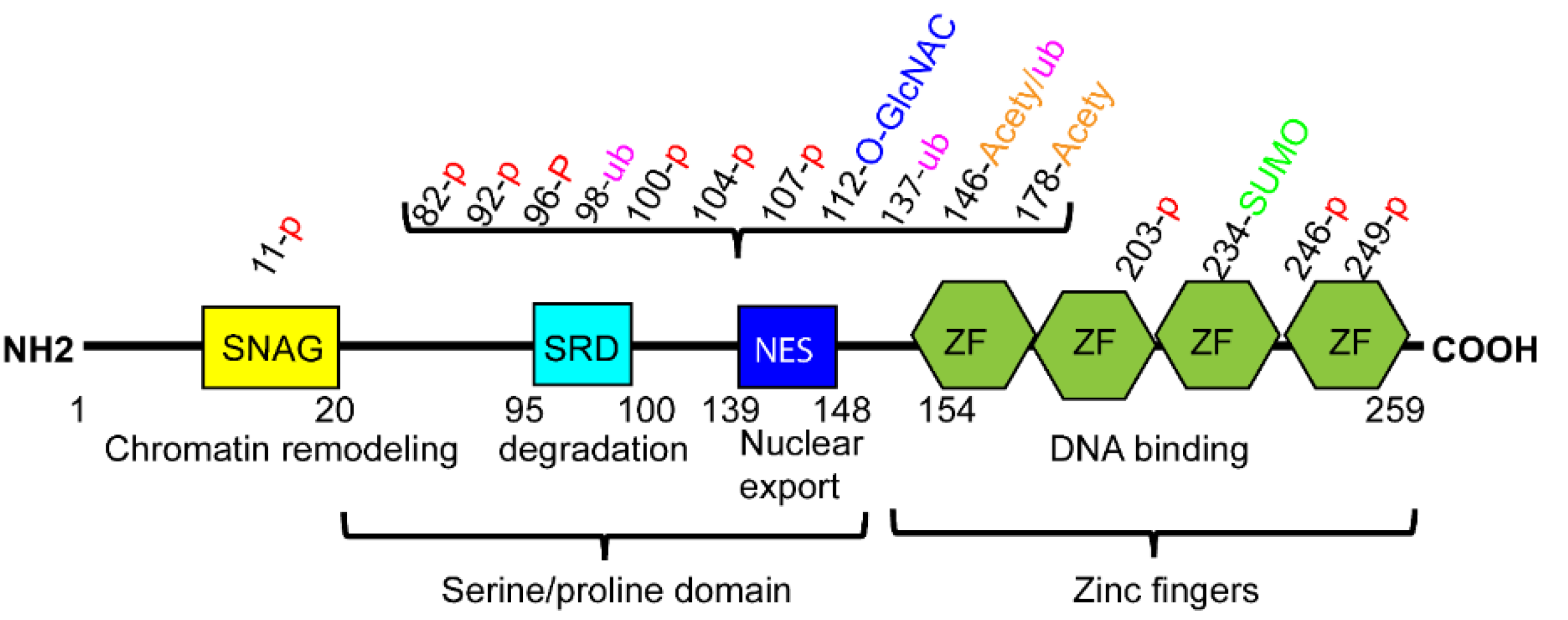
2.1. Structure of SNAI1
2.2. Transcriptional and Post-Transcriptional Regulation
2.3. Post-Translational Regulation
2.3.1. Phosphorylation Regulation
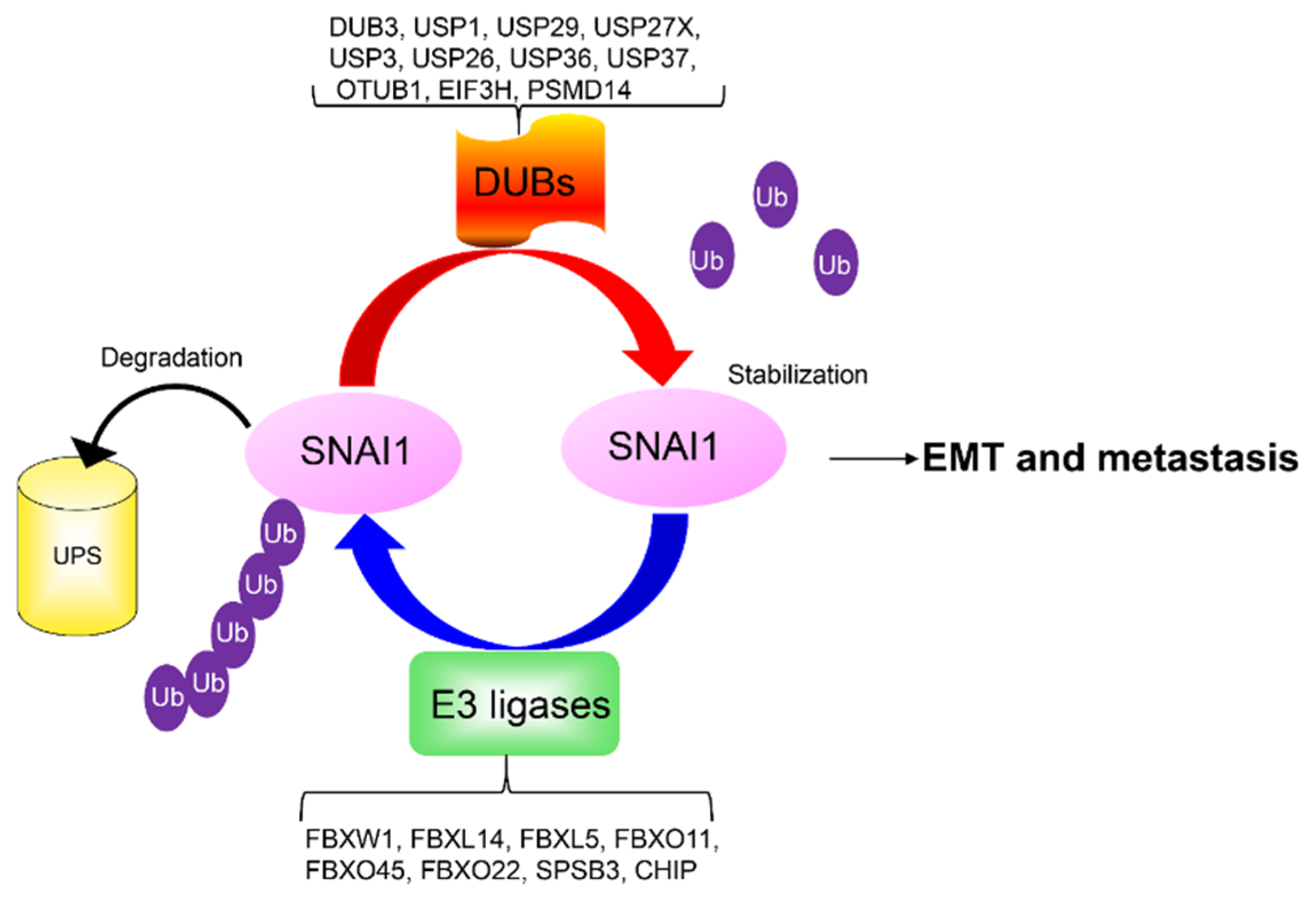
2.3.2. Ubiquitination and Deubiquitination
2.3.3. Other Post-Translational Regulation
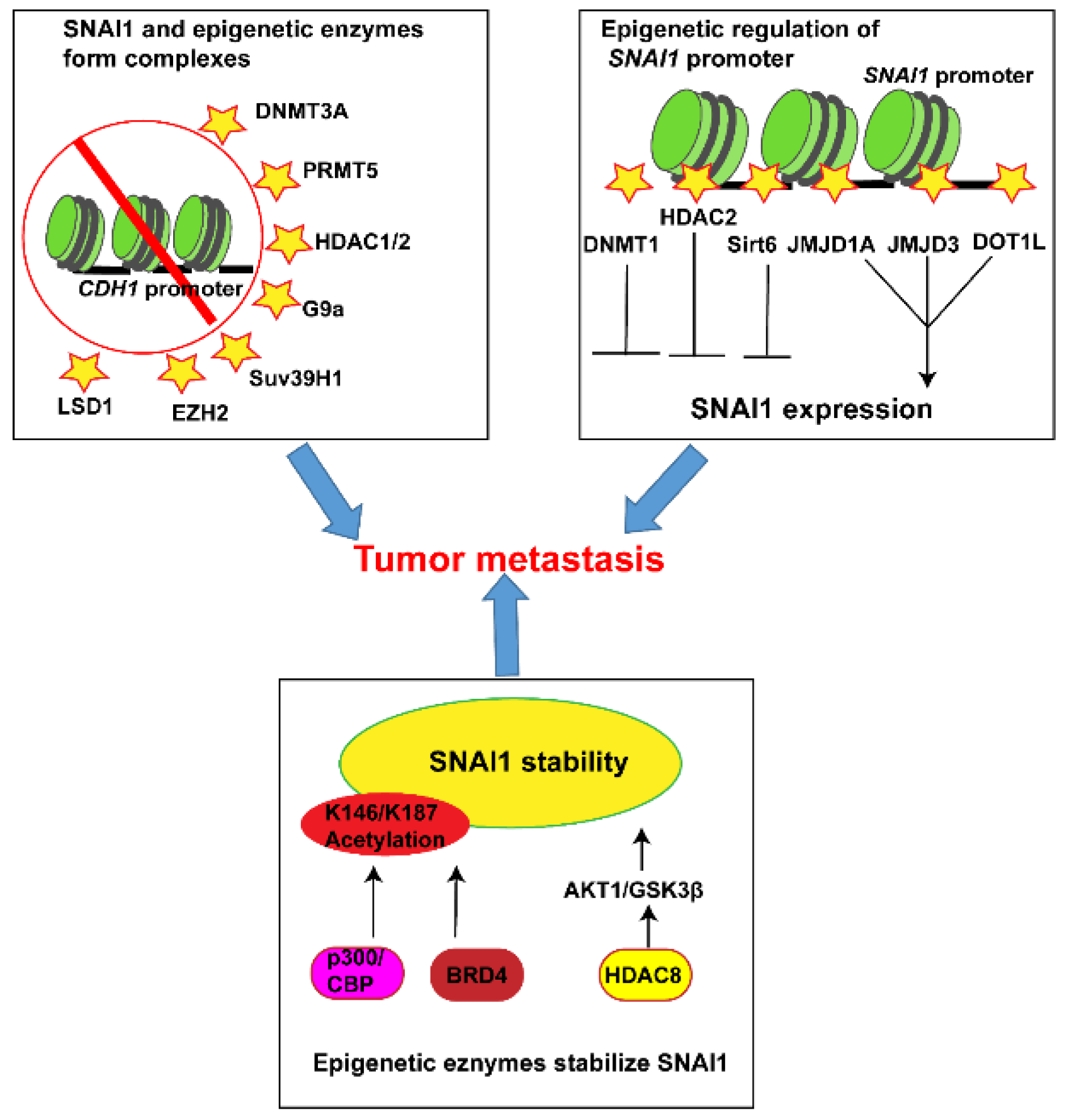
3. The Interplay between SNAI1 and Epigenetic Regulators in Tumor Metastasis
3.1. SNAI1 and DNA Methylation
3.2. SNAI1 and Histone Modification
3.2.1. Acetylation
3.2.2. Deacetylation
3.2.3. Acetylation Readers
3.2.4. Methylation
3.2.5. Demethylation
4. Potential Pharmacological Inhibitors of SNAI1
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, R.L.; Balasas, T.; Callaghan, J.; Coombes, R.C.; Evans, J.; Hall, J.A.; Kinrade, S.; Jones, D.; Jones, P.S.; Jones, R.; et al. A framework for the development of effective anti-metastatic agents. Nat. Rev. Clin. Oncol. 2019, 16, 185–204. [Google Scholar] [CrossRef]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Norgard, R.J.; Stanger, B.Z. Cellular Plasticity in Cancer. Cancer Discov. 2019, 9, 837–851. [Google Scholar] [CrossRef]
- Puisieux, A.; Brabletz, T.; Caramel, J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell Biol. 2014, 16, 488–494. [Google Scholar] [CrossRef]
- Hu, C.-T.; Chang, T.-Y.; Cheng, C.-C.; Liu, C.-S.; Wu, J.-R.; Li, M.-C.; Wu, W.-S. Snail associates with EGR-1 and SP-1 to upregulate transcriptional activation of p15INK4b. FEBS J. 2010, 277, 1202–1218. [Google Scholar] [CrossRef] [PubMed]
- Guaita, S.; Puig, I.; Francí, C.; Garrido, M.; Domínguez, D.; Batlle, E.; Sancho, E.; Dedhar, S.; de Herreros, A.G.; Baulida, J. Snail Induction of Epithelial to Mesenchymal Transition in Tumor Cells Is Accompanied by MUC1 Repression andZEB1 Expression. J. Biol. Chem. 2002, 277, 39209–39216. [Google Scholar] [CrossRef]
- Rembold, M.; Ciglar, L.; Yáñez-Cuna, J.O.; Zinzen, R.; Girardot, C.; Jain, A.; Welte, M.; Stark, A.; Leptin, M.; Furlong, E.E. A conserved role for Snail as a potentiator of active transcription. Genes Dev. 2014, 28, 167–181. [Google Scholar] [CrossRef]
- Hu, C.-T.; Wu, J.-R.; Chang, T.Y.; Cheng, C.-C.; Wu, W.-S. The transcriptional factor Snail simultaneously triggers cell cycle arrest and migration of human hepatoma HepG2. J. Biomed. Sci. 2008, 15, 343–355. [Google Scholar] [CrossRef]
- Ly, T.M.; Chen, Y.-C.; Lee, M.-C.; Hu, C.-T.; Cheng, C.-C.; Chang, H.-H.; You, R.-I.; Wu, W.-S. Snail Upregulates Transcription of FN, LEF, COX2, and COL1A1 in Hepatocellular Carcinoma: A General Model Established for Snail to Transactivate Mesenchymal Genes. Cells 2021, 10, 2202. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-S.; You, R.-I.; Cheng, C.-C.; Lee, M.-C.; Lin, T.-Y.; Hu, C.-T. Snail collaborates with EGR-1 and SP-1 to directly activate transcription of MMP 9 and ZEB1. Sci. Rep. 2017, 7, 17753. [Google Scholar] [CrossRef]
- Kajita, M.; McClinic, K.N.; Wade, P.A. Aberrant Expression of the Transcription Factors Snail and Slug Alters the Response to Genotoxic Stress. Mol. Cell. Biol. 2004, 24, 7559–7566. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Dong, C.; Yuan, T.; Wu, Y.; Wang, Y.; Fan, T.W.; Miriyala, S.; Lin, Y.; Yao, J.; Shi, J.; Kang, T.; et al. Loss of FBP1 by Snail-Mediated Repression Provides Metabolic Advantages in Basal-like Breast Cancer. Cancer Cell 2013, 23, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, B.P. Snail: More than Emt. Cell Adhes. Migr. 2010, 4, 199–203. [Google Scholar] [CrossRef]
- Barrallo-Gimeno, A.; Nieto, M.A. The Snail genes as inducers of cell movement and survival: Implications in development and cancer. Development 2005, 132, 3151–3161. [Google Scholar] [CrossRef]
- Greally, J.M. A user’s guide to the ambiguous word ‘epigenetics’. Nature reviews. Mol. Cell Biol. 2018, 19, 207–208. [Google Scholar]
- Wen, B.; Wu, H.; Shinkai, Y.; Irizarry, R.A.; Feinberg, A.P. Large histone H3 lysine 9 dimethylated chromatin blocks distinguish differentiated from embryonic stem cells. Nat. Genet. 2009, 41, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.L.; Weinberg, R.A. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat. Med. 2013, 19, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 2002, 3, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.A.; Pollard, J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2008, 9, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B.P. The Role of Snail in EMT and Tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef]
- Kaufhold, S.; Bonavida, B. Central role of snail1 in the regulation of emt and resistance in cancer: A target for therapeutic intervention. J. Exp. Clin. Cancer Res. 2014, 33, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Saito, D.; Kyakumoto, S.; Chosa, N.; Ibi, M.; Takahashi, N.; Okubo, N.; Sawada, S.; Ishisaki, A.; Kamo, M. Transforming growth factor-beta1 induces epithelial-mesenchymal transition and integrin alpha3beta1-mediated cell migration of hsc-4 human squamous cell carcinoma cells through slug. J. Biochem. 2013, 153, 303–315. [Google Scholar] [CrossRef][Green Version]
- Nakamura, R.; Ishii, H.; Endo, K.; Hotta, A.; Fujii, E.; Miyazawa, K.; Saitoh, M. Reciprocal expression of Slug and Snail in human oral cancer cells. PLoS ONE 2018, 13, e0199442. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.A.; Geiger, T.R.; Song, J.Y.; Gitelman, I.; Peeper, D.S. A twist-snail axis critical for trkb-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol. Cell Biol. 2009, 29, 3722–3737. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, K.; Majka, M. Interplay among SNAIL Transcription Factor, MicroRNAs, Long Non-Coding RNAs, and Circular RNAs in the Regulation of Tumor Growth and Metastasis. Cancers 2020, 12, 209. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Zhu, W.; Chu, H.; Yu, H.; Wei, P.; Wu, X.; Zhu, H.; Gao, H.; Liang, J.; et al. UDP-glucose accelerates SNAI1 mRNA decay and impairs lung cancer metastasis. Nature 2019, 571, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chai, G.; Wu, Y.; Li, J.; Chen, F.; Liu, J.; Luo, G.; Tauler, J.; Du, J.; Lin, S.; et al. RNA m6A methylation regulates the epithelial mesenchymal transition of cancer cells and translation of Snail. Nat. Commun. 2019, 10, 2065. [Google Scholar] [CrossRef]
- Li, F.; Zhao, H.; Su, M.; Xie, W.; Fang, Y.; Du, Y.; Yu, Z.; Hou, L.; Tan, W. Hnrnp-f regulates emt in bladder cancer by mediating the stabilization of snail1 mrna by binding to its 3’ UTR. EBioMedicine 2019, 45, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.P.; Deng, J.; Xia, W.; Xu, J.; Li, Y.M.; Gunduz, M.; Hung, M.C. Dual regulation of snail by gsk-3beta-mediated phos-phorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 2004, 6, 931–940. [Google Scholar] [CrossRef]
- Mimoto, R.; Taira, N.; Takahashi, H.; Yamaguchi, T.; Okabe, M.; Uchida, K.; Miki, Y.; Yoshida, K. Dyrk2 controls the epithe-lial-mesenchymal transition in breast cancer by degrading snail. Cancer Lett. 2013, 339, 214–225. [Google Scholar] [CrossRef]
- Zheng, H.; Shen, M.; Zha, Y.L.; Li, W.; Wei, Y.; Blanco, M.A.; Ren, G.; Zhou, T.; Storz, P.; Wang, H.Y.; et al. Pkd1 phosphor-ylation-dependent degradation of snail by scf-fbxo11 regulates epithelial-mesenchymal transition and metastasis. Cancer Cell 2014, 26, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Fattet, L.; Tsai, J.H.; Kajimoto, T.; Chang, Q.; Newton, A.C.; Yang, J. Apical-basal polarity inhibits epitheli-al-mesenchymal transition and tumour metastasis by par-complex-mediated snai1 degradation. Nat. Cell Biol. 2019, 21, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Guo, X.; Qian, X.; Wang, H.; Yang, C.; Brinkman, K.L.; Serrano-Gonzalez, M.; Jope, R.S.; Zhou, B.; Engler, D.A.; et al. Activation of the ATM-Snail pathway promotes breast cancer metastasis. J. Mol. Cell Biol. 2012, 4, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Pyun, B.-J.; Seo, H.R.; Lee, H.-J.; Jin, Y.B.; Kim, E.-J.; Kim, N.H.; Kim, H.S.; Nam, H.W.; Yook, J.I.; Lee, Y.-S. Mutual regulation between DNA-PKcs and snail1 leads to increased genomic instability and aggressive tumor characteristics. Cell Death Dis. 2013, 4, e517. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.J.; Park, S.M.; Park, S.H.; Kim, I.K.; Han, H.; Kim, H.J.; Kim, S.H.; Hong, K.S.; Kim, H.; Kim, M.; et al. P38 stabilizes snail by suppressing dyrk2-mediated phosphorylation that is required for gsk3beta-betatrcp-induced snail degradation. Cancer Res. 2019, 79, 4135–4148. [Google Scholar] [CrossRef]
- MacPherson, M.R.; Molina, P.; Souchelnytskyi, S.; Wernstedt, C.; Martin-Pérez, J.; Portillo, F.; Cano, A. Phosphorylation of serine 11 and serine 92 as new positive regulators of human snail1 function: Potential involvement of casein kinase-2 and the camp-activated kinase protein kinase a. Mol. Biol. Cell 2010, 21, 244–253. [Google Scholar] [CrossRef]
- Zhang, K.; Corsa, C.; Ponik, S.; Prior, J.L.; Piwnica-Worms, D.; Eliceiri, K.; Keely, P.J.; Longmore, G.D. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat. Cell Biol. 2013, 15, 677–687. [Google Scholar] [CrossRef]
- Yang, Z.; Rayala, S.; Nguyen, D.; Vadlamudi, R.K.; Chen, S.; Kumar, R. Pak1 Phosphorylation of Snail, a Master Regulator of Epithelial-to-Mesenchyme Transition, Modulates Snail’s Subcellular Localization and Functions. Cancer Res. 2005, 65, 3179–3184. [Google Scholar] [CrossRef]
- Chen, L.; Pan, X.-W.; Huang, H.; Gao, Y.; Yang, Q.-W.; Wang, L.-H.; Cui, X.-G.; Xu, D.-F. Epithelial-mesenchymal transition induced by GRO-α-CXCR2 promotes bladder cancer recurrence after intravesical chemotherapy. Oncotarget 2017, 8, 45274–45285. [Google Scholar] [CrossRef]
- Escriva, M.; Peiro, S.; Herranz, N.; Villagrasa, P.; Dave, N.; Montserrat-Sentis, B.; Murray, S.A.; Franci, C.; Gridley, T.; Virtanen, I.; et al. Repression of pten phosphatase by snail1 transcriptional factor during gamma radiation-induced apoptosis. Mol. Cell Biol. 2008, 28, 1528–1540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Rodriguez-Aznar, E.; Yabuta, N.; Owen, R.J.; Mingot, J.M.; Nojima, H.; Nieto, M.A.; Longmore, G.D. Lats2 kinase potentiates Snail1 activity by promoting nuclear retention upon phosphorylation. EMBO J. 2012, 31, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Dong, B.; Guo, W.; Piotr, R.; Longmore, G.; Yang, X.; Yu, Z.; Deng, J.; Evers, B.M.; Wu, Y. STK39 promotes breast cancer invasion and metastasis by increasing SNAI1 activity upon phosphorylation. Theranostics 2021, 11, 7658–7670. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Evers, B.M.; Zhou, B.P. Small C-terminal Domain Phosphatase Enhances Snail Activity through Dephosphorylation. J. Biol. Chem. 2009, 284, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, J.; Chen, F.; Feng, X.H. C-terminal domain small phosphatase-like 2 promotes epithelial-to-mesenchymal tran-sition via snail dephosphorylation and stabilization. Open Biol. 2018, 8, 170274. [Google Scholar] [CrossRef]
- Yu, Q.; Zhou, B.P.; Wu, Y. The regulation of snail: On the ubiquitin edge. Cancer Cell Microenviron. 2017, 4, e1567. [Google Scholar] [CrossRef][Green Version]
- Sun, R.; Xie, H.-Y.; Qian, J.-X.; Huang, Y.-N.; Yang, F.; Zhang, F.-L.; Shao, Z.-M.; Li, D.-Q. Fbxo22 possesses both protumor-igenic and antimetastatic roles in breast cancer progression. Cancer Res. 2018, 78, 5274–5286. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Zhu, R.; Ding, F.; Li, Y.; Cao, X.; Liu, Z. Spsb3 targets snail for degradation in gsk-3beta phosphoryla-tion-dependent manner and regulates metastasis. Oncogene 2018, 37, 768–776. [Google Scholar] [CrossRef]
- Park, S.; Park, S.; Ryu, K.; Kim, I.-K.; Han, H.; Kim, H.; Kim, S.; Hong, K.; Kim, H.; Kim, M.; et al. Downregulation of CHIP promotes ovarian cancer metastasis by inducing Snail-mediated epithelial–mesenchymal transition. Mol. Oncol. 2019, 13, 1280–1295. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jung, S.M.; Yang, K.-M.; Bae, E.; Ahn, S.G.; Park, J.S.; Seo, D.; Kim, M.; Ha, J.; Lee, J.; et al. A20 promotes metastasis of aggressive basal-like breast cancers through multi-monoubiquitylation of Snail1. Nat. Cell Biol. 2017, 19, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Lin, Y.; Liu, Y.; Wang, Y.; Jia, J.; Singh, P.; Chi, Y.-I.; Wang, C.; Dong, C.; et al. Dub3 inhibition suppresses breast cancer invasion and metastasis by promoting Snail1 degradation. Nat. Commun. 2017, 8, 14228. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yu, J.; Deng, M.; Yin, Y.; Zhang, H.; Luo, K.; Qin, B.; Li, Y.; Wu, C.; Ren, T. Cdk4/6-dependent activation of dub3 regulates cancer metastasis through snail1. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Sonego, M.; Pellarin, I.; Costa, A.; Vinciguerra, G.L.R.; Coan, M.; Kraut, A.; D’Andrea, S.; Dall’Acqua, A.; Castillo-Tong, D.C.; Califano, D.; et al. USP1 links platinum resistance to cancer cell dissemination by regulating Snail stability. Sci. Adv. 2019, 5, eaav3235. [Google Scholar] [CrossRef]
- Qian, W.; Li, Q.; Wu, X.; Li, W.; Li, Q.; Zhang, J.; Li, M.; Zhang, D.; Zhao, H.; Zou, X.; et al. Deubiquitinase USP29 promotes gastric cancer cell migration by cooperating with phosphatase SCP1 to stabilize Snail protein. Oncogene 2020, 39, 6802–6815. [Google Scholar] [CrossRef]
- Lambies, G.; Miceli, M.; Martinez-Guillamon, C.; Olivera-Salguero, R.; Pena, R.; Frias, C.P.; Calderon, I.; Atanassov, B.S.; Dent, S.Y.R.; Arribas, J.; et al. Tgfbeta-activated usp27x deubiquitinase regulates cell migration and chemoresistance via stabilization of snail1. Cancer Res. 2019, 79, 33–46. [Google Scholar] [CrossRef]
- Fan, L.; Chen, Z.; Wu, X.; Cai, X.; Feng, S.; Lu, J.; Wang, H.; Liu, N. Ubiquitin-Specific Protease 3 Promotes Glioblastoma Cell Invasion and Epithelial–Mesenchymal Transition via Stabilizing Snail. Mol. Cancer Res. 2019, 17, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, Y.; Zhu, R.; Ding, F.; Cao, X.; Lin, D.; Liu, Z. OTUB1 promotes esophageal squamous cell carcinoma metastasis through modulating Snail stability. Oncogene 2018, 37, 3356–3368. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, Y.; Zhou, H.; Li, L.; Li, Y.; Ding, F.; Cao, X.; Liu, Z. Deubiquitinating enzyme PSMD14 promotes tumor metastasis through stabilizing SNAIL in human esophageal squamous cell carcinoma. Cancer Lett. 2018, 418, 125–134. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, R.; Luo, A.; Zhou, H.; Ding, F.; Yang, H.; Liu, Z. EIF3H promotes aggressiveness of esophageal squamous cell carcinoma by modulating Snail stability. J. Exp. Clin. Cancer Res. 2020, 39, 175. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Li, M.; Wang, X.; Li, L.; Li, Q.; Hou, Z.; Jia, H.; Liu, S. USP37 Promotes Lung Cancer Cell Migration by Stabilizing Snail Protein via Deubiquitination. Front. Genet. 2020, 10, 1324. [Google Scholar] [CrossRef]
- Li, L.; Zhou, H.; Zhu, R.; Liu, Z. USP26 promotes esophageal squamous cell carcinoma metastasis through stabilizing Snail. Cancer Lett. 2019, 448, 52–60. [Google Scholar] [CrossRef]
- Gudey, S.K.; Sundar, R.; Heldin, C.H.; Bergh, A.; Landstrom, M. Pro-invasive properties of snail1 are regulated by sumoylation in response to tgfbeta stimulation in cancer. Oncotarget 2017, 8, 97703–97726. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, H.S.; Kim, N.H.; Ji, S.; Cha, S.Y.; Kang, J.G.; Ota, I.; Shimada, K.; Konishi, N.; Nam, H.W.; et al. Snail1 is stabilized by o-glcnac modification in hyperglycaemic condition. EMBO J. 2010, 29, 3787–3796. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.S.-S.; Wang, H.-J.; Tai, S.-K.; Chou, C.-H.; Hsieh, C.-H.; Chiu, P.-H.; Chen, N.-J.; Yang, M.-H. Acetylation of Snail Modulates the Cytokinome of Cancer Cells to Enhance the Recruitment of Macrophages. Cancer Cell 2014, 26, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Hu, Y.; Guo, D.; Zhang, A.; Gu, Y.; Zhang, S.; Zhao, C.; Gong, P.; Shen, X.; Li, Y.; et al. DNA methyltransferase 3A isoform b contributes to repressing E-cadherin through cooperation of DNA methylation and H3K27/H3K9 methylation in EMT-related metastasis of gastric cancer. Oncogene 2018, 37, 4358–4371. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Wang, J.; Yumoto, K.; Jung, Y.; Cackowski, F.C.; Decker, A.M.; Li, Y.; Franceschi, R.T.; Pienta, K.J.; Taichman, R.S. DNMT1 Regulates Epithelial-Mesenchymal Transition and Cancer Stem Cells, Which Promotes Prostate Cancer Metastasis. Neoplasia 2016, 18, 553–566. [Google Scholar] [CrossRef]
- Espada, J.; Peinado, H.; Lopez-Serra, L.; Setién, F.; Lopez-Serra, P.; Portela, A.; Renart, J.; Carrasco, E.; Calvo, M.; Juarranz, A.; et al. Regulation of SNAIL1 and E-cadherin function by DNMT1 in a DNA methylation-independent context. Nucleic Acids Res. 2011, 39, 9194–9205. [Google Scholar] [CrossRef]
- Jiang, H.; Cao, H.-J.; Ma, N.; Bao, W.-D.; Wang, J.-J.; Chen, T.-W.; Zhang, E.-B.; Yuan, Y.-M.; Ni, Q.-Z.; Zhang, F.-K.; et al. Chromatin remodeling factor ARID2 suppresses hepatocellular carcinoma metastasis via DNMT1-Snail axis. Proc. Natl. Acad. Sci. USA 2020, 117, 4770–4780. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, R.; Feng, D.; Huang, W.; Huo, M.; Zhang, J.; Leng, S.; Yang, Y.; Yang, T.; Yin, X.; et al. Snail/PRMT5/NuRD complex contributes to DNA hypermethylation in cervical cancer by TET1 inhibition. Cell Death Differ. 2021, 28, 2818–2836. [Google Scholar] [CrossRef]
- Chang, Z.; Zhang, Y.; Liu, J.; Zheng, Y.; Li, H.; Kong, Y.; Li, P.; Peng, H.; Shi, Y.; Cao, B.; et al. Snail promotes the generation of vascular endothelium by breast cancer cells. Cell Death Dis. 2020, 11, 457. [Google Scholar] [CrossRef]
- Peinado, H.; Ballestar, E.; Esteller, M.; Cano, A. Snail Mediates E-Cadherin Repression by the Recruitment of the Sin3A/Histone Deacetylase 1 (HDAC1)/HDAC2 Complex. Mol. Cell. Biol. 2004, 24, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Fujita, N.; Jaye, D.; Kajita, M.; Geigerman, C.; Moreno, C.S.; Wade, P.A. MTA3, a Mi-2/NuRD Complex Subunit, Regulates an Invasive Growth Pathway in Breast Cancer. Cell 2003, 113, 207–219. [Google Scholar] [CrossRef]
- Ren, X.; Yang, X.; Cheng, B.; Chen, X.; Zhang, T.; He, Q.; Li, B.; Li, Y.; Tang, X.; Wen, X.; et al. HOPX hypermethylation promotes metastasis via activating SNAIL transcription in nasopharyngeal carcinoma. Nat. Commun. 2017, 8, 14053. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Jiang, D.-M.; Hu, S.-S.; Zhao, L.; Wang, L.; Yang, M.-H.; Ai, M.-L.; Jiang, H.-J.; Han, Y.; Ding, Y.-Q.; et al. SATB2-AS1 Suppresses Colorectal Carcinoma Aggressiveness by Inhibiting SATB2-Dependent Snail Transcription and Epithelial–Mesenchymal Transition. Cancer Res. 2019, 79, 3542–3556. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Chen, F.; Li, Z.; Ling, Y.; Peng, Y.; Zhang, H.; Li, J.; Chen, Z.; Wang, H. Hdac8 promotes the dissemination of breast cancer cells via akt/gsk-3beta/snail signals. Oncogene 2020, 39, 4956–4969. [Google Scholar] [CrossRef]
- Chen, F.; Ma, X.; Liu, Y.; Ma, D.; Gao, X.; Qian, X. SIRT6 inhibits metastasis by suppressing SNAIL expression in nasopharyngeal carcinoma cells. Int. J. Clin. Exp. Pathol. 2021, 14, 63–74. [Google Scholar]
- Belkina, A.; Denis, G.V. BET domain co-regulators in obesity, inflammation and cancer. Nat. Rev. Cancer 2012, 12, 465–477. [Google Scholar] [CrossRef]
- Qin, Z.-Y.; Wang, T.; Su, S.; Shen, L.-T.; Zhu, G.-X.; Liu, Q.; Zhang, L.; Liu, K.-W.; Zhang, Y.; Zhou, Z.-H.; et al. BRD4 promotes gastric cancer progression and metastasis through acetylation-dependent stabilization of Snail. Cancer Res. 2019, 79, 4869–4881. [Google Scholar] [CrossRef]
- Lu, L.; Chen, Z.; Lin, X.; Tian, L.; Su, Q.; An, P.; Li, W.; Wu, Y.; Du, J.; Shan, H.; et al. Inhibition of brd4 suppresses the ma-lignancy of breast cancer cells via regulation of snail. Cell Death Differ. 2020, 27, 255–268. [Google Scholar] [CrossRef]
- Chen, M.-W.; Hua, K.-T.; Kao, H.-J.; Chi, C.-C.; Wei, L.-H.; Johansson, G.; Shiah, S.-G.; Chen, P.-S.; Jeng, Y.-M.; Cheng, T.-Y.; et al. H3K9 Histone Methyltransferase G9a Promotes Lung Cancer Invasion and Metastasis by Silencing the Cell Adhesion Molecule Ep-CAM. Cancer Res. 2010, 70, 7830–7840. [Google Scholar] [CrossRef]
- Dong, C.; Wu, Y.; Yao, J.; Wang, Y.; Yu, Y.; Rychahou, P.; Evers, B.M.; Zhou, B.P. G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J. Clin. Investig. 2012, 122, 1469–1486. [Google Scholar] [CrossRef]
- Herranz, N.; Pasini, D.; Díaz, V.M.; Francí, C.; Gutierrez, A.; Dave, N.; Escrivà, M.; Hernandez-Muñoz, I.; Di Croce, L.; Helin, K.; et al. Polycomb Complex 2 Is Required for E-cadherin Repression by the Snail1 Transcription Factor. Mol. Cell. Biol. 2008, 28, 4772–4781. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Yu, J.; Dhanasekaran, S.M.; Kim, J.H.; Mani, R.; Tomlins, S.; Mehra, R.; Laxman, B.; Cao, X.; Kleer, C.G.; et al. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene 2008, 27, 7274–7284. [Google Scholar] [CrossRef]
- Tong, Z.-T.; Cai, M.-Y.; Wang, X.-G.; Kong, L.-L.; Mai, S.-J.; Liu, Y.-H.; Zhang, H.-B.; Liao, Y.-J.; Zheng, F.; Zhu, W.-G.; et al. EZH2 supports nasopharyngeal carcinoma cell aggressiveness by forming a co-repressor complex with HDAC1/HDAC2 and Snail to inhibit E-cadherin. Oncogene 2011, 31, 583–594. [Google Scholar] [CrossRef]
- Okada, Y.; Feng, Q.; Lin, Y.; Jiang, Q.; Li, Y.; Coffield, V.M.; Su, L.; Xu, G.; Zhang, Y. hDOT1L Links Histone Methylation to Leukemogenesis. Cell 2005, 121, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Park, J.-H.; Choi, H.-J.; Park, M.-K.; Won, H.-Y.; Park, Y.-J.; Lee, C.H.; Oh, S.-H.; Song, Y.-S.; Kim, H.S.; et al. DOT1L cooperates with the c-Myc-p300 complex to epigenetically derepress CDH1 transcription factors in breast cancer progression. Nat. Commun. 2015, 6, 7821. [Google Scholar] [CrossRef] [PubMed]
- Metzger, E.; Wissmann, M.; Yin, N.; Müller, J.M.; Schneider, R.; Peters, A.H.; Günther, T.; Buettner, R.; Schüle, R. Lsd1 de-methylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 2005, 437, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lan, F.; Matson, C.; Mulligan, P.; Whetstine, J.R.; Cole, P.A.; Casero, R.; Shi, Y. Histone Demethylation Mediated by the Nuclear Amine Oxidase Homolog LSD1. Cell 2004, 119, 941–953. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, Y.; Li, J.; Dong, C.; Ye, X.; Chi, Y.-I.; Evers, B.M.; Zhou, B.P. The SNAG domain of Snail1 functions as a molecular hook for recruiting lysine-specific demethylase 1. EMBO J. 2010, 29, 1803–1816. [Google Scholar] [CrossRef]
- Lin, T.; Ponn, A.; Hu, X.; Law, B.K.; Lu, J. Requirement of the histone demethylase LSD1 in Snai1-mediated transcriptional repression during epithelial-mesenchymal transition. Oncogene 2010, 29, 4896–4904. [Google Scholar] [CrossRef]
- Ramadoss, S.; Chen, X.; Wang, C.-Y. Histone Demethylase KDM6B Promotes Epithelial-Mesenchymal Transition. J. Biol. Chem. 2012, 287, 44508–44517. [Google Scholar] [CrossRef]
- Tang, D.E.; Dai, Y.; Fan, L.L.; Geng, X.Y.; Fu, D.X.; Jiang, H.W.; Xu, S.H. Histone demethylase jmjd1a promotes tumor pro-gression via activating snail in prostate cancer. Mol. Cancer Res. 2020, 18, 698–708. [Google Scholar] [CrossRef]
- Vistain, L.F.; Yamamoto, N.; Rathore, R.; Cha, P.; Meade, T.J. Targeted Inhibition of Snail Activity in Breast Cancer Cells by Using a CoIII-Ebox Conjugate. ChemBioChem 2015, 16, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Shen, G.-N.; Jung, Y.S.; Lee, S.-J.; Chung, J.-Y.; Kim, H.-S.; Xu, Y.; Choi, Y.; Lee, J.-W.; Ha, N.-C.; et al. Antitumor effect of novel small chemical inhibitors of Snail-p53 binding in K-Ras-mutated cancer cells. Oncogene 2010, 29, 4576–4587. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baron, R.; Binda, C.; Tortorici, M.; McCammon, J.A.; Mattevi, A. Molecular Mimicry and Ligand Recognition in Binding and Catalysis by the Histone Demethylase LSD1-CoREST Complex. Structure 2011, 19, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Ferrari-Amorotti, G.; Fragliasso, V.; Esteki, R.; Prudente, Z.; Soliera, A.R.; Cattelani, S.; Manzotti, G.; Grisendi, G.; Dominici, M.; Pieraccioli, M.; et al. Inhibiting Interactions of Lysine Demethylase LSD1 with Snail/Slug Blocks Cancer Cell Invasion. Cancer Res. 2013, 73, 235–245. [Google Scholar] [CrossRef]
- Li, H.M.; Bi, Y.R.; Li, Y.; Fu, R.; Lv, W.C.; Jiang, N.; Xu, Y.; Ren, B.X.; Chen, Y.D.; Xie, H.; et al. A potent cbp/p300-snail in-teraction inhibitor suppresses tumor growth and metastasis in wild-type p53-expressing cancer. Sci. Adv. 2020, 6, eaaw8500. [Google Scholar] [CrossRef]
- Han, D.; Wu, G.; Chang, C.; Zhu, F.; Xiao, Y.; Li, Q.; Zhang, T.; Zhang, L. Disulfiram inhibits tgf-β-induced epitheli-al-mesenchymal transition and stem-like features in breast cancer via erk/nf-κb/snail pathway. Oncotarget 2015, 6, 40907–40919. [Google Scholar] [CrossRef]
- Baritaki, S.; Chapman, A.; Yeung, K.; Spandidos, D.; Palladino, M.; Bonavida, B. Inhibition of epithelial to mesenchymal transition in metastatic prostate cancer cells by the novel proteasome inhibitor, NPI-0052: Pivotal roles of Snail repression and RKIP induction. Oncogene 2009, 28, 3573–3585. [Google Scholar] [CrossRef]
- Burslem, G.; Crews, C.M. Proteolysis-Targeting Chimeras as Therapeutics and Tools for Biological Discovery. Cell 2020, 181, 102–114. [Google Scholar] [CrossRef]
- Samarasinghe, K.T.; Jaime-Figueroa, S.; Burgess, M.; Nalawansha, D.A.; Dai, K.; Hu, Z.; Bebenek, A.; Holley, S.A.; Crews, C.M. Targeted degradation of transcription factors by TRAFTACs: TRAnscription Factor TArgeting Chimeras. Cell Chem. Biol. 2021, 28, 648–661.e5. [Google Scholar] [CrossRef]
- Hu, Z.; Crews, C.M. Recent Developments in PROTAC-Mediated Protein Degradation: From Bench to Clinic. ChemBioChem 2021. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.P.; Chen, J.; Tse, W.K.F. Role of Deubiquitinases in Human Cancers: Potential Targeted Therapy. Int. J. Mol. Sci. 2020, 21, 2548. [Google Scholar] [CrossRef]
- Dong, B.; Qiu, Z.; Wu, Y. Tackle Epithelial-Mesenchymal Transition with Epigenetic Drugs in Cancer. Front. Pharmacol. 2020, 11, 596239. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Han, J.-W. Targeting epigenetics for cancer therapy. Arch. Pharmacal Res. 2019, 42, 159–170. [Google Scholar] [CrossRef] [PubMed]
| Function | Regulation Factor | Phosphorylation Sites |
|---|---|---|
| Degradation | PKD1 | Ser11 |
| αPKC | Ser249 | |
| CK1 | Ser104/Ser107 | |
| GSK3β | Ser96/Ser100/S104/Ser107 | |
| DYRK2 | Ser104 | |
| Stabilization | PTK6 | Tyr342 |
| ATM | Ser100 | |
| CK2 | Ser92 | |
| p38 | Ser107 | |
| Nuclear accumulation and stabilization | ERK | Ser82/Ser104 |
| PAK1 | Ser246 | |
| GROα | ||
| LATS2 | Thr203 | |
| STK39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, B.; Wu, Y. Epigenetic Regulation and Post-Translational Modifications of SNAI1 in Cancer Metastasis. Int. J. Mol. Sci. 2021, 22, 11062. https://doi.org/10.3390/ijms222011062
Dong B, Wu Y. Epigenetic Regulation and Post-Translational Modifications of SNAI1 in Cancer Metastasis. International Journal of Molecular Sciences. 2021; 22(20):11062. https://doi.org/10.3390/ijms222011062
Chicago/Turabian StyleDong, Bo, and Yadi Wu. 2021. "Epigenetic Regulation and Post-Translational Modifications of SNAI1 in Cancer Metastasis" International Journal of Molecular Sciences 22, no. 20: 11062. https://doi.org/10.3390/ijms222011062
APA StyleDong, B., & Wu, Y. (2021). Epigenetic Regulation and Post-Translational Modifications of SNAI1 in Cancer Metastasis. International Journal of Molecular Sciences, 22(20), 11062. https://doi.org/10.3390/ijms222011062





