Expression Patterns of the Heat Shock Protein 90 (Hsp90) Gene Suggest Its Possible Involvement in Maintaining the Dormancy of Dinoflagellate Resting Cysts
Abstract
:1. Introduction
2. Results
2.1. The Full-Length cDNA Sequence of StHsp90
2.2. Structural Characterization of StHsp90
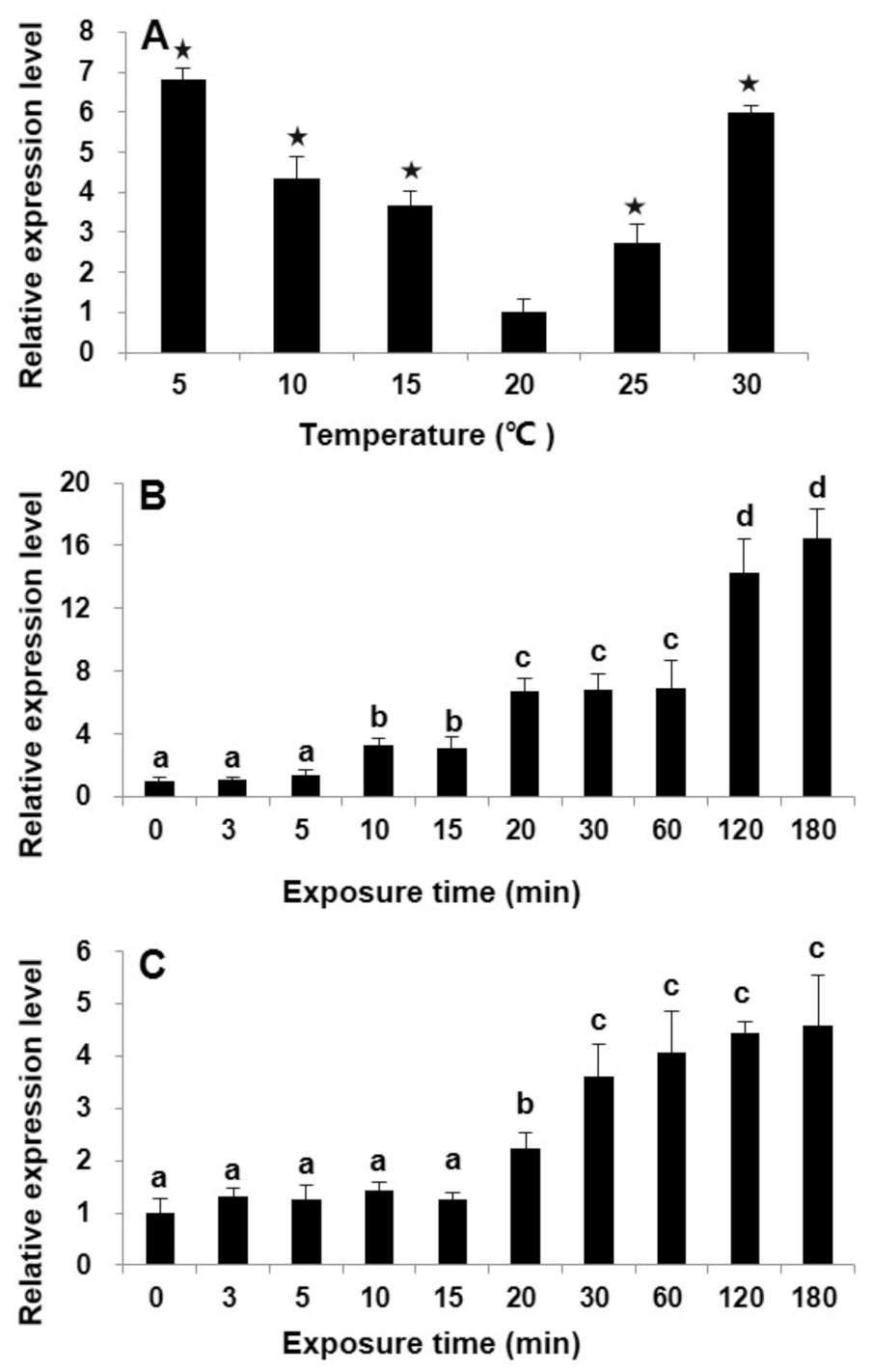
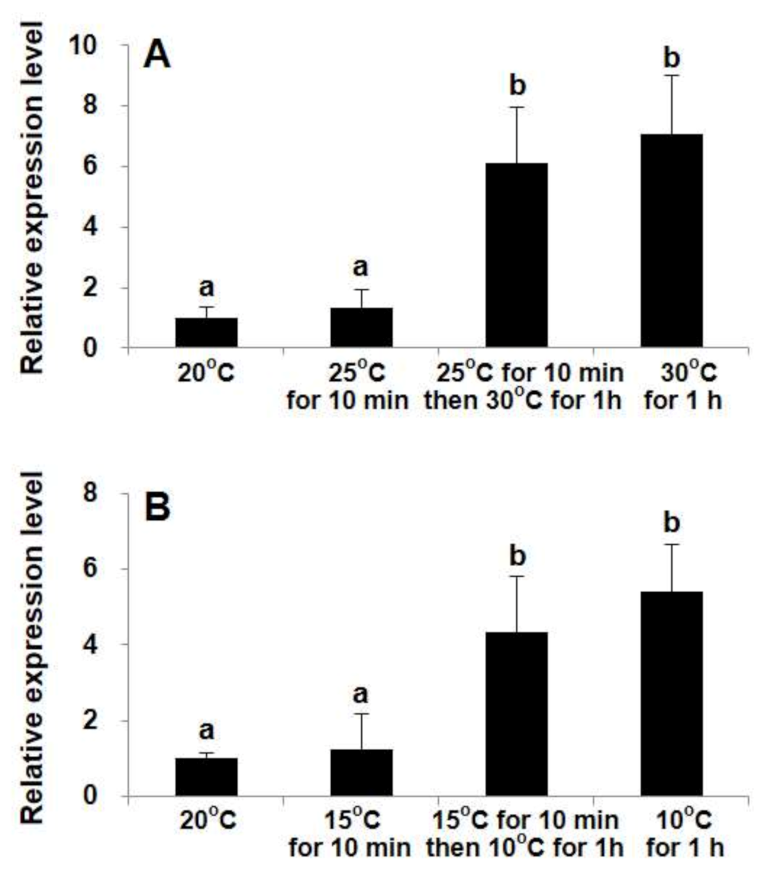
2.3. Differential Transcriptional Responses of StHsp90 in Vegetative cells to Temperature Variations in Laboratory Culture
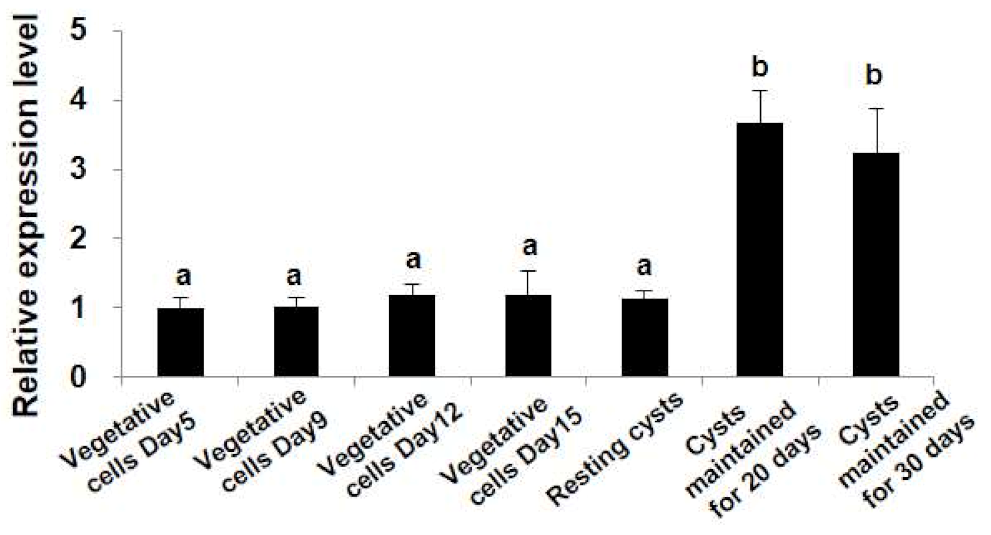
2.4. Differential Transcription Profiles of StHsp90 at Different Stages of Growth and Life Cycle
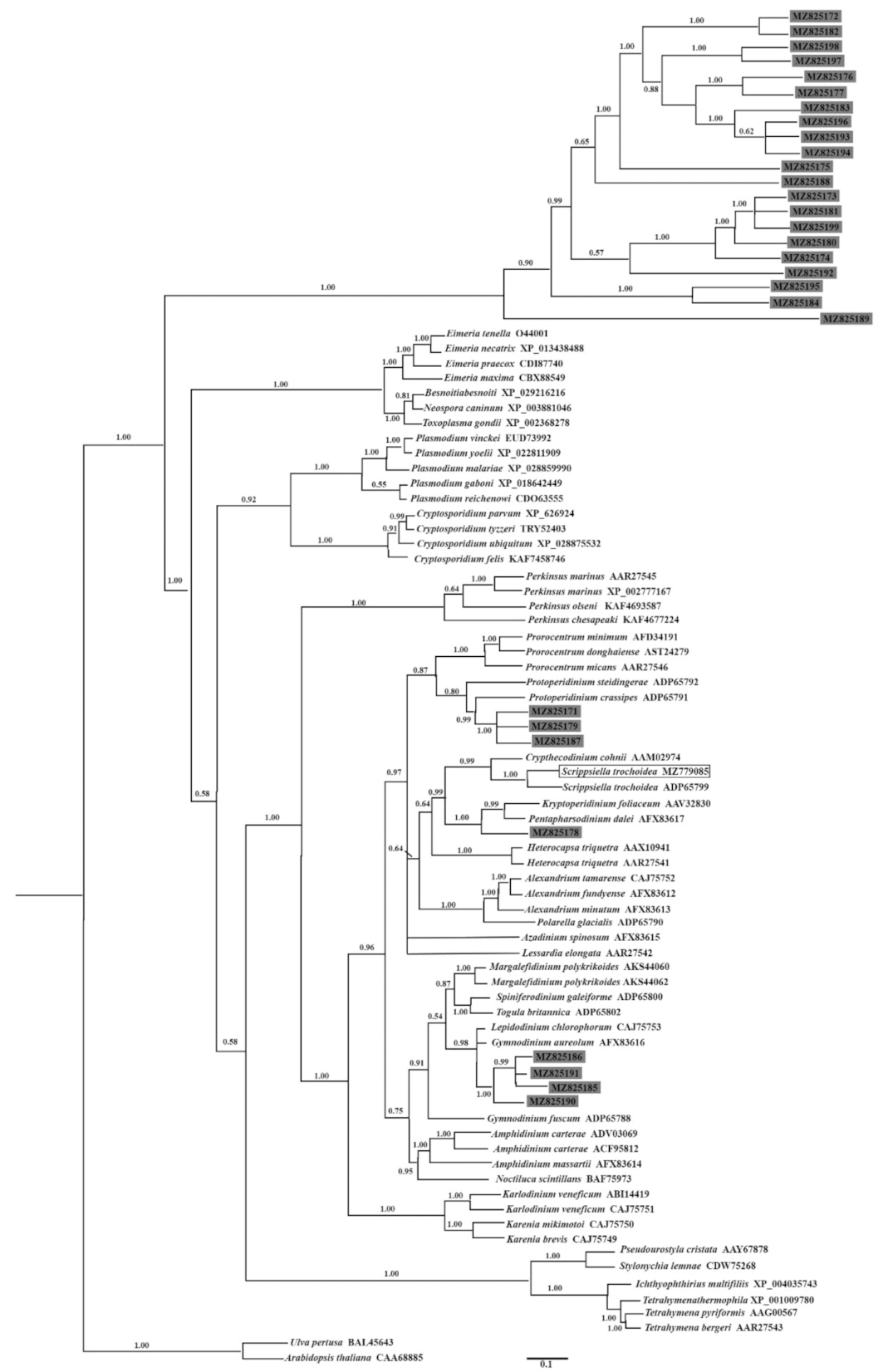
2.5. Identification and Phylogenetic Analysis of Nuclear Dinoflagellate Hsp90 Genes from a Dinoflagellate-Specific e-cDNA Library
3. Discussion
3.1. General Comments on the Newly Isolated Hsp90 Gene from Scrippsiella Trochoidea
3.2. StHsp90 May Be Engaged in the Resistance to Both Heat and Cold Stresses
3.3. Hsp90s Expressions Observed Both in Laboratory Culture and in the Cyst Assemblage in the Field Suggest an Involvement of Hsp90 Chaperones in the Dormancy of Resting Cysts of Dinoflagellates
4. Materials and Methods
4.1. Culture Establishment and Maintenance of Scrippsiella Trochoidea
4.2. Cloning of the Full-Length StHsp90 cDNA
4.3. Analysis of StHsp90 Deduced Amino Acid Sequence
4.4. Transcriptional Profiles of StHsp90 in Responses to Temperature Stress and Alteration of Life Cycle in Laboratory Culture
4.4.1. Samples Collection
Temperature Stress Exposures
Cells at Different Life Stages
4.4.2. Real-Time Quantitative PCR (qPCR)
4.5. Construction of Dinoflagellate-Specific Environmental cDNA (e-cDNA) Library from Marine Sediment
4.5.1. Sediment Collection and Resting Cysts Separation
4.5.2. Construction of e-cDNA Library and PacBio Iso-Seq Sequencing
4.5.3. Data Processing
4.6. Identification of Hsp90 Genes from Dinoflagellate-Specific e-cDNA Library and Phylogenetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and Ecological Physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangster, T.A.; Queitsch, C. The HSP90 chaperone complex, an emerging force in plant development and phenotypic plastic-ity. Curr. Opin. Plant Biol. 2005, 8, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Efeoğlu, B. Heat shock proteins and heat shock response in plants. GUJ Sci. 2009, 22, 67–75. [Google Scholar]
- Biebl, M.M.; Buchner, J. Structure, Function, and Regulation of the Hsp90 Machinery. Cold Spring Harb. Perspect. Biol. 2019, 11, a034017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.C.; Sharma, A.; Mishra, M.; Mishra, R.K.; Chowdhuri, D.K. Heat shock proteins in toxicology: How close and how far? Life Sci. 2010, 86, 377–384. [Google Scholar] [CrossRef]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef]
- Sima, S.; Richter, K. Regulation of the Hsp90 system. Biochim. Biophys. Acta BBA Bioenergy 2018, 1865, 889–897. [Google Scholar] [CrossRef]
- Abassi, S.; Wang, H.; Ki, J.-S. Molecular cloning of heat shock protein 70 and HOP from the freshwater green algae Closterium ehrenbergii and their responses to stress. Cell Stress Chaperone 2020, 25, 1117–1123. [Google Scholar] [CrossRef]
- Prodromou, C. The ‘active life’ of Hsp90 complexes. BBA Mol. Cell. Res. 2012, 1823, 614–623. [Google Scholar] [CrossRef] [Green Version]
- Zabinsky, R.A.; Mason, G.A.; Queitsch, C.; Jarosz, D.F. It’s not magic-Hsp90 and its effects on genetic and epigenetic variation. Semin. Cell Dev. Biol. 2019, 88, 21–35. [Google Scholar] [CrossRef]
- Rutherford, S.L.; Lindquist, S. Hsp90 as a capacitor for morphological evolution. Nature 1998, 396, 336–342. [Google Scholar] [CrossRef]
- Hackett, J.D.; Anderson, D.M.; Erdner, D.L.; Bhattacharya, D. Dinoflagellates: A remarkable evolutionary experiment. Am. J. Bot. 2004, 91, 1523–1534. [Google Scholar] [CrossRef]
- Trench, R.K.; Blank, R.J. Symbiodinium microdriaticum ferudenthal, S. Goreauii sp. nov., S. kawagutii sp. nov. and S. pilosum sp. nov.: Gymnodinioid dinoflagellate symbionts of marine invertebrates. J. Phycol. 1987, 23, 469–481. [Google Scholar] [CrossRef]
- Jeong, H.J.; Kang, H.C.; Lim, A.S.; Jang, S.H.; Lee, K.; Lee, S.Y.; Ok, J.H.; You, J.H.; Kim, J.H.; Lee, K.H.; et al. Feeding diverse prey as an excellent strategy of mixotrophic dinoflagellates for global dominance. Sci. Adv. 2021, 7, eabe4214. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in Understanding Harmful Algal Blooms: Paradigm Shifts and New Technologies for Research, Monitoring, and Management. Annu. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, L.; Wilcox, L.W. Algae; Prentice-Hall: Upper Saddle River, NJ, USA, 2000. [Google Scholar]
- Brosnahan, M.L.; Fischer, A.D.; Lopez, C.B.; Moore, S.K.; Anderson, D.M. Cyst-forming dinoflagellates in a warming climate. Harmful Algae 2000, 91, 101728. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Lv, S.H.; Chen, J.F.; He, L.S.; Xie, L.C.; Qi, Y.Z. The influence of water temperature and salinity on the growth of Scrippsiella trochoidea. Mar. Environ. Sci. 2004, 23, 36–38. [Google Scholar]
- Deng, G.; Li, Y.G.; Hu, H.J.; Qi, Y.Z.; Geng, Y.H.; Li, Z.K. Effects of temperature, light and pH on photosynthesis, and of light-dark cycle on growth rate and biomass of Scrippsiella trochoidea and Alexandrium tamarense. J. Wuhan Bot. Res. 2004, 22, 129–135. [Google Scholar]
- Kim, D.-I.; Matsuyama, Y.; Nagasoe, S.; Yamaguchi, M.; Yoon, Y.-H.; Oshima, Y.; Imada, N.; Honjo, T. Effects of temperature, salinity and irradiance on the growth of the harmful red tide dinoflagellate Cochlodinium polykrikoides Margalef (Dinophyceae). J. Plankton Res. 2004, 26, 61–66. [Google Scholar] [CrossRef]
- Matsubara, T.; Nagasoe, S.; Yamasaki, Y.; Shikata, T.; Shimasaki, Y.; Oshima, Y.; Honjo, T. Effects of temperature, salinity, and irradiance on the growth of the dinoflagellate Akashiwo sanguinea. J. Exp. Mar. Biol. Ecol. 2007, 342, 226–230. [Google Scholar] [CrossRef]
- Ellegaard, M.; Ribeiro, S. The long-term persistence of phytoplankton resting stages in aquatic ‘seed banks’. Biol. Rev. 2018, 93, 166–183. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.Z.; Gu, H.F.; Wang, Z.H.; Liu, D.Y.; Wang, Y.; Lu, D.D.; Hu, Z.X.; Deng, Y.Y.; Shang, L.X.; Qi, Y.Z. Exploration of resting cysts (stages) and relevant aspects of possibly HABs-causing species in China. Harmful Algae 2021, 107, 102050. [Google Scholar] [CrossRef]
- Bravo, I.; Figueroa, R.I. Towards an Ecological Understanding of Dinoflagellate Cyst Functions. Microorganisms 2014, 2, 11–32. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.Z.; Hu, Z.X.; Deng, Y.Y. Characteristical life history (resting cyst) provides a mechanism for recurrence and geographic expansion of harmful algal blooms of dinoflagellates: A Review. Studia Mar. Sin. 2016, 51, 132–154. [Google Scholar]
- Leander, B.S.; Keeling, P.J. Early evolutionary history of dinoflagellates and apicomplexans (alveolata) as inferred from hsp90 and actin phylogenies. J. Phycol. 2004, 40, 341–350. [Google Scholar] [CrossRef]
- Shalchian-Tabrizi, K.; Minge, M.A.; Cavalier-Smith, T.; Nedreklepp, J.M.; Klaveness, D.; Jakobsen, K.S. Combined heat shock protein 90 and ribosomal RNA sequence phylogeny supports multiple replacements of dinoflagellate platastids. J. Eukaryot. Microbiol. 2006, 53, 217–224. [Google Scholar] [CrossRef]
- Fukuda, Y.; Endoh, H. Phylogenetic analyses of the dinoflagellate Noctiluca scintillans based on β-tubulin and Hsp90 genes. Eur. J. Protistol. 2008, 44, 27–33. [Google Scholar] [CrossRef]
- Hoppenrath, M.; Leander, B.S. Dinoflagellate Phylogeny as Inferred from Heat Shock Protein 90 and Ribosomal Gene Sequences. PLoS ONE 2010, 5, e13220. [Google Scholar] [CrossRef] [Green Version]
- Orr, R.J.S.; Murray, S.A.; Stüken, A.; Rhodes, L.; Jakobsen, K.S. When Naked Became Armored: An Eight-Gene Phylogeny Reveals Monophyletic Origin of Theca in Dinoflagellates. PLoS ONE 2012, 7, e50004. [Google Scholar] [CrossRef] [Green Version]
- Han, M.S.; Wang, P.; Kim, J.H.; Cho, S.Y.; Park, B.S.; Kim, J.H.; Katano, T.; Kim, B.H. Morphological and molecular phylo-genetic position of Prorocentrum micans sensu stricto and description of Prorocentrum koreanum sp. nov. from southern coastal waters in Korea and Japan. Protist 2016, 167, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hou, Y.; Miranda, L.; Campbell, D.A.; Sturm, N.R.; Gaasterland, T.; Lin, S. Spliced leader RNA trans-splicing in dinoflagellates. Proc. Natl. Acad. Sci. USA 2007, 104, 4618–4623. [Google Scholar] [CrossRef] [Green Version]
- Leggat, W.; Seneca, F.; Wasmund, K.; Ukani, L.; Yellowlees, D.; Ainsworth, T. Differential Responses of the Coral Host and Their Algal Symbiont to Thermal Stress. PLoS ONE 2011, 6, e26687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, C.D.; Mello, L.V.; Samatar, N.; Martin, L.E.; Montagnes, D.J.; Watts, P.C. The transcriptome of the novel dinoflagellate Oxyrrhis marina (Alveolata: Dinophyceae): Response to salinity examined by 454 sequencing. BMC Genom. 2011, 12, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.Z.; Li, C.; Zhang, Y.; Wang, Y.Y.; He, Z.P.; Lin, L.; Hong, H.S. Quantitative proteomic analysis of differentially ex-pressed proteins in the toxicity-lost mutant of Alexandrium catenella (Dinophyceae) in the exponential phase. J. Proteom. 2012, 75, 5564–5577. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, L.; Wang, M.; Li, C.; Hong, H. Proteomic analysis of a toxic dinoflagellate Alexandrium catenella under different growth phases and conditions. Chin. Sci. Bull. 2012, 57, 3328–3341. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.-Z.; Zhang, Y.-J.; Zhang, S.-F.; Lin, L.; Hong, H.-S. Quantitative Proteomic Analysis of Cell Cycle of the Dinoflagellate Prorocentrum donghaiense (Dinophyceae). PLoS ONE 2013, 8, e63659. [Google Scholar] [CrossRef] [Green Version]
- Barshis, D.J.; Ladner, J.T.; Oliver, T.A.; Palumbi, S.R. Lineage-Specific Transcriptional Profiles of Symbiodinium spp. Unaltered by Heat Stress in a Coral Host. Mol. Biol. Evol. 2014, 31, 1343–1352. [Google Scholar] [CrossRef] [Green Version]
- Gierz, S.L.; Forêt, S.; Leggat, W. Transcriptomic Analysis of Thermally Stressed Symbiodinium Reveals Differential Expression of Stress and Metabolism Genes. Front. Plant Sci. 2017, 8, 271. [Google Scholar] [CrossRef]
- Wang, H.; Kim, H.; Ki, J.-S. Transcriptome survey, molecular identification, and expression analysis of stress-responsive genes in the toxic dinoflagellate Alexandrium pacificum under algicidal agents and metal stresses. J. Appl. Phycol. 2021, 33, 3139–3151. [Google Scholar] [CrossRef]
- Rosic, N.N.; Pernice, M.; Dove, S.; Dunn, S.; Hoegh-Guldberg, O. Gene expression profiles of cytosolic heat shock proteins Hsp70 and Hsp90 from symbiotic dinoflagellates in response to thermal stress: Possible implications for coral bleaching. Cell Stress Chaperone 2011, 16, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Ki, J.-S. Differential transcription of heat shock protein 90 (HSP90) in the dinoflagellate Prorocentrum minimum by copper and endocrine-disrupting chemicals. Ecotoxicology 2012, 21, 1448–1457. [Google Scholar] [CrossRef]
- Guo, R.; Youn, S.H.; Ki, J.S. Heat shock protein 70 and 90 genes in the harmful dinoflagellate Cochlodinium polykrikoides: Ge-nomic structures and transcriptional responses to environmental stresses. Int. J. Genom. 2015, 2015, 484626. [Google Scholar]
- Zhang, C.Y.; Chen, G.F.; Wang, Y.Y.; Zhang, X.D.; Li, C.H. Molecular characterization of heat shock protein 90 from the dinoflagellate Prorocentrum donghaiense and its transcriptional response to thermal, copper and nutrient stresses. Mar. Biol. Res. 2019, 15, 343–356. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Z.; Song, X.; Cao, X.; Zhang, Y. Effects of ammonium and nitrate on encystment and growth of Scrippsiella trochoidea. Chin. Sci. Bull. 2014, 59, 4491–4497. [Google Scholar] [CrossRef]
- Deng, Y.; Hu, Z.; Shang, L.; Peng, Q.; Tang, Y.Z. Transcriptomic Analyses of Scrippsiella trochoidea Reveals Processes Regulating Encystment and Dormancy in the Life Cycle of a Dinoflagellate, with a Particular Attention to the Role of Abscisic Acid. Front. Microbiol. 2017, 8, 2450. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Hu, Z.; Chai, Z.; Tang, Y.Z. Molecular cloning of heat shock protein 60 (Hsp60) and 10 (Hsp10) genes from the cosmopolitan and harmful dinoflagellate Scrippsiella trochoidea and their differential transcriptions responding to temperature stress and alteration of life cycle. Mar. Biol. 2019, 166, 7. [Google Scholar] [CrossRef]
- Deng, Y.; Hu, Z.; Chai, Z.; Tang, Y.Z. Cloning and comparative studies of proliferating cell nuclear antigen (PCNA) genes for nine dinoflagellates. J. Appl. Phycol. 2019, 31, 2969–2979. [Google Scholar] [CrossRef]
- Deng, Y.; Hu, Z.; Chai, Z.; Tang, Y.Z. Cloning and Partial Characterization of a Cold Shock Domain-Containing Protein Gene from the Dinoflagellate Scrippsiella trochoidea. J. Eukaryot. Microbiol. 2019, 66, 393–403. [Google Scholar] [CrossRef]
- Deng, Y.; Hu, Z.; Shang, L.; Chai, Z.; Tang, Y.Z. Transcriptional Responses of the Heat Shock Protein 20 (Hsp20) and 40 (Hsp40) Genes to Temperature Stress and Alteration of Life Cycle Stages in the Harmful Alga Scrippsiella trochoidea (Dinophyceae). Biology 2020, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Hu, Z.; Zhan, Z.; Ma, Z.; Tang, Y. Differential expressions of an Hsp70 gene in the dinoflagellate Akashiwo sanguinea in response to temperature stress and transition of life cycle and its implications. Harmful Algae 2015, 50, 57–64. [Google Scholar] [CrossRef]
- Harry, J.; Williams, K.; Briscoe, D. Sex determination in loggerhead turtles: Differential expression of two hnRNP proteins. Development 1990, 109, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Curci, A.; Bevilacqua, A.; Fiorenza, M.T.; Mangia, F. Developmental regulation of heat-shock response in mouse oogenesis: Identification of differentially responsive oocyte classes during Graafian follicle development. Dev. Biol. 1991, 144, 362–368. [Google Scholar] [CrossRef]
- Lee, Y.K.; Kim, S.H.; Hong, C.B.; Chah, O.-K.; Kim, G.H.; Lee, I.K. Heat-shock protein 90 may be involved in differentiation of the female gametophytes in Griffithsia japonica (Ceramiales, Rhodophyta). J. Phycol. 1998, 34, 1017–1023. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar] [CrossRef]
- Rombel, I.T.; Sykes, K.F.; Rayner, S.; Johnston, S.A. ORF-FINDER: A vector for high-throughput gene identification. Gene 2002, 282, 33–41. [Google Scholar] [CrossRef]
- Letunic, I.; Doerks, T.; Bork, P. SMART 7: Recent updates to the proteindomain annotation resource. Nucleic Acids Res. 2012, 40, D302–D305. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2005. [Google Scholar]
- Geourjon, C.; Deléage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput. Appl. Biosci. 1995, 11, 681–684. [Google Scholar] [CrossRef]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Radonic, A.; Thulke, S.; Mackay, I.M.; Landt, O.; Siegert, W.; Nitsche, A. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2004, 313, 856–862. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Zakrajsek, B.A.; Mills, A.G.; Gorn, V.; Singer, M.J.; Reed, M.W. Quantitative Reverse Transcription–Polymerase Chain Reaction to Study mRNA Decay: Comparison of Endpoint and Real-Time Methods. Anal. Biochem. 2000, 285, 194–204. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolch, C.J.S. The use of sodium polytungstate for the separation and concentration of living dinoflagellate cysts from marine sediments. Phycologia 1997, 36, 472–478. [Google Scholar] [CrossRef]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefing. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Hoehna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Primer Name | Nucleotide Sequences (5′→3′) | Remarks |
|---|---|---|
| P1 | TCTTCATTATGGACGATTGC | fragment cloning |
| P2 | GATGGAGTGCTTCGGATT | fragment cloning |
| P3 | GCACTTGCCAAACTGCTCGTAACATT | 5′ RACE |
| P4 | CACGACGCCCTTGACCATGTTGA | 5′ RACE |
| P5 | ATGGCTGACTCCCCTTGCGTGCTC | 3′ RACE |
| P6 | GAGGTGAATCCGAAGCACTCCATC | 3′ RACE |
| P7 | GCATTCGGAATTTATTGGC | qPCR |
| P8 | ATCTTCGGCTCGTCACCC | qPCR |
| anchor primer | GCTGTCAACGATACGCTACGTAACGGCAT GACAGTGT (18) | cDNA synthesis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Li, F.; Hu, Z.; Yue, C.; Tang, Y.Z. Expression Patterns of the Heat Shock Protein 90 (Hsp90) Gene Suggest Its Possible Involvement in Maintaining the Dormancy of Dinoflagellate Resting Cysts. Int. J. Mol. Sci. 2021, 22, 11054. https://doi.org/10.3390/ijms222011054
Deng Y, Li F, Hu Z, Yue C, Tang YZ. Expression Patterns of the Heat Shock Protein 90 (Hsp90) Gene Suggest Its Possible Involvement in Maintaining the Dormancy of Dinoflagellate Resting Cysts. International Journal of Molecular Sciences. 2021; 22(20):11054. https://doi.org/10.3390/ijms222011054
Chicago/Turabian StyleDeng, Yunyan, Fengting Li, Zhangxi Hu, Caixia Yue, and Ying Zhong Tang. 2021. "Expression Patterns of the Heat Shock Protein 90 (Hsp90) Gene Suggest Its Possible Involvement in Maintaining the Dormancy of Dinoflagellate Resting Cysts" International Journal of Molecular Sciences 22, no. 20: 11054. https://doi.org/10.3390/ijms222011054
APA StyleDeng, Y., Li, F., Hu, Z., Yue, C., & Tang, Y. Z. (2021). Expression Patterns of the Heat Shock Protein 90 (Hsp90) Gene Suggest Its Possible Involvement in Maintaining the Dormancy of Dinoflagellate Resting Cysts. International Journal of Molecular Sciences, 22(20), 11054. https://doi.org/10.3390/ijms222011054







