The Effect of Surface-Modified Gold Nanorods on the Early Stage of Embryonic Development and Angiogenesis: Insight into the Molecular Pathways
Abstract
:1. Introduction
2. Results
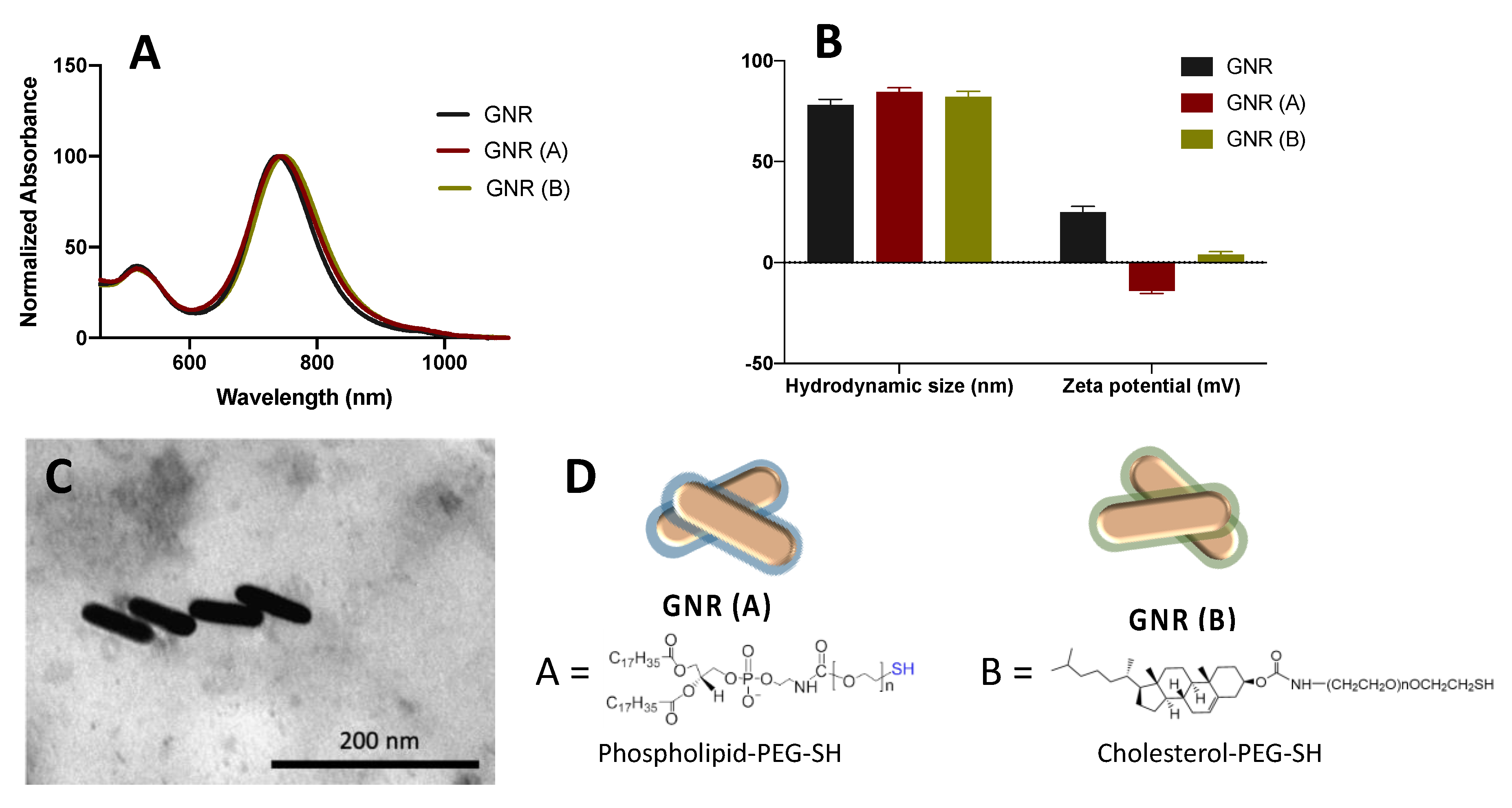
2.1. Synthesis, Functionalization and Characterization of GNR
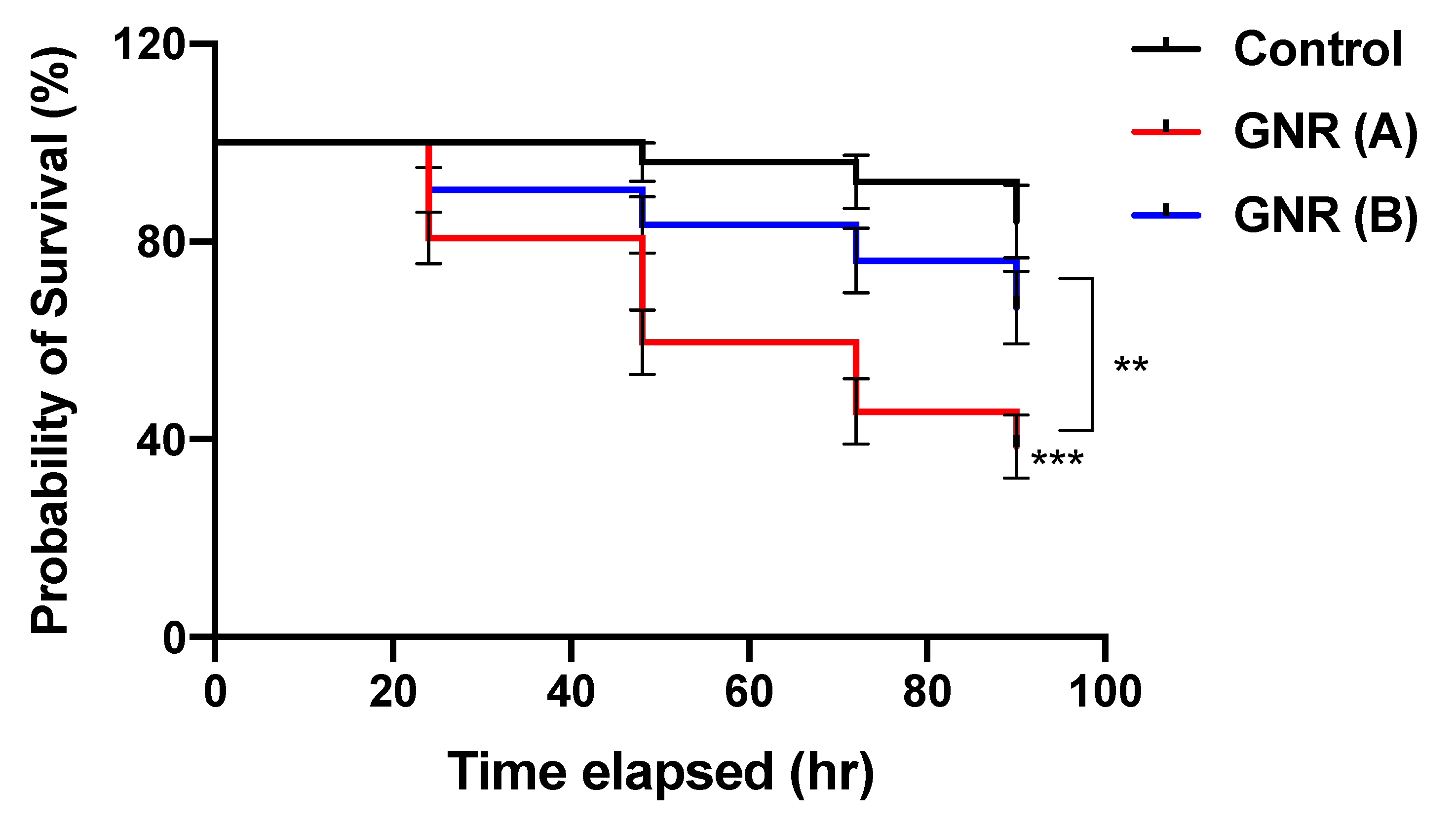
2.2. The Effects of GNR (A) and GNR (B) on the Early Stage of Embryonic Development
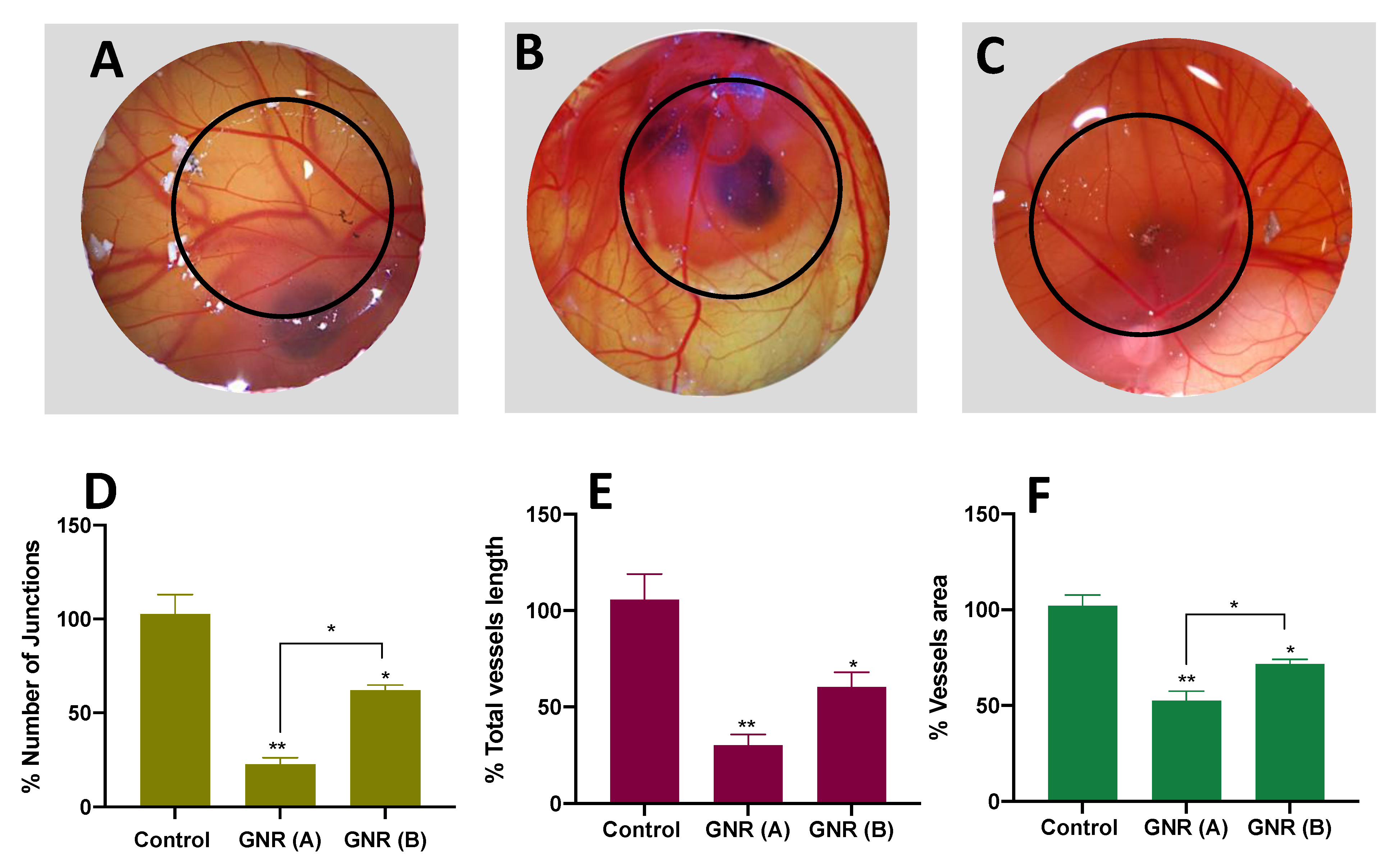
2.3. Angiogenesis Effect of GNR (A) and GNR (B) Using the CAM of the Chicken Embryo Model
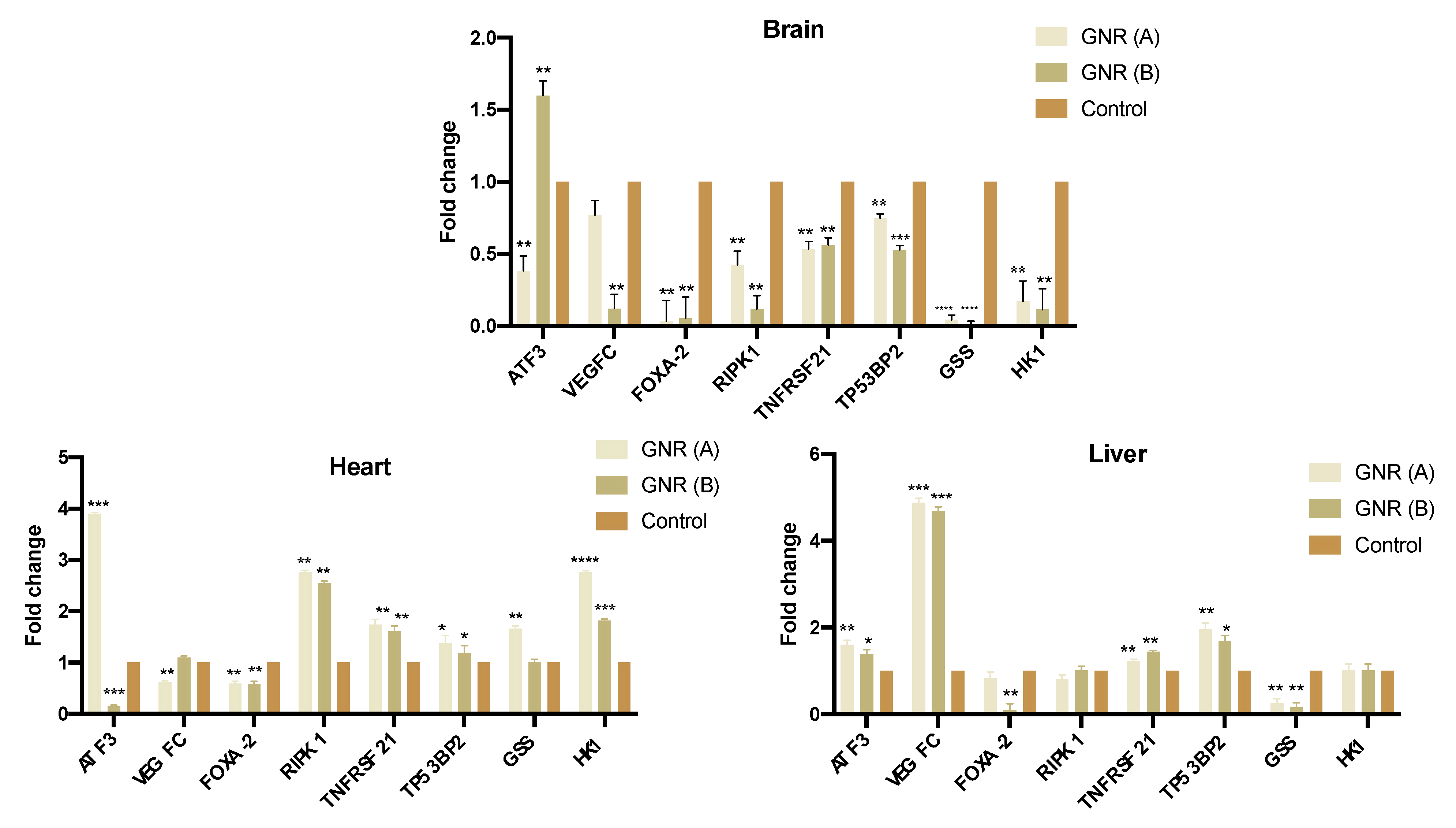
2.4. Effects of GNR (A) and GNR (B) on Gene Expression in Different Tissues from Exposed Chicken Embryos
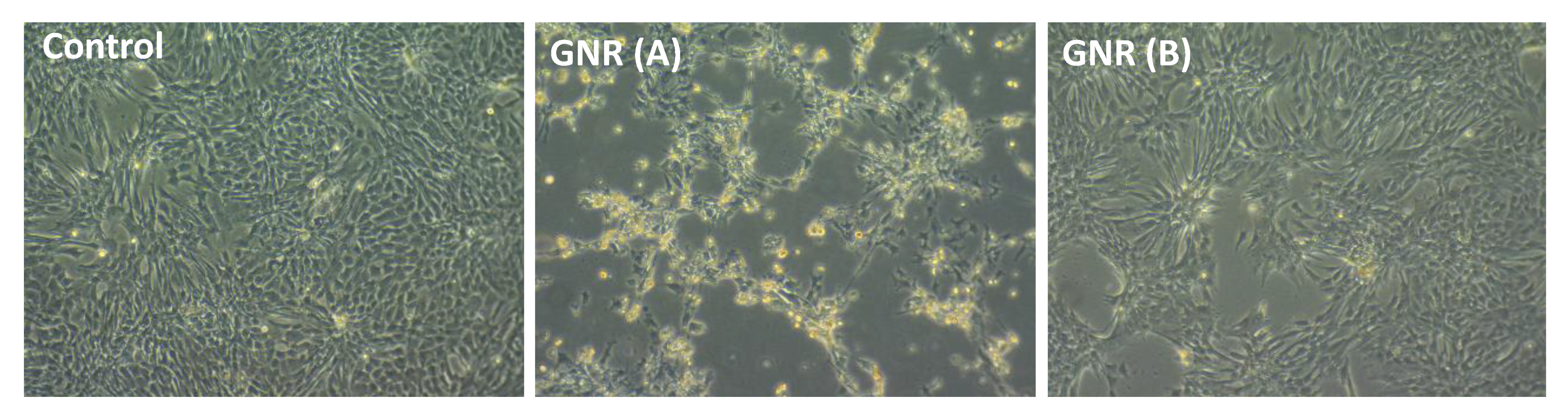
2.5. Effects of GNR (A) and GNR (B) on the Morphology of Cultured Chicken Embryo Fibroblasts
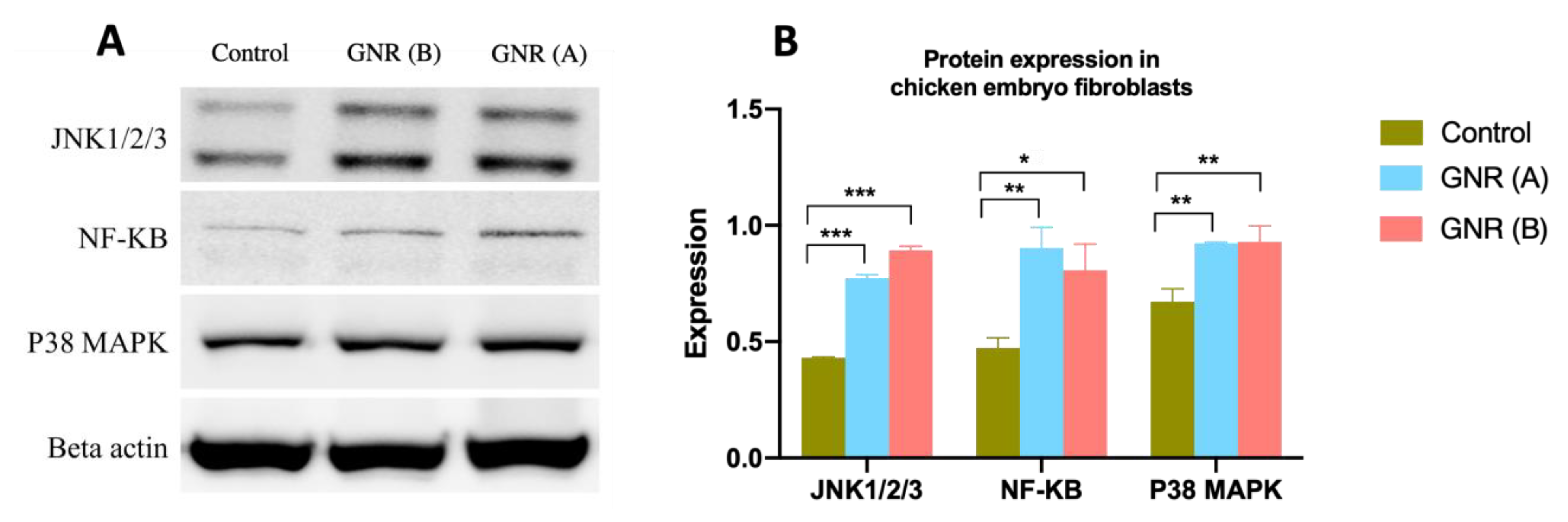
2.6. Effects of GNR (A) and GNR (B) on Protein Expression Patterns Analysis of JNK 1/2/3, NF-KB p65, and P38 MAPK in Embryonic Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Synthesis, Functionalization, and Characterization of GNR
4.2. Evaluating the Effects of GNR (A) and GNR (B) Treatments on the Early Stage of Embryonic Development
4.3. Angiogenesis Assay Using the CAM Model
4.4. Gene Expression by Real Time-PCR (qRT-PCR) Analysis
RNA Extraction
4.5. qRT-PCR
4.6. Microscopic Evaluation for Morphological Changes of Embryonic Fibroblast Cells (EFCs) upon Exposure to GNR Treatments
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelleher, F.C.; Fennelly, D.; Rafferty, M. Common critical pathways in embryogenesis and cancer. Acta Oncol. 2006, 45, 375–388. [Google Scholar] [CrossRef]
- Aiello, N.M.; Stanger, B.Z. Echoes of the embryo: Using the developmental biology toolkit to study cancer. Dis. Model. Mech. 2016, 9, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef] [Green Version]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef]
- Gacche, R.N.; Meshram, R.J. Angiogenic factors as potential drug target: Efficacy and limitations of anti-angiogenic therapy. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2014, 1846, 161–179. [Google Scholar] [CrossRef]
- Huang, D.; Lan, H.; Liu, F.; Wang, S.; Chen, X.; Jin, K.; Mou, X. Anti-angiogenesis or pro-angiogenesis for cancer treatment: Focus on drug distribution. Int. J. Clin. Exp. Med. 2015, 8, 8369–8376. [Google Scholar]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J. Clin. Med. 2019, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D.; Annese, T.; Ruggieri, S.; Tamma, R.; Crivellato, E. Limitations of Anti-Angiogenic Treatment of Tumors. Transl. Oncol. 2019, 12, 981–986. [Google Scholar] [CrossRef]
- Banerjee, D.; Harfouche, R.; Sengupta, S. Nanotechnology-mediated targeting of tumor angiogenesis. Vasc. Cell 2011, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Mioc, A.; Mioc, M.; Ghiulai, R.; Voicu, M.; Racoviceanu, R.; Trandafirescu, C.; Dehelean, C.; Coricovac, D.; Soica, C. Gold Nanoparticles as Targeted Delivery Systems and Theranostic Agents in Cancer Therapy. Curr. Med. Chem. 2019, 26, 6493–6513. [Google Scholar] [CrossRef]
- Liu, Y.; Crawford, B.M.; Vo-Dinh, T. Gold nanoparticles-mediated photothermal therapy and immunotherapy. Immunotherapy 2018, 10, 1175–1188. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Heuer-Jungemann, A.; Fernandes, A.R.; Kanaras, A.G.; Baptista, P.V. Peptide-coated gold nanoparticles for modulation of angiogenesis in vivo. Int. J. Nanomed. 2016, 11, 2633–2639. [Google Scholar] [CrossRef] [Green Version]
- Pan, F.; Li, W.; Yang, W.; Yang, X.Y.; Liu, S.; Li, X.; Zhao, X.; Ding, H.; Qin, L.; Pan, Y. Anterior gradient 2 as a supervisory marker for tumor vessel normalization induced by anti-angiogenic treatment. Oncol. Lett. 2018, 16, 3083–3091. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Li, X.; Liu, S.; Yang, W.; Pan, F.; Yang, X.-Y.; Du, B.; Qin, L.; Pan, Y. Gold nanoparticles attenuate metastasis by tumor vasculature normalization and epithelial-mesenchymal transition inhibition. Int. J. Nanomed. 2017, 12, 3509–3520. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.; Bhattacharya, R.; Wang, P.; Wang, L.; Basu, S.; Nagy, J.A.; Atala, A.; Mukhopadhyay, D.; Soker, S. Antiangiogenic Properties of Gold Nanoparticles. Clin. Cancer Res. 2005, 11, 3530. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM) assay. Reprod. Toxicol. 2017, 70, 97–101. [Google Scholar] [CrossRef]
- Darweesh, R.S.; Ayoub, N.M.; Nazzal, S. Gold nanoparticles and angiogenesis: Molecular mechanisms and biomedical applications. Int. J. Nanomed. 2019, 14, 7643–7663. [Google Scholar] [CrossRef] [Green Version]
- Vimalraj, S.; Ashokkumar, T.; Saravanan, S. Biogenic gold nanoparticles synthesis mediated by Mangifera indica seed aqueous extracts exhibits antibacterial, anticancer and anti-angiogenic properties. Biomed. Pharm. 2018, 105, 440–448. [Google Scholar] [CrossRef]
- Haine, A.T.; Niidome, T. Gold Nanorods as Nanodevices for Bioimaging, Photothermal Therapeutics, and Drug Delivery. Chem. Pharm. Bull. 2017, 65, 625–628. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Yao, C.; Yin, X.; Li, C.; Huang, Y.; Wu, M.; Wang, B.; Guo, X.; Wang, Y.; Wu, M. Size, Shape, and Protein Corona Determine Cellular Uptake and Removal Mechanisms of Gold Nanoparticles. Small 2018, 14, e1801451. [Google Scholar] [CrossRef]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of shape on Cellular Uptake of Gold Nanoparticles in the forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 3827. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.N.; Alhusban, A.A.; Ali, J.I.; Al-Bakri, A.G.; Hamed, R.; Khalil, E.A. Preferential Accumulation of Phospholipid-PEG and Cholesterol-PEG Decorated Gold Nanorods into Human Skin Layers and Their Photothermal-Based Antibacterial Activity. Sci. Rep. 2019, 9, 5796. [Google Scholar] [CrossRef] [Green Version]
- Abu-Dahab, R.; Mahmoud, N.N.; Abdallah, M.; Hamadneh, L.; Hikmat, S.; Zaza, R.; Abuarqoub, D.; Khalil, E.A. Cytotoxicity and Cellular Death Modality of Surface-Decorated Gold Nanorods against a Panel of Breast Cancer Cell Lines. ACS Omega 2021, 6, 15903–15910. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Abu-Dahab, R.; Hamadneh, L.A.; Abuarqoub, D.; Jafar, H.; Khalil, E.A. Insights into the Cellular Uptake, Cytotoxicity, and Cellular Death Modality of Phospholipid-Coated Gold Nanorods toward Breast Cancer Cell Lines. Mol. Pharm. 2019, 16, 4149–4164. [Google Scholar] [CrossRef] [PubMed]
- Dahabiyeh, L.A.; Mahmoud, N.N.; Al-Natour, M.A.; Safo, L.; Kim, D.-H.; Khalil, E.A.; Abu-Dahab, R. Phospholipid-Gold Nanorods Induce Energy Crisis in MCF-7 Cells: Cytotoxicity Evaluation Using LC-MS-Based Metabolomics Approach. Biomolecules 2021, 11, 364. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Sabbah, D.A.; Abu-Dahab, R.; Abuarqoub, D.; Abdallah, M.; Ameerah; Khalil, E.A. Cholesterol-coated gold nanorods as an efficient nano-carrier for chemotherapeutic delivery and potential treatment of breast cancer: In vitro studies using the MCF-7 cell line. RSC Adv. 2019, 9, 12718–12731. [Google Scholar] [CrossRef] [Green Version]
- Hamad, K.M.; Mahmoud, N.N.; Al-Dabash, S.; Al-Samad, L.A.; Abdallah, M.; Al-Bakri, A.G. Fluconazole conjugated-gold nanorods as an antifungal nanomedicine with low cytotoxicity against human dermal fibroblasts. RSC Adv. 2020, 10, 25889–25897. [Google Scholar] [CrossRef]
- Benschop, R.; Wei, T.; Na, S. Tumor necrosis factor receptor superfamily member 21: TNFR-related death receptor-6, DR6. Adv. Exp. Med. Biol. 2009, 647, 186–194. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kajino, S.; Takahashi, N.; Kanazawa, S.; Imai, K.; Hibi, Y.; Ohara, H.; Itoh, M.; Okamoto, T. 53BP2 induces apoptosis through the mitochondrial death pathway. Genes Cells 2005, 10, 253–260. [Google Scholar] [CrossRef]
- Perry, R.R.; Mazetta, J.A.; Levin, M.; Barranco, S.C. Glutathione levels and variability in breast tumors and normal tissue. Cancer 1993, 72, 783–787. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, D.; Yu, G.; Liang, J. Overexpression of metabolic markers HK1 and PKM2 contributes to lymphatic metastasis and adverse prognosis in Chinese gastric cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 9264–9271. [Google Scholar] [PubMed]
- Alkilany, A.M.; Thompson, L.B.; Boulos, S.P.; Sisco, P.N.; Murphy, C.J. Gold nanorods: Their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv. Drug Deliv. Rev. 2012, 64, 190–199. [Google Scholar] [CrossRef]
- Zhang, J.; Mou, L.; Jiang, X. Surface chemistry of gold nanoparticles for health-related applications. Chem. Sci. 2020, 11, 923–936. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Chen, J.; Cao, Y.; Chai, O.J.H.; Xie, J. Ligand Design in Ligand-Protected Gold Nanoclusters. Small 2021, 17, e2004381. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, D.; Li, W.; Yu, J.; Chen, Y. Effect of Size, Shape, and Surface Modification on Cytotoxicity of Gold Nanoparticles to Human HEp-2 and Canine MDCK Cells. J. Nanomater. 2012, 2012, 375496. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, N.N.; Qabooq, H.; Alsotari, S.; Tarawneh, O.A.; Aboalhaija, N.H.; Shraim, S.; Alkilany, A.M.; Khalil, E.A.; Abu-Dahab, R. Quercetin-gold nanorods incorporated into nanofibers: Development, optimization and cytotoxicity. RSC Adv. 2021, 11, 19956–19966. [Google Scholar] [CrossRef]
- Pan, F.; Yang, W.; Li, W.; Yang, X.-Y.; Liu, S.; Li, X.; Zhao, X.; Ding, H.; Qin, L.; Pan, Y. Conjugation of gold nanoparticles and recombinant human endostatin modulates vascular normalization via interruption of anterior gradient 2–mediated angiogenesis. Tumor Biol. 2017, 39, 1010428317708547. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, S.; Bhat, F.A.; Raja Singh, P.; Mukherjee, S.; Elumalai, P.; Das, S.; Patra, C.R.; Arunakaran, J. Gold nanoparticle-conjugated quercetin inhibits epithelial-mesenchymal transition, angiogenesis and invasiveness via EGFR/VEGFR-2-mediated pathway in breast cancer. Cell Prolif. 2016, 49, 678–697. [Google Scholar] [CrossRef]
- Mukherjee, S. Recent progress toward antiangiogenesis application of nanomedicine in cancer therapy. Future Sci. 2018, 4, FSO318. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Gao, F.; Jiang, H.; Niu, L.; Bi, Y.; Young, C.Y.F.; Yuan, H.; Lou, H. Induction of DNA damage and ATF3 by retigeric acid B, a novel topoisomerase II inhibitor, promotes apoptosis in prostate cancer cells. Cancer Lett. 2013, 337, 66–76. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Ge, C.; Liu, Z.; Li, L.; Zhao, F.; Tian, H.; Chen, T.; Li, H.; Yao, M.; Li, J. ATF3 inhibits the tumorigenesis and progression of hepatocellular carcinoma cells via upregulation of CYR61 expression. J. Exp. Clin. Cancer Res. 2018, 37, 263. [Google Scholar] [CrossRef]
- Xie, J.J.; Xie, Y.M.; Chen, B.; Pan, F.; Guo, J.C.; Zhao, Q.; Shen, J.H.; Wu, Z.Y.; Wu, J.Y.; Xu, L.Y.; et al. ATF3 functions as a novel tumor suppressor with prognostic significance in esophageal squamous cell carcinoma. Oncotarget 2014, 5, 8569–8582. [Google Scholar] [CrossRef]
- Rauniyar, K.; Jha, S.K.; Jeltsch, M. Biology of Vascular Endothelial Growth Factor C in the Morphogenesis of Lymphatic Vessels. Front. Bioeng. Biotechnol. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Khromova, N.; Kopnin, P.; Rybko, V.; Kopnin, B.P. Downregulation of VEGF-C expression in lung and colon cancer cells decelerates tumor growth and inhibits metastasis via multiple mechanisms. Oncogene 2012, 31, 1389–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hata, S.; Namae, M.; Nishina, H. Liver development and regeneration: From laboratory study to clinical therapy. Dev. Growth Differ. 2007, 49, 163–170. [Google Scholar] [CrossRef]
- Nurmi, H.; Saharinen, P.; Zarkada, G.; Zheng, W.; Robciuc, M.R.; Alitalo, K. VEGF-C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol. Med. 2015, 7, 1418–1425. [Google Scholar] [CrossRef]
- Wang, B.; Liu, G.; Ding, L.; Zhao, J.; Lu, Y. FOXA2 promotes the proliferation, migration and invasion, and epithelial mesenchymal transition in colon cancer. Exp. Ther. Med. 2018, 16, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Balaguer, A.; Ortiz-Martínez, F.; García-Martínez, A.; Pomares-Navarro, C.; Lerma, E.; Peiró, G. FOXA2 mRNA expression is associated with relapse in patients with Triple-Negative/Basal-like breast carcinoma. Breast Cancer Res. Treat. 2015, 153, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef] [Green Version]
- Kampa, K.M.; Acoba, J.D.; Chen, D.; Gay, J.; Lee, H.; Beemer, K.; Padiernos, E.; Boonmark, N.; Zhu, Z.; Fan, A.C.; et al. Apoptosis-stimulating protein of p53 (ASPP2) heterozygous mice are tumor-prone and have attenuated cellular damage–response thresholds. Proc. Natl. Acad. Sci. USA 2009, 106, 4390. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.D.; Zhang, R.; Han, X.; Kang, K.A.; Piao, M.J.; Maeng, Y.H.; Chang, W.Y.; Hyun, J.W. Involvement of glutathione and glutathione metabolizing enzymes in human colorectal cancer cell lines and tissues. Mol. Med. Rep. 2015, 12, 4314–4319. [Google Scholar] [CrossRef] [Green Version]
- Calmettes, G.; Ribalet, B.; John, S.; Korge, P.; Ping, P.; Weiss, J.N. Hexokinases and cardioprotection. J. Mol. Cell. Cardiol. 2015, 78, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Zhang, F.F.; Wang, F.; Qiu, J.H.; Luo, C.H.; Zhu, G.Y.; Liu, Y.F. TIPE2 Inhibits Lung Cancer Growth Attributing to Promotion of Apoptosis by Regulating Some Apoptotic Molecules Expression. PLoS ONE 2015, 10, e0126176. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Fan, M.; Chen, Y.; Zhao, Q.; Song, C.; Yan, Y.; Jin, Y.; Huang, Z.; Lin, C.; Wu, J. Melatonin Induces Cell Apoptosis in AGS Cells Through the Activation of JNK and P38 MAPK and the Suppression of Nuclear Factor-Kappa B: A Novel Therapeutic Implication for Gastric Cancer. Cell. Physiol. Biochem. 2015, 37, 2323–2338. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef] [Green Version]
- Perkins, N.D.; Gilmore, T.D. Good cop, bad cop: The different faces of NF-κB. Cell Death Differ. 2006, 13, 759–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khandelwal, N.; Simpson, J.; Taylor, G.; Rafique, S.; Whitehouse, A.; Hiscox, J.; Stark, L.A. Nucleolar NF-κB/RelA mediates apoptosis by causing cytoplasmic relocalization of nucleophosmin. Cell Death Differ. 2011, 18, 1889–1903. [Google Scholar] [CrossRef] [Green Version]
- Araki, K.; Kawauchi, K.; Tanaka, N. IKK/NF-κB signaling pathway inhibits cell-cycle progression by a novel Rb-independent suppression system for E2F transcription factors. Oncogene 2008, 27, 5696–5705. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Xu, J.; Rao, H.-M.; Li, X.; Zhang, Y.-K.; Jiang, F.; Wu, W.-X. Mechanism of Apoptosis Induction by Mycoplasmal Nuclease MGA_0676 in Chicken Embryo Fibroblasts. Front. Cell. Infect. Microbiol. 2018, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- Into, T.; Kiura, K.; Yasuda, M.; Kataoka, H.; Inoue, N.; Hasebe, A.; Takeda, K.; Akira, S.; Shibata, K.-i. Stimulation of human Toll-like receptor (TLR) 2 and TLR6 with membrane lipoproteins of Mycoplasma fermentans induces apoptotic cell death after NF-κB activation. Cell. Microbiol. 2004, 6, 187–199. [Google Scholar] [CrossRef]
- Seitz, C.S.; Deng, H.; Hinata, K.; Lin, Q.; Khavari, P.A. Nuclear Factor κB Subunits Induce Epithelial Cell Growth Arrest. Cancer Res. 2000, 60, 4085. [Google Scholar]
- Ye, X.; Zheng, C.; Chen, J.; Gao, Y.; Murray, C.B. Using binary surfactant mixtures to simultaneously improve the dimensional tunability and monodispersity in the seeded growth of gold nanorods. Nano Lett. 2013, 13, 765–771. [Google Scholar] [CrossRef]
- Abdo, G.G.; Kheraldine, H.; Gupta, I.; Rizeq, B.; Elzatahry, A.; Al Moustafa, A.E.; Khalil, A. Significant Toxic Effect of Carbon Nanofibers at the Early Stage of Embryogenesis. J. Biomed. Nanotechnol. 2020, 16, 975–984. [Google Scholar] [CrossRef]
- Zudaire, E.; Gambardella, L.; Kurcz, C.; Vermeren, S. A computational tool for quantitative analysis of vascular networks. PLoS ONE 2011, 6, e27385. [Google Scholar] [CrossRef] [Green Version]
- Al-Asmakh, M.; Bawadi, H.; Hamdan, M.; Gupta, I.; Kheraldine, H.; Jabeen, A.; Rizeq, B.; Al Moustafa, A.E. Dasatinib and PD-L1 inhibitors provoke toxicity and inhibit angiogenesis in the embryo. Biomed. Pharm. 2021, 134, 111134. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2−ΔΔCT method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Mahmoud, N.N.; Abu-Dahab, R.; Abdallah, M.; Al-Dabash, S.; Abuarqoub, D.; Albasha, A.; Khalil, E.A. Interaction of gold nanorods with cell culture media: Colloidal stability, cytotoxicity and cellular death modality. J. Drug Deliv. Sci. Technol. 2020, 60, 101965. [Google Scholar] [CrossRef]
| Group | Sample Size | Mortality Rate (%) on Day 4 of Exposure |
|---|---|---|
| GNR (A) | 56 | 61.4 |
| GNR (B) | 42 | 33.5 |
| Control | 25 | 16.0 |
| No. | Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| 1 | ATF-3 | AAAAGCGAAGAAGGGAAAGG | ATACAGGTGGGCCTGTGAAG |
| 2 | VEGFC | AGGGAACACTCCAGCTCTGA | CTCCAAACTCTTTCCCCACA |
| 3 | FOXA-2 | GACCTCTTCCCCTTCTACCG | AGGTAGCAGCCGTTCTCAAA |
| 4 | RIPK1 | CCGTACAGAATTGCAGCAGA | TTCCATTAGCACACGAGCTG |
| 5 | TNFRSF21 | GTGGGCTGATGGAAGACAC | CAGGAGAGCGGAATTCTCAA |
| 6 | TP53BP2 | GTTGTGTTGAGGTGGGTGTC | CATCACGTCCAACCATCGAC |
| 7 | GSS | AGGGATAGCGACAGATGGTG | TGTTTCTGTGGAGCCTCGAT |
| 8 | HK1 | CATACAGAGCAGCGGAACAC | GTCACTTCTGATGGCAGCAA |
| 9 | GAPDH | CCTCTCTGGCAAAGTCCAAG | CATCTGCCCATTTGATGTTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, N.N.; Zakaria, Z.Z.; Kheraldine, H.; Gupta, I.; Vranic, S.; Al-Asmakh, M.; Al Moustafa, A.-E. The Effect of Surface-Modified Gold Nanorods on the Early Stage of Embryonic Development and Angiogenesis: Insight into the Molecular Pathways. Int. J. Mol. Sci. 2021, 22, 11036. https://doi.org/10.3390/ijms222011036
Mahmoud NN, Zakaria ZZ, Kheraldine H, Gupta I, Vranic S, Al-Asmakh M, Al Moustafa A-E. The Effect of Surface-Modified Gold Nanorods on the Early Stage of Embryonic Development and Angiogenesis: Insight into the Molecular Pathways. International Journal of Molecular Sciences. 2021; 22(20):11036. https://doi.org/10.3390/ijms222011036
Chicago/Turabian StyleMahmoud, Nouf N., Zain Zaki Zakaria, Hadeel Kheraldine, Ishita Gupta, Semir Vranic, Maha Al-Asmakh, and Ala-Eddin Al Moustafa. 2021. "The Effect of Surface-Modified Gold Nanorods on the Early Stage of Embryonic Development and Angiogenesis: Insight into the Molecular Pathways" International Journal of Molecular Sciences 22, no. 20: 11036. https://doi.org/10.3390/ijms222011036
APA StyleMahmoud, N. N., Zakaria, Z. Z., Kheraldine, H., Gupta, I., Vranic, S., Al-Asmakh, M., & Al Moustafa, A.-E. (2021). The Effect of Surface-Modified Gold Nanorods on the Early Stage of Embryonic Development and Angiogenesis: Insight into the Molecular Pathways. International Journal of Molecular Sciences, 22(20), 11036. https://doi.org/10.3390/ijms222011036











