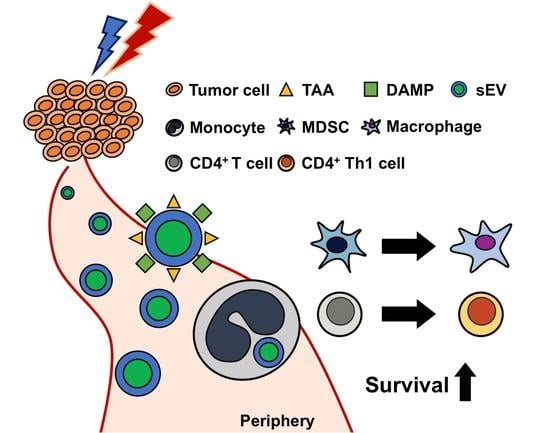
Supplementation with Serum-Derived Extracellular Vesicles Reinforces Antitumor Immunity Induced by Cryo-Thermal Therapy
Abstract
:1. Introduction
2. Results
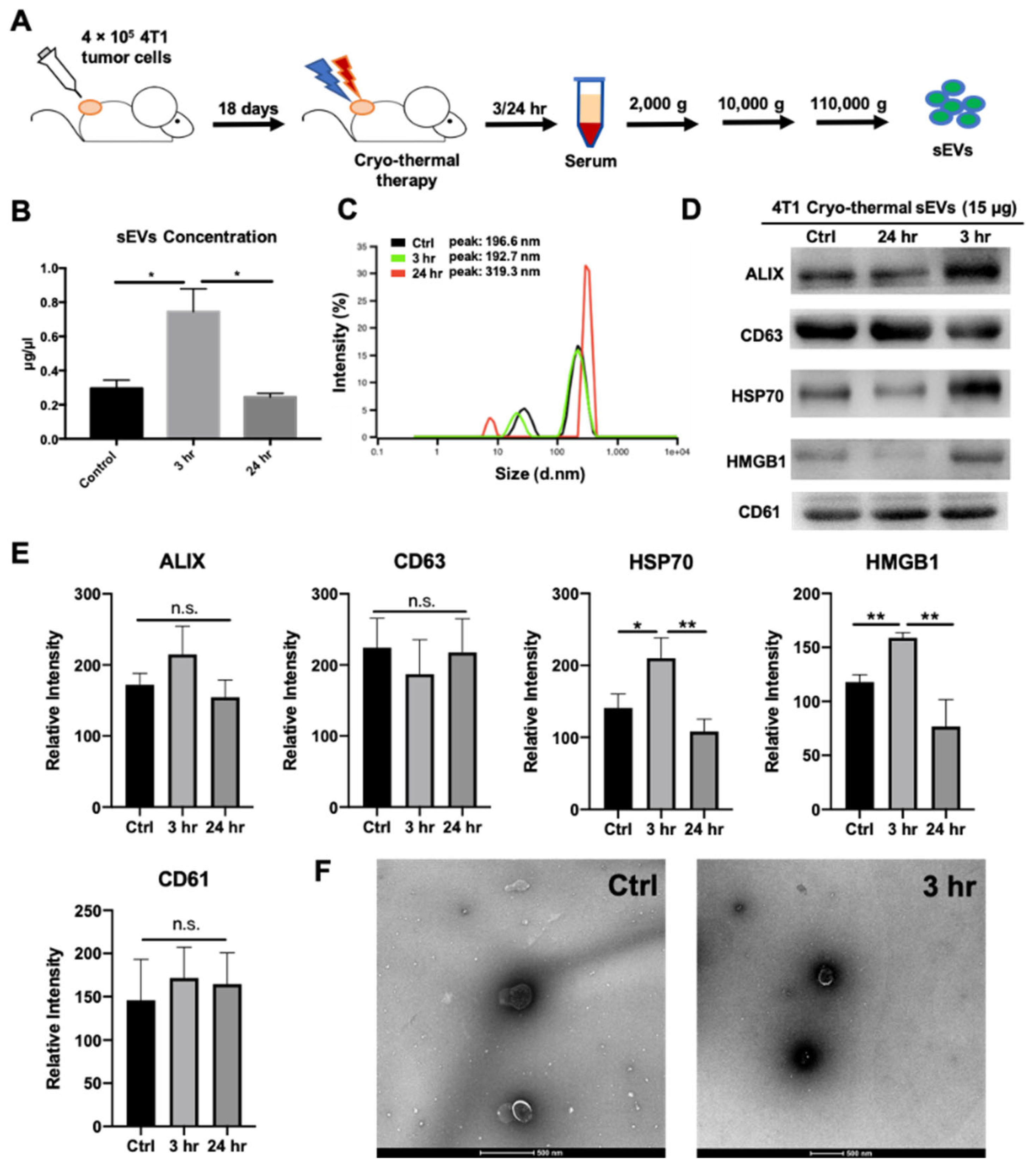
2.1. Cryo-Thermal Therapy Induces Extracellular Vesicle Release into the Peripheral Circulation
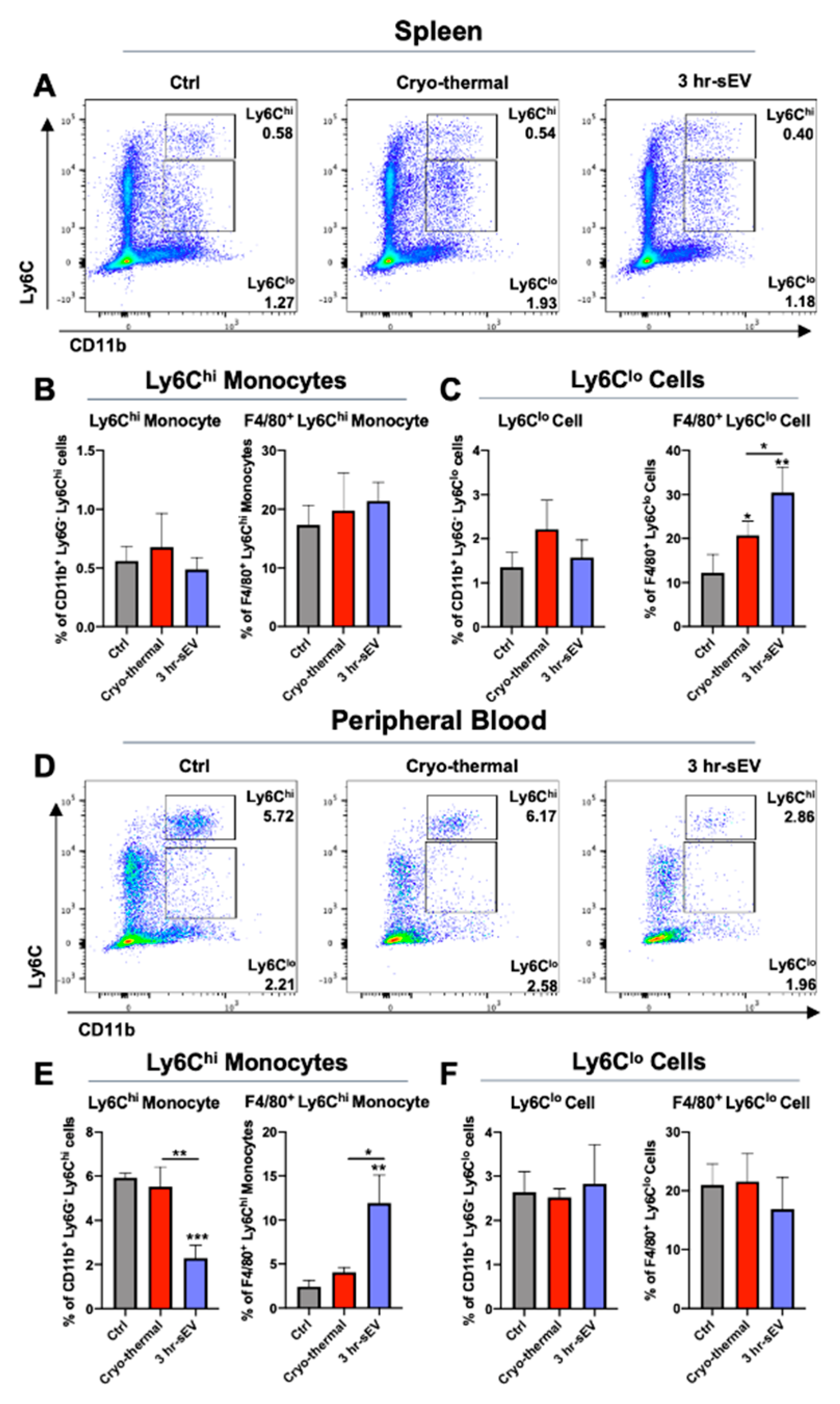
2.2. Uptake of Cryo-Thermal sEVs into Circulating Monocytes and Macrophage-Like Monocytes
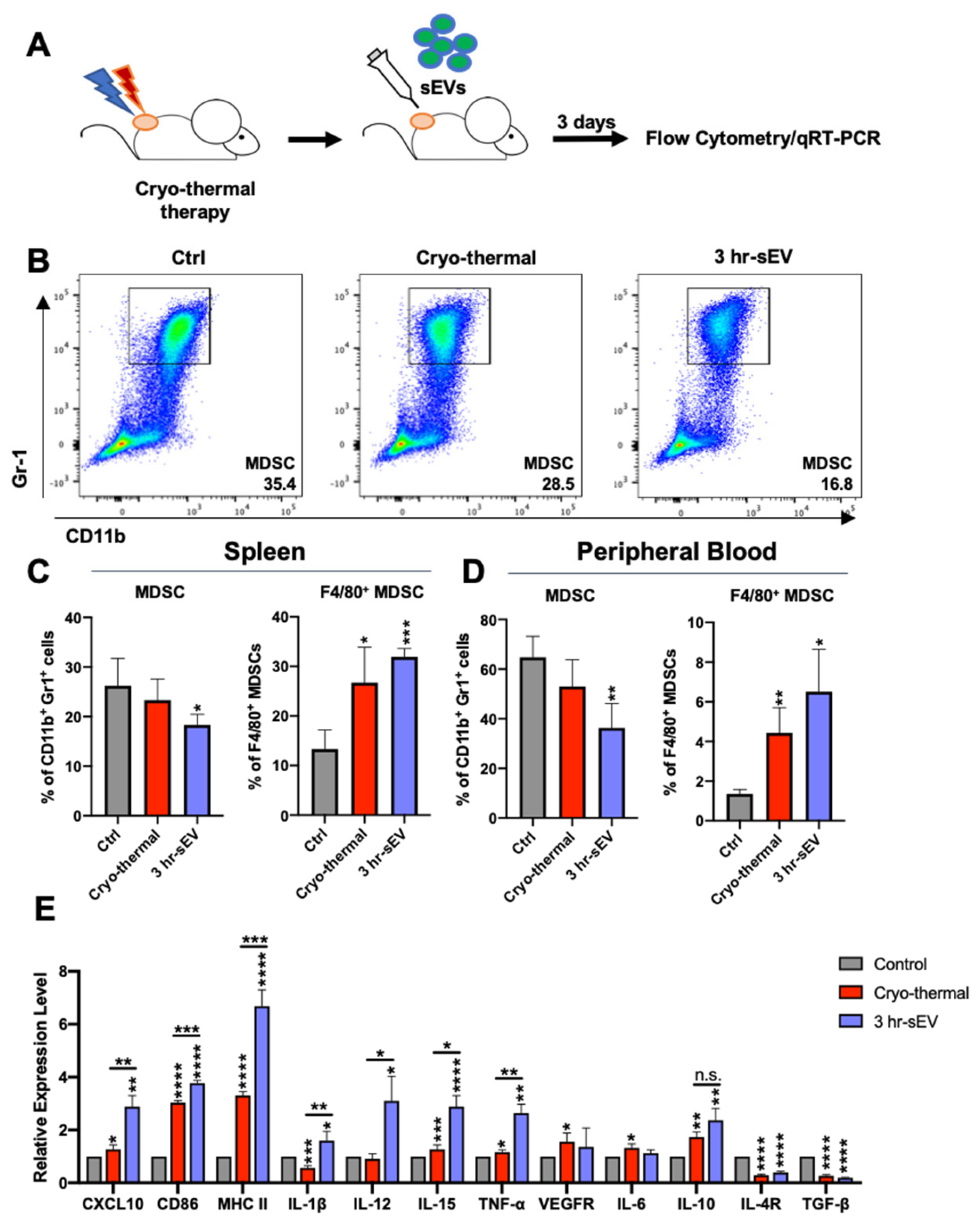
2.3. sEVs Released after Cryo-Thermal Therapy Potentiated the Activation of Innate Immunity Induced by Cryo-Thermal Therapy
2.4. sEVs Released after Cryo-Thermal Therapy Promoted the Differentiation of CD4+ T Cells into the Th1 Subset
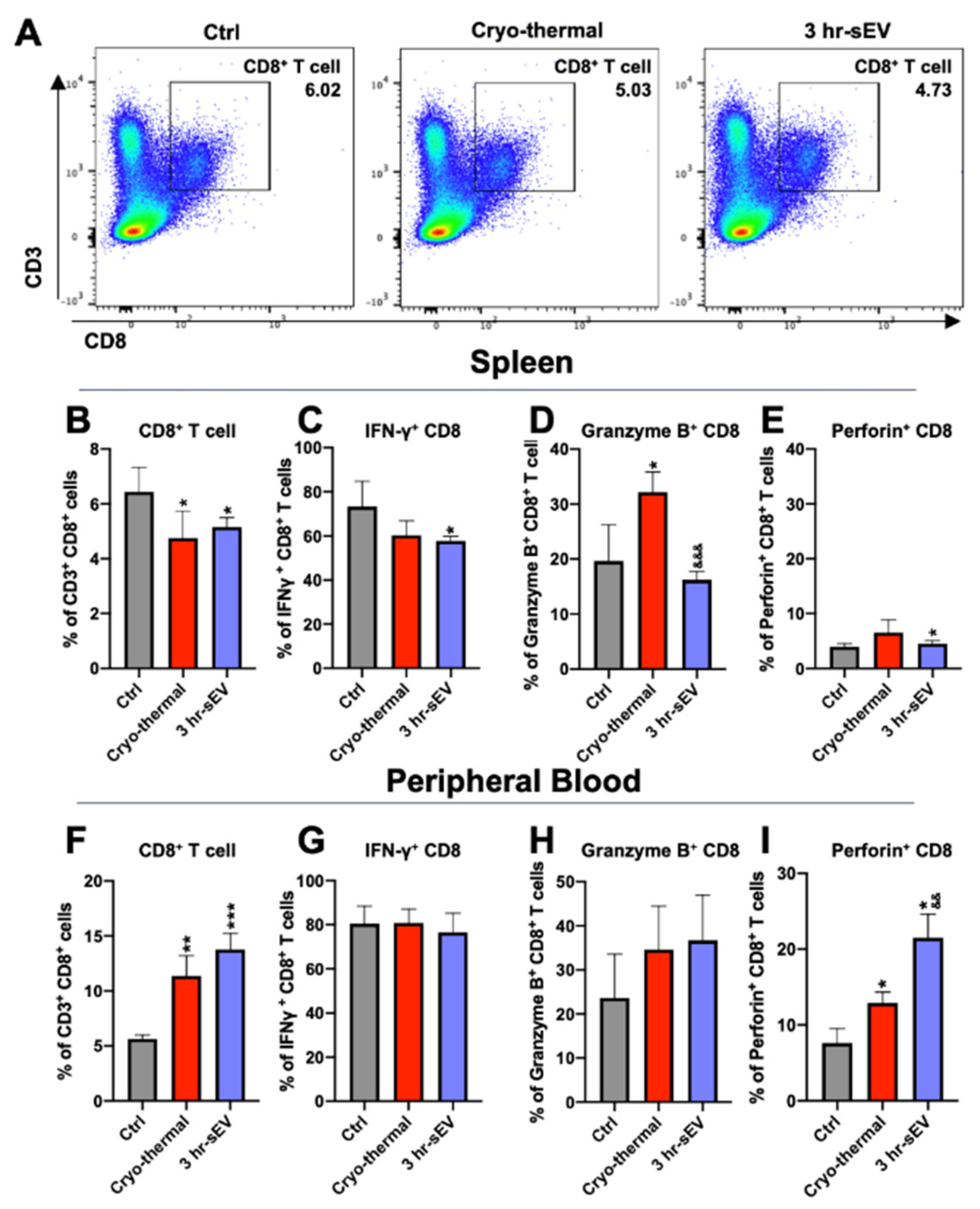
2.5. sEVs Released after Cryo-Thermal Therapy Enhanced the Cytotoxicity of CD8+ T Cells Induced by Cryo-Thermal Therapy
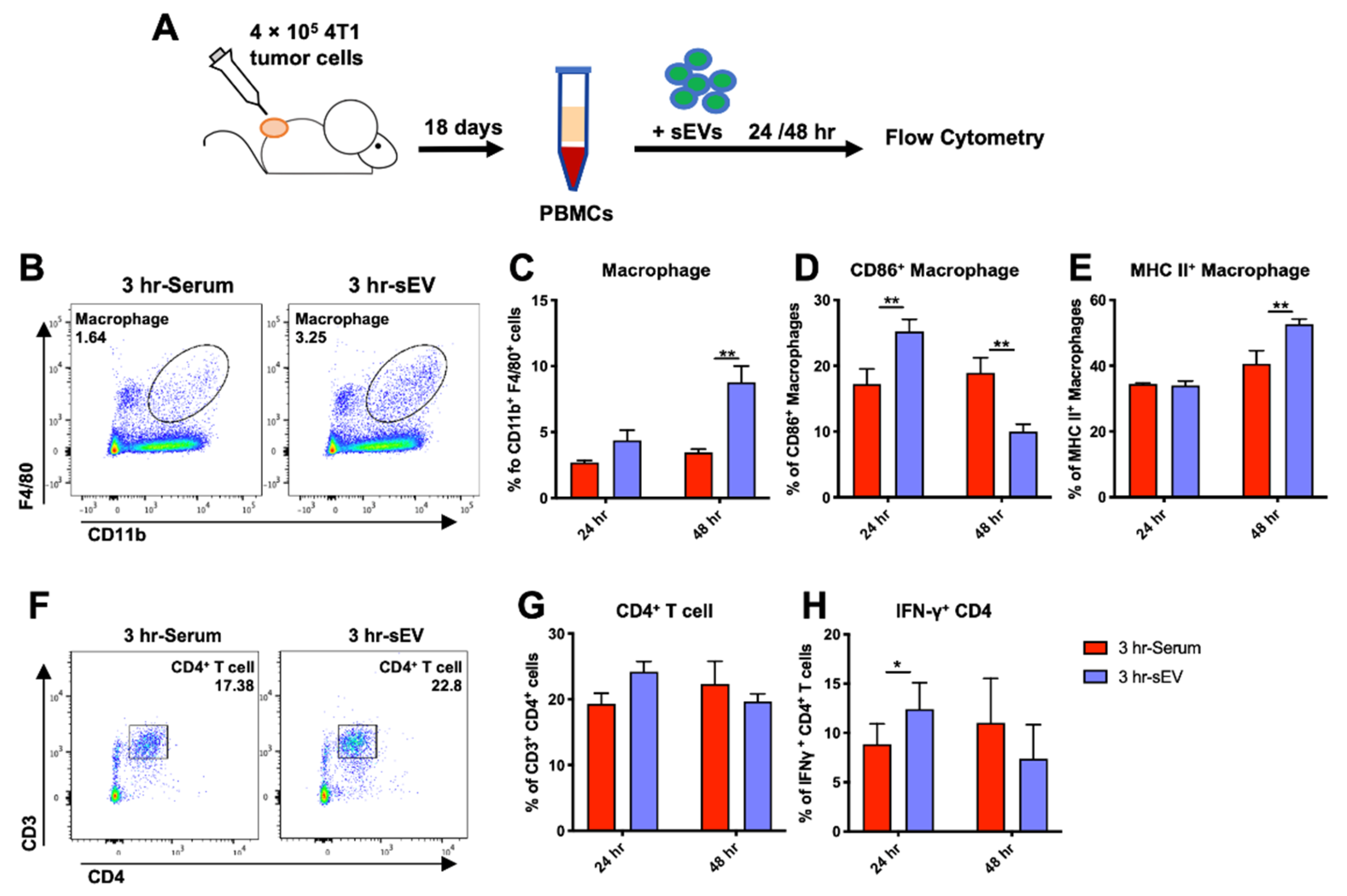
2.6. sEVs Released after Cryo-Thermal Therapy Promoted Macrophage Maturation and Th1 Cell Differentiation In Vitro
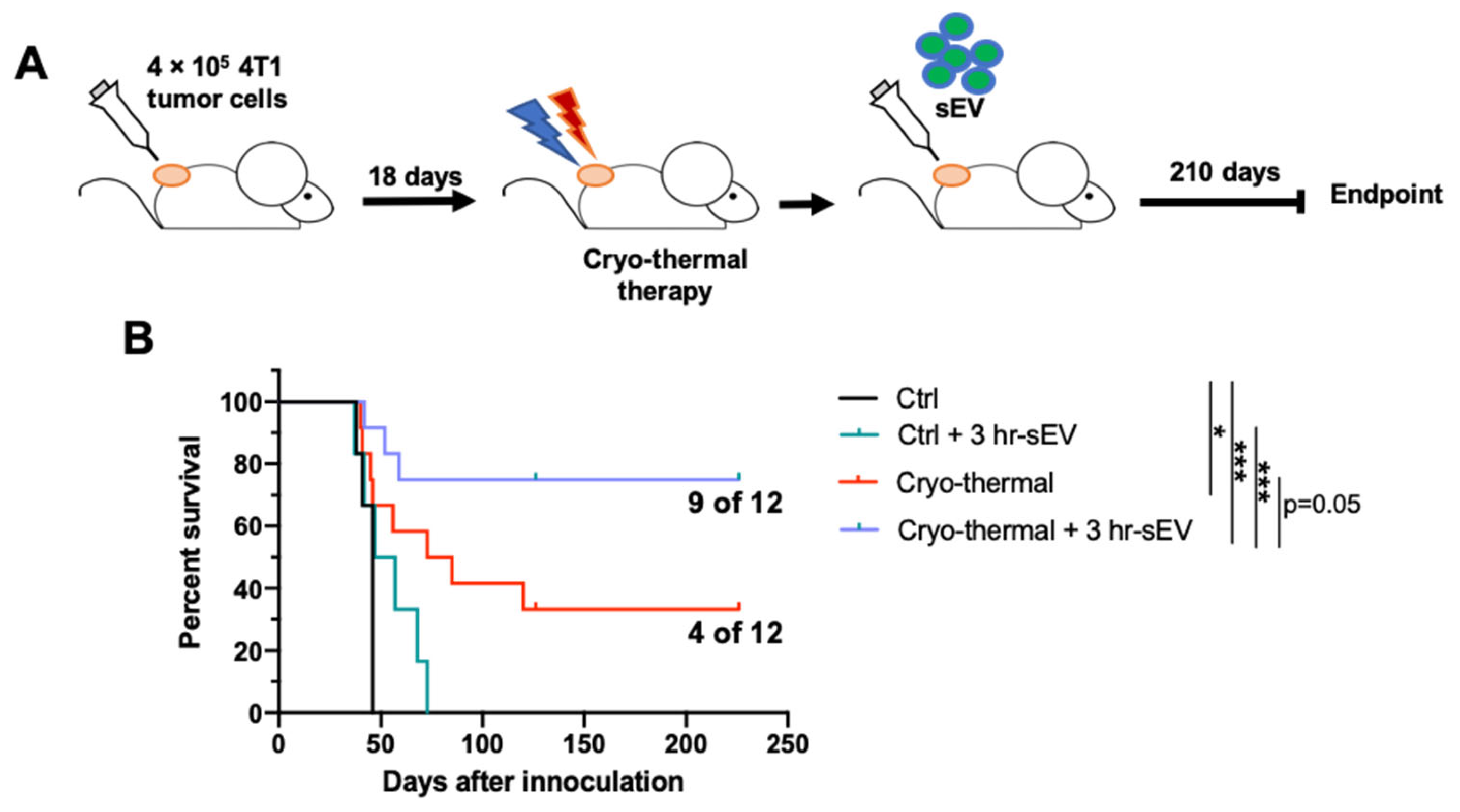
2.7. sEVs Released after Cryo-Thermal Therapy Prolonged the Survival of Mice Treated with Cryo-Thermal Therapy
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Animal Models
4.3. Cryo-Thermal Therapy Procedure
4.4. Isolation of Splenic Macrophages, DCs and MDSCs
4.5. RNA Extraction and Real-Time PCR
4.6. sEVs Isolation and Characterization
4.7. Phagocytosis Assay
4.8. Flow Cytometry Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaked, Y. The pro-tumorigenic host response to cancer therapies. Nat. Rev. Cancer 2019, 19, 667–685. [Google Scholar] [CrossRef]
- Shaked, Y. Balancing efficacy of and host immune responses to cancer therapy: The yin and yang effects. Nat. Rev. Clin. Oncol. 2016, 13, 611–626. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017, 27, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Ceelen, W.; Pattyn, P.; Mareel, M. Surgery, wound healing, and metastasis: Recent insights and clinical implications. Crit. Rev. Oncol. Hematol. 2014, 89, 16–26. [Google Scholar] [CrossRef]
- Baldo, B.A.; Pagani, M. Adverse events to nontargeted and targeted chemotherapeutic agents: Emphasis on hypersensitivity responses. Immunol. Allergy Clin. N. Am. 2014, 34, 565–596. [Google Scholar] [CrossRef]
- Kalbasi, A.; Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Hellmann, M.D. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 2020, 37, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wen, X.; Tian, L.; Li, T.; Xu, C.; Wen, X.; Melancon, M.P.; Gupta, S.; Shen, B.; Peng, W.; et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat. Commun. 2019, 10, 899. [Google Scholar] [CrossRef] [Green Version]
- Frey, B.; Weiss, E.-M.; Rubner, Y.; Wunderlich, R.; Ott, O.J.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Old and new facts about hyperthermia-induced modulations of the immune system. Int. J. Hyperth. 2012, 28, 528–542. [Google Scholar] [CrossRef]
- Multhoff, G.; Pockley, A.G.; Streffer, C.; Gaipl, U.S. Dual role of heat shock proteins (HSPs) in anti-tumor immunity. Curr. Mol. Med. 2012, 12, 1174–1182. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Lee, C.-T.; Ashcraft, K.A. The future of biology in driving the field of hyperthermia. Int. J. Hyperth. 2016, 32, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, A.; Xu, L.X. Evaluation of alternate cooling and heating for tumor treatment. Int. J. Heat Mass Transf. 2008, 51, 5478–5485. [Google Scholar] [CrossRef]
- Sun, J.Q.; Xu, C.C.; Wei, G.H.; Sun, X.G.; Liu, P.; Zhang, A.L.; Xu, L.X. Tumor Treatment System with Alternate Cooling and Heating—Preliminary Results in an Animal Model. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Munich, Germany, 7–12 September 2009; pp. 337–340. [Google Scholar]
- Cai, Z.; Song, M.; Zhang, A.; Sun, J.; Xu, L. Numerical Simulation of a New Probe for the Alternate Cooling and Heating of a Subcutaneous Mouse Tumor Model. Numer. Heat Transf. Part A Appl. 2013, 63, 534–548. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.; Zhang, A.; He, K.; Liu, P.; Xu, L.X. Cryo-thermal therapy elicits potent anti-tumor immunity by inducing extracellular Hsp70-dependent MDSC differentiation. Sci. Rep. 2016, 6, 27136. [Google Scholar] [CrossRef]
- Zhu, J.; Lou, Y.; Liu, P.; Xu, L.X. Tumor-related HSP70 released after cryo-thermal therapy targeted innate immune initiation in the antitumor immune response. Int. J. Hyperth. 2020, 37, 843–853. [Google Scholar] [CrossRef]
- Peng, P.; Hu, H.; Liu, P.; Xu, L.X. Neoantigen-specific CD4 T-cell response is critical for the therapeutic efficacy of cryo-thermal therapy. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Toraya-Brown, S.; Fiering, S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int. J. Hyperth. 2014, 30, 531–539. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.; Shao, C.; Liu, S.; Yu, Y.; Wang, Q.; Cao, X. Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur. J. Immunol. 2006, 36, 1598–1607. [Google Scholar] [CrossRef]
- Chen, T.; Guo, J.; Yang, M.; Zhu, X.; Cao, X. Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J. Immunol. 2011, 186, 2219–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, H.; Yang, Y.; Ma, S.; Xiu, F.; Cai, Z.; Zhao, H.; Du, L. Induction of a tumour-specific CTL response by exosomes isolated from heat-treated malignant ascites of gastric cancer patients. Int. J. Hyperth. 2011, 27, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Chen, Y.; Wang, S.; Yu, L.; Shen, Y.; Zhong, H.; Yang, Y. Exosomes from heat-stressed tumour cells inhibit tumour growth by converting regulatory T cells to Th17 cells via IL-6. Immunology 2018, 154, 132–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, K.; Jia, S.; Lou, Y.; Liu, P.; Xu, L.X. Cryo-thermal therapy induces macrophage polarization for durable anti-tumor immunity. Cell Death Dis. 2019, 10, 216. [Google Scholar] [CrossRef] [Green Version]
- Pulaski, B.A.; Ostrand-Rosenberg, S. Mouse 4T1 breast tumor model. Curr. Protoc. Immunol. 2001. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, D.; Kim, H.-J.; Kurt, R.A.; Bu, W.; Li, Y.; Ma, X. A novel role of hematopoietic CCL5 in promoting triple-negative mammary tumor progression by regulating generation of myeloid-derived suppressor cells. Cell Res. 2013, 23, 394–408. [Google Scholar] [CrossRef]
- Jakubzick, C.V.; Randolph, G.J.; Henson, P.M. Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol. 2017, 17, 349–362. [Google Scholar] [CrossRef]
- Taniuchi, I. CD4 Helper and CD8 Cytotoxic T Cell Differentiation. Annu. Rev. Immunol. 2018, 36, 579–601. [Google Scholar] [CrossRef]
- Durgeau, A.; Virk, Y.; Corgnac, S.; Mami-Chouaib, F. Recent Advances in Targeting CD8 T-Cell Immunity for More Effective Cancer Immunotherapy. Front. Immunol. 2018, 9, 14. [Google Scholar] [CrossRef]
- Belhadj, Z.; He, B.; Deng, H.; Song, S.; Zhang, H.; Wang, X.; Dai, W.; Zhang, Q. A combined “eat me/don’t eat me” strategy based on extracellular vesicles for anticancer nanomedicine. J. Extracell. Vesicles 2020, 9, 1806444. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.-L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-S.; Kim, M.-H.; Yi, H.-S.; Kim, S.Y.; Kim, H.-H.; Kim, J.H.; Yeon, J.E.; Byun, K.S.; Byun, J.-S.; Jeong, W.-I. CXCR1 differentiates F4/80 monocytes into pro-inflammatory F4/80 macrophages in the liver. Sci. Rep. 2018, 8, 15076. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gao, W.; Yuan, J.; Wu, C.; Yao, K.; Zhang, L.; Ma, L.; Zhu, J.; Zou, Y.; Ge, J. Exosomes derived from dendritic cells improve cardiac function via activation of CD4(+) T lymphocytes after myocardial infarction. J. Mol. Cell Cardiol. 2016, 91, 123–133. [Google Scholar] [CrossRef]
- Nanjundappa, R.H.; Wang, R.; Xie, Y.; Umeshappa, C.S.; Chibbar, R.; Wei, Y.; Liu, Q.; Xiang, J. GP120-specific exosome-targeted T cell-based vaccine capable of stimulating DC- and CD4(+) T-independent CTL responses. Vaccine 2011, 29, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yang, J.; Qian, J.; Liu, R.; Huang, E.; Wang, Y.; Luo, F.; Chu, Y. TLR1/TLR2 signaling blocks the suppression of monocytic myeloid-derived suppressor cell by promoting its differentiation into M1-type macrophage. Mol. Immunol. 2019, 112, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Poe, S.L.; Oriss, T.B.; Krishnamoorthy, N.; Yarlagadda, M.; Wenzel, S.E.; Billiar, T.R.; Ray, A.; Ray, P. TLR4/MyD88-induced CD11b+Gr-1 int F4/80+ non-migratory myeloid cells suppress Th2 effector function in the lung. Mucosal. Immunol. 2010, 3, 578–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinetti, T.; Spagnuolo, L.; Mottas, I.; Secondini, C.; Treinies, M.; Rüegg, C.; Hotz, C.; Bourquin, C. TLR7-based cancer immunotherapy decreases intratumoral myeloid-derived suppressor cells and blocks their immunosuppressive function. Oncoimmunology 2016, 5, e1230578. [Google Scholar] [CrossRef] [Green Version]
- Tortola, L.; Jacobs, A.; Pohlmeier, L.; Obermair, F.-J.; Ampenberger, F.; Bodenmiller, B.; Kopf, M. High-Dimensional T Helper Cell Profiling Reveals a Broad Diversity of Stably Committed Effector States and Uncovers Interlineage Relationships. Immunity 2020, 53, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Cantor, H. CD4 T-cell subsets and tumor immunity: The helpful and the not-so-helpful. Cancer Immunol. Res. 2014, 2, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Akpinarli, A.; Maris, C.; Hipkiss, E.L.; Lane, M.; Kwon, E.-K.M.; Muranski, P.; Restifo, N.P.; Antony, P.A. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J. Exp. Med. 2010, 207, 651–667. [Google Scholar] [CrossRef] [Green Version]
- Quezada, S.A.; Simpson, T.R.; Peggs, K.S.; Merghoub, T.; Vider, J.; Fan, X.; Blasberg, R.; Yagita, H.; Muranski, P.; Antony, P.A.; et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 2010, 207, 637–650. [Google Scholar] [CrossRef] [Green Version]
- Mucida, D.; Husain, M.M.; Muroi, S.; van Wijk, F.; Shinnakasu, R.; Naoe, Y.; Reis, B.S.; Huang, Y.; Lambolez, F.; Docherty, M.; et al. Transcriptional reprogramming of mature CD4⁺ helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat. Immunol. 2013, 14, 281–289. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, F.; Meng, F.; Wei, J.; Liu, B. MHC class II restricted neoantigen: A promising target in tumor immunotherapy. Cancer Lett. 2017, 392, 17–25. [Google Scholar] [CrossRef]
- Eisel, D.; Das, K.; Dickes, E.; König, R.; Osen, W.; Eichmüller, S.B. Cognate Interaction with CD4 T Cells Instructs Tumor-Associated Macrophages to Acquire M1-Like Phenotype. Front. Immunol. 2019, 10, 219. [Google Scholar] [CrossRef] [Green Version]
- Schoenberger, S.P.; Toes, R.E.M.; van der Voort, E.I.H.; Offringa, R.; Melief, C.J.M. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature 1998, 393, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Olalekan, S.A.; Cao, Y.; Hamel, K.M.; Finnegan, A. B cells expressing IFN-γ suppress Treg-cell differentiation and promote autoimmune experimental arthritis. Eur. J. Immunol. 2015, 45, 988–998. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Liu, P.; Xu, L.X. The cryo-thermal therapy eradicated melanoma in mice by eliciting CD4 T-cell-mediated antitumor memory immune response. Cell Death Dis. 2017, 8, e2703. [Google Scholar] [CrossRef] [Green Version]
- Keck, S.; Schmaler, M.; Ganter, S.; Wyss, L.; Oberle, S.; Huseby, E.S.; Zehn, D.; King, C.G. Antigen affinity and antigen dose exert distinct influences on CD4 T-cell differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 14852–14857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukamoto, H.; Nishikata, R.; Senju, S.; Nishimura, Y. Myeloid-derived suppressor cells attenuate TH1 development through IL-6 production to promote tumor progression. Cancer Immunol. Res. 2013, 1, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Verma, P.; Verma, R.; Nair, R.R.; Budhwar, S.; Khanna, A.; Agrawal, N.R.; Sinha, R.; Birendra, R.; Rajender, S.; Singh, K. Altered crosstalk of estradiol and progesterone with Myeloid-derived suppressor cells and Th1/Th2 cytokines in early miscarriage is associated with early breakdown of maternal-fetal tolerance. Am. J. Reprod. Immunol. 2019, 81, e13081. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Lenz, L.L.; Harris, R.A. A Breakthrough: Macrophage-Directed Cancer Immunotherapy. Cancer Res. 2016, 76, 513–516. [Google Scholar] [CrossRef] [Green Version]
- Basu, R.; Hatton, R.D.; Weaver, C.T. The Th17 family: Flexibility follows function. Immunol. Rev. 2013, 252, 89–103. [Google Scholar] [CrossRef] [Green Version]
- Veerman, R.E.; Teeuwen, L.; Czarnewski, P.; Akpinar, G.G.; Sandberg, A.; Cao, X.; Pernemalm, M.; Orre, L.M.; Gabrielsson, S.; Eldh, M. Molecular Evaluation of Five Different Isolation Methods for Extracellular Vesicles Reveals Different Clinical Applicability and Subcellular Origin. J. Extracell. Vesicles 2021, 10, e12128. [Google Scholar] [CrossRef]
- Wu, B.; Chen, X.; Wang, J.; Qing, X.; Wang, Z.; Ding, X.; Xie, Z.; Niu, L.; Guo, X.; Cai, T.; et al. Separation and Characterization of Extracellular Vesicles from Human Plasma by Asymmetrical Flow Field-Flow Fractionation. Anal. Chim. Acta 2020, 1127, 234–245. [Google Scholar] [CrossRef]
- Karimi, N.; Cvjetkovic, A.; Jang, S.C.; Crescitelli, R.; Hosseinpour Feizi, M.A.; Nieuwland, R.; Lötvall, J.; Lässer, C. Detailed Analysis of the Plasma Extracellular Vesicle Proteome after Separation from Lipoproteins. Cell Mol. Life Sci. 2018, 75, 2873–2886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möller, A.; Lobb, R.J. The evolving translational potential of small extracellular vesicles in cancer. Nat. Rev. Cancer 2020, 20, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Name | Primer Sequence (5′–3′) |
|---|---|
| GAPDH F | AGGTCGGTGTGAACGGATTTG |
| GAPDH R | GGGGTCGTTGATGGCAACA |
| ITGAV F | CCGTGGACTTCTTCGAGCC |
| ITGAV R | CTGTTGAATCAAACTCAATGGGC |
| ITGB3 F | CCACACGAAACTC |
| ITGB3 R | CTTCAGGTTACATCGGGGTGA |
| ITGAL F | CCAGACTTTTGCTACTGGGAC |
| ITGAL R | GCTTGTTCGGCAGTGATAGAG |
| ICAM1 F | TGCCTCTGAAGCTCGGATATAC |
| ICAM1 R | TCTGTCGAACTCCTCAGTCAC |
| MFGE8 F | CCGCGTCTGGTGACTTCTG |
| MFGE8 R | TCCTCTCTCAGTCTCATTGCAC |
| CXCL10 F | CCAAGTGCTGCCGTCATTTTC |
| CXCL10 R | GGCTCGCAGGGATGATTTCAA |
| CD86 F | GAGCTGGTAGTATTTTGGCAGG |
| CD86 R | GGCCCAGGTACTTGGCATT |
| MHC II F | AGCCCCATCACTGTGGAGT |
| MHC II R | GATGCCGCTCAACATCTTGC |
| IL-1β F | ACAGCAGCACATCAACAAGAG |
| IL-1β R | ATGGGAACGTCACACACCAG |
| IL-12p40 F | TGGTTTGCCATCGTTTTGCTG |
| IL-12p40 R | ACAGGTGAGGTTCACTGTTTCT |
| IL-15 F | AGAGGCCAACTGGATAGATGT |
| IL-15 R | AGAGCACGTTTCTTACTGTTTCA |
| TNFα F | TTCTGTCTACTGAACTTCGGGGTGATCGGTCC |
| TNFα R | GTATGAGATAGCAAATCGGCTGACGGTGTGGG |
| VEGFR2 F | TTTGGCAAATACAACCCTTCAGA |
| VEGFR2 R | GCAGAAGATACTGTCACCACC |
| IL-6 F | GACAAAGCCAGAGTCCTTCAGAGAGATACAG |
| IL-6 R | TTGGATGGTCTTGGTCCTTAGCCAC |
| IL-10 F | GCTCTTACTGACTGGCATGAG |
| IL-10 R | CGCAGCTCTAGGAGCATGTG |
| IL-4R F | CCCCAGCTAGTTGTCATCCTG |
| IL-4R R | CAAGTGATTTTTGTCGCATCCG |
| TGF-β F | CTCCCGTGGCTTCTAGTGC |
| TGF-β R | GCCTTAGTTTGGACAGGATCTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cen, Y.; Lou, Y.; Wang, J.; Wang, S.; Peng, P.; Zhang, A.; Liu, P. Supplementation with Serum-Derived Extracellular Vesicles Reinforces Antitumor Immunity Induced by Cryo-Thermal Therapy. Int. J. Mol. Sci. 2021, 22, 11021. https://doi.org/10.3390/ijms222011021
Cen Y, Lou Y, Wang J, Wang S, Peng P, Zhang A, Liu P. Supplementation with Serum-Derived Extracellular Vesicles Reinforces Antitumor Immunity Induced by Cryo-Thermal Therapy. International Journal of Molecular Sciences. 2021; 22(20):11021. https://doi.org/10.3390/ijms222011021
Chicago/Turabian StyleCen, Yinuo, Yue Lou, Junjun Wang, Shicheng Wang, Peng Peng, Aili Zhang, and Ping Liu. 2021. "Supplementation with Serum-Derived Extracellular Vesicles Reinforces Antitumor Immunity Induced by Cryo-Thermal Therapy" International Journal of Molecular Sciences 22, no. 20: 11021. https://doi.org/10.3390/ijms222011021
APA StyleCen, Y., Lou, Y., Wang, J., Wang, S., Peng, P., Zhang, A., & Liu, P. (2021). Supplementation with Serum-Derived Extracellular Vesicles Reinforces Antitumor Immunity Induced by Cryo-Thermal Therapy. International Journal of Molecular Sciences, 22(20), 11021. https://doi.org/10.3390/ijms222011021